Abstract
Song learning, maintenance and production require coordinated activity across multiple auditory, sensory-motor, and neuromuscular structures. Telencephalic components of the sensory-motor circuitry are unique to avian species that engage in song learning. The song system shows protracted development that begins prior to hatching but continues well into adulthood. The staggered developmental timetable for construction of the song system provides clues of subsystems involved in specific stages of song learning and maintenance. Progressive events, including neurogenesis and song system growth, as well as regressive events such as apoptosis and synapse elimination, occur during periods of song learning and the transitions between stereotyped and variable song during both development and adulthood. There is clear evidence that gonadal steroids influence the development of song attributes and shape the underlying neural circuitry. Some aspects of song system development are influenced by sensory, motor and social experience, while other aspects of neural development appear to be experience-independent. Although there are species differences in the extent to which song learning continues into adulthood, growing evidence suggests that despite differences in learning trajectories, adult refinement of song motor control and song maintenance can require remarkable behavioral and neural flexibility reminiscent of sensory-motor learning.
Introduction
Vocal learning is remarkably similar in songbirds and humans. In both cases, the capacity to learn is constrained by inborn predispositions, relies on hearing, is modulated by social context, and wanes with increasing age. Moreover, even in songbirds that do not normally change their songs in adulthood, the maintenance of song stereotypy, as with speech, is an active process dependent on hearing. Finally, vocal learning in birds and humans relies on a specialized series of brain regions (Doupe and Kuhl, 1999; Kuhl, 2003).
Research over the past two decades has revealed that a substantial amount of avian brain development occurs after hatching and continues well into adulthood. This provides an exceptional opportunity to relate specific developmental neurobiological events to the acquisition and expression of learned vocal behavior. This developmental timetable also raises the possibility that the building and refinement of avian vocal control circuitry is dependent on vocal experience, and there is ample evidence that this is at least partially true. However, growing evidence also suggests that this plasticity is constrained in important ways. The purpose of this review is to provide an outline of what is known about key aspects of brain development as they relate to song learning. While the list of species in which song learning and brain development have been explored is increasing, the most comprehensive analyses have been done on the zebra finch and canary and so much of the work that will be described comes from these two species. However, comparative work incorporating more species has begun to provide important new insights and so, where appropriate, this work will be cited as well.
Timetable for song learning
In nearly all oscine songbirds that have been examined, song learning begins when juveniles memorize the songs of one or more adults. Auditory memories are then used to guide vocal motor development during a sensory-motor stage. The sensory-motor phase progresses through several stages beginning with sub-song, which bears little resemblance to adult song, followed by a plastic song stage where individual notes or syllables can be recognized but are highly variable from one song rendition to the next, followed finally by song crystallization, where notes and, in some species, note sequences, are sung in a highly stereotyped manner (see Williams, 2008 for review).
All songbirds studied to date require auditory experience for normal song development and in some species, exposure to song must occur during an early critical period for it to be copied (Konishi, 2004). There is also evidence for a critical period during which vocal motor practice is essential in the zebra finch (Pytte and Suthers, 2000). Among songbirds, canaries and zebra finches may represent the extremes on a continuum in terms of song learning. While in both species, song is predominantly or exclusively produced by males, and in both species song is initially learned during juvenile life, these species differ with respect to learning trajectories. The zebra finch is often referred to as “close-ended” or “age-limited” in that most of song development is over by 90-120 days after hatching, around the time birds reach sexual maturity (Immelmann, 1969; Arnold, 1975). Zebra finch song is comprised of one to several short, introductory notes, followed by 4-7 individually distinct and more complex ‘syllables’ or note complexes, which are typically produced without repetition and in a fixed sequence. This comprises the bird’s “motif”, which can be sung one-to-several times in rapid succession (referred to as a song bout). After puberty, there is very little change in the acoustic structure of individual notes, but occasional variation in note number and sequence persists (Nordeen and Nordeen, 1992, 1993; Williams and Mehta, 1999; Lombardino and Nottebohm, 2000; Brainard and Doupe, 2001; Williams, 2004; Glaze and Troyer, 2006). In contrast, the canary is considered an “open-ended” learner. Canaries sing up to 30-40 distinct notes, repeating each several times before transitioning to another, and note sequence is not fixed (Guttinger, 1985; Nottebohm et al., 1986). Canaries go through a similar early learning sequence (Marler and Waser, 1977; Marler, 1997), but the process of remodeling song continues throughout life on a seasonal basis. Canaries as old as 4-5 years have been known to learn new song from conspecifics (Guttinger, 1979). Song is remodeled by the addition of new syllables as well as the loss or modification of pre-existing syllables. Canaries also retain some syllables from previous years and there may be strain differences in the amount of new song material learned each year (Nottebohm et al., 1986; Leitner et al., 2001; Voigt and Leitner, 2008).
The open-ended versus age-limited classification of these two species is somewhat inaccurate. While it is true that unlike canaries, normally raised zebra finches cannot (or will not) learn to produce the songs of other conspecifics after song crystallization (Eales, 1985; Zevin et al., 2004), zebra finch song note stereotypy increases with adult age, (Pytte et al., 2007) and relies on auditory feedback (Nordeen and Nordeen, 1992; Wang et al., 1999; Brainard and Doupe, 2000a; Lombardino and Nottebohm, 2000; to be discussed in more detail later). Adult song refinement and maintenance may reflect an active process reminiscent of the later stages of juvenile song learning, whereby slight deviations from a target song are corrected based on auditory feedback. In support of this idea, recent work has shown that naturally occurring drift in adult zebra finch song is corrected by re-exposure to the original tutor song (Funabiki and Funabiki, 2008) and that adult zebra finches will alter note pitch to avoid masking white noise (Tumer and Brainard, 2007; also see Sober and Brainard, 2009) Thus, while zebra finches will normally not learn new songs as adults, it is likely that sensory-motor matching and error correction, used in the later stages of juvenile song development, continue well into adulthood. At the other end of the spectrum, song modification in the open-ended canary appears to decrease with adult age (Nottebohm et al., 1986) and a relatively large fraction of the syllables produced in a breeding season were learned at some earlier (perhaps juvenile?) stage in life (Nottebohm et al., 1986; Leitner et al., 2001; Voigt and Leitner, 2008). Although it is clear that adult vocal plasticity in the canary exceeds that in the zebra finch, it seems equally clear that zebra finches retain vocal flexibility after puberty. Moreover, although canaries do modify songs annually, they recycle some previously learned notes. From these behavioral insights, one might also expect more similarities in adult vocal control system plasticity than is implied by the age-limited/open ended labels.
The Vocal Control System
Arguably the single most important discovery in the history of the songbird field, from a neuroethologist’s perspective, was that there is a discrete series of brain regions dedicated to song learning and production (Nottebohm et al., 1976). This provided an unprecedented opportunity to explore the neurobiological control of a single, well-defined and learned behavior in endothermic vertebrates. Subsequent work has added important brain regions to this circuitry and refined functional distinctions. The vocal control system as well as important auditory structures is illustrated in Figure 1. A timetable for the development of this complete series of brain regions is lacking and so, for the purpose of this review, the focus will be restricted to a few key structures essential for vocal learning and song production. One major pathway, often referred to as the ‘motor’ pathway, includes two pallial (cortical) structures, HVC (used as a proper name) and RA (robust nucleus of the arcopallium). HVC projects to RA and neurons of this type (HVC⇒RA) make up approximately 60% of all HVC neurons (Alvarez-Buylla et al., 1988; Kirn et al., 1991; Kirn et al., 1999). In turn, RA projects directly and indirectly onto brain stem regions innervating respiratory motor neurons, as well as motor neurons innervating the vocal musculature (syrinx). The ‘motor’ designation is based in part on this anatomical configuration, the fact that the temporal pattern of activity in this pathway closely maps onto and precedes sound production, and also on the fact that lesions at any point within this circuit disrupt adult song production, although deficits vary based on lesion placement (Margoliash, 1997). A second major circuit, referred to as the anterior forebrain pathway (AFP), is analogous to basal ganglia-cortico-thalamic circuitry in mammals (reviewed by (Bottjer, 2004). This pathway begins with HVC and a cell population distinct from that synapsing on RA. This latter population (HVC⇒X) projects to a medial striatal region, Area X, which, in turn, projects to the thalamus (DLM, dorsal lateral nucleus of the medial thalamus). DLM then projects to a cortical region called LMAN (lateral magnocellular nucleus of the anterior nidopallium). LMAN projection neurons have bifurcating axons, with one terminal field in Area X and the other in RA (Vates and Nottebohm, 1995). A key feature of these two pathways is that they both have efferent projections originating in HVC that directly or indirectly converge on the dendrites of neurons in RA (Canady et al., 1988; Herrmann and Arnold, 1991; Mooney and Konishi, 1991). Lesions within the AFP have modest, if any, effects on fully stereotyped song but severely disrupt vocal development (Bottjer et al., 1984; Sohrabji et al., 1990; Scharff and Nottebohm, 1991; Nordeen and Nordeen, 1993; Williams and Mehta, 1999; Kao et al., 2005).
Figure 1.
Sagittal views of the major brain regions involved in song learning and production. While there is considerable overlap in the structures depicted in each schematic, the one on the left is designed to emphasize key structures involved in song vocal control whereas the diagram on the right illustrates important auditory structures. Left schematic: The pathway necessary for the production of learned song is demarcated by solid black lines. The anterior forebrain pathway, necessary for song learning, is shown with dashed lines, and dashed lines illustrate sites of convergence between the two pathways. Right Schematic: Key ascending auditory pathways are shown. Abbreviations: AV-nucleus avalanche; CLM-caudal lateral mesopallium; CMM-caudal medial mesopallium; CN-cochlear nucleus; CStcaudal striatum; DM-dorsal medial nucleus; DLM-medial portion of the dorsolateral nucleus of the thalamus; E-entopallium; B-basorostralis; HVC-high vocal center; LLD-lateral lemniscus, dorsal nucleus; LLI-lateral lemniscus, intermediate nucleus; LLV-lateral lemniscus, ventral nucleus; MLd-dorsal lateral nucleus of the mesencephalon; LMAN-lateral subdivision of the magnocellular nucleus of the anterior nidopallium; mMan-medial subdivision of the magnocellular nucleus of the anterior nidopallium; AreaX-Area X of the medial striatum; MO-oval nucleus of the mesopallium; NCM-caudal medial nidopallium; NIF-interfacial nucleus of the nidopallium; nXIIts-XII, tracheosyringeal part; OV-ovoidalis; PAm-paraambiguus; Ram- retroambigualis; RA-robust nucleus of the arcopallium; SO-superior olive; Uva-nucleus uvaeformis;. Nomenclature based on Reiner et al., in press. nAM-nucleus ambiguous. Adapted from (Reiner et al., 2004).
Based on the results from early lesion studies, there has been a strong tendency to parse the two pathways just described into a song production pathway and a learning pathway. While these labels accurately describe essential features of song that require the integrity of the two pathways, several lines of evidence suggest that both pathways are important for sensory-motor learning and song maintenance. For example, there is evidence that both pathways are involved in song production. Neurons throughout both pathways exhibit song-related pre-motor activity and acute, micro-stimulation applied to either pathway during singing alters song structure (Vu et al., 1994; Yu and Margoliash, 1996; Hessler and Doupe, 1999; Kao et al., 2005). There is also evidence that both pathways serve auditory functions, perhaps related to song learning and maintenance. Under appropriate conditions, neurons in both pathways respond to sound stimuli (reviewed by Doupe et al., 2004) and this is even true of the brainstem motor neurons innervating the syrinx (Williams and Nottebohm, 1985). Moreover, many neurons in both pathways are most responsive to playbacks of the bird’s own song (BOS) and in RA, this attribute is dependent on input from HVC and not LMAN (Doupe and Konishi, 1991; Vicario and Yohay, 1993). Further blurring the distinction, the very same neurons that exhibit BOS auditory preferences also display song-related pre-motor activity (Mooney, 2000; Rosen and Mooney, 2003) an attribute that has received new interest due to its similarities to the properties of “mirror neurons” in primates (Prather et al., 2008; Striedter and Charvet, 2008). Finally, select subtypes of neurons in each pathway (to be discussed later) are replaced throughout life-a process that may facilitate learning. Thus, both pathways may contain multiple sensory-motor representations of song and exhibit substantial neural plasticity. Although this poses challenges in terms of delineating the specific functions of different brain regions, it probably also points to the need for multiple stages of sensory-motor computation during vocal learning and production.
Development of the vocal control system
Much work has been devoted to delineating the timetable for construction of vocal control circuitry. This research has been driven by an interest in relating specific developmental neurobiological events to particular stages of song learning or maintenance. In a broader sense, this research may provide clues for how developmental programs have been altered in order to create a vocal control system that is present in songbirds but absent or much diminished in birds that do not learn their species-typical vocalizations.
A. Neurogenesis
The vocal control circuitry represents a network of cell populations distributed throughout the brain. It is, therefore, not surprising that the timetable for production of neurons destined for different vocal control nuclei spans a broad range of ages, from midembryonic stages to adulthood (Figure 2; Alvarez-Buylla et al., 1994). Although a complete characterization of the timetable for production of neurons destined for all nuclei implicated in vocal control is lacking, to date two general trends can be seen. On a coarse temporal scale, the relative timing of neuron production for a particular song system region follows that of surrounding tissue. For example most thalamic regions as well as arcopallium are generated prior to hatching, as is true for most neurons in the embedded song control structures DLM and RA, respectively. In contrast, much of the striatum and nidopallium are generated after hatching as is true for Area X and HVC, respectively. However, at a more fine-grained temporal scale, neuron production for some vocal control regions is delayed or protracted compared to surrounding tissue. This is most evident for DLM, but can also be observed in HVC (Alvarez-Buylla and Ling, 1991; Alvarez-Buylla et al., 1994). These results suggest that the evolution of vocal control regions may have been constrained by the timetable for the generation of major diencephalic and telencephalic subdivisions, but also that delayed or protracted neurogenesis relative to these regions may have contributed to the emergence of the vocal control system.
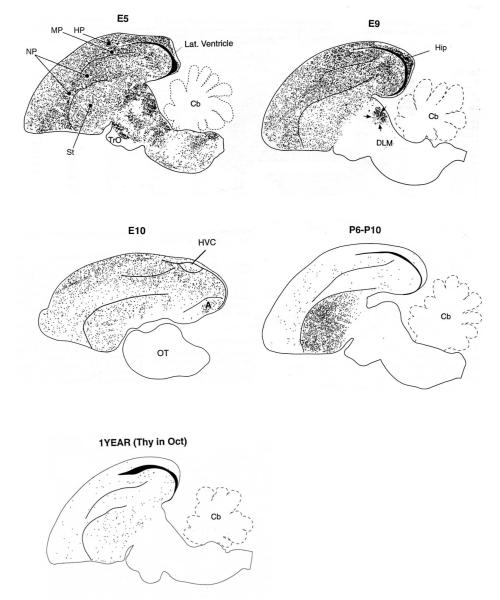
Figure 2.
The distribution of [3H]-labelled neurons in sagittal brain sections from 1-year old canaries following [3H]-thymidine injections at the embryonic (E) ages indicated. The lower map is from a 1-year old adult that received [3H]-thymidine injections in October and was then killed 125 days later. Although there is an overall decrease in neurogenesis (cell production + survival) as development proceeds, the absolute magnitude of the decrease cannot be ascertained from these figures due to several methodological factors, including variation in the amount of time [3H]-thymidine was available for cell labeling (see Alvarez-Buylla et al., 1994). However, developmental shifts in the regional distribution of new neurons are significant. Between E5 and E9, sub-telencephalic neuron addition is virtually over with the notable exception of DLM (medial portion of the dorsolateral nucleus of the thalamus), part of the vocal control system. Injections on E10 labelled cells restricted to the telencephalon, yet few such cells were found in HVC. As with DLM, HVC neurogenesis is delayed relative to surrounding regions. Post-hatching (P6-P10), large numbers of neurons are added to the striatum, including song system nucleus Area X (not shown). The most notable difference between birds injected as juveniles and adults is a greater decline in striatal, relative to extra-striatal, neuron addition in older birds. Nevertheless, neuron addition is widespread even in adults. Dorsal = up and Rostral = left, Modified from (Alvarez-Buylla et al., 1994).
Cells lining the walls of the lateral ventricles give rise to all telencephalic neurons (Goldman and Nottebohm, 1983; Alvarez-Buylla and Nottebohm, 1988; Alvarez-Buylla et al., 1998), and an important question that has not been fully addressed is whether vocal control neurons are formed in discrete locations. In the extreme, is it possible that all telencephalic vocal control neurons arise in the same location within the ventricular zone? This simple model is not supported by the limited data available, at least for neurons generated in juveniles and adults (Stellitano et al., 2003; Scott and Lois, 2007). A complete fate map of the vocal control system may shed important insights concerning the developmental-evolutionary origins of this unique circuitry and the capacity for avian vocal learning.
There is a general trend for projection neurons in the AFP to be produced early. As already mentioned, DLM neurons are formed prior to hatching, as is true for HVC X neurons and neurons destined for LMAN (Alvarez-Buylla et al., 1988; Alvarez-Buylla and Ling, 1991; Alvarez-Buylla and Kirn, 1997). This can be contrasted with the HVC⇒RA neurons, which are generated almost exclusively after hatching (Alvarez-Buylla et al., 1988; Nordeen and Nordeen, 1988; Alvarez-Buylla et al., 1990).
B. Establishment of connectivity
The timetable for the establishment of connections within the vocal control system follows that for neurogenesis. One of the most unanticipated findings was that neurons within the HVC⇒RA pathway, so critical for song motor control (Scharff et al., 2000), are first produced and establishing connections with RA after song vocal development begins and incorporation of this cell type continues throughout song learning in both zebra finch and canary (Fig. 3). Acquisition of a song auditory template (sensory learning) begins at 20-25 days after hatching in the zebra finch and the sensory-motor stage, when birds use auditory memories to guide vocal motor development from sub-song to plastic song and, finally song crystallization, spans the ages of 30-90 days (Immelmann, 1969). In the zebra finch, HVC⇒RA axon terminals do not begin to innervate RA until 30-35 days after hatching (Konishi and Akutagawa, 1985; Mooney and Rao, 1994). In contrast, many of the connections between AFP nuclei develop substantially earlier. For example, HVC⇒X neurons have already formed connections with Area X neurons by day 20 after hatching (Mooney and Rao, 1994). Indeed, many connections in the HVC⇒Area⇒X⇒DLM⇒LMAN⇒RA pathway are in place by day 20, although development of this circuit is far from complete at this age (Johnson and Bottjer, 1992; Mooney, 1992; Sohrabji et al., 1993). These results suggest that the AFP is critically important for early stages of song development.
Figure 3.
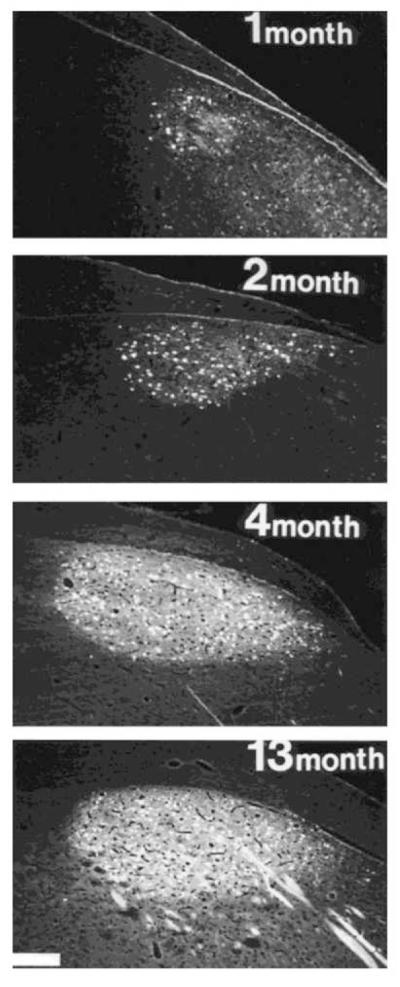
Much of the development of the efferent, HVC⇒RA pathway occurs over the time course of song learning. At the ages indicated, the HVC⇒RA pathway was retrogradely labeled (white) by injecting Fluoro-Gold into RA in male canaries. By 7-12 months, canary song has become fully stereotyped for the first time (Nottebohm and Nottebohm, 1978; Nottebohm et al., 1986). Dorsal = up, Rostral = right. Scale bar = 200μm.
A key question is whether the AFP is the locus of the auditory template used to guide vocal motor development (Doupe et al., 2004; Theunissen et al., 2004). Electrophysiological studies have revealed some AFP cells as well as cells in HVC in juveniles that are equally responsive to tutor song and the bird’s own song (BOS) but cells exclusively responsive to tutor song are rare (Volman, 1993; Solis and Doupe, 2000). Growing evidence suggests that the forebrain auditory area NCM may be one region involved specifically in template formation or storage. Many NCM cells respond preferentially to tutor song and not to BOS and the strength of the response to tutor song correlates with the degree to which birds copy tutor song (Phan et al., 2006). Similar results have been observed using immediate early gene (IEG) expression as a marker of neuronal activity (Bolhuis and Gahr, 2006), and recent work indicates that disruption of IEG expression in NCM and surrounding auditory structures during song tutoring prevents young zebra finches from copying tutor song, without altering performance on auditory perception tasks (London and Clayton, 2008). It is possible that sensory template information is stored in NCM and related auditory structures which relay this information to HVC and the AFP where it is used to guide sensory-motor learning in young juveniles (Bolhuis and Gahr, 2006; Phan et al., 2006).
There is strong evidence that the AFP is critical for much of the sensory-motor stage of song development. This has been confirmed by the findings that lesions of LMAN or Area X prevent normal song development (Bottjer et al., 1984; Sohrabji et al., 1990; Scharff and Nottebohm, 1991). More recent work extends these observations. HVC lesions or transection of HVC⇒RA axons before post hatch day 45 have little effect on juvenile subsong morphology. In contrast, electrolytic lesions or pharmacological inactivation of LMAN before 45 days of age abolishes subsong (Aronov et al., 2008). It is worth noting that HVC lesions would destroy HVC⇒Area X projection neurons, which represent the origin of the AFP. Apparently these cells are not critical for subsong. LMAN inactivation after 45 days of age has more modest effects whereas HVC lesions or transection of the HVC⇒RA pathway interfere with the final stages of song development (Aronov et al., 2008). These results suggest that the earliest song-related vocalizations are controlled by a pathway partially distinct from circuits involved in later stages of song development and mature song production (Aronov et al., 2008). In turn, these results are consistent with the staggered timetable for the development of connectivity within the AFP relative to the HVC⇒RA pathway.
These results point to the development of RA and the confluence of inputs from HVC and LMAN as a locus for synaptic events critical to song learning and maintenance. Synaptic terminals from HVC and LMAN intermingle on the dendrites of RA neurons (Canady et al., 1988; Herrmann and Arnold, 1991) One characteristic of the transition from song development to song stereotypy appears to be a shift in the weighting of RA synapses from these two sources of input. Changes in the weighting of inputs to RA from HVC and LMAN during development are due to dramatic postnatal changes in neuron and synaptic number. There is considerable growth of some song control nuclei after hatching. For example HVC and RA volume nearly triple in size between post-hatch days 12 and 53 in male zebra finches (Bottjer et al., 1985; Bottjer and Arnold, 1997). Increases in HVC size are largely due to protracted neurogenesis (Nordeen and Nordeen, 1988; Kirn and DeVoogd, 1989; Alvarez-Buylla et al., 1992). Few neurons are added to RA post hatch in zebra finches and canaries and so RA growth is due, predominantly, to increases in neuron size and spacing (Kirn and DeVoogd, 1989; Konishi and Akutagawa, 1990). Thus, over the course of song learning the number of HVC neurons, including HVC⇒RA neurons increases dramatically relative to the number of target RA neurons, suggesting progressive increases in RA synapses derived from HVC neurons, a finding supported by ultrastructural counts of synapses (Herrmann and Arnold, 1991). In contrast, LMAN volume decreases dramatically over the same interval. Decreases in LMAN volume are partially due to apoptosis but also the refinement of thalamic input, which initially extends to regions surrounding LMAN, becoming topographically more restricted with increasing age (Bottjer, 2004). Concurrently, dendritic spines on LMAN neurons as well as LMAN axon branches within RA are pruned (Iyengar and Bottjer, 2002a) and LMAN derived synapses in RA are reduced by roughly 80% (Hermann & Arnold, 1991).
These results suggest that inputs to RA from LMAN and HVC are in a dynamic equilibrium, with LMAN inputs dominating during early stages of song development and motor plasticity and HVC inputs dominating as song stereotypy is achieved. This balancing act may continue into adulthood. Recent work has shown that micro-lesions of HVC in adult zebra finches lead to transient song variability, which can be blocked or reversed by LMAN lesions (Thompson and Johnson, 2007). LMAN lesions can also prevent changes in song normally induced either by adult tracheosyringeal nerve transection or deafening (Williams and Mehta, 1999; Brainard and Doupe, 2000b). These results suggest that LMAN is either permissive for song plasticity or actually induces it under appropriate conditions. It would be interesting to see whether the ratio of HVC:LMAN inputs to RA in adult zebra finches changes to favor LMAN after deafening and tracheosyringeal nerve transection.
Inputs to RA from LMAN and HVC are glutamatergic. HVC⇒RA synapses contain a mix of N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazoleproprianate (AMPA) receptors, whereas NMDA receptors predominate at LMAN⇒RA synapses (Mooney and Konishi, 1991; Mooney, 1992; Stark and Perkel, 1999). NMDA receptors have been implicated in Hebbian plasticity in a number of systems (Tsien, 2000) whereby correlated activity between pre- and post-synaptic cells strengthens synapses. Changes in the kinetics of NMDA receptor activation over the course of song development may lend themselves to the trial and error refinement of song motor programs (Nordeen and Nordeen, 2004)). The number of these receptors, their sub-unit distribution and the duration of receptor currents decrease as song learning progresses (Mooney and Konishi, 1991; Livingston and Mooney, 1997; Stark and Perkel, 1999; Singh et al., 2000; Boettiger and Doupe, 2001; Scott et al., 2004; Wang and Hessler, 2006). The developmental change from longer to shorter durations of NMDA receptor activation could facilitate the initial reinforcement and strengthening of synapses associated with production of rough approximations of a target song and then require progressively tighter temporal correlations in activity between inputs, leading to greater refinement of song motor programs. Although the precise mechanisms by which AMPA and NMDA receptors contribute to song learning are still not fully understood (Nordeen and Nordeen, 2004), it is clear that NMDA receptor activation is necessary for song development. Treatment with the NMDA receptor antagonist MK-801 during song tutoring sessions (but not on alternate days when no tutoring occurred) has been shown to disrupt song learning (Aamodt et al., 1996).
By far the largest nucleus in the song system of adults is Area X, also part of the AFP, which has reciprocal connections with LMAN (Vates and Nottebohm, 1995). Between days 25-50, Area X nearly doubles in size and, as with HVC, much of this growth is due to neuron addition (Kirn and DeVoogd, 1989; Burek et al., 1991). However, unlike HVC, Area X cell addition over this time appears to be restricted to interneurons (Sohrabji et al., 1993). Many of the cells added to Area X after hatching express FoxP2 (Rochefort et al., 2007) and knockdown of this gene interferes with song development (Haesler et al, 2007). FoxP2 is of particular interest as it has been implicated in human speech comprehension and production, though its distribution in the brain is widespread among vertebrates (Scharff and Haesler, 2005).
Despite differences in growth trajectories among these song control regions, it is likely that programmed neuron death occurs in HVC, RA, Area X and LMAN at ages encompassing initial song learning, as shown by counts of degenerating cells (Bottjer and Sengelaub, 1989; Kirn and DeVoogd, 1989; Bottjer and Johnson, 1992). The growth and remodeling of these regions by cell addition, loss and synaptic remodeling during juvenile life are likely to have important consequences for song development. Conversely, the incomplete development of these structures at birth opens the door for experience to shape their anatomical and physiological properties.
Neuronal attributes that co-vary with song attributes
Before describing what is known about the relationship between post-hatching experience and vocal control system development, it may be worthwhile to describe the adult song attributes known to co-vary with neuronal structure. Songbird species can be roughly categorized along a continuum where, at one extreme, only males sing, and at the other extreme males and females engage in complex, interactive duets (Brenowitz and Arnold, 1986). There is a relationship between the degree of behavioral sexual dimorphism and the size of vocal control regions, suggesting that the capacity to sing may be constrained by the size and number of neurons in vocal control regions (Brenowitz et al., 1985; Brenowitz and Arnold, 1986). Moreover, it appears that in the case of extreme behavioral sexual dimorphism as with zebra finches where only males sing, the pathway from HVC to RA is much diminished or completely absent (Konishi and Akutagawa, 1985). This brain-behavior relationship is further supported by work in which adult female waterschlager canaries, who normally do not sing, show dramatic growth of the song system when given testosterone in adulthood and this growth is accompanied by the development of a stereotyped, male-like song (Nottebohm, 1980; DeVoogd and Nottebohm, 1981; Gahr and Garcia-Segura, 1996).
Variation between vocal control system size and song behavior has been further explored across individual males within a species. In the first study to address this question it was shown that there is a positive correlation between the volume of HVC, RA and song complexity (as defined by the number of acoustically unique syllables) in adult male canaries (Nottebohm et al., 1981). Subsequent studies have shown a relationship between song complexity and HVC volume across individuals within other species and when comparing species, genera and families (Airey and DeVoogd, 2000; DeVoogd, 2004). In most of these studies, only the volume of HVC (and other regions) was measured and so we do not know the extent to which volume differences reflect variation in neuron number, size, density or some combination of these attributes. Interestingly, a positive correlation was found between HVC neuron number and the level of accuracy with which male zebra finches had copied the songs of their adult ‘tutors’ (Ward et al., 1998). Significant positive correlations have also been found between song complexity and LMAN size (Airey and DeVoogd, 2000). Collectively, these results suggest that there is a relationship between the complexity of a learned task and the amount of brain space devoted to that task. Somewhat surprisingly, there is a negative relationship between song complexity and Area X volume in the zebra finch (Airey and DeVoogd, 2000) and Cassin’s finch (Macdougall-Shackleton et al., 2005; also see Hamilton et al., 1998). While the significance of this finding us unclear, it underscores the value of examining multiple regions with potentially different functions within the vocal control system. Perhaps in at least some species, it is the relative sizes of distinct but interconnected cell populations that is most important with respect to song behavior. In support of this notion, it has been reported that while HVC size is predictive of repertoire size in the zebra finch, the amount of explained variance in song attributes increases significantly when LMAN and Area X are added as co-variates (Airely & DeVoogd, 2000).
Although song system size seems to matter, caution must be used when interpreting its functional significance. One of the earliest reports of a relationship between song system anatomy and song showed a robust positive correlation between amount of singing and the size of HVC and RA in the seasonally breeding canary and this has been replicated in a number of species (Ball et al., 2004; Brenowitz, 2004). This raises the possibility that the amount of vocal motor activity (or auditory feedback from singing), independent of song complexity, is a better correlate of vocal control brain space. However, these two proposals need not be mutually exclusive. An intriguing possibility is that while all canaries show seasonal changes in song system volume in conjunction with amount of singing, those with the most complex repertoires have the largest HVCs. Recent work has replicated seasonal vocal control system size changes using fMRI (Van der Linden et al., 1998; Van Meir et al., 2006; Van der Linden et al., 2007). With further refinement, this method might be used to follow seasonal changes in singing rate, vocal repertoire size and song system volume within the same birds across more than one breeding season.
Neurogenesis and the addition of new neurons to some song system regions continue from early post hatch ages into adulthood and there have been several studies conducted to explore potential links between neuronal replacement (addition and loss) with song attributes. Adult neuron addition has been studied in HVC, Area X in the AFP, and the auditory region NCM (Lipkind et al., 2002; Barnea et al., 2006; Barkan et al., 2007; Adar et al., 2008b). However, the most intensive study has focused on HVC, with particular interest in the replacement of HVC neurons that project to RA. The fact that these cells become incorporated in an otherwise mature brain, manage to synapse on target cells 2-3 mm away, and achieve their sparse and highly specific response properties (Hahnloser et al., 2002) is truly a remarkable developmental feat.
HVC neurogenesis is high throughout song learning and then tapers off dramatically around the time of song crystallization at 90-120 days post hatch in the zebra finch (Wilbrecht and Kirn, 2004). A similar trend occurs with a somewhat different timetable in young canaries (Alvarez-Buylla et al., 1988). In the adult male canary, HVC neuronal replacement, including the addition and loss of HVC⇒RA neurons (Fig. 4) occurs throughout the year. However, replacement varies seasonally, with high rates of neuron addition and loss occurring at times of year when new song learning occurs (Fig. 5, (Alvarez-Buylla et al., 1990; Kirn et al., 1994). Over a six-month period spanning the transition from spring, when singing rates in canaries are high and syllables are stereotyped, to fall, when song is extensively remodeled and song rates are lower, there is a 30-50% loss and replacement of the entire HVC⇒RA pathway (Figure 4; Kirn and Nottebohm, 1993). This raises the possibility that neuronal replacement promotes or enables new song learning (Alvarez-Buylla et al., 1990, Kirn et al., 1994; reviewed by Nottebohm, 2004; Wilbrecht & Kirn, 2004). However, many song-related attributes change seasonally. As previously mentioned, canaries retain some syllables from one year to the next. Between breeding seasons, these notes become variable in structure, and it is at these times that highest rates of neuron addition occur. Thus, perhaps the process of achieving song stereotypy, regardless of when notes are initially learned, is a better correlate of elevated HVC neuron addition. Support for this hypothesis comes from studies of western song sparrows, who learn their entire song as juveniles but show seasonal variation in song stereotypy in adulthood. These birds also show higher rates of HVC neuron addition during periods of song instability that precede and follow the breeding season (Tramontin and Brenowitz, 1999). Another piece of evidence for this hypothesis comes from the zebra finch. Zebra finches continue to receive new HVC neurons throughout life, however rates of neuron addition decline with increasing adult age (Wang et al., 2002). This decline coincides with progressive, post-song crystallization increases in song stereotypy (Pytte et al., 2007) and a decrease in the dependence of song on auditory feedback (figure 6; Lombardino and Nottebohm, 2000; Brainard and Doupe, 2001). Thus, as song becomes more stereotyped, neuronal replacement decreases. The decrease in neuron addition is not accompanied by a change in total HVC neuron number, indicating that as birds get older, both the HVC⇒RA cell population and the song motor program become more stable. Collectively, these results support the hypothesis that highest rates of HVC neuronal addition and replacement are associated with periods when song structure is highly variable but is progressing toward stereotypy. Perhaps ongoing neuronal replacement in HVC provides birds with the motor flexibility to achieve song stereotypy, regardless of when song was initially learned. One model for explaining this process is that when new neurons arrive in HVC, their survival and recruitment depend on the extent to which they contribute to a bird’s achievement of a target song (Wilbrecht and Kirn, 2004). This model implies that there is some basis for evaluation of a neuron’s response properties, which, on a more general level, would require that experience modulates adult neuron addition, a topic to be discussed shortly.
Figure 4.
Neuronal replacement in adult canaries. Upper left: A [3H]-labeled neuron (black dots overlying the nucleus) retrogradely labeled from RA with Fluoro-Gold (white deposits in cytoplasm) indicating an adult-formed HVC⇒RA neuron. Modified from (Kirn et al., 1999). Upper right: A pyknotic, degenerating neuron, also backfilled from RA with Fluoro-Gold. Transfer from long- to short day lengths resulted in a significant increase in the number of degenerating HVC⇒RA neurons in adult canaries and a decrease in the total number of HVC⇒RA neurons. Modified from (Kirn and Schwabl, 1997). Lower panels: HVC⇒RA neurons were labeled with latex microspheres and sacrificed after 20 days in late April (Lower left) or 6 months in October (lower right). This experiment was designed to determine how many HVC⇒RA neurons present in April, when canaries are singing stereotyped song, are still present the following October, when song becomes unstable and new song elements are added. The results suggest that between one-third and one-half of all such neurons are lost and replaced over this interval. Modified from (Kirn and Nottebohm, 1993). Scale bars in upper two panels = 10 μm. Scale bars in lower two panels = 200 μm.
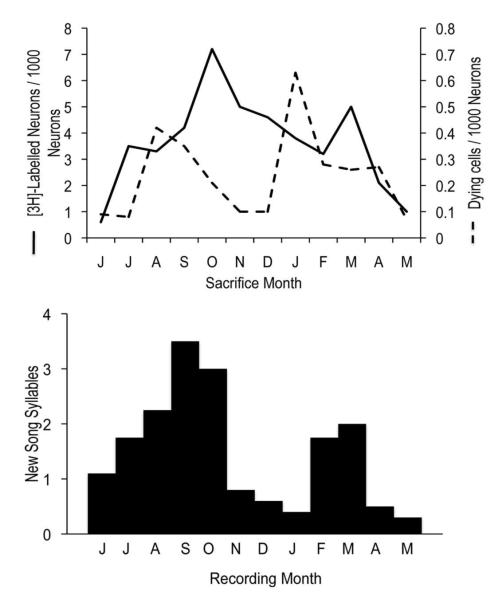
Figure 5.
A relationship between neuronal replacement and song modification in adult canaries. Upper graph: Twelve groups of adult male canaries (one group for each month) were injected with [3H]-thymidine and sacrificed one month later. The mean numbers of [3H]-labeled neurons (solid line) and pyknotic, degenerating cells (dashed line) per 1000 HVC neurons are shown. There were two peaks in new neuron addition, preceded by 2 peaks in cell death. The precise temporal relationship between peaks in addition and loss may not be accurately reflected by these data. Values are plotted by month of sacrifice, rather than month of [3H]-thymidine treatment. Other work indicates that there is a culling of new neurons when they are roughly two weeks old (Kirn et al., 1999). If this culling is responsible for seasonal changes in new neuron number seen at 1-month survivals, then the solid line for [3H]-labeled cell number should be left-shifted by two weeks, revealing greater overlap between death and replacement. Modified from Kirn et al., 1994). Lower graph: In a separate group of adult canaries, song modification was measured between one and two years of age by monthly counts of the mean number of new syllables added per month. Modified from (Nottebohm et al., 1986). Periods of high song modification correlate with periods of high neuronal replacement.
Figure 6.
Adult behavioral and neural plasticity in a close-ended learner. Upper graph: Mean (+SEM) song stereotypy scores-the acoustical similarity across repeated deliveries of the same syllable, as a function of age. Data are for two groups of zebra finch males, “Young Adults” (recorded at 123-140 days, and again at 9-9.5 months and 15-15.5 months) and “Old Adults” (recorded at 3-3 years and 9 months at first recording and then two more times at the same intervals as the younger adults. There was a significant increase in song stereotypy across the recordings for the young, but not older adults. Modified from (Pytte et al., 2007). Middle graph: Adult song maintenance appears to be an active process guided by reference to auditory memories. However, an age-related decline in the reliance on auditory feedback for song maintenance was also found. The data show changes in syllable morphology between songs recorded before and 1-month after deafening performed at various ages (filled circles). For comparison, song similarity scores for age-matched controls are shown (open circles). Deafening at ages between 100-200 days after hatching has a much more dramatic effect on song structure compared to when birds are deafened at older ages (from Brainard and Doupe, 2001). Had longer intervals after deafening been examined, even old adults would eventually show modest song degradation (Lombardino and Nottebohm, 2000). Lower Graph: The number of new neurons (identified using [3H]-thymidine and retrograde labeling from RA (not shown) or Nissl stain) added to HVC declines with increasing age. Each triangle represents the mean number of [3H]-labeled neurons per day of [3H]-thymidine injections for a single bird (modified from Wang et al, 2002). Although precisely comparable ages across data sets are not available, collectively, the results suggest that change occurs at both behavioral and neural levels well beyond song crystallization. Moreover, the data suggest that as song stereotypy increases, the stability of the song motor program as well as the population of HVC⇒RA neurons increases.
There has been much less work exploring potential associations between neuron addition to Area X and song. The age-related increases in song stereotypy reported in adult zebra finches are not associated with changes in Area X neuron addition, suggesting that its control and functions may differ from those for neuron addition to HVC (Pytte et al., 2007). Moreover, the seasonal changes in song stereotypy in sparrows that co-vary with changes in HVC neuron addition are not also accompanied by seasonal changes in Area X neuron addition (Thompson and Brenowitz, 2005). Given that Area X is part of the AFP necessary for song learning, it would be interesting to see whether canaries, which, unlike sparrows, learn some new song material annually, do show seasonal changes in Area X neuron replacement.
The role of androgens in the development of song and the song system
The termination of vocal plasticity at puberty (zebra finch) and at the onset of the breeding season (canary) may be controlled by rising steroid hormones and, in particular, testosterone (Nottebohm, 1989; Bottjer and Hewer, 1992; Bottjer and Johnson, 1997). Testosterone (T) is detectable in male zebra finches during song development (Pröve, 1983; Adkins-Regan et al., 1990), raising the possibility that androgens or related steroids are necessary for regulating the timing of vocal learning. Excess androgens during juvenile life interfere with normal song development, perhaps due to precocious development of one or more song system regions (Korsia and Bottjer, 1991). However, normal song development depends on at least some androgenic stimulation as androgen blockade also disrupts song development (Bottjer and Hewer, 1992). It is tempting to conclude that during normal development, lower levels of androgens or their metabolites are permissive for vocal exploratory behavior and that rising T levels at puberty curtail this plasticity and promote song crystallization and song stereotypy. Peripheral circulating levels of T have been reported to surge at the time of song crystallization in young male zebra finches (Prove, 1983). However, peripheral T levels in younger birds during the plastic song phase are not necessarily lower than in fully mature males (Adkins-Regan et al., 1990). It is possible that more localized changes in hormone secretion within the brain itself orchestrate the timing and sequence of stages that constitute normal song development. Peripheral circulating levels of steroids do not always reflect levels in the brain (Remage-Healey et al., 2008), and it has been shown that all of the enzymes necessary for steroid synthesis are present in brain tissue (Baulieu, 1998, London et al., 2009) (also see Vockel et al., 1990). Therefore, some caution must be used when relating peripheral hormone levels to behavior.
There has been considerable work exploring the potential role of steroids in the establishment of sex differences in the song system, and although early exposure to gonadal steroids promotes the development of a male-like song system in female zebra finches, it has also been shown that neither the gonads, T or estradiol (E) are necessary for the initial development of song control region size and neuron number in male zebra finches (Wade and Arnold, 2004). However, it is possible that within males, more subtle variation in HVC neuron number might be related to early variation in T exposure. There is large inter-clutch variability in egg T levels as well as adult HVC neuron number in zebra finches (Ward et al., 2001), however, a causal link between early T exposure and neuron number has not yet been explored.
In contrast, androgens have been shown to play a pivotal role in the regulation of adult, seasonal plasticity in the song system of some species. In canaries, seasonal peaks in cell death and replacement are preceded by declines in androgen levels (Nottebohm et al., 1987; Kirn et al., 1994), and adult female canaries treated with T exhibit growth of song control regions that accompanies the development of male-like song (Nottebohm, 1980). The initial growth of song system regions in late winter is critically dependent on androgens in white-crowned sparrows and castration early in the breeding season (after HVC has reached maximal size, results in a rapid decline in HVC volume (Tramontin et al., 2000; Thompson et al., 2007). HVC growth and regression reflect steroid influences on neuron addition and survival. An elegant model has been developed suggesting that steroids regulate the trophic factor BDNF, which, in turn, regulates HVC neuron survival (Rasika et al., 1999; Louissaint et al., 2002; Nottebohm, 2004), perhaps by modulating the activity of the caspase family of proteases known to promote programmed cell death (Thompson and Brenowitz, 2008).
When taken together, available data suggest that steroids can influence the development of both song and the song control system. In some species, steroid influences extend well into adulthood. In seasonal breeders, there is a trend for steroids to be highest at times associated with highest singing rates and song stereotypy. Conversely, lower singing rates and increases in song variability and/or new song learning are associated with lower steroid levels. One can see similar trends with respect to steroids and adult seasonal neural plasticity. Seasonal declines in steroids are associated with regression of song system nuclei and an increase in HVC cell death. Subsequent increases in steroids that precede the breeding season promote the replacement of lost neurons, re-growth of HVC, and when birds have high steroid levels and are in full breeding condition, HVC volume and total neuron number are highest and neuronal replacement is low. Interestingly, even in the adult zebra finch, where singing and song variability are not modulated seasonally, the capacity for song modification appears to be influenced by androgens. Unilateral ts (tracheosyringeal) nerve cuts result in varying degrees of song reorganization and the amount of song rearrangement is inversely related to circulating T. Moreover, experimentally induced increases in T inhibit song modification in birds with ts nerve cuts (Williams et al., 2003). These results raise the possibility that steroids play a pivotal role in the regulation of behavioral and neural plasticity both during development and adulthood, and across species with different learning trajectories.
The role of experience in guiding vocal control system development
Due in part to the extensive role of experience in guiding vocal development there have been many experiments designed to test the role of various kinds of experience in the establishment of connectivity, size and number of neurons in the vocal control system. Most of the manipulations that have been employed to study vocal learning on a behavioral level have been co-opted to explore the role of experience in vocal control system development. Perhaps the most profound manipulation involves early deafening, which, depending on when it is done, blocks song model memorization, a bird’s ability to use song memories to guide vocal development, or both (Konishi, 2004). However, deafening deprives birds of all auditory experience. In an attempt to more selectively deprive birds of song-related sensory experience, other work has involved exposure of hearing-intact birds to an impoverished social environment lacking song tutors during development, which can delay the normal closure of the sensitive period for song learning (Eales, 1985). Perhaps the most powerful approach has been to expose birds to different song models in order to see whether variation in song model complexity and the resulting complexity of learned song correlate with song system attributes (Brenowitz et al., 1995; Airey et al., 2000a).
Particular focus has been placed on the AFP and, more specifically, LMAN, its thalamic inputs from DLM, and its projections to RA. Early isolation from potential song models delays the normal decrease in the number of dendritic spines on LMAN neurons, and the regression of axonal arbor of LMAN neurons in RA (Wallhäusser-Franke et al., 1995; Iyengar and Bottjer, 2002a). These results raise the possibility that exuberant connectivity is permissive for song plasticity and that regressive events in the AFP may constrain or terminate behavioral plasticity. The effects of isolation on at least some of these attributes depend on the onset and duration of isolation. Isolation begun on post hatch day 4 and continued until day 55 delays the normal decrease in LMAN spine densities (Nixdorf-Bergweiler et al., 1995). However, social deprivation begun on post hatching day 30 did not delay the normal loss of spines (Heinrich et al., 2005). While it could be that this later onset of deprivation allowed some earlier exposure to key social interactions, this cannot explain the fact that deprivation begun on day 30 did, in fact, delay the closure of the sensitive period for song learning. Thus, developmental decreases in LMAN spine density can be dissociated from closure of the sensitive period for song learning (Heinrich et al, 2005). As already mentioned, the ratio of HVC:LMAN input to RA may be important for song learning and so it would be interesting to see whether social deprivation affects this ratio. It would also be interesting to see how social deprivation affects the maturation of NCM and related auditory structures implicated in auditory template formation.
Deafening or rearing in white noise has also been shown to prevent or delay the normal development in the topography of LMAN terminals in RA (Iyengar and Bottjer, 2002b). Interestingly, normal changes in topography of DLM input to LMAN and between LMAN and Area X were insensitive to this treatment. These results indicate that early experience can alter the normal development and refinement of some AFP neuronal attributes, however, the specific link between developmental changes in these attributes and song learning remains unclear.
The development of HVC volume in zebra finches (Buchanan et al., 2004) and RA volume in song sparrows (Nowicki et al., 2002) are affected by early nutritional stress, indicating, at the very least, that postnatal factors can influence these attributes (but also see (Gil et al., 2006). Moreover, normal variation in HVC volume can be attributed, in part, to early parenting, perhaps including song tutoring (Buchanan et al., 2004). However, the attainment of normal adult volume and total neuron number in these regions is not affected by early deafening in the zebra finch, despite the profound effects deafening has on song development (Burek et al., 1991).
As already mentioned, HVC neurogenesis is high throughout song learning and then declines substantially by the age of 90-120 days post hatch in the zebra finch (Nordeen and Nordeen, 1988b; Wilbrecht and Nottebohm, 2004). Although complete absence of auditory function (by early deafening) has no effect on the attainment of adult HVC volume and neuron number in the zebra finch, potential effects of experience on the dynamic process of cell turnover cannot be assessed without cell birth dating techniques. When neuron replacement is measured, deafening does have an effect in juvenile zebra finches, however this effect is context-dependent. When the tracheosyrngeal nerve innervating the syrinx is transected unilaterally in juveniles, song development progresses with little impairment but relies on the intact, contralateral nerve. Since there is relatively little communication between the two hemispheres “up stream” from the ts nerve, it is possible that this surgery forces the bird to learn song entirely with one hemisphere (Wilbrecht et al., 2002). This manipulation has little effect on HVC neuron addition ipsilateral to nerve transection but there is a dramatic increase in neuron addition and replacement in the contralateral HVC, perhaps resulting from the extra demands placed on the intact hemisphere (Wilbrecht et al., 2002). Under these circumstances, deafening was found to prevent the contralateral increase in neuron addition to HVC. However, deafening alone (without nerve cuts) had no detectable effect on HVC neuron addition in juveniles (Wilbrecht et al., 2002). Given that deafening blocks normal song development in birds with intact and unilateral ts nerve cuts, neither the absence of normal auditory function alone, nor the development of an abnormal song can account for differences between these two experimental conditions. Amount of singing was not explored in this work and so perhaps song-related motor activity or non-auditory proprioceptive feedback differed between these experimental groups.
The effects of deafening in adulthood on HVC size, total neuron number and neuron replacement have been inconsistent. Early work suggested that deafening attenuated the growth of HVC that can be induced by testosterone treatment in female canaries, however, the same group was subsequently unable to replicate this finding (Bottjer and Dignan, 1988; Bottjer and Maier, 1991). Researchers working with other species also failed to detect an effect of deafening on seasonal increases in HVC size (Brenowitz et al., 2007). The first attempt to explore the role of experience in regulating adult neuronal replacement reported a dramatic decrease in new HVC neuron addition after deafening in adult male zebra finches (Wang et al., 1999). However, subsequent attempts to replicate this work were also unsuccessful, and we hypothesize that when deafening does have an effect on neuron replacement, it is contingent on other factors, including differences in the way deafened and hearing birds respond to social context (in preparation). Indeed, in more recent work we found a small but significant increase in HVC neuron incorporation in deafened birds compared to their hearing cage mates (Fig. 7; Hurley et al., 2008). At present, the safest conclusion one can draw from this work is that hearing may be important in some contexts (as was found in juveniles with ts nerve cuts), however, complete absence of normal auditory function, by itself, plays a minor, if any, role in the regulation of HVC growth or neuronal replacement.
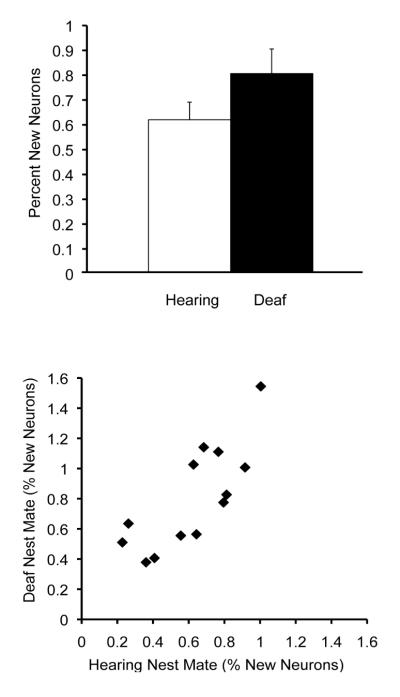
Figure 7.
Potential constraints on adult neuron addition. Nest mate similarities in HVC neuron addition persist even when one member of a nest mate pair is deafened in adulthood. Upper graph: Deafening in adulthood resulted in a significant increase in neuron addition (mean + SEM). Lower graph: However, the mean percentage of [3H]-labeled HVC neurons in nest mate pairs where one member was deafened in adulthood still showed a significant positive correlation. From Hurley et al., 2008.
There is evidence that the act of singing regulates HVC neuron addition in adult canaries. Birds who were not allowed to sing for 8 days had significantly fewer new HVC neurons when compared to birds that sang at high rates in response to playbacks of song (Li et al., 2000). Moreover, there is a positive correlation between naturally occurring variation in amount of singing and new HVC neuron addition (Alvarez-Borda and Nottebohm, 2002). At first glance, these results appear to contradict the finding that in canaries and song sparrows, seasonal increases in neuron addition occur at times of year when singing is very low. However, peaks in HVC neuron addition, in the canary at least, occur as birds are beginning to sing again after a dormant period (Nottebohm et al., 1987; Kirn et al., 1994). Perhaps the laboratory conditions under which singing has been shown to correlate with neuron addition are ones in which increases in singing were encouraged above low baseline levels. These results suggest that singing promotes neuron addition, perhaps by increasing the production of trophic survival factors like Brain-Derived Neurotrophic Factor (BDNF; see Nottebohm, this volume). To the extent that singing influences a bird’s own HVC neuronal replacement, it is tempting to suggest, from all available data, that this is a vocal motor-driven process.
It is likely that adult neuronal replacement in HVC and elsewhere in the song control system is also influenced by the actions and attributes of conspecifics. Social enrichment has been reported to enhance the survival of adult-formed neurons in NC, Area X, HVC and the hippocampus in adult male zebra finches (Lipkind et al., 2002; Barnea et al., 2006; Adar et al., 2008b). In one study, males housed in small, mixed-sex groups were moved to larger cages where a) males were housed singly, b) males were housed individually with an unfamiliar female, or c) males were introduced to large, mixed-sex groups of unfamiliar birds. Males in the latter group showed more new neurons in HVC, NC and Area X compared with the former two groups, which did not differ from one another (Lipkind et al., 2002). It is unlikely that changes in general activity levels or amount of singing could account for these results (Lipkind et al., 2002; Adar et al., 2008a). NC, HVC and Area X have been implicated in auditory perception and memory formation (Scharff et al., 1998; Gentner, 2004; Mello et al., 2004) and the authors concluded that at least one potential way that social enrichment might enhance new neuron survival is by increased demands on systems underlying new auditory memory formation (Adar et al., 2008a). As already mentioned, the role of auditory function in regulating HVC neuron addition is unclear. However, the authors’ interpretation is particularly appealing for NC, which is not directly implicated in song control but serves auditory functions and projects to song control regions (Mello and Clayton, 1994; Mello et al., 2004).
Subsequent work on NC (Adar et al., 2008b) showed that varying the timing and degree of change in social complexity relative to a new neuron’s age impacted that neuron’s chances of survival. It was found that adult-formed neurons that had lived for 3 months prior to manipulation of social complexity were lost in birds transferred from a simple to a more complex social environment whereas this same manipulation enhanced survivorship of neurons that were younger at the time of social change (1 month old). The opposite result was obtained when birds were not transferred from simple to complex environments—older neurons survived longer at the expense of younger neurons. These results suggest that social stability favors survival among pre-existing neurons over newly formed neurons, whereas social change (increases in social complexity) favors survival of new neurons over older ones.
These results provide strong evidence that experience can influence some aspects of juvenile brain development and adult neuron replacement within the vocal control system. However, the relative contributions of song-related auditory and motor experience and other social factors remain unclear.
The relationship between steroids, experience, the development of song and the song system
As already mentioned, steroids can have profound effects on song and the song system. This raises several critical questions. Do steroids have independent, and potentially unrelated effects on song and song system development? This question is not trivial, as many of the experimental manipulations used to explore causal links between song plasticity and brain attributes could also influence circulating steroid levels. Do experience and hormones act synergistically in the development of song and the song system? There is substantial evidence that experience can influence steroid levels (Lehrman, 1964; Goymann et al., 2007). Even when hormone levels are ‘clamped’ by gonadectomy and hormone implants, experience can influence circulating hormone levels, presumably by effects on steroid metabolism (Raouf et al., 2000). It is possible that increases in steroids promote singing, for example, and, in turn, the act of singing might feed back to further augment hormone secretion, and both might be necessary for maximal changes in brain anatomy and function. Finally, the act of singing might have independent effects on brain anatomy/function and steroid levels.
A complete review of this literature is beyond the scope of this chapter (for a more comprehensive review, see Ball et al., 2004; Brenowitz, 2004; Sartor and Ball, 2005). However, some experiments have been conducted to explore (or at least control for) the potential effects of experience and gonadal steroids on song system structure. Recent work has shown that depriving juvenile zebra finches of tutors to delay the end of the critical period for song learning also delays the normal decline in HVC neuron addition without influencing circulating steroid levels (Wilbrecht et al., 2006). In adult white-crowned sparrows, exposure to increasing day length induced large increases in the size of vocal control regions regardless of whether males were housed with or without females, however, growth was greater in males housed with females despite the absence of group differences in circulating T (Tramontin et al., 1999). In adult canaries, a reduction in photoperiod resulted in an increase in the death of HVC⇒RA neurons without affecting circulating gonadal steroids (Kirn and Schwable, 1997). These correlational studies provide evidence for dissociations between circulating T and neural plasticity in juveniles and adults. Interestingly, in the former study (Wilbrecht et al., 2006), the delayed decrease in HVC neuron addition was associated with a delay in the achievement of song stereotypy, and in the latter study (Tramontin et al., 1999), males housed with females and who had larger song control regions also sang more suggesting an additional dissociation between circulating T and song attributes.
There is also strong evidence that T can influence adult HVC size independent of amount of singing or auditory function (Brenowitz et al., 2007). This does not, however, rule out the possibility that singing has independent effects on neural structure/function. Perhaps the strongest evidence for a causal link between singing and neural plasticity that is independent of gonadal steroids comes from previously described work with adult canaries (Li et al., 2000; Alvarez-Borda and Nottebohm, 2002). Birds allowed to sing freely had significantly more new HVC neurons compared to birds prevented from singing for 8 days (Li et al., 2000). Of course, this chronic manipulation could have resulted in reduced T levels in the suppressed group. However, in subsequent work (Alvarez-Borda and Nottebohm, 2002), evidence was presented for independent and, perhaps synergistic effects of singing and T on HVC neuron addition. When comparing castrated and gonadally intact males that sang at comparable levels, neuron addition was nearly three-fold higher in the latter group. However, among castrates, who had undetectable levels of circulating T, there was still a positive correlation between amount of singing and new neuron addition (Alvarez-Borda & Nottebohm, 2002). These results are consistent with a model in which rising steroid levels promote singing and neuron addition but that singing, via T-independent mechanisms, can also enhance neuron addition (Alvarez-Borda & Nottebohm, 2002). Further experiments suggest that both steroids and singing promote HVC neuron survival by the production of BDNF (Nottebohm, 2004). It seems clear from the work summarized that steroids and singing can have independent effects on neuron addition. The relative weighting of these variables in the control of neuron addition may differ, with T having a larger impact than singing (Alvarez-Borda & Nottebohm, 2002)). However, it is also likely that steroids, singing, and possibly other social factors, have additive effects on neuronal replacement. It will be interesting to determine whether BDNF expression is part of the story for all of these variables.
Constraints on vocal control system development
Although there is a large literature devoted to behavioral and song system plasticity, a growing number of studies suggest that there are major constraints on song system development and that variation in song system attributes is regulated by experience-independent factors. As already mentioned, early deafening, which has profound influences on song learning and maintenance, has no detectable effect on the attainment or maintenance of adult HVC volume and total neuron number (Burek et al., 1991). In other studies, it has been shown that where co-variation between neural and behavioral attributes exist, it is more likely that brain structure determines the quality or amount learned, rather than the other way around. For example, western marsh wrens have a much larger song repertoire than their eastern counterparts and western birds have a larger HVC when compared to eastern birds (Canady et al., 1984). When nestlings from the two populations were hand reared and exposed to the same song tutor tapes, western birds still learned a larger repertoire and had a larger HVC compared to their eastern counterparts (Kroodsma and Canady, 1985). Moreover, when western nestling marsh wrens were divided into two groups, one exposed to tape recordings with a large song repertoire from which to learn and the other exposed to recordings with a small repertoire, birds in the former group acquired and produced more complex songs than birds in the latter group, yet there were no group differences in the volume of HVC or RA. Interestingly, within the group that produced a large repertoire, there was a positive correlation between song complexity and vocal control region volume (Brenowitz et al., 1995). Song experience is not entirely without effects, as the number of dendritic spines on HVC neurons was higher in wrens exposed to a larger repertoire (Airey et al., 2000a). Nevertheless, these results strongly suggest that the complexity of learned song does not determine song system size and raise the possibility that song system size constrains the complexity of learned song. It is worth noting that in the very first study to show a relationship between song complexity and the size of song system structures (Nottebohm et al., 1981) the conclusion drawn was that canaries with a large HVC might produce either simple or complex songs, but that the most complex songs required a large HVC. These results suggest a model where variation in HVC volume and total neuron number are largely sculpted by experience-independent factors and that these attributes might place limits on the capacity to learn and/or produce complex songs.
So what regulates the size and number of neurons in vocal control regions? The answer to this question remains unknown but there is growing evidence that these attributes are heritable (Airey et al., 2000b). Zebra finch brothers co-vary in measures of HVC volume and neuron number (Ward et al., 2001; Williams et al., 2003) and this is also true for the size of brain regions with direct projections to and from HVC (Airey et al., 2000b). We cannot rule out a potential role of early life experience (including in ovo exposure to hormones) that has long lasting, perhaps permanent effects on these attributes. For example, as already mentioned, early dietary restriction has been shown to impact HVC volume and in cross-fostering experiments a small but significant amount of the variation in HVC size can be attributed to the environment provided by foster parents (Nowicki et al., 2002; Buchanan et al., 2004). Nevertheless, available data suggest that a significant amount of the variation in the size of HVC, RA and Area X size can be accounted for by variation in genotype.
Several studies have shown changes in HVC neuron addition can occur in the absence of changes in HVC volume or total neuron number (Alvarez-Buylla et al., 1992; Wang et al., 2002; Wilbrecht et al., 2002). However, here too, there appear to be constraints on adult neuron addition that are either heritable or the long-term consequence of early experience. Recent work indicates that adult male zebra finches derived from the same nest have similar rates of neuron addition (Hurley et al., 2008). Similarities among nest mates persisted even when one nest mate of a pair is deafened in adulthood (Fig 7). It is possible that other types of adult experience, like social enrichment, would weaken the co-variation seen among nest mates. However, the wholesale loss and replacement of neurons represents a dramatic (and potentially costly) form of plasticity that is presumably adaptive for modifying neural circuits in response to environmental change. It seems paradoxical that such constraints exist at all. Regardless, if it turns out that nest mate similarities are heritable, this may open the door to a new approach for exploring the functional significance of adult neurogenesis through selective breeding for high and low rates of neuron incorporation.
Experience-independent constraints on total HVC neuron number may also explain the complex nature of the relationship between adult T, singing, and rates of neuron addition. T promotes neuron addition but neuron addition at times of year when circulating T has been high for some time is substantially lower than when T levels have been low for some time (Kirn et al., 1994). Moreover, although singing may promote neuron addition, as already mentioned, neuron addition is low at times of year when birds are naturally singing the most! This has led to a model where available synaptic space in HVC is a major factor in the regulation of neuron addition (see Nottebohm, this volume). In seasonal breeders, the end of the breeding season is marked by a crash in T levels and an increase in cell death (Kirn et al., 1994; Breonowitz, 2004). The increase in cell death has been proposed to free up potential incorporation sites for incoming neurons and this might explain the waves of neuron addition that occur in the fall, when birds are singing little and T is low. However, it is possible that subsequent increases in neuron incorporation are supported by gradual increases in circulating steroids and, perhaps, singing. This scenario would continue until all available space for new neurons in HVC has been occupied. By spring, when T levels and singing are maximal, no further increases in HVC neuron addition would be possible until there was once again a crash in circulating T and an increase in cell death. This model is based almost entirely on the temporal sequence of endocrine, neuronal, and behavioral events in the canary (Nottebohm et al., 1986, 1987; Kirn et al., 1994). While speculative, the relative availability of neuron incorporation sites may explain the complex relationship between T, singing, and HVC neuron addition. Moreover, selective ablation of HVC⇒RA neurons augments their replacement (Scharff et al., 2000) and recent work has shown that treatments that reduce cell death also reduce new HVC neuron addition, lending support to the notion that there is a relationship between neuron addition and the number of preexisting neurons (Thompson & Brenowitz, 2009).
Summary and conclusions
In songbirds, a well-defined series of telencephalic brain regions is dedicated to vocal learning and song production. The staggered developmental timetable for different components within the song system provides evidence for subsystems that are at least partially distinct with respect to function. The AFP appears to be responsible for controlling the earliest stages of vocal motor learning and is necessary for vocal plasticity in adulthood. The HVC⇒RA pathway appears necessary for the last stages of vocal motor development as well as the adult production of learned song. There is substantial growth, regression and sculpting of synaptic inputs after hatching at times when key events in song development occur and future work will likely provide a better understanding of the causal links between the development of brain and behavior. While much is still unknown about the relationship between these steps in brain development and song learning, behavioral observations have informed studies of the underlying neural mechanisms for vocal learning and song production. Conversely, developmental neurobiological studies have forced a reappraisal of some tenets of song development. In particular, the line distinguishing close-ended and open-ended learners has blurred considerably due to recent work suggesting unanticipated neural and behavioral plasticity in the former, potential limits to such plasticity in the latter, and a shared dependence on gonadal steroids for neural and behavioral plasticity. A growing body of research also indicates that although experience alters some aspects of vocal control system development, there are also major constraints limiting the extent to which neural structure is altered as a result of experience, both during development and in adulthood. It is often the case that discoveries of dramatic brain plasticity are met with much more enthusiasm than studies reporting limited plasticity. And yet, an understanding of these constraints may be extremely informative in both an evolutionary context and for a better understanding of proximate neural mechanisms. It has been shown in research from numerous songbird species that predispositions guide and constrain song development, usually in adaptive ways (Gould and Marler, 1987; Marler, 1997; Rose et al., 2004; Gardner et al., 2005). It is likely that such behavioral constraints are mirrored in the avian brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aamodt SM, Nordeen EJ, Nordeen KW. Blockade of NMDA receptors during song model exposure impairs song development in juvenile zebra finches. NeurobiolLearnMem. 1996;65:91–98. doi: 10.1006/nlme.1996.0010. [DOI] [PubMed] [Google Scholar]
- Adar E, Lotem A, Barnea A. The effect of social environment on singing behavior in the zebra finch (Taeniopygia guttata) and its implication for neuronal recruitment. Behav Brain Res. 2008a;187:178–184. doi: 10.1016/j.bbr.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Adar E, Nottebohm F, Barnea A. The relationship between nature of social change, age, and position of new neurons and their survival in adult zebra finch brain. J Neurosci. 2008b;28:5394–5400. doi: 10.1523/JNEUROSCI.5706-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex steroid levels in developing and adult male and female Zebra finches (Poephila guttata) GenCompEndocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- Airey DC, DeVoogd TJ. Greater song complexity is associated with augmented song system anatomy in zebra finches. Neuroreport. 2000;11:2339–2344. doi: 10.1097/00001756-200007140-00054. [DOI] [PubMed] [Google Scholar]
- Airey DC, Kroodsma DE, DeVoogd TJ. Differences in the complexity of song tutoring cause differences in the amount learned and in dendritic spine density in a songbird telencephalic song control nucleus. Neurobiol Learn Mem. 2000a;73:274–281. doi: 10.1006/nlme.1999.3937. [DOI] [PubMed] [Google Scholar]
- Airey DC, Castillo-Juarez H, Casella G, Pollak EJ, DeVoogd TJ. Variation in the volume of zebra finch song control nuclei is heritable: developmental and evolutionary implications. Proc Biol Sci. 2000b;267:2099–2104. doi: 10.1098/rspb.2000.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Borda B, Nottebohm F. Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. J Neurosci. 2002;22:8684–8690. doi: 10.1523/JNEUROSCI.22-19-08684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Ling C-Y. Developmental patterns of neurogenesis in the CNS of the canary. Soc.Neurosci.Abstr. 1991:30. [Google Scholar]
- Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurobiol. 1997;33:585–601. [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Birth of projection neurons in the higher vocal center of the canary forebrain before, during, and after song learning. ProcNatlAcadSciUSA. 1988;85:8722–8726. doi: 10.1073/pnas.85.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990;249:1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Ling C-Y, Nottebohm F. High vocal center growth and its relation to neurogenesis, neuronal replacement and song acquisition in juvenile canaries. JNeurobiol. 1992;23:396–406. doi: 10.1002/neu.480230406. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Ling C-Y, Yu WS. Contribution of neurons born during embryonic juvenile and adult life to the brain of adult canaries: regional specificity and delayed birth of neurons in the song control nuclei. JCompNeurol. 1994;347:233–248. doi: 10.1002/cne.903470207. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Mateo AS, Merchant-Larios H. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J Neurosci. 1998;18:1020–1037. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. The effects of castration on song development in zebra finches (Poephila guttata) JExpZool. 1975;191:261–278. doi: 10.1002/jez.1401910212. [DOI] [PubMed] [Google Scholar]
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann N Y Acad Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- Barkan S, Ayali A, Nottebohm F, Barnea A. Neuronal recruitment in adult zebra finch brain during a reproductive cycle. Dev Neurobiol. 2007;67:687–701. doi: 10.1002/dneu.20379. [DOI] [PubMed] [Google Scholar]
- Barnea A, Mishal A, Nottebohm F. Social and spatial changes induce multiple survival regimes for new neurons in two regions of the adult brain: An anatomical representation of time? Behav Brain Res. 2006;167:63–74. doi: 10.1016/j.bbr.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. doi: 10.1016/s0306-4530(98)00071-7. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Doupe AJ. Developmentally restricted synaptic plasticity in a songbird nucleus required for song learning. Neuron. 2001;31:809–818. doi: 10.1016/s0896-6273(01)00403-2. [DOI] [PubMed] [Google Scholar]
- Bolhuis J, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Bottjer SW. Developmental regulation of basal ganglia circuitry during the sensitive period for vocal learning in songbirds. Ann N Y Acad Sci. 2004;1016:395–415. doi: 10.1196/annals.1298.037. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Dignan TP. Joint hormonal and sensory stimulation modulate neuronal number in adult canary brain. JNeurobiol. 1988;19:624–635. doi: 10.1002/neu.480190705. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Sengelaub DR. Cell death during development of a forebrain nucleus involved with vocal learning in zebra finches. JNeurobiol. 1989;20:609–618. doi: 10.1002/neu.480200702. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Maier E. Testosterone and the incidence of hormone target cells in song-control nuclei of adult canaries. JNeurobiol. 1991;22:512–521. doi: 10.1002/neu.480220507. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Matters of life and death in the songbird forebrain. JNeurobiol. 1992;23:1172–1191. doi: 10.1002/neu.480230909. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Hewer SJ. Castration and antisteroid treatment impair vocal learning in male zebra finches. JNeurobiol. 1992;23:337–353. doi: 10.1002/neu.480230402. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Arnold AP. Developmental plasticity in neural circuits for a learned behavior. Annu Rev Neurosci. 1997;20:459–481. doi: 10.1146/annurev.neuro.20.1.459. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–902. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Glaessner SL, Arnold AP. Ontogeny of brain nuclei controlling song learning and behavior in zebra finches. JNeurosci. 1985;5(no. 6):1556–1562. doi: 10.1523/JNEUROSCI.05-06-01556.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Auditory feedback in learning and maintenance of vocal behaviour. Nat Rev Neurosci. 2000a;1:31–40. doi: 10.1038/35036205. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000b;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Postlearning consolidation of birdsong: stabilizing effects of age and anterior forebrain lesions. J Neurosci. 2001;21:2501–2517. doi: 10.1523/JNEUROSCI.21-07-02501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA. Plasticity of the adult avian song control system. Ann N Y Acad Sci. 2004;1016:560–585. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Arnold AP. Interspecific comparisons of the size of neural song control regions and song complexity in duetting birds: evolutionary implications. JNeurosci. 1986;6:2875–2879. doi: 10.1523/JNEUROSCI.06-10-02875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, Arnold AP, Levin RN. Neural Correlates of Female Song in Tropical Duetting Birds. BrainRes. 1985;343:104–112. doi: 10.1016/0006-8993(85)91163-1. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Lent K, Kroodsma DE. Brain space for learned song in birds develops independently of song learning. JNeurosci. 1995;15:6281–6286. doi: 10.1523/JNEUROSCI.15-09-06281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz EA, Lent K, Rubel EW. Auditory feedback and song production do not regulate seasonal growth of song control circuits in adult white-crowned sparrows. J Neurosci. 2007;27:6810–6814. doi: 10.1523/JNEUROSCI.1248-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan KL, Leitner S, Spencer KA, Goldsmith AR, Catchpole CK. Developmental stress selectively affects the song control nucleus HVC in the zebra finch. Proc Biol Sci. 2004;271:2381–2386. doi: 10.1098/rspb.2004.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burek MJ, Nordeen KW, Nordeen EJ. Neuron loss and addition in developing zebra finch song nuclei are independent of auditory experience during song learning. JNeurobiol. 1991;22:215–223. doi: 10.1002/neu.480220302. [DOI] [PubMed] [Google Scholar]
- Canady RA, Kroodsma DE, Nottebohm F. Population differences in complexity of a learned skill are correlated with the brain space involved. Proc Natl Acad Sci U S A. 1984;81:6232–6234. doi: 10.1073/pnas.81.19.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canady RA, Burd GD, DeVoogd TJ, Nottebohm F. Effect of testosterone on input received by an identified neuron type of the canary song system: A golgi/electron microscopy/degeneration study. JNeurosci. 1988;8(10):3770–3784. doi: 10.1523/JNEUROSCI.08-10-03770.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoogd TJ. Neural constraints on the complexity of avian song. Brain Behav Evol. 2004;63:221–232. doi: 10.1159/000076783. [DOI] [PubMed] [Google Scholar]
- DeVoogd TJ, Nottebohm F. Gonadal hormones induce dendritic growth in the adult brain. Science. 1981;214:202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Konishi M. Song-selective auditory circuits in the vocal control system of the zebra finch. Proc Natl Acad Sci U S A. 1991;88:11339–11343. doi: 10.1073/pnas.88.24.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Solis MM, Kimpo R, Boettiger CA. Cellular, circuit, and synaptic mechanisms in song learning. Ann N Y Acad Sci. 2004;1016:495–523. doi: 10.1196/annals.1298.035. [DOI] [PubMed] [Google Scholar]
- Eales LA. Song learning in Zebra Finches: Some effects of song model availability on what is learnt and when. AnimBehav. 1985;33:1293–1300. [Google Scholar]
- Funabiki Y, Funabiki K. Song retuning with tutor model by adult zebra finches. Dev Neurobiol. 2008;68:645–655. doi: 10.1002/dneu.20597. [DOI] [PubMed] [Google Scholar]
- Gahr M, Garcia-Segura LM. Testosterone-dependent increase of gap-junctions in HVC neurons of adult female canaries. Brain Res. 1996;712:69–73. doi: 10.1016/0006-8993(95)01448-9. [DOI] [PubMed] [Google Scholar]
- Gardner TJ, Naef F, Nottebohm F. Freedom and rules: the acquisition and reprogramming of a bird’s learned song. Science. 2005;308:1046–1049. doi: 10.1126/science.1108214. [DOI] [PubMed] [Google Scholar]
- Gentner TQ. Neural systems for individual song recognition in adult birds. Ann N Y Acad Sci. 2004;1016:282–302. doi: 10.1196/annals.1298.008. [DOI] [PubMed] [Google Scholar]
- Gil D, Naguib M, Riebel K, Rutstein A, Gahr M. Early condition, song learning, and the volume of song brain nuclei in the zebra finch (Taeniopygia guttata) J Neurobiol. 2006;66:1602–1612. doi: 10.1002/neu.20312. [DOI] [PubMed] [Google Scholar]
- Glaze CM, Troyer TW. Temporal structure in zebra finch song: implications for motor coding. J Neurosci. 2006;26:991–1005. doi: 10.1523/JNEUROSCI.3387-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. ProcNatlAcadSciUSA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould JL, Marler P. Learning by Instinct. SciAmer. 1987;256:74–85. [Google Scholar]
- Goymann W, Landys MM, Wingfield JC. Distinguishing seasonal androgen responses from male-male androgen responsiveness-revisiting the Challenge Hypothesis. Horm Behav. 2007;51:463–476. doi: 10.1016/j.yhbeh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Guttinger HR. The integration of learned and genetically programmed behavior: S study of hierarchical organization in songs of canaries, greenfinches and their hybrids. Zeitschrift fur Tierpsychologie. 1979;49:285–303. [Google Scholar]
- Guttinger HR. Consequences of domestication on the song structures in the canary. Behavior. 1985;94:254–278. [Google Scholar]
- Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P, Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in songbird basal ganglia nucleus Area X. PLoS Biol. 2007;5:e321. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Hamilton KS, King AP, Sengelaub DR, West MJ. Visual and song nuclei correlate with courtship skills in brown-headed cowbirds. Anim Behav. 1998;56:973–982. doi: 10.1006/anbe.1998.0848. [DOI] [PubMed] [Google Scholar]
- Heinrich JE, Nordeen KW, Nordeen EJ. Dissociation between extension of the sensitive period for avian vocal learning and dendritic spine loss in the song nucleus lMAN. Neurobiol Learn Mem. 2005;83:143–150. doi: 10.1016/j.nlm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Herrmann K, Arnold AP. The development of afferent projections to the robust archistriatal nucleus in male zebra finches: A quantitative electron microscopic study. JNeurosci. 1991;11:2063–2074. doi: 10.1523/JNEUROSCI.11-07-02063.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J Neurosci. 1999;19:10461–10481. doi: 10.1523/JNEUROSCI.19-23-10461.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley P, Pytte C, Kirn JR. Nest of origin predicts adult neuron addition rates in the vocal control system of the zebra finch. Brain Behav Evol. 2008;71:263–270. doi: 10.1159/000127046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immelmann K. Song development in the zebra finch and other Estrildid finches. In: Hinde RA, editor. Bird vocalizations. Cambridge University press; London: 1969. pp. 61–74. [Google Scholar]
- Iyengar S, Bottjer SW. Development of individual axon arbors in a thalamocortical circuit necessary for song learning in zebra finches. J Neurosci. 2002a;22:901–911. doi: 10.1523/JNEUROSCI.22-03-00901.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Bottjer SW. The role of auditory experience in the formation of neural circuits underlying vocal learning in zebra finches. J Neurosci. 2002b;22:946–958. doi: 10.1523/JNEUROSCI.22-03-00946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Bottjer SW. Growth and regression of thalamic efferents in the song-control system of male zebra finches. J Comp Neurol. 1992;326:442–450. doi: 10.1002/cne.903260309. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal gangliaforebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Kirn JR, DeVoogd TJ. Genesis and death of vocal control neurons during sexual differentiation in the zebra finch. J Neurosci. 1989;9:3176–3187. doi: 10.1523/JNEUROSCI.09-09-03176.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Nottebohm F. Direct evidence for loss and replacement of projection neurons in adult canary brain. JNeurosci. 1993;13:1654–1663. doi: 10.1523/JNEUROSCI.13-04-01654.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Schwabl H. Photoperiod regulation of neuron death in adult canary. JNeurobiol. 1997;33:223–231. doi: 10.1002/(sici)1097-4695(199709)33:3<223::aid-neu2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Kirn JR, Alvarez-Buylla A, Nottebohm F. Production and survival of projection neurons in a forebrain vocal center of adult male canaries. JNeurosci. 1991;11:1756–1762. doi: 10.1523/JNEUROSCI.11-06-01756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, O’Loughlin B, Kasparian S, Nottebohm F. Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song. ProcNatlAcadSciUSA. 1994;91:7844–7848. doi: 10.1073/pnas.91.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Fishman Y, Sasportas K, Alvarez-Buylla A, Nottebohm F. The fate of new neurons in adult canary high vocal center during the first 30 days after their formation. JCompNeurol. 1999;411(3):487–494. [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in birdsong. Ann N Y Acad Sci. 2004:463–475. doi: 10.1196/annals.1298.010. [DOI] [PubMed] [Google Scholar]
- Konishi M, Akutagawa E. Neuronal growth, atrophy and death in a sexually dimorphic song nucleus in the zebra finch brain. Nature. 1985;315:145–147. doi: 10.1038/315145a0. [DOI] [PubMed] [Google Scholar]
- Konishi M, Akutagawa E. Growth and atrophy of neurons labeled at their birth in a song nucleus of the zebra finch. ProcNatlAcadSciUSA. 1990;87:3538–3541. doi: 10.1073/pnas.87.9.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsia S, Bottjer SW. Chronic testosterone treatment impairs vocal learning in male zebra finches during a restricted period of development. JNeurosci. 1991;11:2362–2371. doi: 10.1523/JNEUROSCI.11-08-02362.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroodsma DE, Canady RA. Differences in repertoire size, singing behavior, and associated neuroanatomy amnong marsh wren populations have a genetic basis. The Auk. 1985;102:439–446. [Google Scholar]
- Kuhl PK. Human speech and birdsong: communication and the social brain. Proc Natl Acad Sci U S A. 2003;100:9645–9646. doi: 10.1073/pnas.1733998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman DS. The Reproductive Behavior of Ring Doves. Sci Am. 1964;211:48–54. doi: 10.1038/scientificamerican1164-48. [DOI] [PubMed] [Google Scholar]
- Leitner S, Voigt C, Garcia-Segura LM, Van’t Hof T, Gahr M. Seasonal activation and inactivation of song motor memories in wild canaries is not reflected in neuroanatomical changes of forebrain song areas. Horm Behav. 2001;40:160–168. doi: 10.1006/hbeh.2001.1700. [DOI] [PubMed] [Google Scholar]
- Li XC, Jarvis ED, Alvarez-Borda B, Lim DA, Nottebohm F. A relationship between behavior, neurotrophin expression, and new neuron survival. Proc Natl Acad Sci U S A. 2000;97:8584–8589. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind D, Nottebohm F, Rado R, Barnea A. Social change affects the survival of new neurons in the forebrain of adult songbirds. Behav Brain Res. 2002;133:31–43. doi: 10.1016/s0166-4328(01)00416-8. [DOI] [PubMed] [Google Scholar]
- Livingston FS, Mooney R. Development of intrinsic and synaptic properties in a forebrain nucleus essential to avian song learning. J Neurosci. 1997;17:8997–9009. doi: 10.1523/JNEUROSCI.17-23-08997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardino AJ, Nottebohm F. Age at deafening affects the stability of learned song in adult male zebra finches. J Neurosci. 2000;20:5054–5064. doi: 10.1523/JNEUROSCI.20-13-05054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Remage-Healey L, Schlinger BA. Neurosteroid production in the songbird brain: A re-evaluation of core principles. Front Neuroendocrinol. 2009 doi: 10.1016/j.yfrne.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Jr., Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Macdougall-Shackleton SA, Ball GF, Edmonds E, Sul R, Hahn TP. Age- and sex-related variation in song-control regions in Cassin’s finches, Carpodacus cassinii. Brain Behav Evol. 2005;65:262–267. doi: 10.1159/000084644. [DOI] [PubMed] [Google Scholar]
- Margoliash D. Functional organization of forebrain pathways for song production and perception. J Neurobiol. 1997;33:671–693. doi: 10.1002/(sici)1097-4695(19971105)33:5<671::aid-neu12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Marler P. Three models of song learning: evidence from behavior. J Neurobiol. 1997;33:501–516. [PubMed] [Google Scholar]
- Marler P, Waser MS. Role of auditory feedback in canary song development. J Comp Physiol Psychol. 1977;91(1):8–16. doi: 10.1037/h0077303. [DOI] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. JNeurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Velho TA, Pinaud R. Song-induced gene expression: a window on song auditory processing and perception. Ann N Y Acad Sci. 2004;1016:263–281. doi: 10.1196/annals.1298.021. [DOI] [PubMed] [Google Scholar]
- Mooney R. Synaptic basis for developmental plasticity in a birdsong nucleus. JNeurosci. 1992;12:2464–2477. doi: 10.1523/JNEUROSCI.12-07-02464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. J Neurosci. 2000;20:5420–5436. doi: 10.1523/JNEUROSCI.20-14-05420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Konishi M. Two distinct inputs to an avian song nucleus activate different glutamate receptor subtypes on individual neurons. ProcNatlAcadSciUSA. 1991;88:4075–4079. doi: 10.1073/pnas.88.10.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Rao M. Waiting periods versus early innervation: The development of axonal connections in the zebra finch song system. JNeurosci. 1994;14:6532–6543. doi: 10.1523/JNEUROSCI.14-11-06532.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler BE, Wallhausser-Franke E, DeVoogd TJ. Regressive development in neuronal structure during song learning in birds. J Neurobiol. 1995;27:204–215. doi: 10.1002/neu.480270207. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Nordeen KW. Sex and regional differences in the incorporation of neurons born during song learning in zebra finches. JNeurosci. 1988a;8:2869–2874. doi: 10.1523/JNEUROSCI.08-08-02869.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Projection neurons within a vocal motor pathway are born during song learning in zebra finches. Nature. 1988b;334:149–151. doi: 10.1038/334149a0. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. BehavNeural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Long-term maintenance of song in adult zebra finches is not affected by lesions of a forebrain region involved in song learning. BehavNeural Biol. 1993;59:79–82. doi: 10.1016/0163-1047(93)91215-9. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Synaptic and molecular mechanisms regulating plasticity during early learning. Ann N Y Acad Sci. 2004;1016:416–437. doi: 10.1196/annals.1298.018. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Testosterone triggers growth of brain vocal control nuclei in adult female canaries. BrainRes. 1980;189:429–436. doi: 10.1016/0006-8993(80)90102-x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. From bird song to neurogenesis. SciAmer. 1989;260:74–79. doi: 10.1038/scientificamerican0289-74. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;1016:628–658. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME. Relationship between song repertoire and age in the canary, Serinus canarius. ZTierpsychol. 1978;46:298–305. [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. JCompNeurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Kasparian S, Pandazis C. Brain space for a learned task. BrainRes. 1981;213:99–109. doi: 10.1016/0006-8993(81)91250-6. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane L. Developmental and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behavioral Neural Biol. 1986;46:445–471. doi: 10.1016/s0163-1047(86)90485-1. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane LA, Wingfield JC. Seasonal changes in gonadal hormone levels of adult male canaries and their relation to song. Behavioral Neural Biol. 1987;47:197–211. doi: 10.1016/s0163-1047(87)90327-x. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Searcy WA, Peters S. Brain development, song learning and mate choice in birds: a review and experimental test of the “nutritional stress hypothesis”. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:1003–1014. doi: 10.1007/s00359-002-0361-3. [DOI] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci U S A. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- Pröve E. Hormonal correlates of behavioural development in male zebra finches. Springer-Verlag; Berlin: 1983. [Google Scholar]
- Pytte CL, Suthers RA. Sensitive period for sensorimotor integration during vocal motor learning. J Neurobiol. 2000;42:172–189. doi: 10.1002/(sici)1097-4695(20000205)42:2<172::aid-neu2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Pytte CL, Gerson M, Miller J, Kirn JR. Increasing stereotypy in adult zebra finch song correlates with a declining rate of adult neurogenesis. Dev Neurobiol. 2007;67:1699–1720. doi: 10.1002/dneu.20520. [DOI] [PubMed] [Google Scholar]
- Raouf S, Van Roo B, Sengelaub D. Adult plasticity in hormone-sensitive motoneuron morphology: methodological/behavioral confounds. Horm Behav. 2000;38:210–221. doi: 10.1006/hbeh.2000.1620. [DOI] [PubMed] [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Mello CV, Jarvis ED. Songbirds and the revised avian brain nomenclature. Ann N Y Acad Sci. 2004;1016:77–108. doi: 10.1196/annals.1298.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, He X, Scotto-Lomassese S, Scharff C. Recruitment of FoxP2-expressing neurons to area X varies during song development. Dev Neurobiol. 2007;67:809–817. doi: 10.1002/dneu.20393. [DOI] [PubMed] [Google Scholar]
- Rose GJ, Goller F, Gritton HJ, Plamondon SL, Baugh AT, Cooper BG. Species-typical songs in white-crowned sparrows tutored with only phrase pairs. Nature. 2004;432:753–758. doi: 10.1038/nature02992. [DOI] [PubMed] [Google Scholar]
- Rosen MJ, Mooney R. Inhibitory and excitatory mechanisms underlying auditory responses to learned vocalizations in the songbird nucleus HVC. Neuron. 2003;39:177–194. doi: 10.1016/s0896-6273(03)00357-x. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119:233–244. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. JNeurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F, Cynx J. Conspecific and heterospecific song discrimination in male zebra finches with lesions in the anterior forebrain pathway. J Neurobiol. 1998;36:81–90. [PubMed] [Google Scholar]
- Scharff C, Haesler S. An evolutionary perspective on FoxP2: strictly for the birds? Curr Opin Neurobiol. 2005;15:694–703. doi: 10.1016/j.conb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron. 2000;25:481–492. doi: 10.1016/s0896-6273(00)80910-1. [DOI] [PubMed] [Google Scholar]
- Scott BB, Lois C. Developmental origin and identity of song system neurons born during vocal learning in songbirds. J Comp Neurol. 2007;502:202–214. doi: 10.1002/cne.21296. [DOI] [PubMed] [Google Scholar]
- Scott LL, Singh TD, Nordeen EJ, Nordeen KW. Developmental patterns of NMDAR expression within the song system do not recur during adult vocal plasticity in zebra finches. J Neurobiol. 2004;58:442–454. doi: 10.1002/neu.10300. [DOI] [PubMed] [Google Scholar]
- Singh TD, Basham ME, Nordeen EJ, Nordeen KW. Early sensory and hormonal experience modulate age-related changes in NR2B mRNA within a forebrain region controlling avian vocal learning. J Neurobiol. 2000;44:82–94. doi: 10.1002/1097-4695(200007)44:1<82::aid-neu8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Sober SJ, Brainard MS. Adult birdsong is actively maintained by error correction. Nat Neurosci. 2009 Jul;12(7):927–31. doi: 10.1038/nn.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Characterization of neurons born and incorporated into a vocal control nucleus during avian song learning. Brain Res. 1993;620:335–338. doi: 10.1016/0006-8993(93)90176-n. [DOI] [PubMed] [Google Scholar]
- Solis MM, Doupe AJ. Compromised neural selectivity for song in birds with impaired sensorimotor learning. Neuron. 2000;25:109–121. doi: 10.1016/s0896-6273(00)80875-2. [DOI] [PubMed] [Google Scholar]
- Stark LL, Perkel DJ. Two-stage, input-specific synaptic maturation in a nucleus essential for vocal production in the zebra finch. J Neurosci. 1999;19:9107–9116. doi: 10.1523/JNEUROSCI.19-20-09107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellitano KE, Zollinger S, Kirn JR. Society for Neuroscience. Washington D.C.: 2003. A retroviral analysis of neurons formed in the adult zebra finch brain. [Google Scholar]
- Striedter GF, Charvet CJ. J Comp Neurol. Vol. 507. 2008. Developmental origins of species differences in telencephalon and tectum size: morphometric comparisons between a parakeet (Melopsittacus undulatus) and a quail (Colinus virgianus) pp. 1663–1675. [DOI] [PubMed] [Google Scholar]
- Theunissen FE, Amin N, Shaevitz SS, Woolley SM, Fremouw T, Hauber ME. Song selectivity in the song system and in the auditory forebrain. Ann N Y Acad Sci. 2004;1016:222–245. doi: 10.1196/annals.1298.023. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Brenowitz EA. Seasonal change in neuron size and spacing but not neuronal recruitment in a basal ganglia nucleus in the avian song control system. J Comp Neurol. 2005;481:276–283. doi: 10.1002/cne.20381. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Brenowitz EA. Caspase inhibitor infusion protects an avian song control circuit from seasonal-like neurodegeneration. J Neurosci. 2008;28:7130–7136. doi: 10.1523/JNEUROSCI.0663-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Bentley GE, Brenowitz EA. Rapid seasonal-like regression of the adult avian song control system. Proc Natl Acad Sci U S A. 2007;104:15520–15525. doi: 10.1073/pnas.0707239104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Johnson F. HVC microlesions do not destabilize the vocal patterns of adult male zebra finches with prior ablation of LMAN. Dev Neurobiol. 2007;67:205–218. doi: 10.1002/dneu.20287. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. A field study of seasonal neuronal incorporation into the song control system of a songbird that lacks adult song learning. J Neurobiol. 1999;40:316–326. doi: 10.1002/(sici)1097-4695(19990905)40:3<316::aid-neu4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Wingfield JC, Brenowitz EA. Contributions of social cues and photoperiod to seasonal plasticity in the adult avian song control system. J Neurosci. 1999;19:476–483. doi: 10.1523/JNEUROSCI.19-01-00476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Hartman VN, Brenowitz EA. Breeding conditions induce rapid and sequential growth in adult avian song control circuits: a model of seasonal plasticity in the brain. J Neurosci. 2000;20:854–861. doi: 10.1523/JNEUROSCI.20-02-00854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien JZ. Linking Hebb’s coincidence-detection to memory formation. Curr Opin Neurobiol. 2000;10:266–273. doi: 10.1016/s0959-4388(00)00070-2. [DOI] [PubMed] [Google Scholar]
- Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature. 2007;450:1240–1244. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- Van der Linden A, Van Camp N, Ramos-Cabrer P, Hoehn M. Current status of functional MRI on small animals: application to physiology, pathophysiology, and cognition. NMR Biomed. 2007;20:522–545. doi: 10.1002/nbm.1131. [DOI] [PubMed] [Google Scholar]
- Van der Linden A, Verhoye M, Van Auderkerke J, Peeters R, Eens M, Newman SW, Smulders T, Balthazart J, DeVoogd TJ. Non invasive in vivo anatomical studies of the oscine brain by high resolution MRI microscopy. J Neurosci Methods. 1998;81:45–52. doi: 10.1016/s0165-0270(98)00013-2. [DOI] [PubMed] [Google Scholar]
- Van Meir V, Pavlova D, Verhoye M, Pinxten R, Balthazart J, Eens M, Van der Linden A. In vivo MR imaging of the seasonal volumetric and functional plasticity of song control nuclei in relation to song output in a female songbird. Neuroimage. 2006;31:981–992. doi: 10.1016/j.neuroimage.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Vates GE, Nottebohm F. Feedback circuitry within a song-learning pathway. ProcNatlAcadSciUSA. 1995;92:5139–5143. doi: 10.1073/pnas.92.11.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario DS, Yohay KH. Song-selective auditory input to a forebrain vocal control nucleus in the zebra finch. JNeurobiol. 1993;24:488–505. doi: 10.1002/neu.480240407. [DOI] [PubMed] [Google Scholar]
- Vockel A, Pröve E, Balthazart J. Effects of castration and testosterone treatment on the activity of testosterone-metabolizing enzymes in the brain of male and female zebra finches. Journal of Neurobiology. 1990;21:808–825. doi: 10.1002/neu.480210514. [DOI] [PubMed] [Google Scholar]
- Voigt C, Leitner S. Seasonality in song behaviour revisited: seasonal and annual variants and invariants in the song of the domesticated canary (Serinus canaria) Horm Behav. 2008;54:373–378. doi: 10.1016/j.yhbeh.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Volman SF. Development of neural selectivity for birdsong during vocal learning. J Neurosci. 1993;13:4737–4747. doi: 10.1523/JNEUROSCI.13-11-04737.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu ET, Mazurek ME, Kuo Y-C. Identification of a forebrain motor programming network for the learned song of zebra finches. JNeurosci. 1994;14:6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Ann N Y Acad Sci. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- Wallhäusser-Franke E, Nixdorf-Bergweiler BE, DeVoogd TJ. Song isolation is associated with maintaining high spine frequencies on zebra finch IMAN neurons. NeurobiolLearnMem. 1995;64:25–35. doi: 10.1006/nlme.1995.1041. [DOI] [PubMed] [Google Scholar]
- Wang J, Hessler NA. Coordination of presynaptic and postsynaptic maturation in a zebra finch forebrain motor control nucleus during song learning. Eur J Neurosci. 2006;24:2859–2869. doi: 10.1111/j.1460-9568.2006.05173.x. [DOI] [PubMed] [Google Scholar]
- Wang N, Aviram R, Kirn JR. Deafening alters neuron turnover within the telencephalic motor pathway for song control in adult zebra finches. J Neurosci. 1999;19:10554–10561. doi: 10.1523/JNEUROSCI.19-23-10554.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Hurley P, Pytte C, Kirn JR. Vocal control neuron incorporation decreases with age in the adult zebra finch. J Neurosci. 2002;22:10864–10870. doi: 10.1523/JNEUROSCI.22-24-10864.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BC, Nordeen EJ, Nordeen KW. Individual variation in neuron number predicts differences in the propensity for avian vocal imitation. Proc Natl Acad Sci U S A. 1998;95:1277–1282. doi: 10.1073/pnas.95.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BC, Nordeen EJ, Nordeen KW. Anatomical and ontogenetic factors producing variation in HVc neuron number in zebra finches. Brain Res. 2001;904:318–326. doi: 10.1016/s0006-8993(01)02488-x. [DOI] [PubMed] [Google Scholar]
- Wilbrecht L, Kirn JR. Neuron Addition and Loss in the Song System: Regulation and Function. Ann NY Acad Sci. 2004;1016:659–683. doi: 10.1196/annals.1298.024. [DOI] [PubMed] [Google Scholar]
- Wilbrecht L, Nottebohm F. Age and experience affect the recruitment of new neurons to the song system of zebra finches during the sensitive period for song learning: ditto for vocal learning in humans? Ann N Y Acad Sci. 2004;1021:404–409. doi: 10.1196/annals.1308.049. [DOI] [PubMed] [Google Scholar]
- Wilbrecht L, Crionas A, Nottebohm F. Experience affects recruitment of new neurons but not adult neuron number. J Neurosci. 2002;22:825–831. doi: 10.1523/JNEUROSCI.22-03-00825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbrecht L, Williams H, Gangadhar N, Nottebohm F. High levels of new neuron addition persist when the sensitive period for song learning is experimentally prolonged. J Neurosci. 2006;26:9135–9141. doi: 10.1523/JNEUROSCI.4869-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H. Birdsong and singing behavior. Ann N Y Acad Sci. 2004;1016:1–30. doi: 10.1196/annals.1298.029. [DOI] [PubMed] [Google Scholar]
- Williams H. Birdsong and singing behavior. In: Zeigler HP, Marler P, editors. Neuroscience of birdsong. Cambridge University Press; London, UK: 2008. pp. 32–49. [Google Scholar]
- Williams H, Nottebohm F. Auditory responses in avian vocal motor neurons: a motor theory for song perception in birds. Science. 1985;229:279–282. doi: 10.1126/science.4012321. [DOI] [PubMed] [Google Scholar]
- Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol. 1999;39:14–28. [PubMed] [Google Scholar]
- Williams H, Connor DM, Hill JW. Testosterone decreases the potential for song plasticity in adult male zebra finches. Horm Behav. 2003;44:402–412. doi: 10.1016/j.yhbeh.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Yu AC, Margoliash D. Temporal hierarchical control of singing in birds. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]
- Zevin JD, Seidenberg MS, Bottjer SW. Limits on reacquisition of song in adult zebra finches exposed to white noise. J Neurosci. 2004;24:5849–5862. doi: 10.1523/JNEUROSCI.1891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]