Abstract
Preface
The genetic systems controlling body axis formation trace back as far as the ancestor of diploblasts (corals, hydra, and jellyfish) and triploblasts (bilaterians). Comparative molecular studies provide powerful tools for elucidating the origins of mechanisms for establishing the dorsal-ventral and anterior-posterior axes in bilaterians and reveal differences in the evolutionary pressures acting upon tissue patterning. In this review, we focus on the origins of nervous system patterning and discuss recent comparative genetic studies; these indicate the existence of an ancient molecular mechanism underlying nervous system organization that was probably already present in the bilaterian ancestor.
The evolutionary conservation of genes involved in patterning the primary body axes has revolutionized the fields of both developmental and evolutionary biology, and has spawned the field of EvoDevo. One of the most fertile areas of EvoDevo has been the analysis of neural induction, during which the embryonic ectoderm is partitioned into neural and epidermal domains. Current studies have raised several important questions, including whether a condensed CNS has arisen only once or multiple times during evolution and whether patterning of the CNS by bone morphogenetic proteins (BMPs) in different branches of the phylogenetic tree reflects a conserved ancestral mechanism (homology) or parallel evolution (convergent evolution). Answers to these questions are essential for reconstructing the nature of the nervous system in the common ancestor of bilateral organisms and, more generally, for translating molecular similarities between existing organisms to morphological homologies in the body plans of ancestors. Several technological innovations have aided recent experimental advances, such as imaging multiple gene expression patterns and comparing genome sequences and homologous gene expression patterns in an ever increasing array of organisms from different branches of evolution. In this review, we discuss the key issues pertaining to the role of BMP signaling in establishing pattern within the developing CNS and its evolutionary implications.
Recent phylogenetic analyses suggest that bilaterian organisms can be subdivided into three main branches: the Ecdysozoa (moulting invertebrates such as arthropods and nematodes), Lophotrochozoa (non-moulting invertebrates such as mollusks and annelid worms), and Deuterostoma (including chordates, hemichordates, and echinoderms 1. Among the 37 extant phyla populating these three branches there are many examples of organisms with condensed ventral nerve chords that are partitioned into two or three primary tracts2. This recurring theme argues for evolutionary conservation of the trunk CNS across these lineages. However, there are also scattered examples of bilaterian species with a diffuse CNS, which raises the question of which of the two forms represents the most derived or most primitive state, and when the two different modes of nervous system patterning are likely to have arisen. In principle, it should be possible to distinguish between these alternatives by comparing fossil records for various lineages. However, in practice, due to incomplete fossil data, these questions demand comparative genetic and molecular studies.
Two of the best studied examples of comparative molecular anatomy are the mutually exclusive expression of BMPs and their antagonists in the epidermal and neural ectoderm, respectively, and the conserved relative expression domains of neural identity genes that subsequently subdivide the nervous system along the dorsal-ventral (D/V) axis. There are striking parallels in how the BMP signaling pathway controls D/V patterning and establishes the embryonic neural territory in a variety of organisms3-5. BMP signaling is also involved in the aforementioned subdivision of the neuroectoderm of vertebrates and invertebrates6, and similar expression and regulatory relationships have been found in a primitive polychaete annelid7. These parallels strongly suggest that a conserved genetic system controls neural induction and patterning, and also that the role of BMP4 signaling and that of its fly homologue decapentaplegic (Dpp) in this process traces back to a common bilaterian ancestor with an organized nervous system. Given these recent findings, we argue that the diffuse nervous systems found in a broad array of organisms probably represent derived secondary simplifications, rather than the ancestral bilaterian state.
In this review, we first discuss the evidence for a conserved genetic system controlling neural induction and its subsequent role in neural patterning. We also compare neural patterning in different organisms, including hemichordates. These latter organisms have a diffuse nerve net, which we hypothesize have lost the ability to pattern the neuroectoderm in response to BMPs. Finally, we propose a hypothesis that may help reconcile the various findings and that provides a starting point for comparisons of the regulatory systems that control patterning along the anterior-posterior (A/P) and D/V axes.
St. Hilaire’s hypothesis
The D/V axis is inverted in vertebrates versus invertebrates
In 1822, the prominent comparative anatomist Geoffrey Saint-Hilaire noted that the general organization of the body plan was virtually identical in vertebrates and invertebrates, except that they were inverted along the D/V axis with respect to each other8 (Box 1). The vertebrate heart, for instance, lies along the ventral side, but is located dorsally in invertebrates. Similarly, the nervous system lies dorsally in vertebrates, but ventrally in invertebrates (Fig. 1). As would be expected if the D/V axis were inverted in vertebrates relative to invertebrates, BMP-4 and Dpp are expressed in inverse patterns in these organisms, where they act to define the epidermal ectoderm. Conversely, secreted BMP antagonists, such as Short gastrulation (Sog) in Drosophila melanogaster and its vertebrate homologue Chordin (Chd), are expressed in patterns complementary to Dpp/BMP and function to promote genesis of the nervous system [the relative positioning of the NS in flies/vertebrates has already been mentioned in this paragraph OK]9-11. Although Saint-Hilaire’s proposal was initially rejected, it was resurrected almost two hundred years later, in the light of conserved gene expression patterns and regulatory relationships between BMP/Dpp, Sog/Chd and other pathway components during neural induction in flies and vertebrates12-17.
Box 1: Inversion of the dorsal ventral axis in vertebrates?

A unique feature of the nerve chord in vertebrates is that it forms dorsally whereas in invertebrates it forms ventrally. Since the heart and direction of fluid flow are also reversed in vertebrates versus invertebrates, Geoffrey St. Hilaire suggested that the dorsal-ventral (D/V) axes may have been inverted during evolution. The reversed relative expression patterns of components of the bone morphogenetic protein (BMP) signaling system, such as BMPs, Short gastrulation/Chordin (Sog/Chd), Tolloid/Xolloid (Tld/Xld), and Twisted gastrulation (Tsg), are consistent with an early developmental inversion of the D/V axis. An important evolutionary implication of the D/V inversion hypothesis is that both the vertebrate and invertebrate lineages are likely to have inherited a centralized nervous system from a common ancestor, which was condensed to one side of the body and was subsequently inverted dorso-ventrally in the vertebrate lineage (see panels a, b; blue indicates ventral nervous system defective/NK transcription factor related (vnd/Nkx) domain, green indicates intermediate neuroblasts defective/Genomic screen homeobox (ind/Gsh), red indicates muscle segment homeobox (msh/Msx)).
It should be noted that the assignment ‘ventral’ and ‘dorsal’ is somewhat arbitrary, as the ventral side of both vertebrates and invertebrates is defined by the location of the mouth orifice. Otherwise, one could imagine that D/V inversion was a trivial consequence of a vertebrate ancestor just evolving to swim upside down. A necessary element of the D/V inversion hypothesis is that there was a concomitant shift of the mouth to the opposite side in a vertebrate ancestor, which may have involved the formation of a new oral opening ventrally 87 (panel b). Another possibility to account for the relative organization of the trunk versus mouth is that there was a 180° rotation of the trunk relative to the head (panel c). This hypothesis has the advantage of providing an explanation for an otherwise puzzling feature of the vertebrate nervous systems, which is that many sensory systems project to the opposite side of the brain. An alternative hypothesis for the relative location of the mouth is that the nervous system evolved independently in vertebrate and invertebrate lineages from a more primitive and diffuse net of nerve cells76,88-90, and only later became centralized on opposite sides, either dorsally in vertebrates, or ventrally in invertebrates (panel d). As mentioned above, based on the known similarities in gene expression patterns across phylogenetic lineages, we argue that the common bilaterian ancestor had an organized centralized nerve chord, thus favoring the D/V inversion hypothesis.
The description goes from panels ‘a,b’ to ‘d’ then ‘c’. We could just switch the positions of panels ‘d’ and ‘c’ in the figure so that we describe panels in alphabetical order. Would that be ok? We prefer not to change the figure since it would disrupt its organization. We moved the relevant section of text instead.
Image modified with permission from 91
Figure 1. Neurulation in flies and vertebrates.
a) Cross-sectional diagram of the dorsal ectoderm of a vertebrate embryo showing the neural plate (blue) and adjacent epidermal ectoderm (yellow) during invagination of the neural plate to form the neural tube (from left to right). As this process proceeds, the mesoderm (red) becomes partitioned into the notochord and somites and the original dorsal midline (d.m.l.) of the embryo becomes the ventral midline (v.m.l.) of the neural tube, thereby inverting the dorsal-ventral (D/V) orientation of cells in the neural tube (NT) with respect to the body axis.
b) Cross-sectional diagram of Drosophila melanogaster embryo as it gastrulates. The ventral mesoderm (red) invaginates, resulting in the joining of the two lateral neuroectodermal domains along the future ventral midline (v.m.l.) of the embryo. Following mesoderm invagination, neuroblasts delaminate from the neuroectoderm to form the CNS. This mechanism for generating neuroblasts preserves their D/V positions with respect to the overall body axis.
Modified with permission from 91
Please indicate the tissue labeling of the final RH diagram.
NB this diagram is modified from Mizutani and Bier and therefore this source should be cited in the reference list.
An important element of the D/V inversion hypothesis is to show that the similar final patterns of neural gene expression in vertebrates and invertebrates are not just the result of fortuitous evolutionary convergence, but that this developmental process is broadly shared across different organisms. As we discuss below, there is a growing body of evidence indicating that the latter is indeed the case and that the BMP signaling pathway, which is responsible for organizing gene expression in the neuroectoderm, may once have controlled patterning along the entire D/V extent of the ectoderm.
The conserved BMP signaling pathway
The BMP signaling pathway consists of both intracellular and extracellular components that are conserved and exert similar functions from fruitflies to humans 18. The extracellular factors interact to establish the availability of ligand in graded patterns throughout tissues, whereas the intracellular signaling transduction components regulate target gene expression (see Box 2).
Box 2: The Conserved BMP signaling pathway.
In Drosophila melanogaster, there are six diffusible extracellular ligands belonging to the Transforming growth factor-β (TGF-β) superfamily of growth factors, including the Bone Morphogenetic Protein (BMP) ligands Decapentaplegic (Dpp), Screw (Scw) and Glass-bottom-boat (Gbb)92-94, whereas in vertebrates there are thirty TGF-β ligands, including BMP-2 and BMP-4, the orthologues of Dpp (reviewed by 18). BMP ligands are likely to be secreted by expressing cells in the form of homodimers (for example, Dpp/Dpp) or heterodimers (such as Dpp/Scw), and activate specific combinations of tetrameric receptor complexes formed by type I and type II Serine-Threonine kinase receptors 95. In the diagram, the names of pathway components are given for D. melanogaster; the human orthologues are indicated here in parentheses. Dpp dimers (BMP2/BMP4) bind to heterotetrameric receptors consisting of the two Thick veins (Tkv) (BMPR1A/B) type-I chains and two Punt (Put) (Activin receptor type II, ACTRII) type II chains (revised figure accordingly); by contrast, Dpp/Scw heterodimers signal through one Tkv type I chain, one Saxophone (Sax) (Activin receptor-like kinase, ALK1/2) type I chain, and two Put type II chains (revised figure accordingly)95-98. The type II chains of the activated receptor phosphorylate the type I chains, which in turn phosphorylate and activate Mothers against dpp (MAD) (SMAD1/5/8/9) to generate phospho-MAD (pMAD)99,100. pMAD forms a complex with the co-MAD Medea (SMAD4), and enters the nucleus to alter gene expression. Formation of the pMAD-coSMAD complex can be inhibited by I-MADs such as Daughters against dpp (Dad) (SMAD6/7), which negatively regulate signaling by either targeting active receptors (not shown), or at the level of transcription 18. Once in the nucleus, high levels of the pMAD-co-SMAD complex activate expression of epidermal genes dorsally. pMAD and Medea can also form a trimeric complex with Schnurri (Shn), which efficiently represses expression of neural genes in the lateral neuroectoderm where Dpp levels are much lower than dorsally. The levels of Mad and Medea that are required for repression of target genes are much less than those required for activation of epidermal genes. Another transcription factor that regulates BMP signaling is Brinker (Brk), which binds to sequences that overlap those of pMAD and blocks the response to BMP signaling27,101-103.
In addition to the intracellular regulatory control of BMP signaling, there is a tight control of the distribution and availability of the ligands in the extracellular space. The BMP antagonist short gastrulation (Sog) binds to Dpp/Scw heterodimers and prevents the ligand from gaining access to receptors [note that in the diagram the dimer captured by Sog is a non-Dpp-containing homodimer - should we make one of the spheres red?/Yes]]. Sog can also bind Dpp homodimers when in a trimeric complex with Twisted gastrulation (Tsg; not shown). The metalloprotease Tolloid (Tld) can cleave Sog, releasing Dpp to signal; Tld can also process Sog into forms that have broader BMP inhibitory activities.
No permission required.
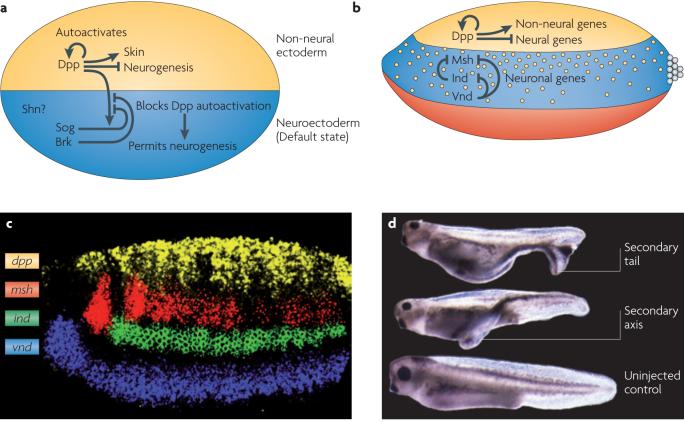
In the early embryo, the first step in neural development is the subdivision of the ectoderm into neural and epidermal domains (Fig. 2a). This process is typically referred to as neural induction4. Subsequently, the neural ectoderm is partitioned into three non-overlapping D/V stripes that express different homeobox transcription factors known as neural identity genes19 (Fig. 2b,c). The resulting tripartite neuroectoderm gives rise to three primary rows of neuroblasts that differentiate into distinct neuronal progeny. These two steps of neural development appear to be highly conserved in all three bilaterian branches and depend on two distinct modes of BMP signaling.
Figure 2. Neural induction in flies and vertebrates.
a) In the embryonic epidermis, signaling by Decapentaplegic (Dpp in flies) or bone morphogenetic protein-4 (BMP4 in vertebrates) activates expression of epidermal genes, including itself (autoactivation) and also represses expression of neural genes (see Fig. 2 for details of the BMP signaling pathway). Dpp (BMP4) can diffuse ventrally into the neuroectoderm, where it would induce its own expression via autoactivation were it not prevented from doing so by extracellular BMP antagonists such as Short gastrulation (Sog; Chordin (Chd) in vertebrates) or intracellular transcriptional repressors such as Brinker (Brk). By preventing Dpp from autoactivating in the neuroectoderm Sog and Brk allow cells to follow their default preference to develop as neuroectoderm. Schnurri (Shn) may also function in patterning the neuroectoderm by forming a trimeric complex with Medea and phosphorylated MAD (mothers against dpp) and mediating dose-dependent repression of neural target genes (see Box 2).
b) Diagram showing Dpp expression in the dorsal ectoderm of a fly embryo and expression of the neural identity genes Ventral nervous system defective (Vnd), Intermediate neuroblasts defective (Ind) and Muscle segment homeobox (Msh) in the neuroectoderm. Also depicted is ventral dominant cross-regulation among the neural identity genes wherein Vnd inhibits expression of ind and msh, and Ind inhibits expression of msh.
c) Expression of Dpp (yellow), msh (red), ind (green), and vnd (blue) in a blastoderm stage Drosophila melanogaster embryo.
d) Injection of sog mRNA into ventral cells of a frog embryo results in embryos with a duplicated neural axis (arrows).
Drosophila melanogaster gene names are shown in the figure; vertebrate homologues are listed in parenthesis in the legend. In all panels dorsal is at the top and anterior to the left.
Panel c reproduced from 125
Panel d reproduced from 17
All-or-none role of BMPs in neural induction
During the early phase of neural induction, the primary role of BMP signaling is to divert cells in epidermal regions from adopting the neural fate. This all-or-none role of BMP signaling is achieved by repressing neural gene expression and activating ectodermal genes9-11. In the neural ectoderm, BMP antagonists such as Sog/Chd bind to BMPs and prevent them from gaining access to their receptors, thereby allowing these cells to adopt the default neural fate. This process is highly conserved at the molecular level since fly Sog can act as a neural inducer in vertebrate embryos (Fig. 2d)15,17,20 and Chd can block BMP signaling in flies15,20,21.
BMPs accumulate to high levels in future epidermal regions, whereas BMP antagonists are either expressed in the neural ectoderm (for example, Sog in D. melanogaster) or diffuse into the neural ectoderm from the adjacent dorsal mesoderm (such as Chd in vertebrates)5,22. In flies, diffusion of Sog/Chd towards the dorsal region is thought to form a reciprocal BMP activity gradient in the epidermal ectoderm (Box 3). An intricate extracellular system, which includes additional antagonists and proteases that degrade Sog and thereby free BMPs from inhibitory complexes (Box 2), results in graded receptor activation5.
Box 3: Graded BMP signaling patterns dorsal non-neural ectoderm in Drosophila melanogaster.

Extracellular modulation of high levels of bone morphogenetic protein (BMP) signaling is crucial for establishing the BMP morphogenetic gradient in the dorsal region of the Drosophila melanogaster embryo. Although the genes encoding the ligands decapentaplegic (dpp) and screw (scw) are transcribed uniformly, they elicit different cell responses in a dosage-dependent manner. Cells near the dorsal midline receive peak BMP levels and form an extra-embryonic tissue known as the amnioserosa (panel a)104. Cells located more ventrally experience relatively lower levels of signaling and differentiate as epidermal tissue.
One key component for creating the BMP gradient is diffusion of Short gastrulation (Sog) dorsally from its source in the ventral neuroectoderm, resulting in a concentration gradient with high levels in the ventral side of the epidermal ectoderm [this is how it is labeled in the figure] and progressively lower levels towards the dorsal midline (panels a,b)105. Because Sog binds to BMPs and prevents receptor activation, its asymmetric distribution across the epidermal domain results in a reverse gradient of BMP activity (panel a). The influx of Sog is regulated by the metalloprotease Tolloid (Tld), which, like Dpp, is expressed exclusively in the dorsal epidermal ectoderm106. Tld functions as a local sink to produce a steady-state accumulation of Sog in the dorsal region105 (panel b). In addition, by cleaving Sog bound to BMPs, Tld frees the ligands to activate their receptors107.
Another BMP antagonist, Twisted-gastrulation (Tsg), participates in this process by forming a ternary complex with Sog and Dpp108-112. This binding enhances the capacity of Sog to inhibit BMP 95. The presence of Tsg can also alter the Sog cleavage sites targeted by Tld, leading to the formation of Sog fragments that can inhibit Dpp directly21. In addition to their inhibitory activity, Sog and Tsg enhance the BMP signaling at a long-range distance in dorsal midline cells 113. It has been suggested that such enhancement could be achieved by protecting Dpp from receptor-mediated degradation114, and also by carrying Dpp to the dorsal midline and concentrating it there95,115,116. BMP-promoting forms of processed Sog have also been identified which may participate in this long-range activation function20. Vertebrate homologues of Tsg 109 and Tld 117 also share similar biochemical properties as in flies 111,112,117-119, and there is also evidence that Chordin (Chd) may act in a dose dependent fashion in epidermal regions of vertebrate embryos120-122. It is not yet clear, however, whether these proteins are involved in a similar mechanism to patterning the epidermal domain in vertebrates.
No figure permission required.
A high level of BMP signaling in the dorsal epidermal ectoderm has two effects: it represses expression of all neural genes and activates expression of epidermal genes in a dose dependent fashion (see Boxes 2 and 3). A key gene activated by high levels of BMP signaling is dpp itself23 (see Fig. 3a). The combination of Dpp diffusion and autoactivation can result in the invasive spread of Dpp signaling into the neighbouring neuroectoderm. However, this potentially invasive positive feedback cycle is blocked in the neuroectoderm by the orthologous BMP antagonists Sog and Chd in flies and vertebrates, respectively, as well as by Noggin and DAN (Differential screening-selected gene aberrative in neuroblastoma) family members in vertebrates16,23-26. Acting in parallel with extracellular antagonists, BMP signaling is also blocked at the transcriptional level in the D. melanogaster neuroectoderm by the repressor Brinker (Brk)27. By preventing high level BMP signaling from repressing expression of neural genes, these various ‘neural-inducing’ factors thereby permit neurogenesis to proceed.
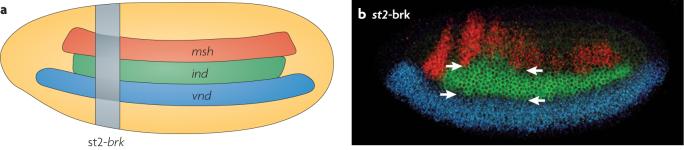
Figure 3. Patterning the neuroectoderm in flies. [ok to delete ‘vertebrates?’]Yes.
The two panels illustrate the design (a) and consequences (b) of inhibiting bone morphogenetic protein (BMP) signaling is in a localized pattern of the Drosophila melanogaster embryo by expressing the repressor Brinker (Brk) in a narrow stripe of cells (vertical gray bar in a) under the control of the even-skipped stripe-2 (st2) promoter. Indicated in horizontal stripes are expression domains of three neural identity genes: ventral nervous system defective (vnd), intermediate neuroblasts defective (ind), and muscle segment homeobox (msh) before (a) and after (b) Brk misexpression.Note the significant dorsal shift in the dorsal border of the ind expression domain and a smaller shift in the vnd/ind border. Normal borders are indicated with carets.
No permission required.
BMPs repress expression of neural genes
Recent work has identified intracellular components that mediate the repressive branch of BMP signaling in an all-or-none fashion in epidermal regions and in a graded fashion within the neuroectoderm. An important component required for BMP-mediated repression is Schnurri (Shn), which encodes a zinc-finger co-repressor protein that is expressed across the embryo in early stages28-30. Shn is recruited to specific DNA elements by the BMP signal transducers Mad and Medea to bind Dpp-dependent silencer elements31,32 (see Box 2). For example, this type of silencer element participates in the Dpp-mediated repression of gooseberry, a gene involved later in neural development32. During the blastoderm stage, however, Schnurri expression becomes largely excluded from the neuroectoderm28-30 so it is not clear how it regulates expression of neural identity genes that are repressed by BMP signaling at this stage. This reduction in Shn levels in the neuroectoderm may be necessary to allow expression of neural genes in this region28,33. It is not known whether elimination of both maternal and zygotic Schnurri function causes defects in the neuroectoderm in addition to defects in dorsal ectodermal patterning28-30. Another transcription factor involved in regulating BMP signaling in the neuroectoderm is Brk, which is expressed in a broad lateral pattern similar to sog and can block Dpp-mediated repression of neural identity genes6. In other developmental contexts, such as the wing imaginal disc, Brk blocks Dpp signaling by binding to the regulatory region of Dpp-target genes34, and suppression of Brk expression by Dpp signaling is dependent on Schnurri. Since a similar repressive trimeric MAD-Medea-Shn complex mediates potent repression of genes during neurogenesis and wing development32 it is possible that a general mechanism underlies BMP-mediated activation versus repression of target gene expression.
Dose dependent patterning of the neuroectoderm
There is evidence that low levels of BMPs can diffuse into the neural domain and contribute to the subsequent subdivision of that region into three abutting territories6. In contrast to the total repression of all neural genes observed in the non-neural-ectoderm, where BMP levels are high, the much lower BMP levels that enter the neighboring neuroectoderm repress neural genes in a dose-dependent fashion. Patterning arises as a consequence of neural genes that are more sensitive to BMP repression being expressed only in ventral-most regions of the neuroectoderm, far from the source of BMPs, whereas genes less sensitive to BMP repression are expressed closer to the epidermal source in dorsal regions.
The neuroectoderm of flies and vertebrates is subdivided into three non-overlapping domains expressing the homeobox transcription factors Drop/muscle segment homeobox (msh) (= Msx1,2,3 in vertebrates) in the domain nearest the epidermis, intermediate defective neuroblasts (ind) (= Genomic screen homeobox, Gsh1,2 in vertebrates), in the intermediate domain, and ventral nervous system defective (vnd) (=NK transcription factor-related, Nkx2.2 and/or Nkx6.1 in vertebrates), in cells adjacent to the CNS midline19 (Fig. 2b, c). These three genes are referred to as neural identity genes since in flies they are required to establish cell fates of neuroblasts in each of these domains, which divide to produce distinct neuronal cell lineages35-41. Cells expressing msh/Msx form the border between the neuroectoderm and the ectoderm, and are exposed to the highest levels of BMP signaling, while cells expressing ind/Gsh and vnd/Nkx are located progressively further away from the BMP source and receive correspondingly lower levels of BMPs (Fig. 2b,c). It is important to note that although low levels of BMPs can repress neural gene expression, they are insufficient to activate epidermal genes in the neuroectoderm owing to the fact that much higher levels of BMP signaling are required to activate than repress target gene expression23.
The broad range in sensitivity to high versus low levels of BMP signaling permits the two branches of BMP signaling to be used in different regions of the embryo (Fig. 2b, Box 3). By restricting the activation and repression branches of the BMP pathway to dorsal versus ventral domains, this single signaling system can establish multiple cell fates along the entire embryonic D/V axis (Box 3a).
Threshold-dependent repression of neural gene expression in flies
When BMP signaling is locally compromised within the D. melanogaster neuroectoderm, the borders of neural identity gene expression domains shift dorsally (Fig. 3a, b), revealing an important role of BMP signaling in establishing pattern within in the neuroectoderm6. BMPs exert their strongest influence on genes nearest to the dorsal ectoderm source of secretion. Thus, the border between msh and ind, located closest to the BMP source, shifts dorsally several cell diameters, resulting in ind expanding into the normal msh domain (Fig. 4b), whereas the border between vnd and ind, located 10-12 cell diameters from the ectoderm, shifts only by one or two cells. This concerted shift in gene expression is the result of two processes: dose-dependent repression of msh versus ind by low level BMP signaling, and ‘ventral dominant’ cross-regulatory inhibition among neural identity genes33,42, wherein more ventrally expressed transcription factors repress the expression of more dorsal genes (Fig. 3b).
Figure 4. BMP patterning in diverse organisms.
a) Evolutionary tree showing the three major branches of metazoa (ecdysozoa, lophotrochozoa, and deuterostomes - we ought to add a label for ‘deuterosteomes’ on the tree OK) as well as their relationship to diploblasts (cnidaria). b-d) Opposing Decapentaplegic/Bone morphogenetic protein (Dpp/BMP) and Short gastrulation/Chordin (Sog/Chd) expression in: a diploblast (coral) embryo (BMP4 expression in blue - Chd not shown)(b - please indicate which colours show which expression pattern here (is only BMP expression shown?; also, would you like us to add in the arrow? OK); a fly embryo (c; dpp expression in blue and sog in brown), and in frog embryos (d). Do panels b-d show RNA in situs?Yes
e) Relative gene expression patterns in flies, vertebrates, polychaete annelids, and hemichordates. The position of expression domains of neural genes between vertebrates and annelids is more similar to each other than between flies and vertebrates, although they all share a basic conserved arrangement of neural domains. By contrast, most of the nervous system patterning along the D/V axis has been lost in the hemichordate.
Panel b reproduced from 71
Image shown in panel b kindly supplied by Eldon Ball and David Hayward, Australian National University, Canberra, Australia.
Panel c reproduced from 14
Panel d, upper panel reproduced from 126
Panel d, lower panel reproduced from 127
Normally, BMPs act in concert with other patterning systems to establish borders between neural identity genes (see Box 4). One example is the Dorsal morphogenetic gradient in flies, which predetermines the territories of primary D/V tissues. However, under experimental conditions, BMPs alone can also generate neural patterning, albeit not as precisely as in wild-type embryos. Experiments performed in embryos where graded Dpp signaling could be uncoupled from patterning mediated by the Dorsal gradient revealed that less BMP signaling is required to inhibit expression of ind than msh6. Threshold-dependent repression of ind and msh results in ind expression being excluded from cells near the Dpp source. A consequence of eliminating ind expression from dorsal-most cells of the neuroectoderm by BMP signaling is to relieve the ventral dominant repression of msh by Ind, and resulting in the apparent activation of msh in those cells. This double negative mechanism effectively segregates cells into adjacent non-overlapping domains of neural gene expression.
Box 4: Organism-specific ventral patterning cues.

Organism-specific ventral patterning systems act in concert with conserved graded Bone Morphogenetic Protein (BMP) signaling to pattern the neuroectoderm (see Figure). In D. melanogaster (left-hand panels in the figure), the potent maternal dorsal/ventral (D/V) morphogen Dorsal activates genes in a threshold dependent fashion in ventral regions of the embryo, whereas in vertebrates (right-hand panels in the figure), the secreted morphogen Sonic Hedgehog (Shh) helps pattern ventral elements of the neural tube.
Ventral neural patterning in D. melanogaster
Dorsal encodes an Nuclear Factor κ-B (NFκ-B) type transcription factor that activates Short gastrulation (Sog) and represses Decapentaplegic (Dpp) in lateral regions, keeping those genes in separate domains123(middle panel). Intermediate Dorsal levels in lateral regions activate expression of other neuroectodermal (NE) genes as well, whereas high Dorsal levels in the ventral region activate mesodermal (MES) genes, such as snail and twist. These genes in turn repress the expression of neuroectodermal genes and define the ventral limit of the neuroectoderm. Comparison of the enhancers of intermediate neuroblasts defective (ind) and ventral nervous system defective (vnd) suggests that the separation between the two expression domains can be induced by Dorsal alone. The ind enhancer contains many closely spaced high affinity binding sites for Dorsal33, which allows it to be activated within the entire width of the neuroectoderm; vnd contains fewer binding sites for Dorsal and therefore is only activated in the ventral region of the neuroectoderm, where Dorsal levels are higher. Because Vnd is a repressor of ind, its early activation excludes the expression of ind from the ventral domain42. In contrast, muscle segment homeobox (msh) regulation is independent of Dorsal124.
Ventral neural patterning in vertebrates
In vertebrates, the secreted morphogen Shh diffuses from the notochord and floorplate of the neural tube to control cell fates in ventral and lateral portions of the CNS56,61. Shh signaling regulates the production of activating versus inhibitory forms of the Glioma-associated oncogene homolog (Gli) family transcription factors. High levels of Shh result in the production of activating forms of Gli (Gli2 in figure), whereas in regions of low level signaling the repressive form (Gli3) predominates. As described in the main text, severe ventral patterning defects are observed when only the activating branch of the pathway is eliminated, whereas much of the pattern is restored when the entire signaling system is eliminated. BMP antagonists such as Chordin (Chd) and Noggin emanating from the notochord act together with Shh to establish ventral cell fates by inhibiting long-range BMP signaling ventrally67,68.
The domains of neural gene expression in the bottom panel are indicated as: blue (Vnd/Nkx2.1), green (Ind/Gsh/Pax6) and red (Msh/Msx).
No permission required.
Similar patterning mechanisms in flies and vertebrates
In vertebrates, a wealth of evidence indicates that BMPs function as dorsal morphogens within the neural tube, defining distinct cell fates by different levels of signaling6,43-51. The conserved expression of neural identity genes relative to the BMP source in flies and vertebrates raises the possibility that BMPs function by similar mechanisms to regulate neural genes in these two species. However, the prevailing view in vertebrates has been that high levels of BMPs promote the expression of genes such as Msx1 in dorsal regions of the neural tube, while lower levels of BMPs activate the expression of intermediate neural genes45,48. This model is opposite to the observed neural repressive role of BMPs in flies and is based primarily on experiments showing that increasing levels of BMP expression leads to a ventral expansion of the Msx domain and a concomitant reduction of intermediate cell fates.
These apparently contrasting modes of regulatory function of BMPs in vertebrates versus flies may be deceiving as nearly all the vertebrate data could be equally well explained by a double negative mechanism similar to that described above for D. melanogaster. Thus, the expansion of Msx expression may not reflect direct activation by BMP signaling, but rather may result indirectly from inhibiting expression of a repressor of Msx. Consistent with this hypothesis, studies in zebrafish suggest that high levels of BMP signaling, corresponding to those acting in the epidermis, can suppress Msx1,2 expression50, paralleling the situation observed in flies. In addition, BMP signaling can repress the expression of intermediate genes, such as Developing brain homeobox (Dbx1, Dbx2) and Paired box (Pax6)52-55. To distinguish between these two models, it will be crucial to determine whether cross-regulation among vertebrate neural identity genes follows a ventral dominant pattern as has been demonstrated in D .melanogaster. It will also be important to test whether BMP-dependent repression of neural genes is a general mechanism operating across vertebrates.
Another parallel with fruit flies is the fact that BMP signaling in vertebrates is sufficient to pattern the neuroectoderm when this tissue is isolated from other patterning cues. In apolar chick neural plate explants that have been adjusted to generate uniform ventralized cell fates, addition of BMPs can cause a ventral-to-dorsal shift in the expression of neural genes6. Moreover, BMPs may be able to pattern the entire D/V axis of the neural tube in the complete absence of the ventral Shh patterning system56 (See below and Box 4).
Similar BMP-mediated patterning has also been observed in primitive polychaete annelids7. The sufficiency of the BMP signaling in creating neural pattern and its highly conserved activity in the three primary phylogenetic lineages of metazoa provides compelling evidence that the bilaterian ancestor already employed this signaling pathway to subdivide a highly organized nervous system, which was inherited in many descendent branches. These findings are difficult to reconcile with the alternative model that organized condensed nerve chords evolved independently in these three lineages, as would be the case if a diffuse nervous system were the primitive state in their most recent common ancestor.
Ventral patterning cues in the neuroectoderm
In conjunction with BMP gradients emanating dorsally, ventral patterning cues also help to establish neuronal fates. In D. melanogaster, there is a ventral-to-dorsal gradient of the Dorsal morphogen, while in vertebrates, Sonic Hedgehog (Shh) diffuses dorsally from the ventral notochord and floorplate of the neural tube (see Box 4). The opposing gradients of BMPs and ventral morphogens may more reliably create sharp boundaries of gene expression within a large field of cells than a single gradient. The recruitment of these additional cues appears to be species-specific, since neither NFκ-B related homologues of Dorsal in vertebrates nor Hedgehog in flies participate in D/V patterning of the nervous system. It is also possible that one of these pathways was ancestral and was lost from the other lineage. For example, it has been reported that a hedgehog homologue is expressed along the ventral midline in a mollusc embryo57. However, it remains to be determined whether this signaling pathway is involved in neuroectoderm patterning in these invertebrate organisms as well.
Dpp and Dl cooperate in flies
In flies, the Dorsal and Dpp gradients act in concert during neuroectodermal patterning. When a gradient of BMP signaling is produced in isolation from the Dorsal gradient, the resulting separation of ind and msh expression is not nearly as sharp as the endogenous mutually exclusive expression of these genes6. There are two possible mechanisms by which the Dorsal gradient may help sharpen borders within the neuroectoderm. First, as mentioned above, Dorsal directly activates ventral and lateral genes in a threshold-dependent fashion6,42 (See Box 4). Second, Dorsal dynamically regulates expression of the BMP antagonists Sog and Brk58,59, which initially are expressed throughout the entire neuroectoderm, but then fade dorsally as the maternal Dorsal gradient collapses during late blastoderm stages14,27. The graded distribution of these BMP antagonists may refine the BMP gradient formed within the neuroectoderm, thereby providing more precision to cellular responses.
The role of Shh in neural tube patterning
Interestingly, experimental assays in vertebrate systems suggest that BMPs alone can generate pattern across most of the D/V axis in the neural tube. In normal embryos, Shh is secreted from the notochord and floor plate and regulates Class I (intermediate genes such as Pax6, Iroquois related homeobox 3 (Irx3), Dbx1/2 and Pax7) and Class II genes (ventral genes such as Nkx2.2, Oligodendrocyte lineage transcription factor 2 (Olig2), Nkx6.1 and Nkx6.2)60,61. In mice mutant that lack Shh function, there is a ventral expansion of intermediate fates and a concomitant loss of ventral cell fates. There is also a loss of ventral fates in mutants for the Gli2 repressor, which mediates Shh signaling in ventral regions of the neural tube, while loss of function of Gli3 alters intermediate cell fates (see Box 4). Surprisingly, expression of most ventral markers that are lost or greatly reduced in Shh single mutants (except for Nkx2.2), can be restored in double mutants for Shh; Gli3, albeit with less regularity than in wild-type individuals56,62-66. This finding indicates that the main role of Shh signaling is to block the repressive activity of Gli3, and that most of ventral neural patterning can be elaborated in the absence of the Shh gradient. Consistent with this hypothesis, BMP mutants exhibit defects spanning the entire D/V axis of the neural tube43,48 and BMP antagonists expressed ventrally in the notochord act synergistically with Shh67,68, suggesting that inhibition of BMPs near the midline normally plays a role in ventral patterning.
These various observations strongly suggest that the BMP gradient effectively reaches the ventral most regions of the neural tube to provide cell fate cues. The ability of BMPs to pattern over such large distances and the conserved roles they function in neural patterning suggest that this pathway may once have been sufficient to pattern the entire D/V axis. The proposal that a single morphogen could pattern the entire D/V axis of the nervous system is in agreement with the speculation that the bilaterian ancestor living in Precambrian times was very small69,70. As organisms grew markedly in size during the Cambrian period, perhaps additional ventral cues were recruited independently in vertebrate and invertebrate lineages to create more robust and reliable patterning in regions far from the dorsal source of BMPs.
Neural patterning in other organisms
As argued above, the similarity of neuronal gene expression patterns and their regulation by the highly conserved BMP signaling pathway in flies (Box 2) and vertebrates (Fig. 4e) strongly suggests that this process was inherited from a common ancestor. An alternative possibility is that similar patterns of gene expression evolved independently in the vertebrate and Drosophila lineages, which is consistent with the lack of clear fossil data for a bilaterian ancestor with a condensed nervous system. One way to distinguish between conserved versus convergent evolutionary processes is to examine pattern formation in a broad variety of organisms (Fig. 4a). Although analysis of neurogenesis across phylogeny is still in its nascent phase, interesting conclusions can already be gleaned from studies in diploblasts, hemichordates, arthropods, and annelids (Figure 4b-d Note that all figure panels should be cited - c,d were missing) OK.
The antiquity of the BMP/Sog-Chd system is revealed by the localized expression of these genes in embryos of diploblasts, such as corals71,72 (Fig. 4b), jellyfish73, and the sea anemone74,75, along an axis orthogonal to the longitudinal body axis expressing nested patterns of Homeobox (Hox) genes. It is not clear, however, whether there is a necessary link between the localized expression of BMP/Sog-Chd genes and neural development. Recent analysis of the hemichordate Saccoglossus kowalevskii 76, has shown that although BMP and Sog/Chd are expressed in opposing domains, misexpression of BMPs does not suppress neuronal development and neural identity genes are not expressed in a restricted fashion along the D/V axis as they are in flies or vertebrates. However, these organisms are nearly rotationally symmetrical, so it is difficult to establish dorsoventrality in the hemichordate ectoderm, which consists of a nearly uniform epidermis with a diffuse net of nerve cells.
It is clear that the neural suppressive activity of BMPs and their inhibition by Sog/Chd in the neuroectoderm in flies is shared with other arthropods, such as spiders77 and beetles78. One missing model from the phylogenetic comparisons until recently was analysis of organisms from the third major branch of bilaterians, namely the lophotrochozoa. This branch includes annelids, mollusks, and brachiopods (Fig. 4a). Such data has now been obtained for a polychaete annelid7, which is thought to have retained many primitive characteristics present in the bilaterian ancestor. Consistent with studies in flies and vertebrates, BMPs also differentially regulate expression of neural genes in this primitive annelid. Remarkably, functional studies verified that the progeny of neurons arising from different D/V positions have similar patterns of connectivity, expression of neurotransmitters, and similar physiological properties in annelids and vertebrates7. Indeed, the conservation in gene expression patterns and cell types between the annelid worm and vertebrates is even greater than between Drosophila and vertebrates. For example, motor neurons arise only from a ventral domain in both vertebrates and annelids, while flies also produce some motor neurons from lateral and dorsal rows of neuroblasts. These detailed similarities in a third branch of the bilaterian lineage strongly suggest that the ancestor not only had a condensed nerve chord with defined cell types arising from stereotyped positions, but also employed distinct thresholds of BMP signaling to establish those cell fates79. The simplest explanation to account for absence of this pattern in S. kowalevskii is that the response to the BMP/Sog-Chd patterning system was lost in that lineage, consistent with the nearly radially symmetrical organization of the hemichordate body plan, which would gain no obvious benefit from restricting neurons to one side of the body.
Together, the existing comparative molecular data are most consistent with the bilaterian ancestor having a condensed nerve chord with at least three primary subdivisions. This view is also in agreement with the ubiquity of extant organisms having two or three ventral nerve chords (Fig. 4e). According to this view, the morphotype of a simple dispersed CNS, which is also observed broadly across phylogeny, is probably the result of frequent secondary loss of the ancestral organized body plan. This interpretation is supported by the high rate of gene loss during evolution, which has become appreciated by comprehensive comparisons of fully sequenced genomes3,80.
Regulatory treadmilling: A hypothesis for the conservation of A/P and D/V patterning genes
One of the most striking findings in developmental biology in the past two decades has been the evolutionary conservation of basic patterning mechanisms that define the body plan. These mechanisms can be exemplified by HOX genes specifying cell fates along the A/P axis81 and the complementary deployment of the BMP and Sog/Chd genes to establish conserved gene expression along the D/V axis9-11. It has generally been assumed that the conserved protein coding sequences of genes acting early in genetic hierarchies, such as the Hox or neural identity genes, reflects the conserved function of these genes in regulating shared sets of downstream effector genes.
An alternative hypothesis can be entertained regarding the function of high order regulatory factors. One could imagine, for example, that Hox genes define only an abstract positional code that is interpreted differently in morphologically diverse organisms82. This view is particularly compelling in the case of the A/P axis, which has undergone extensive morphological transformation during evolution, with different structures forming in different positions along the body in one taxon compared to another. A potential difficulty with this class of models is that it does not immediately account for the high degree of evolutionary conservation of the patterning genes. If the downstream targets differ, what constrains changes so effectively in the regulatory factor?
One possible explanation for the strong conservation of higher order regulatory factors such as the Hox genes, which may act to define only abstract positional codes, is that in any given organism they control large numbers of genes, albeit these sets of targets differ across phylogeny. According to this model, change in such a regulatory factor is constrained during evolution simply because at any given point during evolution the regulator controls many targets. The identity of downstream target genes could turn over during evolution wherein some ancestral target genes lose their regulatory dependence on the regulatory factor while others newly acquire such regulation. Although, with time, the constituency of the controlled set of genes would change, large numbers of target genes would nonetheless always be under control of the regulatory factor in any organism (Fig. 5a). We refer to this process of target gene turnover here as “regulatory treadmilling”.
Figure 5. Regulatory treadmilling.
a) Localized expression of a Homeobox (Hox) gene along the anterior/posterior (A/P) axis (left) results in expression of large sets of virtually non-overlapping genes in flies versus vertebrates (right) that are involved in generating organism-specific structures. Right panels depict hypothetical gene arrays with Hox target genes indicated by filled red circles. b) Localized expression of the Paired box 6 (Pax6) gene (eyeless/twin-of-eyeless in D. melanogaster) in the brain (left) activates two large non-overlapping sets of genes in flies versus vertebrates (right, genes indicated in purple), which, like Hox genes, are involved in generating organism specific structures. In addition there is a smaller overlapping set of genes (brown) induced by Pax6 in both species that are involved in specifying the eye field. Genes defining the eye field provide an interesting predicted exception to the A/P versus dorsal/ventral (D/V) treadmilling rate dichotomy. This module of genes may turn over less frequently, as in the case of primary tissue types. We would predict, therefore, that Pax6 target genes would consist of a rapidly treadmilling component (those genes mediating general A/P positional information in the brain) and a slower treadmilling component required for specifying the eye field. c) Expression of a neural identity gene (e.g., Ventral nervous system defective/NK transcription factor-related 2.2 (Vnd/Nkx2.2)) in a subdomain of the neuroectoderm along the D/V axis. In this case, most/virtually all target genes are in common as they are involved in specifying conserved cell/tissue types.
No permission required.
The crux of the hypothesis is that regulatory treadmilling occurs at a considerably slower pace along the D/V axis (Fig. 5c), which is subdivided into a series of well conserved tissues, than along the A/P axis, where morphological diversification is greater and less tied to specific cell types (Fig. 5a, b). We would therefore predict that two patterns would be evident for divergent species undergoing different rates of morphological diversification. First, the rate of regulatory treadmilling for Hox target genes along the A/P axis (defined as the fraction of target genes shared between the ancestral organism and it descendents) would be higher for the rapidly evolving branch with greater morphological divergence. Second, for both branches, the rate of treadmilling would be lower for D/V patterning genes functioning at an equivalent hierarchical level, such as the neural identity genes (Fig. 5c). Existing data on downstream targets of Hox genes versus tissue determining genes are too fragmentary to draw firm conclusions, but some trends are worth analyzing further. For example, the diversity of Hox target genes observed in different species is striking, with few genes being identified in more than one species 82; conversely, there are some notable examples of similarities in D/V patterning genes between species, such as genes encoding related types of ion channels and transporters in the heart 83, genes encoding proteins involved in transmitter synthesis and release in the nervous system 84, and genes involved in cell migration in the mesoderm (reviewed in 85).
In addition to the experimental determination of directly regulated targets of Hox genes or D/V identity genes (see ref 82 for an excellent discussion of rigorous criteria for establishing genes as being direct regulatory targets), intraspecific germline transplantation experiments may also be helpful in assessing the rate of A/P versus D/V treadmilling. For instance, in an attempt to create hybrids between two distantly related Drosophila species, Lawrence and colleagues transplanted germ cells from D. rajasekari into sterile (OvoD) D. melanogaster female hosts 86, which were then crossed to D. melanogaster males. In rare hybrid rajasekari/melanogaster larvae resulting from this cross, it was noted that the cuticle had normal D/V morphological structures, but showed substantial defects in the head and tail formation. Thus, it appears that one of the first departures in related species harboring a significant degree of morphological changes is to have the patterning along the A/P axis altered, while still maintaining a very similar D/V patterning system. Future analysis of intraspecific hybrid embryos with molecular markers may reveal differences in the degree of conservation in A/P versus D/V regulatory hierarchies.
Conclusions
One of the best models for understanding the evolution of developmental mechanisms is the role of BMP signaling in neural induction and subsequent patterning within the neuroectoderm. Detailed analysis of these processes in the three principal branches of bilaterian organisms has revealed striking similarities in specification and patterning of the CNS. During the early, ‘neural induction’ phase, BMPs act in an all-or-none fashion to inhibit neural gene expression in epidermal domains. Subsequently, BMPs have a dose-dependent role in determining cell fates within the neuroectoderm. In flies, this later phase of patterning relies on the same fundamental mechanism that operates during neural induction, namely repression of neural gene expression. However, in the neuroectoderm, where BMP levels are limiting, gene repression is dosage dependent. BMPs also have a dosage sensitive function in patterning the neuroectoderm of vertebrates and polychaete annelids, but it has not been clearly established whether the mechanism is the same as the one observed in flies.
Although current day flies and vertebrates employ ventral patterning cues in addition to dorsally derived BMPs, we propose a model in which BMPs had an ancestral function in patterning the full D/V axis of the neuroectoderm based on the common elements of gene regulation observed in flies, vertebrates, and annelids. The model is based in part on the premise that high-order regulatory genes control similar sets of downstream target genes in corresponding cell types in diverse organisms. We further suggest that this conserved regulation of target genes may be a more salient property of D/V patterning genes, which define conserved cell types, than of A/P patterning genes, which may define a more abstract positional code.
Abbreviations
- BMPs
Bone morphogenetic proteins
- Dbx1/2 and dbx
Developing brain homeobox
- Dlx and dlx
Distal-less homeobox
- ey
eyeless
- Gsh
Genomic screen homeobox
- ind
intermediate neuroblasts defective
- msh
muscle segment homeobox
- Msx
Muscle segment homeobox
- Nkx2.2, Nkx6.1, Nkx6.2 and nkx6
Nk transcription factor-related
- Pax6 and Pax3/7
Paired box
- sim
single-minded
- vnd
ventral nervous system defective
GLOSSARY
- HEMICHORDATES
(from greek: hemi-, half; from latin: chorda-, cord) Marine wormlike animals that can be slow burrowers (acorn worms, for example) or sessile (pterobranchs, for example). The hemichordata phylum is closely related to echinodermata and chordata phyla; together they comprise the deuterostomata superphylum.
- ECHINODERMS
(from greek: ekhinos-, spiny; derma-, skin) Marine deuterostome animals that include sea stars, sea cucumbers and sea urchins. They possess bilateral symmetry during larval stages, but in adult life they become radially symmetrical.
- IMAGINAL DISC
Single layer of epithelial cells that forms a sac-like structure in the larvae and gives rise to adult appendages after metamorphosis (for example, wings, legs, antenna, eyes and genitalia).
- CUTICLE
A rigid layer of sclerotized chitin and cuticular proteins that covers the insect larval and adult epidermis and constitutes the exoskeleton.
Autobiographies
Ethan Bier received his Ph.D. training in the laboratory of Allan Maxam at Harvard University Medical School. He did his postdoctoral training in the laboratory of Lily Jan and Yuh-Nung Jan at the University of California, San Francisco (UCSF) and then took a faculty position at UC San Diego. Research in his laboratory has focused on understanding the evolutionarily conserved mechanism of early neural induction in Drosophila melanogaster embryos and on the formation of the vein pattern of the adult wing during larval and pupal development. More recently, his group has become interested in using the fly as a tool to address unresolved problems in human genetics (for example, see http://superfly.ucsd.edu/homophila/).
Claudia Mieko Mizutani is a graduate of the Institute of Biophysics at the Federal University of Rio de Janeiro (Brazil), where she had done previous work on Drosophila melanogaster development in the laboratory of Eliana Abdelhay with Helena Araujo as co-advisor. She was a recipient of a CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) PhD fellowship and did part of her graduate training at the University of California, San Diego (USA) in the laboratory of Ethan Bier, where she remained as post-doctoral researcher. Her work has been focused on the genetic and molecular mechanisms of nervous system development in the embryo and quantitative analysis of morphogen gradients in D. melanogaster. She is now joining the faculty of Case Western Reserve University (Ohio, USA) as an Assistant Professor.
Footnotes
- Signaling by bone morphogenetic proteins (BMPs)acts in a conserved all-or-none fashion to repress the expression of all neural genes in the epidermal ectoderm of bilaterian embryos.
- The dorsal-ventral (D/V) axis of vertebrate embryos is likely to be inverted with respect to that of invertebrates.
- BMP signaling represses expression of neural genes in dorsal regions of the CNS in a threshold-dependent manner in the neural ectoderm of Drosophila melanogaster embryos.
- BMPs also act in a dose-dependent manner to pattern the CNS of vertebrates and annelid worms.
- Neural patterning mediated by threshold-dependent BMP repression may be ancestral to all metazoans.
- D. melanogaster and vertebrates have different ventral patterning systems :flies use a gradient of the Dorsal transcription factor whereas a gradient of secreted Sonic hedgehog is employed in vertebrates.
- Patterning systems acting in ventral regions of the CNS act in concert with dorsally produced BMPs to refine and sharpen D/V patterning, and may have evolved independently in vertebrate and invertebrate lineages.
- Genes — such as Hox genes — that control the initial steps in establishing anterior-posterior (A/P) cell fates may define abstract positional codes that allow for rapid morphological diversification; by contrast, comparable genes acting along the D/V axis may define conserved cell types.
- To account for the conservation of both A/P and D/V regulators we propose a hypothesis, referred to as regulatory treadmilling, in which gene targets of A/P regulators turnover (or treadmill) more rapidly than those of D/V regulators.
-
Online links: We will link any gene and protein names, as well as diseases, to the relevant online databases, but please provide the names and URLs of any online resource (databases, online tutorials, freely available software, lab pages etc) that could be added to your article.TO BE ADDED LATER.OK.Flybase: http://flybase.org/Interactive Fly: http://www.sdbonline.org/fly/aimain/1aahome.htmSDB-Reaserch Links/Other Organisms: http://www.sdbonline.org/index.php?option=content&task=view&id=14&Itemid=19
-
A point by point explanation of how you have addressed the comments of the referees.I HAVE ATTACHED THIS IN A SEPARATE DOCUMENT. Thanks.
Highlighted references
- 6.Mizutani CM, Meyer N, Roelink H, Bier E. Threshold-Dependent BMP-Mediated Repression: A Model for a Conserved Mechanism That Patterns the Neuroectoderm. PLoS Biology. 2006;4:e313. doi: 10.1371/journal.pbio.0040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shows that BMPs act in a dose-dependent fashion to repress the expression of neural genes in dorsal and lateral regions of the Drosophila melanogaster embryo; its also proposes that this may be a conserved mechanism for neural patterning.
- 7.Denes AS, et al. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell. 2007;129:277–88. doi: 10.1016/j.cell.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Reveals remarkable similarities in the dorsal-ventral organization of cell markers and cell types in the CNS of annelid worms and vertebrates.
- 8.Geoffroy St.-Hilaire E. Considérations générales sur la vertèbre (Translation: General considerations on vertebrates) Mém. Mus. Hist. Nat. 1822;9:89–119. [Google Scholar]
- Suggests that the dorsal-ventral axis in invertebrates is inverted with respect to that of vertebrates.
- 12.Arendt D, Nubler-Jung K. Inversion of dorsoventral axis? Nature. 1994;371:26. doi: 10.1038/371026a0. [DOI] [PubMed] [Google Scholar]
- Resuscitates the argument of St.-Hilaire and A. Dohrn regarding common origins of the dorsal-ventral axis in vertebrates and invertebrates.
- 22.Biehs B, Francois V, Bier E. The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev. 1996;10:2922–34. doi: 10.1101/gad.10.22.2922. [DOI] [PubMed] [Google Scholar]
- Shows that the BMP antagonist short gastrulation (Sog) prevents BMPs from repressing neural gene expression in Drosophila melanogaster.
- 41.Cowden J, Levine M. Ventral dominance governs sequential patterns of gene expression across the dorsal-ventral axis of the neuroectoderm in the Drosophila embryo. Dev Biol. 2003;262:335–49. doi: 10.1016/s0012-1606(03)00395-6. [DOI] [PubMed] [Google Scholar]
- Provides evidence that neural identity genes act in a hierarchical repressive cascade wherein more ventrally expressed transcription factors repress the expression of more dorsal genes.
- 42.Barth KA, et al. Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development. 1999;126:4977–87. doi: 10.1242/dev.126.22.4977. [DOI] [PubMed] [Google Scholar]
- Shows that BMPs can act over long distances to pattern the dorsal-ventral axis of the zebrafish neural tube and that high level signaling in the epidermis can inhibit expression of dorsal markers such as muscle segment homeobox (Msx) genes.
- 65.Liem KF, Jr., Jessell TM, Briscoe J. Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development. 2000;127:4855–66. doi: 10.1242/dev.127.22.4855. [DOI] [PubMed] [Google Scholar]
- Shows that the BMP antagonist Chordin, which is secreted by the ventrally located notochord, acts in concert with Sonic hedgehog (Shh) to promote ventral cell fates, revealing the long range action of dorsally produced BMPs.
- 74.Lowe CJ, et al. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 2006;4:e291. doi: 10.1371/journal.pbio.0040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Examines the expression and function of BMPs in hemichordate embryos; it finds that while BMPs and Sog/Chd are expressed in opposing domains along the D/V axis, BMPs do not repress formation of diffusely distributed neurons in the nearly rotationally symmetrical ectoderm.
- 80.Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- Reviews the role of Hox genes in patterning the A/P axis and suggests that they act by defining abstract positional codes.
- 85.Dohrn A. Der ursprung der wirbelthiere und das princip des functionwecshels (Translation: The origin of vertebrates and the principle of successions of functions) Verlag von Wilhelm Engelman; Leipzig: 1875. [Google Scholar]
- Re-examines the relationship between the D/V axes of vertebrate and invertebrate embryos and suggests that the invertebrate body plan represents the ancestral state and that a new ventral oral opening may have formed during this process.
References
Please note that the references should be formatted according to our journal style: journal abbreviation should be in italics, volume number in bold, and page numbers provided in full (ie 123-127 rather than 123-7). We are using Endnote and there s currently no Nat. Reviews Genet. template. The closest we could find was Nat. Medicine. Do you have a NGR template we can add to Endnote or would some other Nature journal template work?
THE FORMAT WILL BE CORRECTED LATER. THERE IS ONE REF. ON PAGE 15 TO BE INCLUDED LATER (NEDERBRAGT ET AL 2002). Ok, thanks.
- 1.Dunn CW, et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–9. doi: 10.1038/nature06614. [DOI] [PubMed] [Google Scholar]
- 2.Valentine JW. On The Origin of Phyla. Vol. 614. University of Chicago Press; Chicago: 2004. [Google Scholar]
- 3.De Robertis EM. Evo-devo: variations on ancestral themes. Cell. 2008;132:185–95. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–93. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mizutani CM, Meyer N, Roelink H, Bier E. Threshold-Dependent BMP-Mediated Repression: A Model for a Conserved Mechanism That Patterns the Neuroectoderm. PLoS Biology. 2006;4:e313. doi: 10.1371/journal.pbio.0040313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denes AS, et al. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell. 2007;129:277–88. doi: 10.1016/j.cell.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Geoffroy St.-Hilaire E. Considérations générales sur la vertèbre (Translation: General considerations on vertebrates) Mém. Mus. Hist. Nat. 1822;9:89–119. [Google Scholar]
- 9.Bier E. Anti-neural-inhibition: a conserved mechanism for neural induction. Cell. 1997;89:681–4. doi: 10.1016/s0092-8674(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 10.De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson EL. Conservation of dorsal-ventral patterning in arthropods and chordates. Curr Opin Genet Dev. 1996;6:424–31. doi: 10.1016/s0959-437x(96)80063-3. [DOI] [PubMed] [Google Scholar]
- 12.Arendt D, Nubler-Jung K. Inversion of dorsoventral axis? Nature. 1994;371:26. doi: 10.1038/371026a0. [DOI] [PubMed] [Google Scholar]
- 13.Francois V, Bier E. Xenopus chordin and Drosophila short gastrulation genes encode homologous proteins functioning in dorsal-ventral axis formation. Cell. 1995;80:19–20. doi: 10.1016/0092-8674(95)90446-8. [DOI] [PubMed] [Google Scholar]
- 14.Francois V, Solloway M, O’Neill JW, Emery J, Bier E. Dorsal-ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994;8:2602–16. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- 15.Holley SA, et al. A conserved system for dorsal-ventral patterning in insects and vertebrates involving sog and chordin. Nature. 1995;376:249–53. doi: 10.1038/376249a0. [DOI] [PubMed] [Google Scholar]
- 16.Sasai Y, et al. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–90. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt J, Francois V, Bier E, Kimelman D. Drosophila short gastrulation induces an ectopic axis in Xenopus: evidence for conserved mechanisms of dorsal-ventral patterning. Development. 1995;121:4319–28. doi: 10.1242/dev.121.12.4319. [DOI] [PubMed] [Google Scholar]
- 18.Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–82. doi: 10.1038/nrm2297. [DOI] [PubMed] [Google Scholar]
- 19.Cornell RA, Ohlen TV. vnd/Nkx, ind/Gsh, and msh/Msx: conserved regulators of dorsoventral neural patterning? Curr Opin Neurobiol. 2000;10:63–71. doi: 10.1016/s0959-4388(99)00049-5. [DOI] [PubMed] [Google Scholar]
- 20.Yu K, et al. Cysteine repeat domains and adjacent sequences determine distinct BMP modulatory activities of the Drosophila Sog protein. Genetics. 2004;166:1323–36. doi: 10.1534/genetics.166.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu K, et al. Processing of the Drosophila Sog protein creates a novel BMP inhibitory activity. Development. 2000;127:2143–54. doi: 10.1242/dev.127.10.2143. [DOI] [PubMed] [Google Scholar]
- 22.De Robertis EM. Spemann’s organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biehs B, Francois V, Bier E. The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev. 1996;10:2922–34. doi: 10.1101/gad.10.22.2922. [DOI] [PubMed] [Google Scholar]
- 24.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell. 1996;86:589–98. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamb TM, et al. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–8. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- 26.Smith WC, McKendry R, Ribisi S, Jr., Harland RM. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- 27.Jazwinska A, Rushlow C, Roth S. The role of brinker in mediating the graded response to Dpp in early Drosophila embryos. Development. 1999;126:3323–34. doi: 10.1242/dev.126.15.3323. [DOI] [PubMed] [Google Scholar]
- 28.Arora K, et al. The Drosophila schnurri gene acts in the Dpp/TGF beta signaling pathway and encodes a transcription factor homologous to the human MBP family. Cell. 1995;81:781–90. doi: 10.1016/0092-8674(95)90539-1. [DOI] [PubMed] [Google Scholar]
- 29.Grieder NC, Nellen D, Burke R, Basler K, Affolter M. schnurri is required for Drosophila Dpp signaling and encodes a zinc finger protein similar to the mammalian transcription factor PRDII-BF1. Cell. 1995;81:791–800. doi: 10.1016/0092-8674(95)90540-5. [DOI] [PubMed] [Google Scholar]
- 30.Staehling-Hampton K, Laughon AS, Hoffmann FM. A Drosophila protein related to the human zinc finger transcription factor PRDII/MBPI/HIV-EP1 is required for Dpp signaling. Development. 1995;121:3393–403. doi: 10.1242/dev.121.10.3393. [DOI] [PubMed] [Google Scholar]
- 31.Muller B, Hartmann B, Pyrowolakis G, Affolter M, Basler K. Conversion of an extracellular Dpp/BMP morphogen gradient into an inverse transcriptional gradient. Cell. 2003;113:221–33. doi: 10.1016/s0092-8674(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 32.Pyrowolakis G, Hartmann B, Muller B, Basler K, Affolter M. A simple molecular complex mediates widespread BMP-induced repression during Drosophila development. Dev Cell. 2004;7:229–40. doi: 10.1016/j.devcel.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Stathopoulos A, Levine M. Localized repressors delineate the neurogenic ectoderm in the early Drosophila embryo. Dev Biol. 2005;280:482–93. doi: 10.1016/j.ydbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Affolter M, Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet. 2007;8:663–74. doi: 10.1038/nrg2166. [DOI] [PubMed] [Google Scholar]
- 35.Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–45. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 36.Chu H, Parras C, White K, Jimenez F. Formation and specification of ventral neuroblasts is controlled by vnd in Drosophila neurogenesis. Genes Dev. 1998;12:3613–24. doi: 10.1101/gad.12.22.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isshiki T, Takeichi M, Nose A. The role of the msh homeobox gene during Drosophila neurogenesis: implication for the dorsoventral specification of the neuroectoderm. Development. 1997;124:3099–109. doi: 10.1242/dev.124.16.3099. [DOI] [PubMed] [Google Scholar]
- 38.Jimenez F, et al. vnd, a gene required for early neurogenesis of Drosophila, encodes a homeodomain protein. Embo J. 1995;14:3487–95. doi: 10.1002/j.1460-2075.1995.tb07355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald JA, et al. Dorsoventral patterning in the Drosophila central nervous system: the vnd homeobox gene specifies ventral column identity. Genes Dev. 1998;12:3603–12. doi: 10.1101/gad.12.22.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skeath JB, Panganiban GF, Carroll SB. The ventral nervous system defective gene controls proneural gene expression at two distinct steps during neuroblast formation in Drosophila. Development. 1994;120:1517–24. doi: 10.1242/dev.120.6.1517. [DOI] [PubMed] [Google Scholar]
- 41.Weiss JB, et al. Dorsoventral patterning in the Drosophila central nervous system: the intermediate neuroblasts defective homeobox gene specifies intermediate column identity. Genes Dev. 1998;12:3591–602. doi: 10.1101/gad.12.22.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowden J, Levine M. Ventral dominance governs sequential patterns of gene expression across the dorsal-ventral axis of the neuroectoderm in the Drosophila embryo. Dev Biol. 2003;262:335–49. doi: 10.1016/s0012-1606(03)00395-6. [DOI] [PubMed] [Google Scholar]
- 43.Barth KA, et al. Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development. 1999;126:4977–87. doi: 10.1242/dev.126.22.4977. [DOI] [PubMed] [Google Scholar]
- 44.LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;125:2403–14. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- 45.Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–94. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- 46.Marchant L, Linker C, Ruiz P, Guerrero N, Mayor R. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol. 1998;198:319–29. [PubMed] [Google Scholar]
- 47.Neave B, Holder N, Patient R. A graded response to BMP-4 spatially coordinates patterning of the mesoderm and ectoderm in the zebrafish. Mech Dev. 1997;62:183–95. doi: 10.1016/s0925-4773(97)00659-x. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen VH, et al. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127:1209–20. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- 49.Timmer JR, Wang C, Niswander L. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development. 2002;129:2459–72. doi: 10.1242/dev.129.10.2459. [DOI] [PubMed] [Google Scholar]
- 50.Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–52. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
- 51.Wilson PA, Lagna G, Suzuki A, Hemmati-Brivanlou A. Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development. 1997;124:3177–84. doi: 10.1242/dev.124.16.3177. [DOI] [PubMed] [Google Scholar]
- 52.Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–12. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- 53.Golden JA, et al. Ectopic bone morphogenetic proteins 5 and 4 in the chicken forebrain lead to cyclopia and holoprosencephaly. Proc Natl Acad Sci U S A. 1999;96:2439–44. doi: 10.1073/pnas.96.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartley KO, Hardcastle Z, Friday RV, Amaya E, Papalopulu N. Transgenic Xenopus embryos reveal that anterior neural development requires continued suppression of BMP signaling after gastrulation. Dev Biol. 2001;238:168–84. doi: 10.1006/dbio.2001.0398. [DOI] [PubMed] [Google Scholar]
- 55.Pierani A, Brenner-Morton S, Chiang C, Jessell TM. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell. 1999;97:903–15. doi: 10.1016/s0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- 56.Jacob J, Briscoe J. Gli proteins and the control of spinal-cord patterning. EMBO Rep. 2003;4:761–5. doi: 10.1038/sj.embor.embor896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nederbragt AJ, van Loon AE, Dictus WJ. Evolutionary biology: hedgehog crosses the snail’s midline. Nature. 2002;417:811–2. doi: 10.1038/417811b. [DOI] [PubMed] [Google Scholar]
- 58.Markstein M, et al. A regulatory code for neurogenic gene expression in the Drosophila embryo. Development. 2004;131:2387–94. doi: 10.1242/dev.01124. [DOI] [PubMed] [Google Scholar]
- 59.Stathopoulos A, Van Drenth M, Erives A, Markstein M, Levine M. Whole-genome analysis of dorsal-ventral patterning in the Drosophila embryo. Cell. 2002;111:687–701. doi: 10.1016/s0092-8674(02)01087-5. [DOI] [PubMed] [Google Scholar]
- 60.Gomez-Skarmeta JL, Campuzano S, Modolell J. Half a century of neural prepatterning: the story of a few bristles and many genes. Nat Rev Neurosci. 2003;4:587–98. doi: 10.1038/nrn1142. [DOI] [PubMed] [Google Scholar]
- 61.Ruiz i Altaba A, Nguyen V, Palma V. The emergent design of the neural tube: prepattern, SHH morphogen and GLI code. Curr Opin Genet Dev. 2003;13:513–21. doi: 10.1016/j.gde.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103–15. doi: 10.1016/s1534-5807(03)00394-0. [DOI] [PubMed] [Google Scholar]
- 63.Lei Q, Zelman AK, Kuang E, Li S, Matise MP. Transduction of graded Hedgehog signaling by a combination of Gli2 and Gli3 activator functions in the developing spinal cord. Development. 2004;131:3593–604. doi: 10.1242/dev.01230. [DOI] [PubMed] [Google Scholar]
- 64.Litingtung Y, Chiang C. Specification of ventral neuron types is mediated by an antagonistic interaction between Shh and Gli3. Nat Neurosci. 2000;3:979–85. doi: 10.1038/79916. [DOI] [PubMed] [Google Scholar]
- 65.Persson M, et al. Dorsal-ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 2002;16:2865–78. doi: 10.1101/gad.243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wijgerde M, McMahon JA, Rule M, McMahon AP. A direct requirement for Hedgehog signaling for normal specification of all ventral progenitor domains in the presumptive mammalian spinal cord. Genes Dev. 2002;16:2849–64. doi: 10.1101/gad.1025702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liem KF, Jr., Jessell TM, Briscoe J. Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development. 2000;127:4855–66. doi: 10.1242/dev.127.22.4855. [DOI] [PubMed] [Google Scholar]
- 68.McMahon JA, et al. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–52. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conway Morris S. Early metazoan evolution: reconciling paleontology and molecular biology. Amer. Zool. 1998;38:867–877. [Google Scholar]
- 70.Conway-Morris S. The Cambrian “explosion” of metazoans and molecular biology: would Darwin be satisfied? Int J Dev Biol. 2003;47:505–15. [PubMed] [Google Scholar]
- 71.Hayward DC, et al. Localized expression of a dpp/BMP2/4 ortholog in a coral embryo. Proc Natl Acad Sci U S A. 2002;99:8106–11. doi: 10.1073/pnas.112021499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samuel G, Miller D, Saint R. Conservation of a DPP/BMP signaling pathway in the nonbilateral cnidarian Acropora millepora. Evol Dev. 2001;3:241–50. doi: 10.1046/j.1525-142x.2001.003004241.x. [DOI] [PubMed] [Google Scholar]
- 73.Reber-Muller S, et al. BMP2/4 and BMP5-8 in jellyfish development and transdifferentiation. Int J Dev Biol. 2006;50:377–84. doi: 10.1387/ijdb.052085sr. [DOI] [PubMed] [Google Scholar]
- 74.Rentzsch F, et al. Asymmetric expression of the BMP antagonists chordin and gremlin in the sea anemone Nematostella vectensis: implications for the evolution of axial patterning. Dev Biol. 2006;296:375–87. doi: 10.1016/j.ydbio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Finnerty JR, Pang K, Burton P, Paulson D, Martindale MQ. Origins of bilateral symmetry: Hox and dpp expression in a sea anemone. Science. 2004;304:1335–7. doi: 10.1126/science.1091946. [DOI] [PubMed] [Google Scholar]
- 76.Lowe CJ, et al. Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 2006;4:e291. doi: 10.1371/journal.pbio.0040291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akiyama-Oda Y, Oda H. Axis specification in the spider embryo: dpp is required for radial-to-axial symmetry transformation and sog for ventral patterning. Development. 2006;133:2347–57. doi: 10.1242/dev.02400. [DOI] [PubMed] [Google Scholar]
- 78.van der Zee M, Stockhammer O, von Levetzow C, Nunes da Fonseca R, Roth S. Sog/Chordin is required for ventral-to-dorsal Dpp/BMP transport and head formation in a short germ insect. Proc Natl Acad Sci U S A. 2006;103:16307–12. doi: 10.1073/pnas.0605154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arendt D, Denes AS, Jekely G, Tessmar-Raible K. The evolution of nervous system centralization. Philos Trans R Soc Lond B Biol Sci. 2008;363:1523–8. doi: 10.1098/rstb.2007.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raible F, Arendt D. Metazoan evolution: some animals are more equal than others. Curr Biol. 2004;14:R106–8. [PubMed] [Google Scholar]
- 81.Lohmann I, McGinnis W. Hox Genes: it’s all a matter of context. Curr Biol. 2002;12:R514–6. doi: 10.1016/s0960-9822(02)01025-4. [DOI] [PubMed] [Google Scholar]
- 82.Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1726. [DOI] [PubMed] [Google Scholar]
- 83.Ocorr K, et al. Genetic control of heart function and aging in Drosophila. Trends Cardiovasc Med. 2007;17:177–82. doi: 10.1016/j.tcm.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu MN, Bellen HJ. Genetic dissection of synaptic transmission in Drosophila. Curr Opin Neurobiol. 1997;7:624–30. doi: 10.1016/s0959-4388(97)80081-5. [DOI] [PubMed] [Google Scholar]
- 85.Bier E. Drosophila, the golden bug, emerges as a tool in human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 86.Lawrence PA, Ashburner M, Johnston P. An attempt to hybridize Drosophila species using pole cell transplantation. Genetics. 1993;134:1145–8. doi: 10.1093/genetics/134.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dohrn A. Der ursprung der wirbelthiere und das princip des functionwecshels (Translation: The origin of vertebrates and the principle of successions of functions) Verlag von Wilhelm Engelman; Leipzig: 1875. [Google Scholar]
- 88.Chang T, Mazotta J, Dumstrei K, Dumitrescu A, Hartenstein V. Dpp and Hh signaling in the Drosophila embryonic eye field. Development. 2001;128:4691–704. doi: 10.1242/dev.128.23.4691. [DOI] [PubMed] [Google Scholar]
- 89.Holland ND. Early central nervous system evolution: an era of skin brains? Nat Rev Neurosci. 2003;4:617–27. doi: 10.1038/nrn1175. [DOI] [PubMed] [Google Scholar]
- 90.Lowe CJ. Molecular genetic insights into deuterostome evolution from the direct-developing hemichordate Saccoglossus kowalevskii. Philos Trans R Soc Lond B Biol Sci. 2008;363:1569–78. doi: 10.1098/rstb.2007.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mizutani CM, Bier E. Graded BMP signaling in the neuroectoderm. In: Squire L, Kintner C, editors. The New Encyclopedia of Neuroscience. 2008. G.L. [Google Scholar]
- 92.Arora K, Levine MS, O’Connor MB. The screw gene encodes a ubiquitously expressed member of the TGF-beta family required for specification of dorsal cell fates in the Drosophila embryo. Genes Dev. 1994;8:2588–601. doi: 10.1101/gad.8.21.2588. [DOI] [PubMed] [Google Scholar]
- 93.Padgett RW, St Johnston RD, Gelbart WM. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-beta family. Nature. 1987;325:81–4. doi: 10.1038/325081a0. [DOI] [PubMed] [Google Scholar]
- 94.Wharton KA, Thomsen GH, Gelbart WM. Drosophila 60A gene, another transforming growth factor beta family member, is closely related to human bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1991;88:9214–8. doi: 10.1073/pnas.88.20.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shimmi O, Umulis D, Othmer H, O’Connor MB. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell. 2005;120:873–86. doi: 10.1016/j.cell.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brummel TJ, et al. Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell. 1994;78:251–61. doi: 10.1016/0092-8674(94)90295-x. [DOI] [PubMed] [Google Scholar]
- 97.Letsou A, et al. Drosophila Dpp signaling is mediated by the punt gene product: a dual ligand-binding type II receptor of the TGF beta receptor family. Cell. 1995;80:899–908. doi: 10.1016/0092-8674(95)90293-7. [DOI] [PubMed] [Google Scholar]
- 98.Penton A, et al. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a Decapentaplegic receptor. Cell. 1994;78:239–50. doi: 10.1016/0092-8674(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 99.Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM. Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics. 1995;139:1347–58. doi: 10.1093/genetics/139.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raftery LA, Twombly V, Wharton K, Gelbart WM. Genetic screens to identify elements of the decapentaplegic signaling pathway in Drosophila. Genetics. 1995;139:241–54. doi: 10.1093/genetics/139.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Winter SE, Campbell G. Repression of Dpp targets in the Drosophila wing by Brinker. Development. 2004;131:6071–81. doi: 10.1242/dev.01538. [DOI] [PubMed] [Google Scholar]
- 102.Rushlow C, Colosimo PF, Lin MC, Xu M, Kirov N. Transcriptional regulation of the Drosophila gene zen by competing Smad and Brinker inputs. Genes Dev. 2001;15:340–51. doi: 10.1101/gad.861401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kirkpatrick H, Johnson K, Laughon A. Repression of dpp targets by binding of brinker to mad sites. J Biol Chem. 2001;276:18216–22. doi: 10.1074/jbc.M101365200. [DOI] [PubMed] [Google Scholar]
- 104.Sutherland DJ, Li M, Liu XQ, Stefancsik R, Raftery LA. Stepwise formation of a SMAD activity gradient during dorsal-ventral patterning of the Drosophila embryo. Development. 2003;130:5705–16. doi: 10.1242/dev.00801. [DOI] [PubMed] [Google Scholar]
- 105.Srinivasan S, Rashka KE, Bier E. Creation of a Sog morphogen gradient in the Drosophila embryo. Dev Cell. 2002;2:91–101. doi: 10.1016/s1534-5807(01)00097-1. [DOI] [PubMed] [Google Scholar]
- 106.Shimell MJ, Ferguson EL, Childs SR, O’Connor MB. The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell. 1991;67:469–81. doi: 10.1016/0092-8674(91)90522-z. [DOI] [PubMed] [Google Scholar]
- 107.Marques G, et al. Production of a DPP activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell. 1997;91:417–26. doi: 10.1016/s0092-8674(00)80425-0. [DOI] [PubMed] [Google Scholar]
- 108.Mason ED, Konrad KD, Webb CD, Marsh JL. Dorsal midline fate in Drosophila embryos requires twisted gastrulation, a gene encoding a secreted protein related to human connective tissue growth factor. Genes Dev. 1994;8:1489–501. doi: 10.1101/gad.8.13.1489. [DOI] [PubMed] [Google Scholar]
- 109.Oelgeschlager M, Larrain J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–63. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Oelgeschlager M, et al. The pro-BMP activity of Twisted gastrulation is independent of BMP binding. Development. 2003;130:4047–56. doi: 10.1242/dev.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ross JJ, et al. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410:479–83. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- 112.Scott IC, et al. Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signalling. Nature. 2001;410:475–8. doi: 10.1038/35068572. [DOI] [PubMed] [Google Scholar]
- 113.Ashe HL, Levine M. Local inhibition and long-range enhancement of Dpp signal transduction by Sog. Nature. 1999;398:427–31. doi: 10.1038/18892. [DOI] [PubMed] [Google Scholar]
- 114.Mizutani CM, et al. Formation of the BMP activity gradient in the Drosophila embryo. Dev Cell. 2005;8:915–24. doi: 10.1016/j.devcel.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eldar A, et al. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature. 2002;419:304–8. doi: 10.1038/nature01061. [DOI] [PubMed] [Google Scholar]
- 116.Wang YC, Ferguson EL. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature. 2005;434:229–34. doi: 10.1038/nature03318. [DOI] [PubMed] [Google Scholar]
- 117.Piccolo S, et al. Cleavage of Chordin by Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–16. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chang C, et al. Twisted gastrulation can function as a BMP antagonist. Nature. 2001;410:483–7. doi: 10.1038/35068583. [DOI] [PubMed] [Google Scholar]
- 119.Larrain J, et al. Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 2001;128:4439–47. doi: 10.1242/dev.128.22.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Blitz IL, Shimmi O, Wunnenberg-Stapleton K, O’Connor MB, Cho KW. Is chordin a long-range- or short-range-acting factor? Roles for BMP1-related metalloproteases in chordin and BMP4 autofeedback loop regulation. Dev Biol. 2000;223:120–38. doi: 10.1006/dbio.2000.9740. [DOI] [PubMed] [Google Scholar]
- 121.Hama J, Weinstein DC. Is Chordin a morphogen? Bioessays. 2001;23:121–4. doi: 10.1002/1521-1878(200102)23:2<121::AID-BIES1018>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 122.Hammerschmidt M, Serbedzija GN, McMahon AP. Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev. 1996;10:2452–61. doi: 10.1101/gad.10.19.2452. [DOI] [PubMed] [Google Scholar]
- 123.Stathopoulos A, Levine M. Whole-genome expression profiles identify gene batteries in Drosophila. Dev Cell. 2002;3:464–5. doi: 10.1016/s1534-5807(02)00300-3. [DOI] [PubMed] [Google Scholar]
- 124.von Ohlen T, Doe CQ. Convergence of dorsal, dpp, and egfr signaling pathways subdivides the Drosophila neuroectoderm into three dorsal-ventral columns. Dev Biol. 2000;224:362–72. doi: 10.1006/dbio.2000.9789. [DOI] [PubMed] [Google Scholar]
- 125.Kosman D, et al. Multiplex detection of RNA expression in Drosophila embryos. Science. 2004;305:846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
- 126.Schmidt JE, von Dassow G, Kimelman D. Regulation of dorsal-ventral patterning: the ventralizing effects of the novel Xenopus homeobox gene Vox. Development. 1996;122:1711–21. doi: 10.1242/dev.122.6.1711. [DOI] [PubMed] [Google Scholar]
- 127.Schmidt JE, Suzuki A, Ueno N, Kimelman D. Localized BMP-4 mediates dorsal/ventral patterning in the early Xenopus embryo. Dev Biol. 1995;169:37–50. doi: 10.1006/dbio.1995.1124. [DOI] [PubMed] [Google Scholar]