Abstract
Purpose
To determine if extracorporeal shock wave lithotripsy (ESWL) at 60 shock waves (SWs)/min reduces renal damage and hemodynamic impairment compared to treatment at 120 SWs/min.
Materials and Methods
One kidney in each of 19 juvenile pigs (7–8 weeks old) was treated at 120 or at 60 SWs/min (2000 SWs, 24kV) with an unmodified HM-3 lithotripter (Dornier Medical Systems, Kennesaw, Ga, USA). Renal function was determined before and after ESWL treatment by inulin clearance, extraction and clearance of para-aminohippuric acid. Both kidneys were then removed to measure parenchymal lesion size by sectioning the entire kidney and quantifying the size of the hemorrhagic lesion in each slice.
Results
ESWL at 60 SWs/min significantly reduced the size of the acute morphological lesion compared to 120 SWs/min (0.42% vs 3.93% of functional renal volume, (P = 0.011) and blunted the decrease in glomerular filtration rate and renal plasma flow normally seen after treatment at 120 SWs/min.
Conclusions
Treatment at a firing rate of 60 SWs/min produces less morphological injury and causes less alteration in renal hemodynamics than treatment at 120 SWs/min in the pig model of ESWL-induced renal injury.
Keywords: Extracorporeal shock wave lithotripsy, Renal lesion size, Animal models
INTRODUCTION
ESWL produces acute renal injury in most, if not all, patients who receive a shock wave (SW) dose large enough to comminute stones [1,2]. This injury is primarily vascular, causing hemorrhage into the tissue, and can affect a broad range of blood vessels. In some cases, this parenchymal bleeding can be severe enough to produce subcapsular hematomas and more serious consequences such as acute renal failure and even kidney loss [3]. While there have been numerous reports of hematoma formation in ESWL [2,4], there is little agreement on the significance of subcapsular bleeding, as it has been reported that most hematomas resolve with no lasting effect [5]. However, few clinical or experimental studies have attempted to assess the long-term consequences of the vascular injury in ESWL, but those that have indicate that parenchymal hemorrhage is followed by inflammation, leading to scar formation with permanent loss of functional renal tissue [6–8]. In addition to the adverse effects mentioned above, other serious long-term effects of SWL have recently come to light, e.g. new-onset hypertension [9], exacerbation of stone disease after multiple lithotripsy [10,11], and an increased incidence of diabetes mellitus [12]. Because of all these serious acute and long-term consequences related to SW exposure, it is extremely important to find treatment strategies to reduce the renal injury associated with ESWL [13,14].
Previous work has shown that the size of the renal lesion caused by ESWL can be greatly reduced when SWs are delivered at 30 SWs/min [15] instead of at the more common rate of 120 SWs/min. However, slowing the SW delivery rate to 30 SWs/min increases the duration of treatment such that is prohibitively long for use in clinical practice. With this limitation in mind, we assessed the renal response to treatment at 60 SWs/min in a pig model.
MATERIALS AND METHODS
The present study was carried out with an unmodified HM-3 lithotripter (Dornier Medical Systems, Kennesaw, GA, USA) at the Methodist Hospital, Indianapolis, IN, USA. This lithotripter has an 80 nF capacitor and a focal zone (F2) of 1.0–1.2 cm in diameter and 2.5 cm long. Refurbished spark plugs (Healthtronics, Kennesaw, GA, USA) were used for all experiments and were discarded after 1000 shots.
The experimental protocol used in this study was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and was approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine. Nineteen female farm pigs, (7–8 weeks old; Hardin Farms, Danville, IN, USA), were assigned to receive either 2000 SWs at 120 SWs/min (11 pigs) or 2000 SWs at 60 SWs/min (eight). All SWs were delivered at 24 kV. At every 500 SWs the treatment session was briefly halted to check the position of the SW focus (on a lower pole calyx). A small amount of contrast medium (Renografin 60%, Bracco Diagnostics, Princeton, NJ, USA) was injected through the ureteric catheter into the urinary collection system of the kidney being treated. Once the position was confirmed by fluoroscopy, the treatment recommenced.
Surgical procedures for placing the vascular and ureteic catheters were described previously [16], as were procedures for measuring renal function and protocols for the use of the HM-3 lithotripter [17]. Both kidneys were perfusion-fixed in-situ with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH = 7.4)[18] at the end of the experiment, and then removed and submerged in fresh fixative for subsequent determination of lesion size.
Urine and plasma samples were analyzed for inulin and para-aminohippuric acid (PAH) concentrations, as previously described [19]. Inulin clearances, PAH clearance and PAH extraction (EPAH) were determined and used to calculate the GFR and renal plasma flow (RPF). These data were normalized to baseline values and expressed as the mean (SEM) of the percentage decline from baseline renal function. The data were then analyzed by paired t-tests for differences within groups (between baseline and 1 hour values) and two-sample t-tests (between the 120 and 60 SWs/min groups). The criterion for statistical significance was set at P < 0.05.
Kidneys used to measure lesion size were processed according to our previously published protocol [20]. The size of the hemorrhagic lesion in each slice from the SW-treated kidneys was measured and summed to determine the lesion volume as a percent of the total functional volume (FRV). The mean (SEM) was then calculated in each group of pigs, and assessed using the Kruskal-Wallis test, a non-parametric ANOVA for non-normally distributed data, with the criterion for statistical significance at P < 0.05.
RESULTS
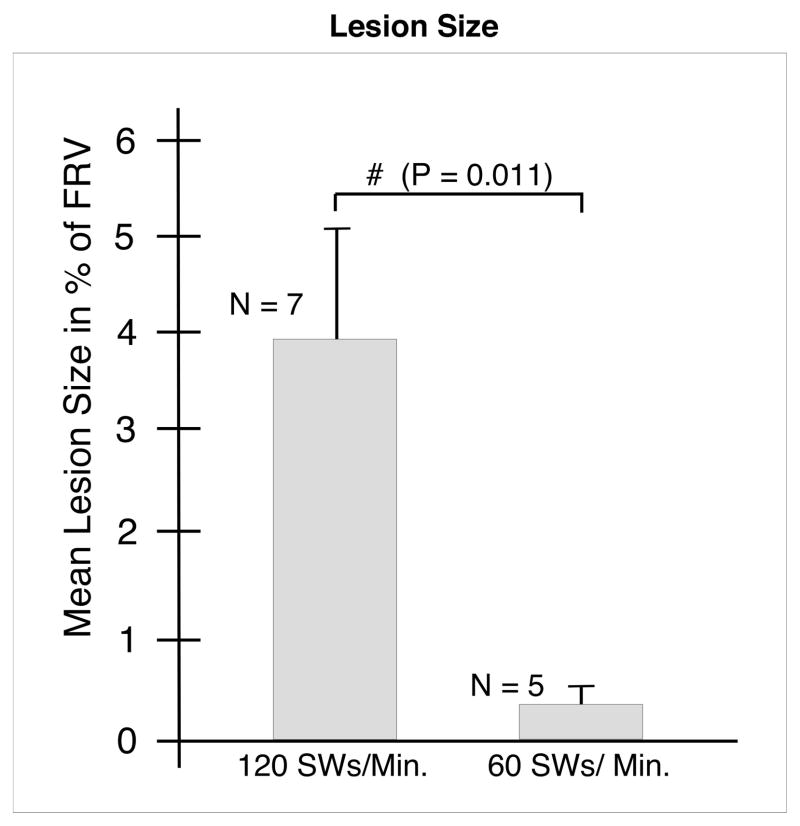
Body weights and baseline blood pressures were not significantly different between the groups of pigs (Table 1). The mean blood pressure was also similar in both groups over the course of the experiment. Figure 1 shows a digitized and colored cross-section of a kidney from each of the two treatment groups. Pigs treated with 120 SWs/min had a mean lesion size of 3.93 (1.29)% FRV (Figure 2). These kidneys had many areas of intraparenchymal bleeding that were localized within the focal volume of the lithotripter and involved both the cortex and medulla (Figure 1). In some cases, the hemorrhage extended all the way from the papillary tip to the renal capsule. All of the treated kidneys in the 120 SWs/min group had subcapsular hematomas. However, only three of eight treated kidneys from pigs in the 60 SWs/min group had surface hematomas. In addition, the sites of intraparenchymal hemorrhage in these kidneys were small, and primarily in the medulla. The lesion size for the 60 SWs/min group was 0.42 (0.23)% FRV (Figure 2), which was significantly smaller than that in the 120 SWs/min group.
Table 1.
Body weight and mean blood pressure (mean ± SEM) of the 120 SWs/minute and 60 SWs/minute treatment groups.
| Group | Body Weight (kg) | Mean Blood Pressure (mmHg) |
|
|---|---|---|---|
| Baseline | + 1 Hour | ||
| 120 SWs/min | 14.8 ± 0.5 | 69.7 ± 11.0 | 66.6 ± 3.0 |
| 60 SWs/min | 13.3 ± 0.5 | 68.2 ± 1.9 | 64.0 ± 1.8 |
FIG. 1.
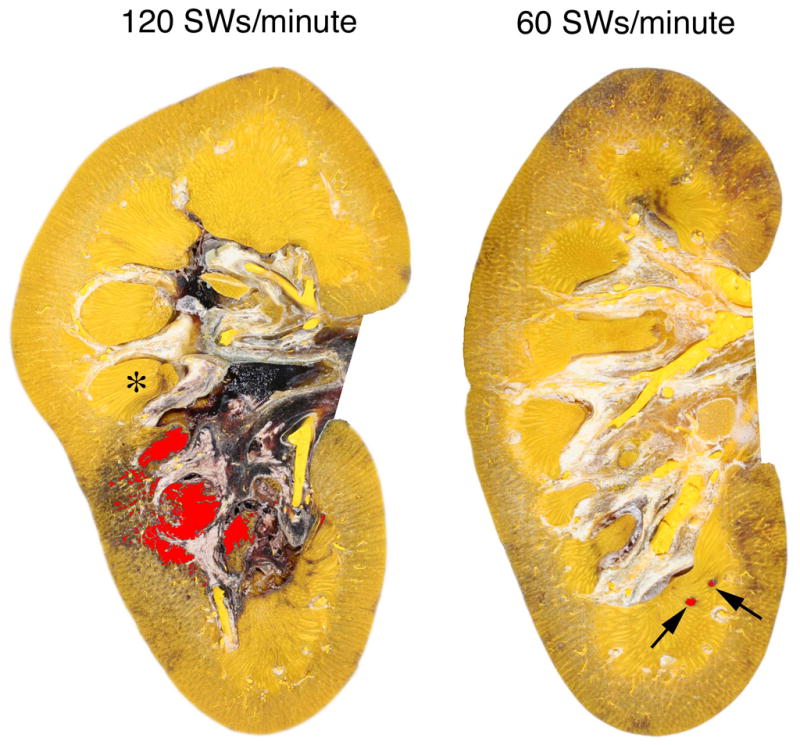
Representative cross-sections from pig kidneys treated at 120 or 60 SWs/min (2000 SWs, 24 kV) with an unmodified Dornier HM-3 lithotripter. The lesions have been coloured (red) to visualize regions of hemorrhage. Quantitation of the lesion was restricted to the renal parenchyma and did not include areas of bleeding into the renal pelvis, seen here as black deposits in the renal pelvicalyceal system. One papilla is marked with an asterisk (*). Damage to the kidney treated at 120 SWs/min involves several renal papillae and extends from the medulla to the cortex. The subcapsular hematoma in this kidney is beyond the plane of section. The lesion in the kidney treated at 60 SWs/min is restricted to small foci in the medulla (arrows).
FIG. 2.
Effect of 2000 SWs at 120 or 60 SWs/min with an unmodified Dornier HM-3 lithotripter on lesion size (percent functional renal volume). The data are expressed as mean ± SEM. N indicates the number of individual kidneys sectioned and quantified in each group. The ampersand (#) indicates a significant difference between the groups.
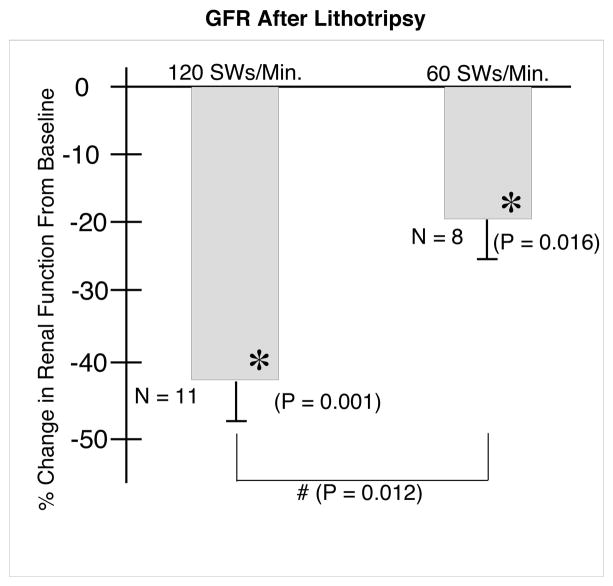
The GFR (Figure 3) decreased significantly (from baseline) in the treated kidneys from both groups at 1 hour after lithotripsy. However, the 19.8 (6.4)% decrease in the 60 SWs/min group was significantly smaller than the 42.2±5.0% decrease in kidneys treated at 120 SWs/min.
FIG. 3.
Effect of 2000 SWs at 120 or 60 SWs/min on glomerular filtration rate. N indicates the number of animals in each group. The asterisks (*) indicates that renal function after ESWL was significantly different in that group from pre-ESWL baseline values. The Ampersand (#) indicates that the two groups were significantly different 1 hour after ESWL.
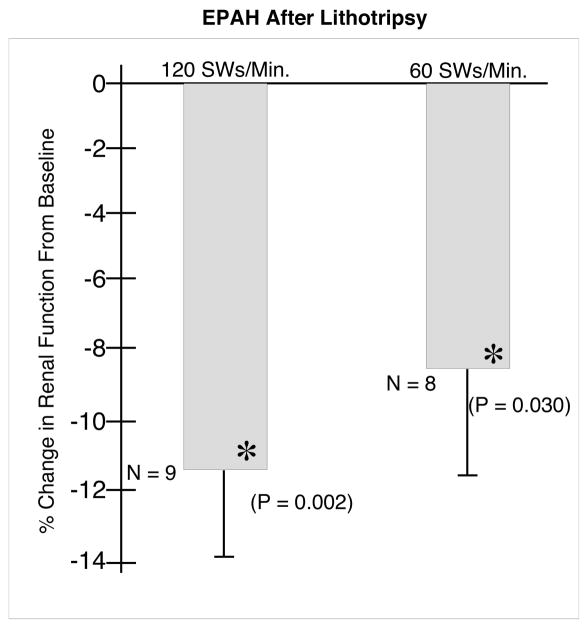
The EPAH (Figure 4) decreased significantly by 8.5 (3.0)% in the kidneys from the 60 SWs/min group at 1 hour after ESWL. This change was not significantly different from the 11.4 (2.3)% decrease in kidneys treated at 120 SWs/min.
FIG. 4.
Effect of 2000 SWs at 120 or 60 SWs/min on EPAH. N indicates the number of animals in each group. The asterisks (*) indicates that renal function was significantly different after ESWL in that group from pre-ESWL baseline values. The two groups were not significantly different from each other 1 hour after ESWL.
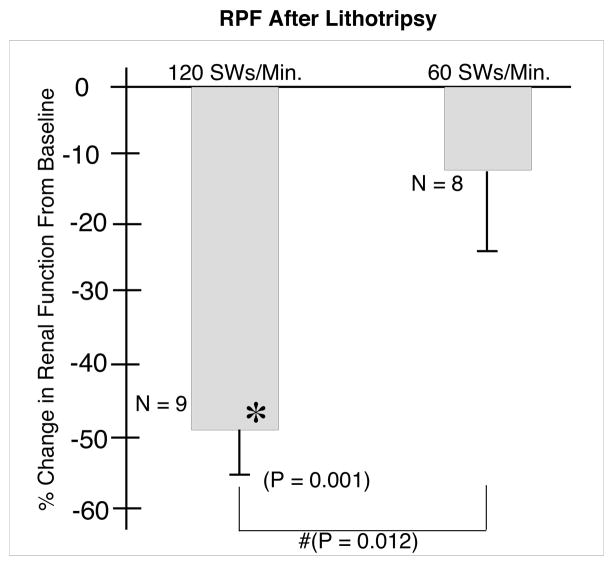
Finally, the RPF (Figure 5) did not change significantly at 1 hour after ESWL in kidneys of the 60 SWs/min group, at −11.9 (10.6)%, vs −47.7 (7.2)% in the kidneys of pigs treated at 120 SWs/min. This response was significantly different from both baseline (P = 0.001) and from the 60 SWs/min group response (P = 0.012).
FIG. 5.
Effect of 2000 SWs at 120 or 60 SWs/min on renal plasma flow. N indicates the number of animals in each group. The asterisks (*) indicates that renal function after ESWL was significantly different in that group from pre-ESWL baseline values. The ampersand (#) indicates that the two groups were significantly different 1 hour after ESWL.
DISCUSSION
The present data show that ESWL at 60 SWs/min significantly reduces the size of the acute hemorrhagic lesion compared to treatment at 120 SWs/min in this porcine model. This result is similar to that previously reported for treatment at 30 SWs/min (0.08% FRV) using the same experimental protocol [15].
While we do not fully understand the mechanism that reduces tissue injury when SW rate is reduced, blood vessel constriction probably plays little or no role in this response. We previously suggested that if vasoconstriction occurs during ESWL, an increase in vessel wall tension would result, which could act to interfere with the cavitation bubble expansion-collapse cycle, thereby reducing the chance of vessel rupture. However, a recent study from our laboratory using measurements of resistive index to monitor renal vasoconstriction in pigs showed that, under condition of continuous SW exposure at 120 SWs/min, vasoconstriction did not occur during SW delivery, but occurred well after ESWL treatment ended [21]. These data suggest that there is no increase in vessel wall tension during the time that SWs are being administered at 120 SWs/min. We suspect, but have not tested, that there will be a similar lack of change in vessel wall tension at 60 SWs/min.
One possible explanation for the decrease in lesion size when SW rate is slowed to 60 SWs/min comes from Freund et al. [22], who used numerical modeling to show that stress can accumulate within tissue if the SW rate exceeds the displacement-relaxation time of that tissue. Their model predicts that shear stress accumulates at SW rates of >≈ 60 SWs/min, and that the degree of shear deformation will be different for different regions of the kidney, such that the renal papilla, particularly the region near the tip of the papilla, will undergo the greatest strain. In previous studies we observed that the renal papilla is more susceptible to SW damage than is the cortex, and this appears to be the case also in the present study [15,23]. The model of Freund et al. proposes a role for a direct effect of SWs on tissue injury, in which shear causes vessel rupture. This initial insult would act to seed sites of hemorrhage where cavitation can occur, in turn leading to expansion of the lesion. The pattern of tissue damage observed in the present study, in which injury at 60 SWs/min was limited to the renal papilla (Fig. 1), seems consistent with such a scenario, but clearly more work is needed to determine the precise mechanisms at play.
In addition to measuring changes in lesion sizes at different SW repetition rates, the present study also sheds light on the functional consequences of treatment at 60 SWs/min. We showed previously that applying 2000 SWs at 120 SWs/min (using same experimental protocol as the present study) with the HM-3 lithotripter significantly reduced GFR, EPAH and RPF [16,23]. The present results indicate that treatment at 60 SWs/min blunts part of this functional impairment. The reduction of GFR at 60 SWs/min was only about half that at 120 SWs/min. In addition, RPF in the 60 SWs/min group did not decrease significantly after ESWL, in contrast to the decline seen after treatment at 120 SWs/min. The mechanism underlying this blunting of the hemodynamic impairment at the lower SW rate is unclear, but might be related to the smaller lesion created at 60 SWs/min or to a difference in the amount of vasoconstriction between different treatment protocols, like that reported previously [21].
While GFR and RPF did not decrease as much with treatment at 60 than 120 SWs/min, the decline in EPAH was comparable between the groups. ESWL at 60 SWs/min did not reduce the impairment in EPAH despite the fact that the hemorrhagic lesion was greatly reduced in that group. This observation would suggest that changes in EPAH are independent of measures of hemorrhagic lesion size. This disparity between lesion size and functional decline might be related to the way lesion size is determined. Calculations of EPAH are based on measurements of tubular epithelial cell transport function, while measurements of ESWL-induced lesion size are based on the histological detection of blood in the parenchyma. As such, there might be injury to tubular cells and transporters unrelated to vascular hemorrhage, and thus undetectable by our morphological techniques.
There is added benefit to treatment at reduced SW rate. Several prospective clinical trials showed better stone breakage at 60 than 120 SWs/min [24–26]. These findings are further supported by an independent meta-analysis concluding that despite differences in methods for assessing success, and that different lithotripters were used in the various studies, treatment outcomes were significantly better at 60 than at 120 SWs/min [27].
In conclusion, the present findings show that a SW rate of 60 SWs/min greatly reduces the acute morphological injury associated with lithotripsy, and blunts the reductions in GFR and RPF that occur with treatment at 120 SWs/min. These observations, in a well-established animal model of ESWL-induced renal injury, suggest that slowing the SW rate to 60 SWs/min is a viable strategy to improve the safety of ESWL.
Acknowledgments
This project was supported by grants from the National Institutes of Health (P01-DK43881 and R01-DK67133). The authors are indebted to Kelli Barns and Cynthia Johnson for their expert assistance.
References
- 1.Evan AP, Willis LR, Connors BA, McAteer JA, Lingeman JE. Renal injury by extracorporeal shock wave lithotripsy. J Endourol. 1991;5:25–35. [Google Scholar]
- 2.Kaude JV, Williams CM, Millner MR, Scott JN, Finlayson B. Renal morphology and function immediately after extracorporeal shock wave lithotripsy. Am J Roentgenol. 1985;145:305–13. doi: 10.2214/ajr.145.2.305. [DOI] [PubMed] [Google Scholar]
- 3.McAteer JA, Evan AP. The acute and long-term adverse effects of shock wave lithotripsy. Semin Nephrol. 2008;28:200–13. doi: 10.1016/j.semnephrol.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orozc Fainas R, Iglesias Prieto JI, Massarrah Halabi J, Mancebo Gomez JM, Perez-Castro Ellendt E. Renal hematoma after extracorporeal shockwave lithotripsy in a series of 324 consecutive sessions with the Doli-S lithotripter: incidents, characteristics, multifactorial analysis and review. Arch Esp Urol. 2008;61:889–914. doi: 10.4321/s0004-06142008000800006. [DOI] [PubMed] [Google Scholar]
- 5.Krishnamurthi V, Streem SB. Long-term radiographic and functional outcome of extracorporeal shock wave lithotripsy induced perirenal hematomas. J Urol. 1995;154:1673–5. [PubMed] [Google Scholar]
- 6.Morris JS, Husmann DA, Wilson WT, et al. A comparison of renal damage induced by varying modes of shock wave generation. J Urol. 1991;145:864–867. doi: 10.1016/s0022-5347(17)38479-3. [DOI] [PubMed] [Google Scholar]
- 7.Lechevallier E, Siles S, Ortega JC, Coulange C. Comparison by SPECT of renal scars after extracorporeal shock wave lithotripsy and percutaneous nephrolithotomy. J Endourol. 1993;7:465–7. doi: 10.1089/end.1993.7.465. [DOI] [PubMed] [Google Scholar]
- 8.Evan AP, Willis LR, Lingeman JE, McAteer JA. Renal trauma and the risk of long-term complications in shock wave lithotripsy. Nephron. 1998;78:1–8. doi: 10.1159/000044874. [DOI] [PubMed] [Google Scholar]
- 9.Janetschek G, Frauscher F, Knapp R, Hofle G, Peschel R, Bartsch G. New onset hypertension after extracorporeal shock wave lithotripsy: age related incidence and prediction by intrarenal resistive index. J Urol. 1997;158:346–51. doi: 10.1016/s0022-5347(01)64475-6. [DOI] [PubMed] [Google Scholar]
- 10.Parks JH, Worcester EM, Coe FL, Evan AP, Lingeman JE. Clinical implications of abundant calcium phosphate in routinely analyzed kidney stones. Kidney Int. 2004;66:777–85. doi: 10.1111/j.1523-1755.2004.00803.x. [DOI] [PubMed] [Google Scholar]
- 11.Parks JH, Coe FL, Evan AP, Worcester EM. Urine pH in renal calcium stone formers who do and do not increase stone phosphate content with time. Nephrol Dial Transplant. 2009;24:130–6. doi: 10.1093/ndt/gfn420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krambeck AE, Gettman MT, Rohlinger AL, Lohse CM, Patterson DE, Sequra JW. Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years followup. J Urol. 2006;175:1742–7. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 13.McAteer JA, Evan AP, Willis LR, et al. Shock wave injury to the kidney in SWL: review and perspective. In: Evan AP, Lingeman JE, Williams JC, editors. Renal Stone Disease: Proceedings of the First International Urolithiasis Research Symposium. Vol. 900. Melville, New York: American Institute of Physics Conference Proceedings; 2007. pp. 287–301. [Google Scholar]
- 14.McAteer JA, Evan AP, Willis LR, et al. Treatment protocols to reduce injury and improve stone breakage in SWL. In: Evan AP, Lingeman JE, Williams JC, editors. Renal Stone Disease 2: Proceedings of the Second International Urolithiasis Research Symposium. Vol. 1049. Melville, New York: American Institute of Physics Conference Proceedings; 2008. pp. 243–8. [Google Scholar]
- 15.Evan AP, McAteer JA, Connors BA, Blomgren PM, Lingeman JE. Renal injury during shock wave lithotripsy is significantly reduced by slowing the rate of shock wave delivery. BJU Int. 2007;100:624–8. doi: 10.1111/j.1464-410X.2007.07007.x. [DOI] [PubMed] [Google Scholar]
- 16.Willis LR, Evan AP, Connors BA, Blomgren PM, Fineberg NS, Lingeman JE. Relationship between kidney size, renal injury, and renal impairment induced by shock wave lithotripsy. J Am Soc Nephrol. 1999;10:1753–62. doi: 10.1681/ASN.V1081753. [DOI] [PubMed] [Google Scholar]
- 17.Evan AP, McAteer JA, Connors BA, et al. Independent assessment of a wide-focus, low-pressure electromagnetic lithotripter: absence of renal bioeffects in the pig. BJU Int. 2007;101:382–8. doi: 10.1111/j.1464-410X.2007.07231.x. [DOI] [PubMed] [Google Scholar]
- 18.Shao Y, Connors BA, Evan AP, Willis LR, Lifshitz DA, Lingeman JE. Morphological changes induced in the pig kidney by extracorporeal shock wave lithotripsy: nephron injury. Anat Rec. 2003;275A:979–89. doi: 10.1002/ar.a.10115. [DOI] [PubMed] [Google Scholar]
- 19.Willis LR, Evan AP, Connors BA, Handa RK, Blomgren PM, Lingeman JE. Prevention of lithotripsy-induced renal injury by pretreating kidneys with low-energy shock waves. J Am Soc Nephrol. 2006;17:663–73. doi: 10.1681/ASN.2005060634. [DOI] [PubMed] [Google Scholar]
- 20.Blomgren PM, Connors BA, Lingeman JE, Willis LR, Evan AP. Quantitation of shock wave lithotripsy-induced lesion in small and large pig kidneys. Anat Rec. 1997;249:341–8. doi: 10.1002/(SICI)1097-0185(199711)249:3<341::AID-AR4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 21.Handa RK, Bailey MR, Paun M, et al. Pretreatment with low-energy shock waves induces renal vasoconstriction during standard SWL: a treatment protocol known to reduce lithotripsy-induced renal injury. BJU Int. 2008 doi: 10.1111/j.1464–410X.2008.08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freund JB, Colonius T, Evan AP. A cumulative shear mechanism for tissue damage initiation in shock-wave lithotripsy. Ultrasound Med Biol. 2007;33:1495–503. doi: 10.1016/j.ultrasmedbio.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connors BA, Evan AP, Willis LR, Blomgren PM, Lingeman JE, Fineberg NS. The effect of discharge voltage on renal injury and impairment caused by lithotripsy in the pig. J Am Soc Nephrol. 2000;11:310–8. doi: 10.1681/ASN.V112310. [DOI] [PubMed] [Google Scholar]
- 24.Madbouly K, El-Tiraifi AM, Seida M, El-Faqih SR, Atassi R, Talic RF. Slow versus fast shock wave lithotripsy rate for urolithiasis: a prospective randomized study. J Urol. 2005;173:127–30. doi: 10.1097/01.ju.0000147820.36996.86. [DOI] [PubMed] [Google Scholar]
- 25.Pace KT, Ghiculete D, Harju M, Honey RJ. Shock wave lithotripsy at 60 or 120 shocks per minute: a randomized, double-blind trial. J Urol. 2005;174:595–9. doi: 10.1097/01.ju.0000165156.90011.95. [DOI] [PubMed] [Google Scholar]
- 26.Kato Y, Yamaguchi S, Hori J, Okuyama M, Kakizaki H. Improvement of stone comminution by slow delivery rate of shock waves in extracorporeal lithotripsy. Int J Urol. 2006;13 :1461–5. doi: 10.1111/j.1442-2042.2006.01609.x. [DOI] [PubMed] [Google Scholar]
- 27.Semins MJ, Trock BJ, Matlaga BR. The effect of shock wave rate on the outcome of shock wave lithotripsy: a meta-analysis. J Urol. 2008;179:194–7. doi: 10.1016/j.juro.2007.08.173. [DOI] [PubMed] [Google Scholar]