Abstract
Implantation of the embryo into the uterine compartment is a multistep event involving attachment of the embryo to the endometrial epithelia, followed by invasion of the embryo through the endometrial stroma. RHOA, RAC1, and CDC42 are members of the Rho GTPase family of proteins, which control cell functions such as cell migration and cytoskeletal reorganization. Herein, using a heterologous in vitro coculture model, we show that implantation of mouse blastocysts into human endometrial stromal cells (hESCs) is regulated by Rho GTPase activity in hESCs. Whereas iRNA-mediated silencing of RAC1 expression in hESCs led to inhibition of embryo implantation, silencing of either RHOA or CDC42 in hESCs promoted embryo implantation in coculture assays. Analysis of downstream signaling pathways demonstrated that RAC1 silencing was associated with decreased focal adhesion disassembly and resulted in large focal adhesion complexes in hESCs. In contrast, RHOA or CDC42 silencing resulted in perturbed focal adhesion assembly, leading to a decrease in the number of focal adhesions observed. Furthermore, inhibition of Rho signaling using a Rho kinase inhibitor, Y27632, led to decreased activation of protein tyrosine kinase 2 (PTK2, also called focal adhesion kinase) and decreased focal adhesion assembly. Importantly, perturbation of focal adhesion turnover in hESCs, mediated by PTK2 silencing, resulted in inhibition of embryo implantation into hESC monolayers. These findings suggest that Rho GTPase-PTK2-dependent remodeling of the endometrial stromal cell compartment may be critical for successful embryo implantation.
Keywords: developmental biology, early development, embryo, endometrium, implantation, pregnancy, Rho GTPases
The GTPases RHOA, RAC1, and CDC42 in human endometrial stromal cells have opposing roles in mediating focal adhesion turnover and embryo invasion in an in vitro implantation model.
INTRODUCTION
Embryo implantation into the endometrium is a critical multistage process required for the establishment of pregnancy. Implantation failure is a major cause of infertility and presents a significant barrier to the success of assisted reproductive technology [1]. It is now well recognized that complicated signaling networks, involving both the embryo and the endometrium, are required to achieve blastocyst competency and uterine receptivity [2]. The embryo-uterine dialogue involves a range of growth factors, cytokines, transcription factors, and lipid mediators, which collectively allow attachment of the embryo to the uterine epithelium and the subsequent invasion of the embryo through the endometrial stroma [3–5]. Although a number of approaches have been employed to examine mouse (for the most part) and human (to a lesser extent) embryo implantation in vitro [6], the precise events that underpin the implantation process remain unclear.
The endometrium is a highly dynamic organ that undergoes extensive remodeling and repair throughout the menstrual cycle [7]. This remodeling, which occurs in response to ovarian hormones, cytokines, and growth factors, shares many features with general tissue repair mechanisms and is characterized by a migratory phenotype, cytoskeletal reorganization, and cell-matrix-dependent signaling. A number of proteolytic enzymes, growth factors, and chemokines, for example, are present in the endometrium and are believed to be important for tissue regeneration and repair following menstruation. In particular, human endometrial stromal cell (hESC) migration and proliferation have been shown to be enhanced by molecules such as platelet-derived growth factor [8], epidermal growth factor [9], heparin-binding epidermal growth factor [10], and fibroblast growth factor [11].
Due to the inaccessible nature of human implantation sites, studies addressing the extent and nature of endometrial stromal remodeling during the implantation event and pregnancy have been limited. Recent gene array studies have demonstrated that hESCs exhibit significant changes in gene expression following contact with trophoblast cells [12] or exposure to trophoblast-conditioned medium [13]. Furthermore, the in vitro differentiation of hECSs into decidual cells has been shown to be associated with changes in the expression of extracellular matrix genes [14–16] and with cytoskeletal reorganization [17].
Recently, we have shown that invasion of the human embryo into endometrial stroma in vitro involves motility of hESCs and that this motility is dependent on the activity of the Rho GTPase RAC1 [18]. In contrast, it was shown that RHOA activity in hESCs inhibited hESC migration and hence embryo invasion. The Rho family of GTPases has a crucial role in the control of processes such as cell movement, cell migration, and cell proliferation [19]. Many of these processes are mediated by changes in the actin and microtubule cytoskeleton and overall changes in cell architecture.
Herein, using a heterologous coculture in vitro model for implantation, we have investigated the invasion of mouse embryos into hESCs to determine if hESC motility is a common feature required for mouse embryo invasion. In addition, we sought to determine whether Rho GTPase-dependent focal adhesion turnover in hESCs is required for successful implantation.
MATERIALS AND METHODS
Ethics
All samples of endometrial tissue were obtained with informed consent in accord with the requirements of the Central Oxford Research Ethics Committee. The experiments were performed with ethical approval from the Oxfordshire Research Ethics Committee.
Materials
Tissue samples and embryos.
Endometrial tissue samples were obtained from fertile patients aged 20–46 yr undergoing sterilization or hysterectomy for benign conditions. All patients had a regular 26–33-day menstrual cycle and had received no hormonal medication in the preceding 3 mo. B6CBF1 mice were superovulated and mated with B6CBF1 males (colony maintained by Biomedical Services, John Radcliffe Hospital). Two-cell embryos were subsequently recovered from oviduct flushings at Day 1.5 postcoitum and placed into M2 medium (Sigma).
Reagents.
Toxin B from Clostridium difficile and the Rho kinase (ROCK) inhibitor Y27632 were obtained from Calbiochem (Merck Chemicals Ltd.). The following primary antibodies were used: anti-RhoA mouse monoclonal (Clone 26C4; Santa Cruz Biotechnology), anti-CDC42 rabbit polyclonal (Clone P1; Santa Cruz Biotechnology), anti-PTK2 rabbit polyclonal (C-20; Santa Cruz Biotechnology), anti-vinculin rabbit polyclonal (V4139l; Sigma), anti-β-actin (ACTB) mouse monoclonal (Sigma), anti-pPTK2 rabbit polyclonal (pTyr397; Cell Signaling Technology), and anti-RAC1 mouse monoclonal (Clone 23A8; Upstate). The secondary antibodies used were donkey anti-mouse fluorescein isothiocyanate (FITC), donkey anti-rabbit FITC (Jackson ImmunoResearch Laboratories Inc.), sheep anti-mouse horseradish peroxidase (HRP) (Sigma), and goat anti-rabbit HRP (Cell Signaling Technology). Texas Red-conjugated phalloidin was obtained from Molecular Probes.
Methods
Tissue and embryo culture.
The isolation, culture, and decidualization of hESCs were performed as described previously [20]. Mouse embryos (2-cell stage) were cultured in M16 culture medium (Sigma) overlaid with mineral oil (Sigma) until the blastocyst stage. Assisted hatching was performed if necessary using acidified Tyrode solution (Sigma). For coculture experiments, hatched blastocysts were cultured with confluent monolayers of hESCs for 48 h as described previously [20].
Toxin B and ROCK inhibitor treatments.
Decidualized hESCs were cultured in the presence of toxin B for 1 h and were then washed extensively before analysis or embryo coculture. Similarly, for ROCK inhibition, decidualized hESCs were cultured in the presence of Y27632 for 16 h and were then washed extensively before analysis or embryo coculture.
Immunohistochemistry and microscopy.
Cells or whole mounts were fixed, permeabilized, and blocked as described previously [20]. Cells were incubated with primary antibody (diluted 1:100) in 0.1% bovine serum albumin overnight at 4°C, followed by the appropriate conjugated secondary antibody for 1 h at room temperature. Mouse/rabbit IgGs were used as controls where appropriate and were shown to be negative. Texas Red-conjugated phalloidin was added during the secondary incubation to visualize actin, and cells were mounted in Vectashield Mounting Media containing 4′,6′-diamidino-2-phenylindole (DAPI) (Vector Laboratories). Whole mounts and cells were viewed using a Leica DMRBE microscope, and images were captured with a Hamamatsu Orca C4742‐95 digital camera and Openlab software (Improvision).
Western blotting.
Western blotting of culture lysates was performed as described previously [18]. Briefly, protein lysates from hESCs were separated on standard SDS-PAGE gels (Invitrogen) and were transferred to polyvinylidene fluoride membranes and blocked in 5% nonfat dry milk. Membranes were incubated in primary antibody (diluted 1:500) overnight at 4°C, followed by 1-h incubation with the appropriate HRP-conjugated secondary antibody. Immunoreactive bands were visualized with an ECL detection kit (Amersham).
siRNA transfections.
Transfection of hESCs with siRNAs directed against human RHOA (RhoA-6 and RhoA-7), RAC1 (RAC1‐5 and RAC1‐6), CDC42 (CDC42‐7 and CDC42‐10), and PTK2 (PTK2‐1 and PTK2‐2) and control nonsilencing siRNAs (Qiagen) were used according to the manufacturer's instructions. Briefly, cells were transfected with 25 nM siRNA using HiPerfect Transfection Reagent (Qiagen). Media were changed after 24 h, and cells were washed in PBS before use in coculture assays or for analysis of protein expression.
RESULTS
Inhibition of hESC Rho GTPase Signaling Inhibits the Invasion of Mouse Blastocysts into hESC Monolayers
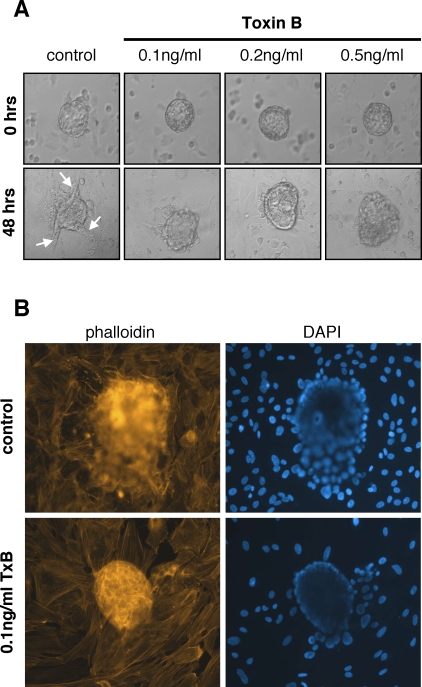
The requirement of Rho GTPase activity in hESCs for mouse blastocyst invasion in vitro was determined by inhibiting Rho GTPase signaling in hESCs with toxin B from C. difficile [21]. Human ESCs were pretreated with toxin B before embryo coculture to avoid inhibiting Rho GTPases in the embryo. Analysis of embryo invasion (Fig. 1A) demonstrated that, as shown previously for human blastocysts [18], toxin B pretreatment of hESCs had no effect on the attachment of mouse embryos to hESCs. Embryo invasion, however, was inhibited in a dose-dependent manner. Embryos cocultured with control cells exhibited extensive trophoblast outgrowth and spreading into the hESC monolayer compared with embryos cocultured with toxin B-pretreated cells. Immunohistochemistry of whole mounts (Fig. 1B) showed clearly that, even at the lowest concentration of toxin B examined (0.1 ng/ml), embryo invasion into hESC monolayers was reduced compared with embryo invasion into control cells.
FIG. 1.
Inhibition of RHO GTPase signaling in hESCs prevents invasion of mouse blastocysts into hESC monolayers. A) Mouse blastocysts were cocultured with control hESCs or hESCs pretreated with the indicated concentration of C. difficile toxin B. Blastocyst morphology and invasion were monitored by phase-contrast microscopy before and after 48 h of coculture. Arrows indicate trophoblast projections. B) Blastocyst-hESC cocultures were fixed after 48 h of culture and stained with Texas Red-conjugated phalloidin and DAPI. Original magnification x200.
iRNA-Mediated Silencing of hESC RAC1 Expression Inhibits Mouse Embryo Invasion, Whereas hESC RHOA or CDC42 Silencing Enhances Embryo Invasion
The identity of individual Rho family members that mediate embryo invasion was investigated by the use of iRNA to silence the expression of specific Rho GTPases in hESCs. Previous findings from our group [18] suggested that RAC1 in hESCs is crucial for hESC cell motility and invasion of the human embryo into hESCs. Therefore, we analyzed the effects of RAC1 silencing in hESCs on mouse embryo invasion during hESC-embryo coculture.
Levels of RAC1 in hESCs were reduced following transfection with RAC1-directed siRNAs by 24 h after transfection and remained so up to 72 h after transfection as shown by both Western blotting and immunohistochemistry (Supplemental Fig. S1, A and B; all Supplemental Data are available at www.biolreprod.org). In accord with previous data obtained using human embryos [18], silencing of RAC1 in hESCs with the more effective siRNA (RAC1‐6) inhibited the subsequent invasion of mouse embryos into hESC monolayers (Fig. 2A), confirming that hESC RAC1 is required for stromal cell function during mouse embryo invasion.
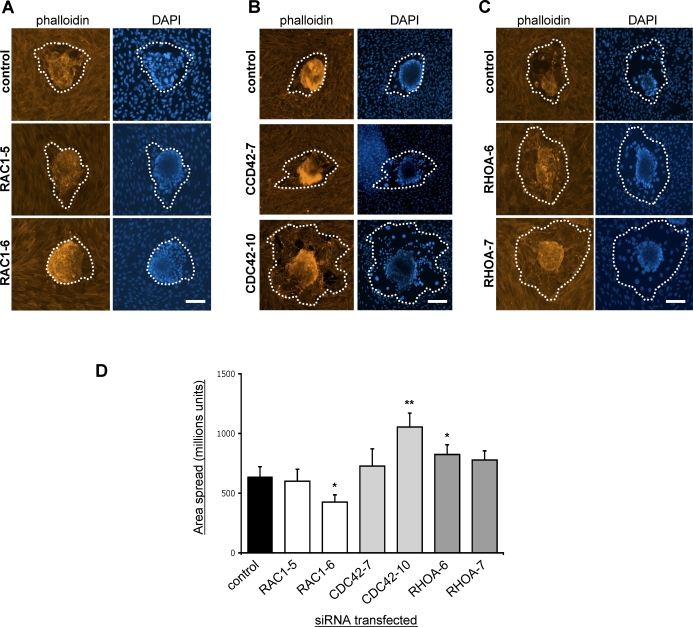
FIG. 2.
RAC1, CDC42, and RHOA have opposing roles during embryo implantation. Mouse blastocysts were cocultured for 48 h with hESCs transfected with NS control siRNA and one of the following: RAC1 siRNAs (RAC1‐5 and RAC1‐6; A), CDC42-directed siRNAs (CDC42‐7 and CDC42‐10; B), or RHOA-directed siRNAs (RHOA‐6 and RHOA-7; C). Whole mounts were then fixed and stained with Texas Red-conjugated phalloidin and DAPI. The area of spread trophoblast is indicated by a dotted line. Bar = 50 μm. D) Quantification of blastocyst spreading following RAC1, CDC42, or RHOA silencing in hESCs. Results shown are the mean areas of embryo spread ± SEM (n = 6 blastocysts). Significant differences between cells transfected with control nonsilencing siRNA are indicated by *P < 0.05 and **P < 0.01.
We analyzed the function of CDC42 and RHOA in hESCs using a similar iRNA-mediated approach. CDC42 expression was reduced in hESCs following transfection with the CDC42-directed siRNAs CDC42‐7 and CDC42‐10. Western blotting analysis and immunohistochemistry of transfected cells demonstrated a reduction in CDC42 expression levels 24 and 72 h after transfection (Supplemental Fig. S1, C and D). Embryo invasion into hESCs transfected with CDC42-directed siRNAs was increased compared with invasion of embryos into control cells (Fig. 2B). The more effective CDC42 siRNA (CDC42‐10) particularly caused a dramatic increase in the extent of trophoblast spreading into the hESC monolayer.
The expression of RHOA in hESCs was also successfully silenced following transfection of hESCs with two different siRNAs, RHOA-6 and RHOA-7. Both siRNAs used were effective and reduced RHOA expression to undetectable levels as observed by Western blotting and immunohistochemistry (Supplemental Fig. S1, E and F). Furthermore, as seen previously with human embryos, the silencing of RHOA in hESCs resulted in increased invasion of mouse embryos into hESC monolayers during coculture (Fig. 2C).
Quantification of the extent of embryo spreading observed following the silencing of hESC Rho GTPases was performed to compare their relative effects on embryo invasion. These analyses (Fig. 2D) demonstrated that RAC1 silencing in hESCs using the RAC1‐6 siRNA resulted in a statistically significant decrease in the area of embryo spread (from approximately 593 × 106 ± 39 to 426 × 106 ± 60 U). In contrast, silencing of CDC42 or RHOA led to statistically significant increases in the area of embryo spreading. Silencing of CDC42 with the CDC42‐10 siRNA resulted in a mean embryo spread area of 1054 × 106 ± 116 U, approximately two-fold higher than that observed following coculture of embryos with control cells. Similarly, silencing of RHOA with the RHOA-6 siRNA led to an increased mean embryo spread area of 824 × 106 ± 82 U. These results suggest that, during mouse embryo-hESC coculture, RAC1 in hESCs is required to facilitate embryo invasion, whereas both CDC42 and RHOA inhibit invasion.
Inhibition of hESC Rho Kinase Signaling Accelerates Mouse Embryo Invasion
We sought to reinforce the putative involvement of the hESC RHOA signaling pathway during embryo invasion. The ROCK inhibitor Y27632 was used as an alternative approach to inhibit RHOA signaling in hESCs. This cell-permeable inhibitor acts as a potent and specific inhibitor of ROCK, one of the major downstream effectors of RHOA [22].
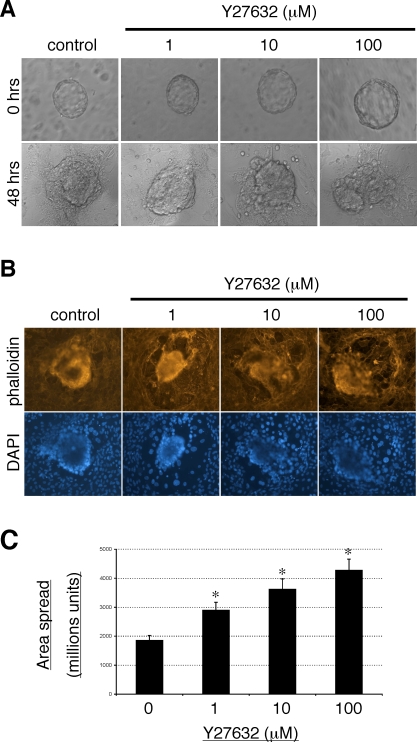
Pretreatment of hESCs with Y27632 before coculture with mouse embryos resulted in a dose-dependent increase in the subsequent extent of embryo invasion as shown by phase-contrast microscopic images of cocultures after 48 h (Fig. 3A). In addition, staining of hESC-embryo whole mounts with Texas Red-conjugated phalloidin and DAPI (Fig. 3B) demonstrated that embryos cultured with Y27632-treated hESCs displayed a greater extent of trophoblast spreading. This is consistent with previous data demonstrating that Y27632 pretreatment of hESCs resulted in an increase in human trophoblast viability and human chorionic gonadotropin secretion during coculture [18].
FIG. 3.
Inhibition of hESC ROCK signaling enhances mouse blastocyst invasion into hESCs. A) Mouse blastocysts were cocultured with control hESCs or hESCs pretreated with the indicated concentrations of Y27632. Invasion was analyzed by phase-contrast microscopy after 48 h of coculture. B) Whole mounts were fixed and stained with Texas Red-conjugated phalloidin and DAPI to analyze trophoblast spreading. Original magnification ×200. C) Graph showing effects of hESC Y27632 treatment on area of trophoblast spread. Results shown are the mean areas of embryo spread ± SEM (n = 6 blastocysts). Significant differences between control untreated cells are indicated by *P < 0.05.
Quantification of the extent of embryo spreading (Fig. 3C) showed that Y27632 treatment of hESCs led to a statistically significant and dose-dependent increase in the area of embryo spreading observed following coculture. This, together with the observation that RHOA silencing in hESCs led to an increase in embryo invasion (Fig. 2), confirms the role of RHOA in hESCs as a negative regulator of implantation.
Focal Adhesions in hESCs Are Differentially Regulated by Rho GTPases
Our previous data showed that RAC1-mediated hESC motility is essential during invasion of the human embryo into hESCs and that restriction of hESC motility inhibits embryo invasion [18]. Conversely, increased hESC motility following RHOA inhibition resulted in increased human embryo invasion. The results already presented suggest that these findings translate to the mouse embryo and that cooperation between hESC motility and trophoblast invasion is also required for successful invasion of mouse embryos. Therefore, we analyzed signaling pathways downstream of Rho GTPases to identify differentially regulated pathways involved in hESC motility, focusing on the expression and function of focal adhesion proteins, which have a central function in cell motility.
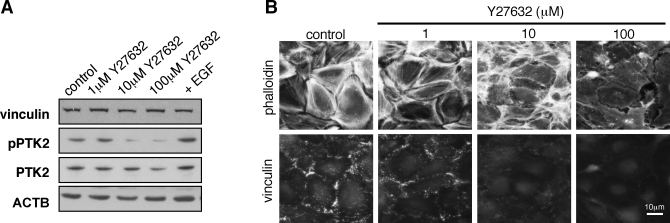
Human ESCs were treated with the ROCK inhibitor Y27632, and the expression of the focal adhesion proteins vinculin and protein tyrosine kinase 2 (PTK2, also called focal adhesion kinase) and the activity of PTK2 were analyzed by Western blotting (Fig. 4A). Y27632 treatment had no effect on the levels of vinculin and PTK2 expressed in hESCs; however, the extent of PTK2 activation was reduced following Y27632 treatment. Analysis of Y27632-treated hESCs by immunohistochemistry revealed a dose-dependent disruption of actin stress fibers and a loss of vinculin localization to focal adhesions (Fig. 4B). As seen previously for other cell types [23], these observations thus highlight an important function for RHOA signaling in the assembly and maintenance of focal adhesions in hESCs.
FIG. 4.
The hESC focal adhesions are disrupted following ROCK inhibition. A) Western blot analysis of control hESCs, hESCs treated with the indicated concentrations of Y27632, and epidermal growth factor (EGF)-stimulated hESCs (100 ng/ml for 10 min). Vinculin, PTK2, and phoshorylated PTK2 were detected, and ACTB was used as a loading control. FAK, focal adhesion kinase. B) The hESCs were treated with the indicated concentrations of Y27632, and vinculin localization was examined by immunohistochemistry. Cells were also counterstained with Texas Red-conjugated phalloidin.
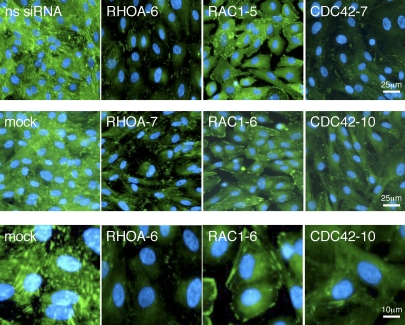
The effect of RHOA silencing on focal adhesion structures was confirmed by performing immunohistochemistry of hESCs transfected with RHOA-directed siRNAs as shown in Figure 5. Silencing of CDC42 also caused an overall reduction in the numbers of focal adhesions (Fig. 5). In contrast, silencing of RAC1 expression in hESCs caused an increase in focal adhesions and, in particular, resulted in a number of large adhesion complexes at the cell periphery. These studies indicate that both RHOA and CDC42 are required for hESC focal adhesion assembly, whereas RAC1 may be involved in hESC focal adhesion turnover.
FIG. 5.
Rho GTPases have opposing roles on focal adhesion disassembly. The hESCs were transfected with siRNAs directed against RHOA, RAC1, and CDC42 or with control nonsilencing siRNA (ns siRNA). Vinculin localization was analyzed by immunohistochemistry 48 h after transfection. High-power magnification panels from the siRNAs that gave the most significant response with regard to embryo invasion (RHOA-6, RAC1‐6, and CDC42‐10) are shown to demonstrate the effects on focal adhesions.
Focal Adhesion Turnover During Embryo Implantation
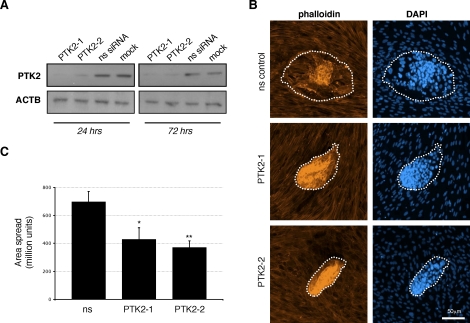
We further investigated the involvement of focal adhesion turnover during embryo implantation by using an iRNA-mediated approach to silence PTK2 expression in hESCs. The expression of PTK2 was reduced in hESCs following transfection with the PTK2-directed siRNAs PTK2‐1 and PTK2‐2 (Fig. 6A and Supplemental Fig. S2A). Consistent with previous investigations performed on Ptk2-null fibroblasts [24], silencing of PTK2 led to an increase in focal adhesions seen at the periphery of cells (Supplemental Fig. S2B), confirming the role of PTK2 in focal adhesion disassembly and turnover. This phenotype was similar to that observed following silencing of RAC1 in hESCs (Fig. 5). Importantly, PTK2 silencing in hESCs resulted in a dramatic decrease in the subsequent extent of embryo invasion into hESCs (Fig. 6B). Embryos attached to hESC monolayers but exhibited minimal spreading. Quantification of the area of embryo spread (Fig. 6C) demonstrated that PTK2 silencing in hESCs (using either of the two PTK2-directed siRNAs) resulted in a significant reduction in the extent of embryo invasion and trophoblast spreading.
FIG. 6.
Effects of PTK2 silencing on blastocyst implantation. A) The hESCs were transfected with PTK2-directed siRNAs (PTK2‐1 and PTK2‐2) or with control nonsilencing siRNA (ns siRNA). PTK2 expression was evaluated 24 and 72 h after transfection by Western blotting. Mock-transfected cells were also analyzed, and ACTB was used as a loading control. B) Mouse blastocysts were cocultured for 48 h with hESCs that had been transfected with nonsilencing control siRNA or PTK2 siRNAs (PTK2‐1 and PTK2‐2). Whole mounts were then fixed and stained with Texas Red-conjugated phalloidin and DAPI. The approximate area of embryo spread is indicated by a dotted line. C) Quantification of blastocyst spreading following PTK2 silencing in hESCs. Results shown are the mean areas of embryo spread ± SEM (n = 6). Significant differences between cells transfected with control nonsilencing siRNA are indicated by *P < 0.05 and **P < 0.01. FAK, focal adhesion kinase.
DISCUSSION
The mechanisms by which the endometrial environment controls embryo implantation remain poorly understood. Herein, we have shown that Rho GTPase activity in hESCs modulates the extent of embryo invasion into hESC monolayers in vitro. Our main findings are that 1) silencing in hESCs of RAC1 inhibits, whereas silencing of RHOA and CDC42 enhances, mouse embryo invasion; 2) perturbation of RHOA, RAC1, and CDC42 expression modulates focal adhesion assembly and disassembly; and 3) silencing of PTK2 in hESCs perturbs focal adhesion disassembly and subsequently limits the extent of embryo invasion. These findings suggest that RAC1 and PTK2 activity in hESCs is critical during invasion of the embryo into the endometrial stroma, whereas both RHOA and CDC42 activities in hESCs restrict the extent of embryo invasion during the implantation process.
We have previously shown that impaired motility of hESCs, achieved either through silencing of RAC1 expression or by inhibition of RAC1 activity, inhibits their capacity to support invasion of the human embryo into the endometrial stroma. The studies presented herein highlight the importance of hESC motility during invasion of the mouse embryo into the stroma. This suggests that this may be a common and conserved mechanism by which hESCs respond to an implanting embryo and subsequently adapt to both accommodate and restrict the invading trophoblast. The precise interactions occurring within our in vitro implantation model (for example, any integrin-mediated contacts) between the trophoblast cells of the embryo and hESCs remain to be investigated. It is likely that the embryo-uterine interactions observed in vivo are more complex due to the three-dimensional environment. In addition, the soft matrix provided by the uterus may induce more dynamic focal contacts and adhesions than those observed at the two-dimensional hESC-glass interface [25]. Nonetheless, despite their two-dimensional nature, the experiments presented herein demonstrate that alterations to hESCs and hence the uterine environment can have a dramatic effect on embryo implantation.
Insufficient trophoblast invasion can lead to a number of placental complications such as preeclampsia [26], whereas excessive invasion is associated with disorders such as placenta creta [27]. A finely tuned balance between individual RHO signaling pathways may therefore contribute to the mechanism by which the endometrium controls the appropriate level of trophoblast invasion. We [18] and others [28–30] have shown that there is a balance between RHOA and RAC1 signaling pathways. In addition, recent investigations have shown that RHOA and CDC42 signaling pathways converge during cell invasion via cooperation between their respective downstream kinases ROCK and CDC42BP (previously known as MRCK) [31]. The results presented herein suggest that CDC42 signaling may act in concert with RHOA in hESCs during the process of embryo implantation. Whether CDC42-CDC42BP signaling is also able to antagonize RAC1 signaling in hESCs remains to be investigated.
Cell migration and motility are perturbed by disruption of focal adhesion assembly or disassembly. Fibroblasts from PTK2-null mice exhibit impaired cell motility and formation of the leading edge during migration [32]. Similarly, silencing of PTK2 expression in a variety of cell types [33] using siRNAs leads to impaired migration and motility. PTK2 activity also has a critical role in numerous other cell signaling pathways such as cell growth, cell survival, and cell adhesion. Based on our previous data [18], however, it is likely that the decreased embryo invasion observed following PTK2 silencing is due to alteration of the migratory phenotype of hESCs and not due to changes in their signaling properties.
PTK2 activity has previously been implicated in hESC function. Expression of PTK2 has been reported to be higher in ovarian endometriotic tissues compared with normal endometrium, suggesting that PTK2-mediated changes in endometrial cell migration may contribute to the pathogenesis of endometriosis [34, 35]. Elevated levels of PTK2 have been observed in a number of human tumor samples and cell lines derived from human tumors [36]. Increased levels of PTK2 correlate with increased proliferation, migration, and invasiveness of tumor cells [37, 38]. Notably, elevated PTK2 levels have been observed in endometrial hyperplasia and carcinomas [39], reinforcing the notion that PTK2 has a key role in regulating cell migration in the endometrium.
PTK2 activity has been reported to increase during in vitro decidualization, accompanied by a redistribution of focal adhesions [17]. Furthermore, decidualization is associated with a decrease in hESC migration, suggesting that the decidualization event restricts or controls the extent of embryo invasion, although the activities of RHO GTPases during decidualization are unknown.
Focal adhesion turnover during implantation has been recently reported in other implantation model systems. Disassembly of endometrial epithelial cell focal adhesions occurs in early pregnancy in the rat, as indicated by the redistribution of the focal adhesion proteins talin and paxillin [40]. Thus, a loss of epithelial cell focal adhesions may facilitate removal of these cells away from the point of interaction between the embryo and the epithelium during the early implantation process. In contrast, enhanced endometrial epithelial cell focal adhesion assembly appears to occur during the later stages of ovine pregnancy [41]. Modulation of focal adhesion assembly and disassembly at the maternal-embryo interface may therefore be required to adapt to increasing forces exerted by the growing fetus.
Focal adhesion assembly occurs when the constituent protein components are sequestered at the inner cell membrane in response to the interaction of integrins with specific extracellular matrix components on the outside of the cell. Integrins (such as α5β1) and extracellular matrix proteins (such as the α5β1 ligand fibronectin) have previously been shown to be present in abundance in the endometrial stroma and colocalize with focal adhesions [41]. Our findings suggest that turnover of focal adhesions in hESCs may have an important function in embryo implantation. Aberrant integrin expression in the endometrial stroma has been implicated with a number of endometrial complications, including endometriosis [42, 43] and unexplained infertility [44, 45]. We suggest that perturbations of endometrial integrin-PTK2-Rac signaling may compromise endometrial function, including embryo implantation.
In summary, the findings presented herein suggest that RHO GTPase activity in the endometrial stromal cell compartment may have a crucial function in regulating the extent of mouse embryo invasion through the stroma. Our findings, in the context of observations previously reported, suggest that RHO GTPases and their downstream signaling pathways modulate remodeling of the endometrial stroma by regulating stromal cell migration. This may in part be mediated by the effect of RHO GTPases on focal adhesion dynamics, or vice versa. This in turn regulates embryo invasion, as we have shown herein for the mouse and previously for the human embryo [18]. Our results from this and previous [18] functional experimental model systems of embryo implantation suggest that RHO GTPases may have a critical function in implantation that is conserved across species.
Supplementary Material
Acknowledgments
We are grateful to the biomedical sciences staff at the University of Oxford for assistance with mouse embryo work and to the patients who donated endometrial tissue for research.
Footnotes
1Supported by the Wellcome Trust and the Medical Research Council.
REFERENCES
- Edwards RG.Human implantation: the last barrier in assisted reproduction technologies? Reprod Biomed Online 2006; 13: 887–904. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Minas V.Mechanisms of implantation. Reprod Biomed Online 2007; 14: 102–109. [DOI] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Kayisli UA, Taylor HS.The role of growth factors and cytokines during implantation: endocrine and paracrine interactions. Semin Reprod Med 2009; 27: 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Daikoku T, Guo Y, Wang H, Dey SK.Molecular complexity in establishing uterine receptivity and implantation. Cell Mol Life Sci 2005; 62: 1964–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mourik MS, Macklon NS, Heijnen CJ.Embryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leukoc Biol 2009; 85: 4–19. [DOI] [PubMed] [Google Scholar]
- Mardon H, Grewal S, Mills K.Experimental models for investigating implantation of the human embryo. Semin Reprod Med 2007; 25: 410–417. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA.Tissue injury and repair in the female human reproductive tract. Reproduction 2003; 125: 301–311. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Nasu K, Nishida M, Ito H, Bing S, Miyakawa I.Regulation of proliferation, motility, and contractility of human endometrial stromal cells by platelet-derived growth factor. J Clin Endocrinol Metab 2005; 90: 3560–3567. [DOI] [PubMed] [Google Scholar]
- Cavaille F, Neau E, Vouters M, Bry-Gauillard H, Colombel A, Milliez J, Le Bouc Y.IGFBP-1 inhibits EGF mitogenic activity in cultured endometrial stromal cells. Biochem Biophys Res Commun 2006; 345: 754–760. [DOI] [PubMed] [Google Scholar]
- Chobotova K, Muchmore ME, Carver J, Yoo HJ, Manek S, Gullick WJ, Barlow DH, Mardon HJ.The mitogenic potential of heparin-binding epidermal growth factor in the human endometrium is mediated by the epidermal growth factor receptor and is modulated by tumor necrosis factor-alpha. J Clin Endocrinol Metab 2002; 87: 5769–5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SJ, Wu MH, Chen HM, Chuang PC, Wing LY.Fibroblast growth factor-9 is an endometrial stromal growth factor. Endocrinology 2002; 143: 2715–2721. [DOI] [PubMed] [Google Scholar]
- Popovici RM, Betzler NK, Krause MS, Luo M, Jauckus J, Germeyer A, Bloethner S, Schlotterer A, Kumar R, Strowitzki T, von Wolff M.Gene expression profiling of human endometrial-trophoblast interaction in a coculture model. Endocrinology 2006; 147: 5662–5675. [DOI] [PubMed] [Google Scholar]
- Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, Fazleabas AT, Fisher SJ, et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod 2007; 76: 102–117. [DOI] [PubMed] [Google Scholar]
- Brar AK, Handwerger S, Kessler CA, Aronow BJ.Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics 2001; 7: 135–148. [DOI] [PubMed] [Google Scholar]
- Popovici RM, Kao LC, Giudice LC.Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology 2000; 141: 3510–3513. [DOI] [PubMed] [Google Scholar]
- Tierney EP, Tulac S, Huang ST, Giudice LC.Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics 2003; 16: 47–66. [DOI] [PubMed] [Google Scholar]
- Ihnatovych I, Hu W, Martin JL, Fazleabas AT, de Lanerolle P, Strakova Z.Increased phosphorylation of myosin light chain prevents in vitro decidualization. Endocrinology 2007; 148: 3176–3184. [DOI] [PubMed] [Google Scholar]
- Grewal S, Carver JG, Ridley AJ, Mardon HJ.Implantation of the human embryo requires RAC1-dependent endometrial stromal cell migration. Proc Natl Acad Sci U S A 2008; 105: 16189–16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A.RHO GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21: 247–269. [DOI] [PubMed] [Google Scholar]
- Carver J, Martin K, Spyropoulou I, Barlow D, Sargent I, Mardon H.An in-vitro model for stromal invasion during implantation of the human blastocyst. Hum Reprod 2003; 18: 283–290. [DOI] [PubMed] [Google Scholar]
- Voth DE, Ballard JD.Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 2005; 18: 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S.Calcium sensitization of smooth muscle mediated by a RHO-associated protein kinase in hypertension. Nature 1997; 389: 990–994. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Ookawara S.RHO-kinase dependent organization of stress fibers and focal adhesions in cultured fibroblasts. Genes Cells 2007; 12: 623–638. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T.Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature 1995; 377: 539–544. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL.Tissue cells feel and respond to the stiffness of their substrate. Science 2005; 310: 1139–1143. [DOI] [PubMed] [Google Scholar]
- van den Brule F, Berndt S, Simon N, Coulon C, Le Goarant J, Munaut C, Noel A, Frankenne F, Foidart JM.Trophoblast invasion and placentation: molecular mechanisms and regulation. Chem Immunol Allergy 2005; 88: 163–180. [DOI] [PubMed] [Google Scholar]
- Tantbirojn P, Crum CP, Parast MM.Pathophysiology of placenta creta: the role of decidua and extravillous trophoblast. Placenta 2008; 29: 639–645. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K.RHO and Rac take center stage. Cell 2004; 116: 167–179. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Hartwig JH, Stossel TP.FilGAP, a RHO- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat Cell Biol 2006; 8: 803–814. [DOI] [PubMed] [Google Scholar]
- Tsuji T, Ishizaki T, Okamoto M, Higashida C, Kimura K, Furuyashiki T, Arakawa Y, Birge RB, Nakamoto T, Hirai H, Narumiya S.ROCK and mDia1 antagonize in RHO-dependent Rac activation in Swiss 3T3 fibroblasts. J Cell Biol 2002; 157: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Paterson HF, Marshall CJ.CDC42-MRCK and RHO-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol 2005; 7: 255–261. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Klingbeil CK, Schlaepfer DD.Focal adhesion kinase functions as a receptor-proximal signaling component required for directed cell migration. Immunol Res 2000; 21: 293–303. [DOI] [PubMed] [Google Scholar]
- Li S, Hua ZC.FAK expression regulation and therapeutic potential. Adv Cancer Res 2008; 101: 45–61. [DOI] [PubMed] [Google Scholar]
- Mu L, Zheng W, Wang L, Chen X, Yang J.Focal adhesion kinase expression in ovarian endometriosis. Int J Gynaecol Obstet 2008; 101: 161–165. [DOI] [PubMed] [Google Scholar]
- Mu L, Zheng W, Wang L, Chen XJ, Zhang X, Yang JH.Alteration of focal adhesion kinase expression in eutopic endometrium of women with endometriosis. Fertil Steril 2008; 89: 529–537. [DOI] [PubMed] [Google Scholar]
- Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, Liu ET, Cance WG.Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res 1995; 55: 2752–2755. [PubMed] [Google Scholar]
- Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT.Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene 2001; 20: 1152–1163. [DOI] [PubMed] [Google Scholar]
- Wang D, Grammer JR, Cobbs CS, Stewart JE, Jr, Liu Z, Rhoden R, Hecker TP, Ding Q, Gladson CL.p125 Focal adhesion kinase promotes malignant astrocytoma cell proliferation in vivo. J Cell Sci 2000; 113(pt 23):4221–4230. [DOI] [PubMed] [Google Scholar]
- Livasy CA, Moore D, Cance WG, Lininger RA.Focal adhesion kinase overexpression in endometrial neoplasia. Appl Immunohistochem Mol Morphol 2004; 12: 342–345. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Lindsay LA, Murphy CR.Focal adhesions disassemble during early pregnancy in rat uterine epithelial cells. Reprod Fertil Dev 2008; 20: 892–899. [DOI] [PubMed] [Google Scholar]
- Burghardt R, Burghardt J, Taylor J, III, Reeder A, Nguen B, Spencer T, Bayless K, Johnson G.Enhanced focal adhesion assembly reflects increased mechanosensation and mechanotransduction at maternal-conceptus interface and uterine wall during ovine pregnancy. Reproduction 2009; 137: 567–582. [DOI] [PubMed] [Google Scholar]
- Lessey BA.Implantation defects in infertile women with endometriosis. Ann N Y Acad Sci 2002; 955: 265–280,.293–295, 396–406. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL.Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab 1994; 79: 643–649. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Sawin SW, Sun J.Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril 1995; 63: 535–542. [PubMed] [Google Scholar]
- Skrzypczak J, Mikolajczyk M, Szymanowski K.Endometrial receptivity: expression of alpha3beta1, alpha4beta1 and alphaVbeta1 endometrial integrins in women with impaired fertility. Reprod Biol 2001; 1: 85–94. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.