Abstract
Background
Roux-en-Y gastric bypass surgery (RYGB) benefits patients with type 2 diabetes mellitus (T2DM) and BMI>35 kg/m2, but its effectiveness in diabetics with BMI<35 kg/m2 is unclear. Asian Indians suffer high risks of T2DM and cardiovascular disease (CVD) at relatively low body mass index (BMI) levels.
Objectives
We examined the safety and efficacy of RYGB in Indian patients with T2DM and a BMI of 22–35 kg/m2.
Setting
Tertiary care medical center.
Methods
Fifteen consecutive patients with T2DM and BMI 22–35 kg/m2 underwent RYGB. Data were prospectively collected before surgery and at 1, 3, 6, and 9 months afterward.
Results
Pre-operative subject characteristics were: male to female 8:7, age 45.6±12 years (mean ± SD), BMI 28.9±4.0 kg/m2, body weight 78.7±12.5 kg, waist circumference 100.2±6.8 cm, duration of diabetes 8.7±5.3 years. At baseline, 80% of subjects were on insulin, the rest on oral hypoglycemic medications. BMI decreased post-operatively by 20%, from 28.9±4.0 to 23.0±3.6 (P<0.001). All anti-diabetes medications were discontinued by 1 month post-surgery in 80% of subjects; by 3 months and thereafter, 100% were euglycemic off diabetes medications. Fasting blood glucose (FBG) decreased from 233±87 to 89±12 mg/dL (P<0.001), while hemoglobin A1c (HbA1c) fell from 10.1±2.0 to 6.1±0.6% (P<0.001). Waist circumference, dyslipidemia, and hypertension improved significantly. Predicted 10-year CVD risk (calculated using UKPDS equations) decreased substantially for fatal and non-fatal coronary heart disease (CHD) and strokes. There was no mortality, major surgical morbidity, or excessive weight loss.
Conclusions
RYGB safely and effectively eliminated T2DM in Asian Indians with BMI<35 kg/m2. Larger, longer-term studies are needed to confirm this benefit.
Keywords: gastric bypass, metabolic surgery, bariatric surgery, diabetes, mortality, cardiovascular risk, ghrelin, coronary heart disease
Introduction
Obesity, which affects >250 million people worldwide, is a major public health problem contributing to morbidity and mortality. Average body weights and the prevalence of obesity are rising so rapidly that the World Health Organization (WHO) has declared a global obesity epidemic.1 Because excess adiposity predisposes to T2DM , the spread of obesity is driving a parallel pandemic of diabetes, a disease that increases CVD risk by 200–400%.2 Type 2 diabetes is a particular problem in east Asia and the Indian subcontinent, where its prevalence has recently burgeoned 3–6 fold within 20–25 years.3 It is predicted that cases of diabetes will increase from 240 million in 2007 to 380 million in 2025, and ~60% of those cases will be in Asia.3
In Asian populations, the risks associated with diabetes and CVD occur at lower BMI levels than in Caucasians.4–6 This is attributed in part to differences in body composition and insulin sensitivity. Persons of Asian descent, such as Asian Indians, typically have a higher proportion of body fat than do Caucasians. For any given BMI, body fat percentage is 3–5% higher in Asians than in Caucasians, despite average absolute BMI levels being 3–4 kg/m2 lower.7,8 Furthermore, Indians store a greater proportion of their body fat viscerally, a distribution that confers increased insulin resistance and CVD risk.9,10 The higher percentage and central distribution of body fat among Asian Indians places them at particular risk for developing T2DM and CVD.6,11 It is not uncommon for Indian people to have clinically significant insulin resistance and T2DM at BMI levels that would be considered lean in other populations.6 Consequently, the WHO advocates lower values to define normal BMI in Asian Indians than in Caucasians. Among Indians, the proposed classification for overweight adult BMI is only 23–25 kg/m2, and for obesity it is >25 kg/m2 (vs. >30 kg/m2 for other populations).5
When lifestyle modifications and medications fail to promote adequate weight loss and glycemic control, surgical interventions can provide substantial, long-term benefits. In particular, RYGB is one of the most effective operations to ameliorate obesity and associated metabolic diseases in severely obese individuals. It is well documented that in patients with T2DM and BMI>35 kg/m2, RYGB promotes marked and sustained weight loss, with clear improvements in hyperglycemia, dyslipidemia, hypertension, other co-morbidities, and all-cause mortality.12–15 Consequently, RYGB is considered appropriate for individuals with BMI>35 kg/m2 and serious obesity-related co-morbidities, such as diabetes.16 Approximately 84% of obese diabetic patients who undergo RYGB experience full remission of T2DM, maintaining euglycemia off of all diabetes medications for at least 14 years after surgery.12,17,18 Increasing evidence indicates that these remarkable effects result not only from weight loss but also from weight-independent anti-diabetic mechanisms.19
Accordingly, use of RYGB to treat T2DM in less obese or even non-obese patients is increasingly being considered,20–22 especially among populations with enhanced diabetes risk at lower degrees of adiposity, such as Asians.4–8,11 However, the benefits of RYGB in patients with T2DM and BMI<35 kg/m2 are not well documented. Therefore, we conducted a prospective study to evaluate the impact of RYGB on T2DM and estimated CVD risk factors in Asian Indian patients with BMI values between 22 and 35 kg/m2.
Patients and Methods
Between December 2006 and December 2007, we studied 15 consecutive patients with T2DM who were scheduled to undergo RYGB and had a BMI between 22 and 35 kg/m2. To ensure that we did not inadvertently study patients with type 1 diabetes, study candidates were excluded if they had any of the following features: positive anti-GAD or anti-islet-cell antibodies, C-peptide <1 ng/ml, a family history of diabetes diagnosed before 30 years of age, or evidence of maturity-onset diabetes of the young (MODY) in the individual or family. Enrolled subjects were examined prospectively for 9 months following surgery. This study was approved by the Institutional Ethics Committee of the Ruby Hall Medical Center, Pune, India, and written informed consent was obtained from each participant.
Laparoscopic Roux-en-Y Gastric Bypass
All RYGB operations were performed laparoscopically by the same surgical team, using a 5-trocar approach. This procedure is executed as follows. Under general anesthesia, a pneumoperitoneum is created by inserting an optic trocar left of the midline, above the umbilicus. After insufflation, additional 12-mm working trocars are introduced under direct visualization. A Roux loop of jejunum and a jejuno-jejunostomy are constructed. The procedure begins by making a 25–30 cc gastric pouch from the proximal portion of the stomach, leaving the left gastric artery blood supply intact. The greater omentum is divided in the center, and the jejunum is divided 50 cm distal to the ligament of Treitz. The distal jejunal end is anastomosed to the gastric pouch (linear stapling followed by hand-sewn gastrojejunostomy closure) through an antecolic, antegastric route. Using a stapler, ~150 cm of the alimentary limb is anastomosed (ante-colic, ante-gastric jejuno-jejunostomy) with 50–70 cm of the proximal jejunum (biliopancreatic limb), completing the Roux-en-Y configuration. Dilute methylene blue is used to test the anastomoses. The mesenteric defect and the space behind the Roux limb are closed, leaving a drain in the left hypochondrium. Average duration of surgery for this study was 1.5 hours. Following a gastrograffin contrast study performed 24 hours after the operation, enteral feeding was initiated with clear liquids. Average hospital stay was 3–4 days.
Postoperative Management
All study participants received daily multivitamins, calcium citrate, and mineral supplements, and they were placed on a high-protein, low-fat, calorie-restricted diet. Participants also received vitamin B12 and cholecalciferol injections. Iron supplements were prescribed for premenopausal women.
Subjects were evaluated postoperatively in the clinic every week for 1 month and again at 3, 6, and 9 months. Each follow-up visit included a complete medical and laboratory workup, with evaluation by a surgeon, internist, and dietitian. Diabetes management was performed by an endocrinologist.
UKPDS Risk Engine
Following the recognition that hyperglycemia is a risk factor for CHD, the UKPDS risk engine was introduced in 2001, incorporating glycemic parameters (HbA1c and duration of diabetes) into a model to calculate the risk of CHD and stroke.23 We used this methodology to predict CVD risk before and after surgery, as the model uses diabetes-specific equations and corrects for ethnic features of Asian Indians. The risk engine is on the website of the Diabetes Trials Unit, Oxford University Centre for Diabetes, Endocrinology, and Metabolism (http://www.dtu.ox.ac.uk/index.php?maindoc=/riskengine/).
Statistical Analyses
Continuous data are presented as mean ± SD. Changes from baseline in efficacy and risk parameters were evaluated with Students t-tests. Statistical analyses were conducted using SPSS for Windows, version 16.0. P values of <0.05 were considered significant.
Results
The study group consisted of 7 females and 8 males, with the following mean pre-operative characteristics: age 45.6±12 years, BMI 28.9±4.0 kg/m2, body weight 78.7±12.5 kg, waist circumference 100.2±6.8 cm. The average duration of T2DM in study subjects was 8.7±5.3 years. Baseline glycemic indices and other metabolic parameters are summarized in Table 1.
Table 1.
Baseline Pre-Operative Metabolic Parameters
| Parameter | Values * |
|---|---|
| Duration of diabetes (years) | 8.7 ± 5.3 |
| Fasting blood glucose (mg/dL) | 233 ± 87 |
| HbA1C (%) | 10.1 ± 2.0 |
| Subjects on insulin (n) | 12 (80%) |
| Subjects on oral anti-diabetic medicines (n) |
3 (20%) |
| Subjects with hypertension (n) | 9 (60%) |
| Systolic blood pressure (mm hg) | 136 ± 20 |
| Subjects with dyslipidemia (n) | 14 (93%) |
| Total cholesterol (mg/dL) | 175 ± 22 |
| High density lipoproteins, HDL (mg /dL) | 37 ± 9 |
Continuous data presented as mean ± standard deviation.
The average duration of surgery was 1.5 hours. Subjects were ambulated in the evening of their operation, given water orally within 12 hours post-operatively, and they stayed in the hospital for 3–4 days. There were no major surgical complications or mortality in the series.
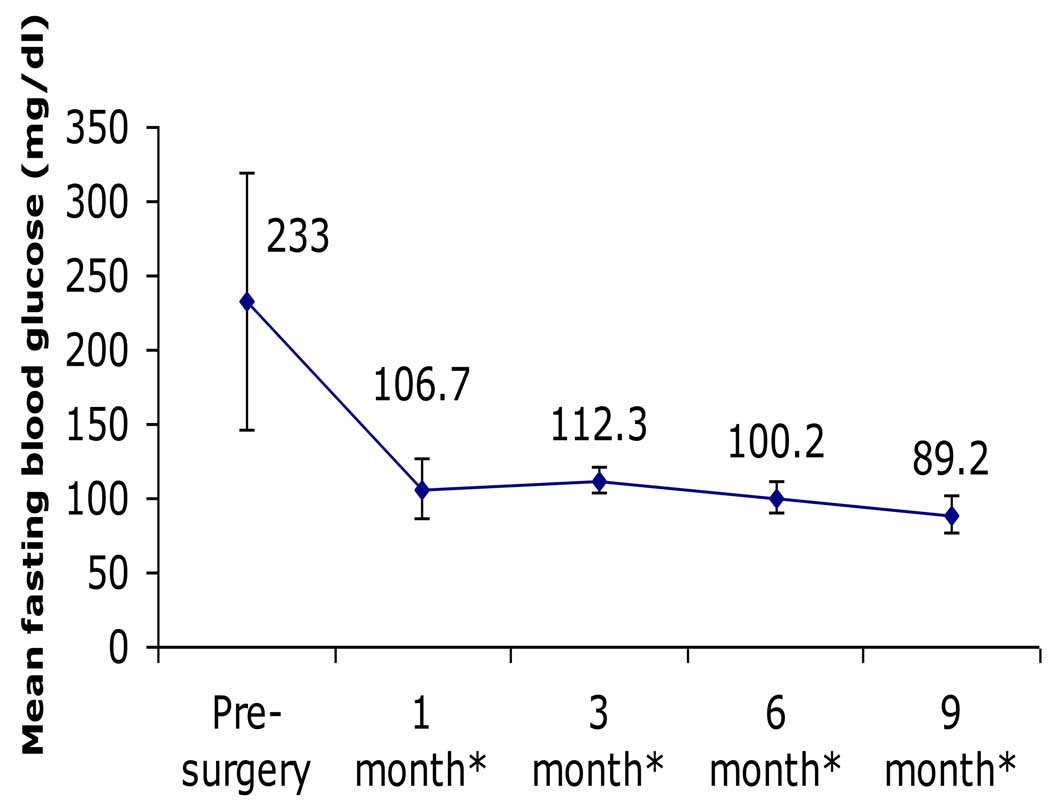
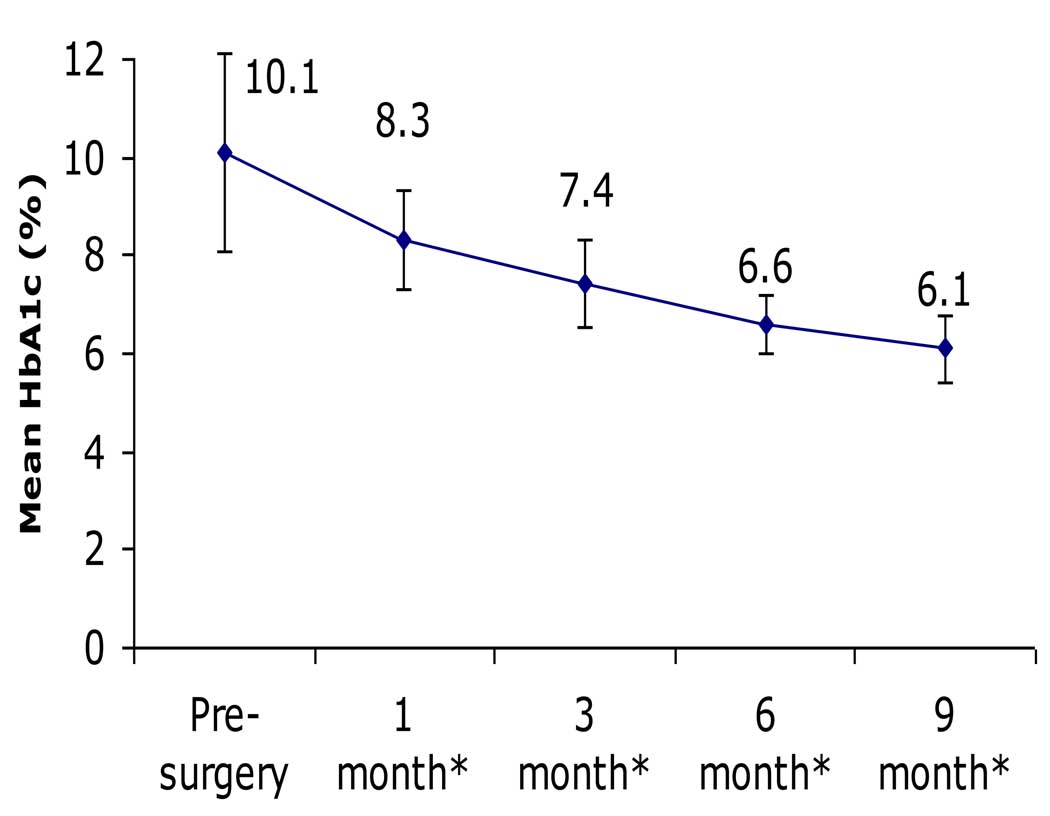
There were significant and sustained improvements among all participants in glycemic parameters, which changed from values consistent with poorly controlled diabetes to values in the normal, non-diabetic range. For example, FBG decreased from 233±87 to 89±12 mg/dL (P<0.001), while HbA1c decreased from 10.1±2.0 to 6.1±0.6% (P<0.001; Table 2, Fig. 1 & Fig. 2). BMI decreased 20%, from 28.9±4.0 to 23.0±3.6 kg/m2 (P<0.001). There were also significant improvements in body weight, waist circumference, blood pressure, and lipid profiles (Table 2).
Table 2.
Change in Glycemia and Other Parameters Following Surgery
| Parameter | Baseline (n=15) |
Follow-up (n=15) |
Change | Significance (compared with baseline) |
|---|---|---|---|---|
| BMI (kg/m2) | 28.9 | 23.0 | − 5.9 | P <0.001 |
| Body weight (kg) | 78.7 | 62.7 | − 16.0 | P <0.001 |
| Waist circumference (cm) | 100.2 | 83.3 | − 16.9 | P <0.001 |
| Fasting blood glucose (mg/dL) | 233 | 89 | − 144 | P <0.001 |
| HbA1c (%) | 10.1 | 6.1 | − 4.0 | P <0.001 |
| Systolic blood pressure (mm hg) | 136 | 116 | − 20 | P <0.001 |
| Total cholesterol (mg/dL) | 175 | 135 | − 40 | P <0.001 |
| High density lipoproteins (mg/dl) | 37 | 50 | + 13 | P <0.001 |
P is statistically significant at ≤0.05.
Figure 1. Change in fasting blood glucose following RYGB.
There was significant and sustained reduction in FBG throughout the 9 months following RYGB (* after surgery).
Figure 2. Change in HbA1c following RYGB.
There was significant and sustained reduction in HbA1c throughout the 9 months following RYGB (* after surgery).
Although all 15 subjects had pre-operative diabetic-level FBG values (range 150–410 mg/dL) while taking anti-diabetes medications, at just 1 month following surgery, 80% (12 of 15) had discontinued all of their anti-diabetes medications, and 93% (14 of 15) had FBG levels in the normal, non-diabetic range. By 3 months, 100% were euglycemic off of any diabetes medications, and every subject remained so throughout the duration of the study (i.e., at the 6- and 9-month post-operative follow-up visits). At 9 months, hypertension was corrected in 67% of previously hypertensive individuals (6 of 9), and dyslipidemia was corrected in all 14 (100%) of the previously dyslipidemic patients.
In short, even though these patients had severe baseline T2DM – with known disease for an average of nearly a decade, poor pre-operative glycemic control despite medical therapy, and insulin usage in 80% of cases – every individual experienced complete remission of T2DM by 3 months after RYGB, most of them by just one month. Thereafter they all manifested normal FBG and HbA1c values off of diabetes medications throughout the study.
The 10-year CHD risk, calculated using the UKPDS risk engine, was estimated to be 14.9% before surgery, and it decreased significantly to 4.7% after surgery (P=0.001, Table 3). The risk of fatal CHD also fell markedly from 9.8% pre-operatively to 2.5% post-operatively (P=0.004). Similarly, the risk of stroke dropped from 3.7% before surgery to 2.5% afterward (P=0.03), and the risk of fatal stroke decreased from 0.6% to 0.3% (P=0.03).
Table 3.
UKPDS 10-Year Cardiovascular Risk Predictions Before and After RYGB
| Baseline (n=15) Mean Risk (%) ± SD |
Follow-Up (n=15) Mean Risk (%) ± SD |
Absolute % Risk Reduction |
95% Confidence Interval |
Relative % Risk Reduction |
P-Value* | |
|---|---|---|---|---|---|---|
| CHD | 14.9 ± 12.7 | 4.7 ± 3.8 | − 10.1 | 5.1–15.2 | 69 | 0.001 |
| Fatal CHD | 9.8 ± 11.1 | 2.5 ± 2.6 | − 7.3 | 2.7–12.0 | 75 | 0.004 |
| Stroke | 3.7 ± 4.4 | 2.5 ± 2.7 | − 1.2 | 0.2–2.2 | 32 | 0.03 |
|
Fatal Stroke |
0.6 ± 0.7 | 0.3 ± 0.3 | − 0.3 | 0.03–0.5 | 50 | 0.03 |
P is statistically significant at ≤0.05.
An instinctive concern about performing “bariatric” surgery in less obese or merely overweight patients is that this might cause excessive weight loss with consequent malnutrition; however, we did not observe this problem in our study. Despite the group having a mean pre-operative BMI of just 28.9 kg/m2, only 4 subjects’ BMI levels fell to <22 kg/m2 by the end of the observation period. Of these patients, only one – who had the lowest baseline BMI of the cohort (22 kg/m2) – displayed a post-operative BMI below the healthy range, as defined by the WHO.5 Although this individual’s final BMI ended in the “mildly undernourished”5 range (17–18.5 kg/m2), a thorough workup failed to reveal any evidence of malnutrition. Specifically, the following blood values remained normal: albumin, total protein, globulins, vitamin D, parathyroid hormone, electrolytes (including calcium), vitamins (including B12 and folate), minerals (including iron indices), and thyroid function tests. It is worth noting that the average BMI for adult rural Indians is 19.0 kg/m2, which is only slightly higher than the range dubbed “mildly undernourished” for all populations by the WHO.5
Discussion
Because RYGB typically promotes complete remission of T2DM in severely obese patients,12,17,18 and because mounting evidence indicates that this results from hormonal and metabolic mechanisms beyond just those related to weight loss,19 use of RYGB to treat T2DM in less obese patients is increasingly being considered.20–22 This concept is particularly germane for populations with enhanced risks of diabetes and CVD at lower BMI levels, such as Asian Indians.4–8,11 Accordingly, we examined the effects of RYGB in a series of Indian patients with T2DM and BMI between 22 and 35 kg/m2. These subjects had BMI levels below the minimal cutoff for bariatric surgery according to the 1991 NIH Consensus Conference,16 but they would generally be deemed overweight or mildly to moderately obese by Indian-specific WHO criteria.5
Our subjects experienced marked, rapid improvements in glycemia, allowing them, without exception, to discontinue all diabetes medications and thereafter manifest non-diabetic FBG and HbA1c levels. This complete remission of diabetes, which had occurred in every subject by 3 months following surgery (typically by only 1 month), was particularly remarkable because the study cohort had relatively severe baseline T2DM. They had a mean duration of known disease of 9 years, poor initial glycemic control (mean pre-operative HbA1c of 10.1%), and insulin usage in 80% of cases (with oral diabetes medications in the remainder). The impressive improvement in glycemic control we observed was accompanied by substantial reductions in other obesity-related co-morbidities, including hypertension and dyslipidemia. These metabolic benefits produced major reductions in predicted CVD risk, including the probabilities of fatal and non-fatal CHD and strokes. Our salutary results were achieved without mortality, significant surgical morbidity, excessive weight loss, or malnutrition, emphasizing the safety and efficacy of RYGB in Indian patients with T2DM and BMI<35 kg/m2.
Although we did not directly compare RYBG in diabetic patients below vs. above the traditional BMI cutoff of 35 kg/m2, comparison of our results with historical controls suggests that this operation is at least as effective against diabetes among less obese persons as among patients obese enough to qualify for bariatric surgery under existing NIH guidelines.16 Patients with BMI>35 kg/m2 typically lose ~1/3 of total body weight, experiencing complete T2DM remission in ~84% of cases.12,13,17,18 Although our subjects with BMI<35 kg/m2 lost only ~1/5 of body weight during the follow-up period, they experienced 100% T2DM remission (and all have since remained euglycemic, without weight regain, during the period when this paper was being prepared and reviewed). For patients with BMI>35 kg/m2, the few whose diabetes does not remit after RYGB are characterized by longer duration of disease, for example >5 years.24 All of our subjects with BMI<35 kg/m experienced complete T2DM remission, despite an average 8.7-year duration of known disease. Our observation that diabetes improves at least as much or more among less obese patients with lesser weight loss, compared to more obese patients with greater weight loss, suggests that weight loss is not the only determinant of T2DM improvement following RYGB and that additional weight-independent anti-diabetes mechanisms are engaged.19 Consistent with this assertion, we found no correlations between the amount of weight lost and the degree of glycemic improvement – i.e., the magnitude of decrease in FBG or HbA1c – or the rapidity of T2DM remission (data not shown).
One of our most important findings was that RYGB markedly improved predicted CVD risk, as calculated with the UKPDS risk engine. Framingham risk scores have also been utilized to predict CVD risk, but this method has important limitations.25 The UKPDS risk equations are specific for T2DM patients, and they correct for glycemic control and Indian ethnicity.23,26 Thus, UKPDS risk predictions should provide more accurate estimates for our study population. Our subjects’ improvement in metabolic parameters additional to glycemia, such as total and HDL cholesterol, are included in UKPDS risk equations, and they contributed to the decrease in CVD risk. It has been demonstrated that in patients with BMI>35 kg/m2, RYGB reduces predicted CVD risk and actual observed long-term mortality.14,15,27 Our findings extend these observations to show reductions in predicted CVD risk also among patients with BMI<35 kg/m2, at least in Asian Indians with T2DM.
Our positive findings regarding RYGB in diabetic patients with BMI<35 kg/m2 complement the small extant literature on bariatric surgery performed in less obese patients with metabolic disease. Other preliminary investigations of RYGB in patients with BMI<35 kg/m2 have also found favorable effects on T2DM and dyslipidemia in Chinese21 and Brazilian20,22 populations, both of which have relatively high metabolic disease risks. Importantly, as in our cohort, excessive post-RYGB weight loss was not observed in these studies of less obese patients. Early explorations of gastric banding, biliopancreatic diversion, and experimental gastrointestinal operations to treat T2DM among patients with BMI<35 kg/m2 have also reported desirable results,20,28–30 although further studies are required to judge the balance of risks and benefits among these approaches.
Notable limitations of our work include the modest sample size, absence of control groups, and relatively short duration of follow-up. To our knowledge, however, this is the first study to evaluate RYGB as a treatment for T2DM among Asian Indian patients with BMI<35 kg/m2, and we feel that its promising results help justify larger and longer-term investigations in this area.
If corroborated by more definitive clinical trials, our findings would have important implications for diabetes care, at least among persons of Indian descent. Diet, exercise, and medications remain the cornerstones of primary T2DM therapy. However, the long-term adherence and success rates of lifestyle modifications can be disappointing, and despite an ever-increasing armamentarium of pharmacotherapeutics, adequate glycemic control often remains elusive. Moreover, most diabetes medications promote weight gain, and using them to achieve tight glycemic control increases the risk of hypoglycemia. In cases where behavioral and pharmacologic strategies prove insufficient, surgery offers a powerful alternative. Among severely obese patients, RYGB causes profound weight loss and ameliorates virtually all obesity-related co-morbidities, with acceptable surgical mortality and complication rates of <1% and 10–15%, respectively, and decreased long-term mortality.12,14,15,31 Such encouraging results in patients with T2DM and BMI>35 kg/m2, along with the continuing evolution of minimally invasive techniques with lower risks, have prompted consideration of RYGB in less obese diabetic individuals.20–22
This is of special relevance in populations such as Asian Indians, who accumulate more body fat, especially visceral fat, than Caucasians do at a comparable BMI levels.4–8,11 Our data support the use of RYGB for Indian patients with T2DM and BMI<35 kg/m2, although independent corroborations of our findings are required before considering widespread changes in clinical practice.
Conclusions
Our study was impelled by three prior observations. (1) RYGB is highly beneficial in patients with T2DM and BMI>35 kg/m2, causing diabetes remission in 84% of cases and reducing diabetes-related mortality by 92%.12,15 (2) The impressive anti-diabetic effects of RYGB result from mechanisms beyond just weight loss.19 (3) Asian Indians carry significant risks of T2DM and CVD at lower BMI levels than do Caucasians,4–8,11 yet the latter population was primarily used to establish the existing threshold of >35 kg/m2 for use of RYGB in patients with T2DM.
We therefore examined the effects of RYGB among Indian patients with T2DM and BMI<35 kg/m2. The operation caused rapid, complete remission of diabetes in all subjects, even though they had severe, longstanding disease preoperatively. Other metabolic syndrome features also improved, including dyslipidemia and hypertension, and these benefits markedly decreased predicted CVD risks from CHD and strokes. There was no mortality, untoward surgical morbidity, or excessive weight loss.
Our study suggests that RYGB is a safe, effective procedure to ameliorate T2DM and associated co-morbidities, thereby reducing CVD risk, in Asian Indian patients with T2DM and BMI<35 kg/m2. Given the technical expertise required to perform this operation, further data are required from larger and longer-term trials before routinely recommending RYGB in patients with BMI<35 kg/m2. However, our favorable preliminary findings help justify such studies designed to clarify whether the indications for RYGB should be broadened, especially in ethnic groups with enhanced T2DM risk, and whether this operation might be viewed primarily as “metabolic”, rather than “bariatric”, surgery.
Abbreviations
- BMI
body mass index
- CHD
coronary heart disease
- CVD
cardiovascular disease
- FBG
fasting blood glucose
- HbA1c
hemoglobin A1c
- MODY
maturity-onset diabetes of the young
- RYGB
Roux-en-Y gastric bypass
- T2DM
type 2 diabetes mellitus
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None of the authors has any conflicts of interest to declare. This manuscript has been read and approved by all authors.
References
- 1.James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008;32 Suppl 7:S120–S126. doi: 10.1038/ijo.2008.247. [DOI] [PubMed] [Google Scholar]
- 2.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998;21:1138–1145. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 3.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. Jama. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 4.Wen CP, David Cheng TY, Tsai SP, et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr. 2009;12:497–506. doi: 10.1017/S1368980008002802. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 6.Yajnik CS, Joglekar CV, Lubree HG, et al. Adiposity, inflammation and hyperglycaemia in rural and urban Indian men: Coronary Risk of Insulin Sensitivity in Indian Subjects (CRISIS) Study. Diabetologia. 2008;51:39–46. doi: 10.1007/s00125-007-0847-1. [DOI] [PubMed] [Google Scholar]
- 7.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat percent relationship. Obes Rev. 2002;3:141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 8.Anjana M, Sandeep S, Deepa R, et al. Visceral and central abdominal fat and anthropometry in relation to diabetes in Asian Indians. Diabetes Care. 2004;27:2948–2953. doi: 10.2337/diacare.27.12.2948. [DOI] [PubMed] [Google Scholar]
- 9.Lapidus L, Bengtsson C, Larsson B, et al. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Brit Med J. 1984;289:1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson B, Svardsudd K, Welin L, et al. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed) 1984;288:1401–1404. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vikram NK, Misra A, Pandey RM, et al. Anthropometry and body composition in northern Asian Indian patients with type 2 diabetes: receiver operating characteristics (ROC) curve analysis of body mass index with percentage body fat as standard. Diabetes Nutr Metab. 2003;16:32–40. [PubMed] [Google Scholar]
- 12.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 13.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 14.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 15.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 16.Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992;55:615S–619S. doi: 10.1093/ajcn/55.2.615s. [DOI] [PubMed] [Google Scholar]
- 17.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. discussion 50-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thaler JP, Cummings DE. Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–2525. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 20.DePaula AL, Macedo AL, Rassi N, et al. Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index less than 35. Surg Endosc. 2008;22:706–716. doi: 10.1007/s00464-007-9472-9. [DOI] [PubMed] [Google Scholar]
- 21.Lee WJ, Wang W, Lee YC, et al. Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BMI>35 and <35 kg/m2. J Gastrointest Surg. 2008;12:945–952. doi: 10.1007/s11605-007-0319-4. [DOI] [PubMed] [Google Scholar]
- 22.Cohen R, Pinheiro JS, Correa JL, Schiavon CA. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m(2): a tailored approach. Surg Obes Relat Dis. 2006;2:401–404. doi: 10.1016/j.soard.2006.02.011. discussion 4. [DOI] [PubMed] [Google Scholar]
- 23.Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56) Clin Sci (Lond) 2001;101:671–679. [PubMed] [Google Scholar]
- 24.Alexandrides TK, Skroubis G, Kalfarentzos F. Resolution of diabetes mellitus and metabolic syndrome following Roux-en-Y gastric bypass and a variant of biliopancreatic diversion in patients with morbid obesity. Obes Surg. 2007;17:176–184. doi: 10.1007/s11695-007-9044-z. [DOI] [PubMed] [Google Scholar]
- 25.Schlendorf KH, Nasir K, Blumenthal RS. Limitations of the Framingham risk score are now much clearer. Prev Med. 2009;48:115–116. doi: 10.1016/j.ypmed.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Guzder RN, Gatling W, Mullee MA, Mehta RL, Byrne CD. Prognostic value of the Framingham cardiovascular risk equation and the UKPDS risk engine for coronary heart disease in newly diagnosed Type 2 diabetes: results from a United Kingdom study. Diabet Med. 2005;22:554–562. doi: 10.1111/j.1464-5491.2005.01494.x. [DOI] [PubMed] [Google Scholar]
- 27.Batsis JA, Sarr MG, Collazo-Clavell ML, et al. Cardiovascular risk after bariatric surgery for obesity. Am J Cardiol. 2008;102:930–937. doi: 10.1016/j.amjcard.2008.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Angrisani L, Favretti F, Furbetta F, et al. Italian Group for Lap-Band System: results of multicenter study on patients with BMI < or =35 kg/m2. Obes Surg. 2004;14:415–418. doi: 10.1381/096089204322917963. [DOI] [PubMed] [Google Scholar]
- 29.Scopinaro N, Papadia F, Marinari G, Camerini G, Adami G. Long-term control of type 2 diabetes mellitus and the other major components of the metabolic syndrome after biliopancreatic diversion in patients with BMI < 35 kg/m2. Obes Surg. 2007;17:185–192. doi: 10.1007/s11695-007-9045-y. [DOI] [PubMed] [Google Scholar]
- 30.Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 31.Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142:621–632. doi: 10.1016/j.surg.2007.07.018. discussion 32-5. [DOI] [PubMed] [Google Scholar]