Abstract
Soy-based infant formulas are widely used in the United States and some other countries. These formulas contain high levels of the estrogenic isoflavone genistein, leading to concern that neonatal genistein exposure could cause acute and/or long-term adverse effects on reproductive and other organs. However, previous work to assess genistein effects in rodent models has not typically replicated the route of delivery and/or serum genistein concentrations reported for soy formula-fed human infants. Our objective was to develop a mouse model that more closely mimics the oral genistein exposure and total serum genistein concentrations observed in soy formula-fed infants. Mouse pups were dosed orally with genistein in a soy formula-corn oil emulsion from Postnatal Day (PND) 1 to PND 5, then effects on reproductive and nonreproductive organs were assessed after dosing and during subsequent development. Neonatal treatment resulted in changes both at the completion of dosing (PND 5) and in adult animals. At PND 5, neonatal genistein treatment caused increased relative uterine weight and down-regulation of progesterone receptor in uterine epithelia. Estrogenic effects of genistein were also seen in the neonatal ovary and thymus, which had an increase in the incidence of multioocyte follicles (MOFs) and a decrease in thymic weight relative to body weight, respectively. The increased incidence of MOFs persisted into adulthood for neonatally treated genistein females, and estrous cycle abnormalities were seen at 6 mo of age despite normal fertility in these mice. The immediate and long-term effects in this neonatal animal model raise concerns that high serum concentrations of genistein are estrogenic and could potentially impact the development of human infants fed soy formula.
Keywords: female reproductive tract, genistein, infant, soy formula, toxicology
In a physiological mouse model, neonatal exposure to genistein at concentrations seen in soy formula-fed infants results in morphological and functional changes in neonatal and adult reproductive and nonreproductive organs.
INTRODUCTION
Approximately 25% of all formula-fed infants in the United States are fed soy-based infant formula [1], and over 20 million infants in the United States have been fed soy formula [2]. Worldwide usage varies widely, from over 30% in Israel [3] and 13% in New Zealand to under 10% in the U.K., Italy, and France [4], with minimal usage in Asian countries. Lower usage in other countries may reflect warnings from governmental and scientific bodies in Europe, Australia, and New Zealand regarding soy formula [4]. However, the American Academy of Pediatrics has regarded this as a safe feeding alternative [1] but only specifically recommends soy formula use over cow milk formulas for infants with galactosemia or primary lactase deficiency, or for families preferring a vegetarian lifestyle. Nonetheless, the percentage of U.S. infants using soy formula greatly exceeds the percentage of infants with any condition that would provide a medical basis for usage of soy formula [1].
Though current preparations of soy formulas support normal growth [5, 6], there have been long-standing concerns that the high phytoestrogen content in soy formulas could have unintended negative consequences in the developing infant [7]. Phytoestrogens are plant-derived chemicals that have the ability to bind and signal through estrogen receptors [8]. The predominant phytoestrogen in soy and derived products is the isoflavone genistein, which accounts for approximately two thirds of the soy isoflavone content [9]. Genistein binds estrogen receptor 1 (ESR1) but has greater affinity for estrogen receptor 2 [8]. Soy also contains the isoflavone daidzein, but because both its estrogenic potency [8] and its abundance are less than those of genistein [9, 10], it is a relatively minor contributor to the total estrogenic effects of soy. Infants consuming soy-based formulas have plasma concentrations of total genistein that are an order of magnitude greater than those of adults eating high soy diets and several hundredfold higher than those of adults consuming Western diets [9, 11]. These total plasma genistein concentrations in infants fed soy-based formulas are approximately 10-fold higher than the plasma concentrations of isoflavones necessary to disrupt the menstrual cycle in premenopausal women [12].
This high exposure to phytoestrogens occurs during a stage of life when endogenous estrogens are low in both sexes [10], raising concerns that these soy isoflavones could cause reproductive and other effects during neonatal and/or adult life. Though human data in this area are minimal, a handful of studies have associated soy formula consumption with aberrant development. Soy formula consumption was identified as one risk factor in Puerto Rican infants showing precocious breast development [13]. Other studies suggested a reduced response to childhood vaccinations in soy formula-fed infants [14], but a later, more extensive study disputed this claim [15, 16]. A telephone survey of 128 young women fed soy formula as infants found no significant changes in a number of parameters related to fertility and pubertal maturation, although the data suggest these women were more likely to have menstrual abnormalities and multiple births [2].
Although two prospective human clinical studies have begun [5, 17–19], these will not generate data on adult consequences of neonatal genistein exposure for decades. To address whether there are acute and/or long-term effects of the genistein in soy formula on human infants, a neonatal rodent model that replicates human exposure via oral administration is needed. However, genistein is minimally incorporated into the dam's milk following dietary administration of genistein, and therefore it is not possible to mimic human infant serum concentrations in rodent pups through lactational exposure [20]. Therefore, many neonatal rodent studies have examined the effects of injecting genistein directly into the pup. These studies demonstrated an increased incidence of multioocyte follicles (MOFs) [21], increased uterine size, disrupted estrous cycling, decreased fertility, and other effects consistent with estrogenization [22]. However, the ability of these animal studies to provide definitive data regarding potential human risk has been questioned because injected genistein bypasses first-pass metabolism in the gut and thus has different pharmacokinetics than oral administration; also, genistein administered at higher doses produces peak serum genistein concentrations exceeding those in soy formula-fed infants [23–25].
To address some of the limitations of previous genistein rodent studies, we have developed a model involving oral administration of genistein in suspension within a soy formula-corn oil emulsion. The emulsion is delivered directly to pups' mouths with a pipette tip that triggers the suckling reflex and leads to ingestion of the dose. Oral administration allows for first-pass metabolism, and the dose used achieves total genistein serum concentrations comparable to those of soy formula-fed infants. Female pups exposed to genistein through this method show clear estrogenic effects on reproductive and nonreproductive organs in the neonatal period as well as effects on the ovary and estrous cycle that persist into adulthood.
MATERIALS AND METHODS
Animal Care
C57BL/6 mice were purchased from Harlan. Mice were housed at 25°C in conventional polycarbonate cages with 3.2 mm corncob bedding (Harlan) on a 12L:12D cycle. Breeder mice were given water and Teklad Rodent Diet 8604 (Harlan) ad libitum, and the day dams gave birth, they were switched to phytoestrogen-free Teklad Rodent Diet 2016 (Harlan). Pups remained on Diet 2016 for the remainder of the experiment and were weaned at Postnatal Day (PND) 21. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Illinois and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Genistein Suspension in Soy Formula-Corn Oil Emulsion
The vehicle emulsion for the genistein dosing was prepared in a 50-ml tube by adding 2.4 g of powdered soy infant formula (Similac Isomil Advanced soy formula with iron; Ross Products Laboratory, Abbott Laboratories) and 1 μL of corn oil into 10 ml of double distilled H2O, then vortexing (Genie2; Fisher Scientific) at maximum speed for 15 sec. The genistein dosage was prepared as a suspension by adding genistein to a 2-ml volume of the vehicle so that the final concentration was 25 mg genistein/ml of emulsion. Both vehicle and genistein stocks were prepared fresh weekly in 5-ml Eppendorf tubes and stored at 4°C. Genistein was purchased from Indofine Chemical Company, and had greater than 98% purity (confirmed by HPLC and 1H nuclear magnetic resonance at the University of Illinois).
Dosing
On the day of birth (designated PND 1), litters were adjusted to a maximum of eight pups, with approximately equal numbers of males and females. Pups were dosed once daily from PND 1 to PND 5. Each pup was weighed and dosed with genistein at 2 μl/g body weight (bw), whereas control pups were given vehicle alone. Genistein is relatively insoluble in aqueous media and forms a suspension that settles quickly. To ensure uniform distribution of genistein in suspension, the Eppendorf tube of vehicle or genistein was kept warmed in a water bath and then vortexed at maximal speed for 20 sec to disperse clumps and promote even distribution of genistein in the suspension. Before dosing of each pup, the Eppendorf tube was vortexed an additional 5–7 sec and quickly inverted 2–3 times, then immediately a 20 μl pipette (Rainen Gilson) equipped with a 20–200-μl-capacity tip (Midsci) was used to withdraw the appropriate amount from the inner corner Eppendorf lid for dosing. To determine the reliability and consistency of the genistein dose obtained in this manner, samples of the suspension were analyzed by HPLC, and an average of 88.9% ± 15.2% (percentage coefficient of variation; n = 3) of the expected genistein was measured in the aliquots of the genistein suspension.
Placing the pipette in the pup's mouth stimulated the suckling response, and the pup suckled the contents from the pipette tip. Care was taken to ensure the pup did not displace the delivered dose from its mouth. To promote ingestion of vehicle or genistein, pups were held ventral side up and their abdomens were gently stroked until they swallowed the dose.
Analysis of Serum Genistein Concentrations
Serum samples (10–50-μl aliquots) from mice were analyzed for total and aglycone genistein, with and without enzymatic deconjugation of glucuronides and sulfates, respectively, using liquid chromatography with tandem-mass spectrometry as previously validated for isoflavones with modifications [26]. The method detection limit for genistein was approximately 0.050 μM, accuracy was in the range of 88%–96%, and precision was in the range of 3%–8%.
Body, Thymic, and Uterine Weights
From nine separate litters, one randomly chosen female pup was weighed daily on PND 1–5 and subsequently on PND 7, 14, and 21. Thymus and uterus of some pups were excised and weighed at PND 5, whereas other pups were kept for later studies. Thymic weight from 10 vehicle- and 15 genistein-treated litters and uterine weight from 9 vehicle- and 14 genistein-treated litters are reported.
Histological Evaluation of Follicle Numbers
At selected time points (PND 5 and 4 mo old), ovaries were collected from vehicle- and genistein-treated animals for histological evaluation as described below [27]. PND 5 was selected because it is immediately after genistein exposure. Four-month-old animals were selected to assess adult ovarian morphology.
Ovaries were collected and fixed in Dietrick solution (5% formalin, 37% ethanol, and 1.25% glacial acetic acid), serially sectioned (8 μm), mounted on glass slides, and stained with hematoxylin and eosin. A stratified sample (8–10 sections of the neonatal ovary and 20–30 sections of the adult ovary) consisting of every 10th section was used to estimate the total number of MOFs. All sections were evaluated by a blinded observer.
Immunohistochemistry for Progesterone Receptor and Microscopy
Uteri collected at PND 5 were immersion-fixed in 10% (v/v) neutral buffered formalin. After fixation, tissues were embedded in paraffin, sectioned at 5 μm, and mounted on glass slides and incubated at 37°C overnight. Tissue sections were deparaffinized in xylene, rehydrated through a graded series of ethanols, and washed in tap water. Antigen retrieval was performed by boiling slides in 10 mM sodium citrate buffer (pH 6.0) for 10 min and then cooling the slides to room temperature. Endogenous peroxidase activity was quenched by incubation with 0.3% H2O2 for 20 min. Washes between steps (three times for 5 min each) were done using PBS with 0.05% Triton X. Nonspecific binding was inhibited using serum blocking solution (Vectastain Elite ABC kit; Vector Laboratories). After the serum block, sections were incubated overnight with a polyclonal rabbit anti-human progesterone receptor (PGR; DakoCytomation, 1:500 in PBS solution). Labeling was visualized by incubation with a biotinylated secondary antibody (Vectastain Elite ABC kit) for 1 h followed by incubation with an avidin and biotin horseradish peroxidase complex (Vectastain Elite ABC kit) for 30 min, then sections were covered in 3,3′-diaminobenzidine solution (Vector Laboratories) until the chromogen was visible. Slides then were counterstained with Mayer hematoxylin (Sigma-Aldrich), dehydrated in graded alcohols and xylene, and mounted using Permount (Fisher Scientific). Photomicrographs were taken using bright-field illumination with 20× objective and 2× magnification ring using an Olympus BX51 microscope (Olympus Corp.).
Measurement of Estrous Cycling, Breeding, and Fertility Studies
At 6 mo, estrous cycle stages were assessed from 12 genistein- and 15 vehicle-treated females [28] for a period of 25 days using daily lavage. Later, these females were bred for 7 days to proven breeders, and were checked daily for copulation plugs. On Day 7, females were individually caged and were monitored twice daily until parturition. Litter size and sex ratio were recorded.
Statistics
An unpaired t-test was used to compare MOFs as well as thymic and uterine weights. Thymic and uterine weights were normalized to body weight. A two-tailed two-way ANOVA was used to compare pharmacokinetics of total and aglycone genistein. Growth curve was analyzed using a repeated-measures ANOVA using the Proc Mixed procedure with a Tukey-Kramer adjustment for equal or unequal sample size (SAS 9.1; SAS Institute Inc.). For qualitative data, the Fisher Exact Probability test was performed (SAS 9.1). All data were expressed as mean ± SEM, except for the estrous and pregnancy data, which were expressed as mean alone. A P < 0.05 was considered statistically significant.
RESULTS
In order to identify a dose that produced serum genistein concentrations mimicking those in soy formula-fed human infants, in initial work we determined serum genistein concentrations (total and aglycone) in pups that had been dosed orally once daily from PND 1 to PND 5 with genistein at 5, 20, 50, and 100 mg/kg bw or vehicle alone. Initial results showed that the 50 mg/kg bw dose produced the desired serum genistein concentrations, and thus this study focuses exclusively on the 50 mg kg−1 day −1 dose. The 5 and 20 mg/kg doses yielded genistein concentrations substantially below the desired range (data not shown). The 100 mg/kg bw dose gave only a modest increase in genistein serum concentrations (data not shown) relative to 50 mg/kg and was problematic to administer because of the difficulty in extruding the more concentrated genistein suspension from the feeding pipette.
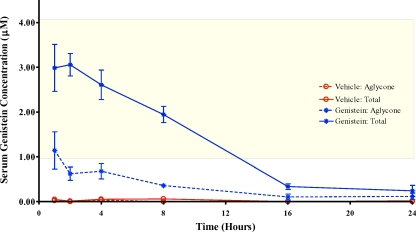
Total circulating serum genistein reached over 3 μM at 1 h following genistein dosing at 50 mg/kg (Fig. 1), and peak concentrations were obtained 2 h following administration. Genistein concentrations at 4 and 8 h decreased relative to those at 2 h but remained between 2 and 3 μM. By 16 h after dosing, total serum genistein concentrations had returned back to nearly baseline levels, and remained low at 24 h after dosing. Total genistein concentrations in serum of pups fed vehicle alone were minimal at all time points examined. The aglycone fraction in genistein-treated pups ranged from about 20% to 40% of the total serum genistein concentration (Fig. 1), and the fraction of the genistein present as the aglycone did not vary over the observed time.
FIG. 1.
Pharmacokinetics of orally administered genistein in PND 5 female mice. Serum was collected at the indicated times after the final dosing on PND 5 from pups. The yellow shaded area indicates total genistein (GEN) serum concentrations reported for 4-mo-old soy formula-fed infants [9]. After oral dosing of genistein, PND 5 pups have between 8 and 16 h of total genistein serum concentrations comparable to that of infants fed soy-based formula. n = 6–10 pups/time point. Data are shown as mean ± SEM.
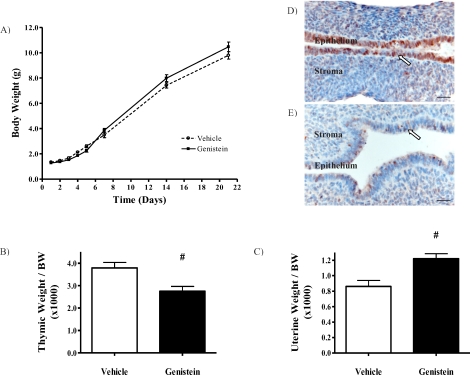
A critical question is whether neonatal genistein exposure changes growth and development of individual organs or the overall animal. Body weights for pups treated with genistein were comparable to those of the corresponding vehicle group from birth until weaning (Fig. 2A), and this pattern continued through adulthood (data not shown). This indicates that genistein treatment does not significantly alter body weight in developing or adult animals.
FIG. 2.
Orally administered genistein affects estrogen-sensitive targets in the neonate. A) Body weights from PND 1 to PND 21. Body weights in the two groups were comparable. Vehicle: n = 9; genistein: n = 9. B) Thymic weight normalized to body weight. Thymic weight:body weight ratio decreased by 28% in genistein-treated pups. Vehicle (white bar): n = 10; genistein (black bar): n = 15. C) Uterine weight normalized to body weight. Uterine weight:body weight ratio increased by 41% in genistein-treated pups. Vehicle (white bar): n = 9; genistein (black bar): n = 14. Data are shown as mean ± SEM. #P < 0.01. D) Control animals showed robust epithelial progesterone receptor expression (arrow), whereas this was nearly absent in neonatally exposed genistein-treated animals (E). Bar = 50 μm. B–E were collected and measured from PND 5 pups. For all results shown, n represents one animal from a different litter. n = 4 for both treatment groups.
The thymus is critical for immune function, and exposure of neonatal or adult mice to estrogens is capable of inducing thymic atrophy [29, 30]. Neonatal genistein treatment resulted in a 28% decrease in relative thymic weight at PND 5 in treated pups (Fig. 2B, P < 0.01 vs. controls).
Neonatal uterus is highly responsive to estrogens and functions as a biomarker for estrogenic exposure [31]. Neonatal genistein exposure resulted in a 41% increase in uterine weight:body weight ratio (Fig. 2C; P < 0.01). We next asked whether, in addition to hypertrophic effects of genistein on the uterus, there were other functional changes in response to genistein. Immunohistochemical analysis of neonatal uteri showed marked down-regulation of epithelial PGR expression in genistein-treated mice compared to controls (Fig. 2D, arrows).
Timing of vaginal opening has been shown to be sensitive to neonatal estrogen exposure [32]. Mice treated neonatally with genistein were monitored for timing of vaginal opening, but no significant difference was noted in control versus genistein-treated animals (data not shown).
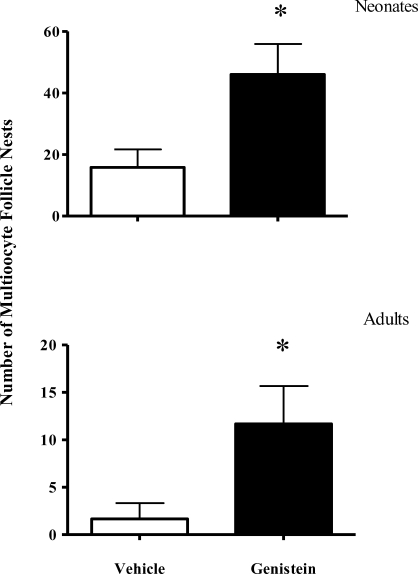
In the rodent ovary, neonatal administration of exogenous estrogen results in disruption of follicular development and increases the incidence of MOFs [21]. We therefore asked whether orally administered genistein increased the incidence of MOFs in the ovary both at the termination of genistein dosing (PND 5) and in adulthood (4 mo old). In the neonate, the incidence of MOFs increased approximately 3-fold in genistein-treated animals compared to controls (Fig. 3 top, P < 0.05 vs. controls). Of potentially greater significance, the higher incidence of MOFs seen in animals dosed neonatally with genistein persisted into adulthood, with an approximate 7-fold increased incidence of MOFs (Fig. 3 bottom, P < 0.05 vs. controls).
FIG. 3.
Neonatal genistein increases the incidence of MOFs in neonates and adults. Top: PND 5 ovaries; vehicle: n = 12; genistein: n = 10. Bottom: 4-mo-old adult ovaries; n = 6 for both vehicle and genistein. Each n represents one animal from a different litter. Data are shown as mean ± SEM. *P < 0.05.
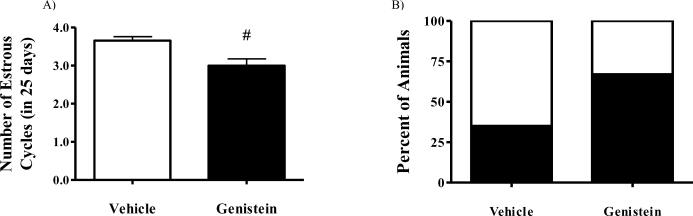
Neonatal genistein injections have been previously demonstrated to alter adult estrous cycles in rodents [33, 34]. Given this previous finding and our present observation regarding persistent increased incidence in MOFs in adult ovaries of genistein-treated animals, we next asked whether the adult estrous cycle was impacted by neonatal genistein. Our results showed that neonatal genistein exposure significantly decreased the number of complete estrous cycles over a given 25-day observational period in 6-mo-old mice (Fig. 4A, P = 0.002 vs. controls); this appeared to result from an extension of the diestrous stage in a great proportion of the genistein-treated animals (Fig. 4B and Supplemental Fig. S1; all Supplemental Data are available online at www.biolreprod.org). Despite these changes in estrous cycle length, the fertility of females neonatally treated with genistein was not compromised. Vehicle- and genistein-treated females had similar rates of pregnancies and numbers of live births; however, genistein-treated animals had a slightly greater, though not statistically significant, increase in the number of stillbirths (Supplemental Figs. S2A and S2B).
FIG. 4.
Neonatal genistein exposure decreases the overall number of estrous cycles in 6-mo-old females because of prolonged diestrous phase. A) Mice treated neonatally with genistein had a significant reduction in the number of cycles (#P = 0.002) over a 25-day period. B) Stacked bar graph showing the percentage of animals with prolonged diestrous phase, which was defined as diestrous phase lasting more than 3 days. White bar indicates percentage of animals with normal diestrous phase, and black bar represents the percentage of animals with prolonged diestrous phase. Vehicle: n = 12; genistein-treated: n = 20. Data are presented as the mean.
DISCUSSION
We have developed a minimally invasive oral dosing regimen for neonatal mice that closely mimics genistein exposure in human infants fed soy-based formula. This system retains critical features of human exposure to soy formula, such as first-pass metabolism, that determine the aglycone fraction responsible for biological responses.
Earlier studies examining developmental consequences of neonatal genistein exposure relied on injection into pups or lactational transfer through treated dams. Injected genistein produces reproductive and nonreproductive effects in neonates and adults [33], but in some cases serum genistein concentrations were not measured or injections produced supraphysiological genistein concentrations. In addition, genistein injection results in higher proportions of free aglycone than does genistein ingestion [35], because ingested genistein is subject to first-pass metabolism. Although genistein injection can produce total serum genistein concentrations comparable to those obtained by oral ingestion by infants fed soy formula, the higher proportions of circulating biologically active genistein (aglycone) has the potential to produce different biological effects. Limited maternal to pup transfer through the mother's milk [36] makes it impractical to expose pups to genistein concentrations seen in soy-fed infants through lactational transfer.
Our model produced peak serum genistein concentrations of just over 3 μM, almost identical to the total genistein concentrations of 2.53 ± 1.64 μM (and a range of approximately 1–4 μM) originally reported in soy formula-fed human infants [9] as well as the 3.6 μM median genistein concentration reported in a more recent study [18]. For 8–16 h after dosing, total serum genistein concentrations in neonatal pups remained in the range seen in soy formula-fed infants, but were minimal by 24 h. This contrasts with human infants, who have relatively constant circulating genistein concentrations over a 24-h period because of repeated feedings. Furthermore, mouse pups were exposed to genistein for approximately a quarter of the preweaning period, which is less than human infants, who may be exposed to soy formula for the entire nursing period. Thus, mouse pups in our model may be exposed to slightly lower cumulative doses of genistein. The ability of this regimen to impact reproductive and other organ systems emphasizes the potential of higher and longer genistein exposure in soy formula-fed infants to produce biological effects.
In initial studies utilizing oral administration of genistein to newborn rodents [37, 38], serum genistein concentrations were not measured, precluding comparisons of genistein concentrations between treated pups and soy-fed human infants. Following initial submission of our work describing our genistein dosing model, another report by Jefferson et al. [39] was published that involves oral delivery of either genistein or genistin to neonatal pups, and subsequent examination of acute and chronic effects of this treatment. However, the dose in this model for which the genistein pharmacokinetics were evaluated produced peak serum genistein concentrations of almost 20 μM, which is 8- and 6-fold greater, respectively, than the 2.5 [9] and 3.6 μM [18] genistein concentrations reported in soy formula-fed human infants. Thus, our model may have some advantages over this system by more accurately exposing the neonatal mouse to the same concentrations of genistein seen in soy formula-fed human infants rather than those that exceed the human values by several fold.
In the first 8 h after genistein administration, the aglycone fraction in genistein-treated pups was approximately 25% of the total plasma genistein concentration, considerably higher than the 3%–5% aglycone fraction in adult rodents following oral genistein exposure [20]. This is consistent with reports showing higher aglycone fractions in rodent pups in comparison to adults following genistein injection, as well as another recently described model for oral administration of genistein and genistin to neonatal mice [39]. The differing ratios of aglycone to total genistein concentrations between neonates and adults have been suggested to result from immaturity of neonatal uridine 5′-diphospho glucuronosyltransferases [36]. Aglycone levels for soy formula-fed human infants have not been reported, but others have presumed isoflavones exist in human infants largely as conjugated glucuronides based on human adult metabolism of genistein and other phytoestrogens [10]. However, our present results suggest the possibility that a similarly increased ratio of aglycone to total genistein may exist in soy formula-fed human infants in comparison to adults consuming soy. In conjunction with the minimal affinity of genistein for serum binding proteins [40] and alpha fetoprotein [41], which normally binds and inactivates endogenous estrogens such as 17β-estradiol in newborn humans, this potential exposure to a significant aglycone fraction emphasizes the plausibility of biological effects in soy formula-fed infants.
We sought to determine whether total serum genistein concentrations found in human infants consuming soy formula had reproductive and/or nonreproductive effects in our mouse model. Body weight of genistein-treated pups was comparable to that of the vehicle control during genistein administration and subsequent life. This is consistent with the normal growth seen in infants fed soy formula [6], and the reported lack of differences in weight or height in adults fed soy formula as infants compared to non-soy formula-fed adults [2].
The thymus regulates T cell and overall immune development, and young animals are especially susceptible to thymolytic effects of estrogen [29, 30]. Our results indicate that exposure of mouse pups to total serum concentrations of genistein similar to those in soy formula-fed human infants significantly inhibits thymic weight. Whether this impacts neonatal or adult immune function remains to be established, but these developmental changes could result in either reduced immune function or immune hypersensitivity and increased susceptibility to autoimmune disorders during adulthood. Immune function studies offer conflicting data on vaccine response in soy formula-fed infants [14–16], but more extensive recent studies using present soy-based infant formulations [15, 16] did not identify clear immune deficits in soy-fed infants. In the sole study of adults who had consumed soy formula as infants, the only parameter examined related to immune function (use of asthma or allergy medications) was increased in adults who had consumed soy formula as infants [2]. Although definitive immune changes in infants or adults in response to soy formula have not been shown, the decrease of thymic weight in response to genistein in neonatal mice suggests that this question may warrant continued exploration.
Our dose for neonatal exposure to genistein produces a clear estrogenization of neonatal mice, as shown by increased uterine weight and decreased epithelial PGR expression. In uteri of ovariectomized adult mice, signaling through ESR1 induces down- and up-regulation of epithelial and stromal PGR, respectively [42]. PGR expression in uterine epithelium appears neonatally in rats, whereas PGR is not seen in uterine stroma of rats until Day 12 postnatally, and estrogen treatment does not induce precocious PGR expression in uterine stroma [43]. Thus, only epithelial PGR is expressed at the age we are examining, which likely explains the lack of genistein stimulation of stromal PGR despite well-known effects of estrogens on PGR in adult uterine stroma.
These results suggest that genistein exposure seen in soy formula-fed infants may be sufficient to produce estrogenic changes in reproductive and other organs. These results are compatible with recent preliminary data from human infants that indicated that neonatal girls given soy formula had changes in vaginal cytology at 6 mo of age consistent with estrogenization [17] and changes in breast development during their second year of life [44].
Our data demonstrate that transient neonatal genistein exposure increases the incidence of ovarian MOFs in neonates that persist into adulthood. In rodent ovaries, neonatal estrogen treatment disrupts follicular development and increases MOF incidence [21]. MOFs have also been described in girls [45] and women [46], although a link with early estrogen exposure and MOFs in women has not been established. It has been postulated that normal oocyte-follicle maturation in mice requires the postpartum decrease in estrogen and progesterone and that this process is disrupted by the estrogenic actions of genistein administered neonatally [47], leading to MOFs. Another possibility for MOFs is that estrogens interfere with ovarian activin signaling in neonatal mice [48]. In rodents and other wildlife, MOFs are correlated with reduced fertility and embryonic survival rates [21, 49]. Previous work utilizing neonatal genistein injections and genistein administered orally has shown that this treatment increases MOFs, and this is associated with decreased fertility as well as premature reproductive senescence [33, 37, 39].
Soy formula-fed infants consume approximately 10-fold more genistein per body weight basis than amounts necessary to disrupt menstrual cycles in women [12]. Other studies demonstrated that neonatal injections or oral administration of genistein altered adult estrous cycles of mice [33, 39] and rats [34]. Similarly, others have shown aberrant cycling in Sprague-Dawley rats treated perinatally or chronically with genistein (up to approximately 40 mg [kg bw]−1 day−1) [50]. These findings raised the question of whether neonatal genistein exposure at concentrations mimicking those in soy formula-fed infants would alter the adult estrous cycle in our model. Our results indicate that early genistein exposure results in adult ovarian cycle abnormalities at 6 mo of age. Despite changes in estrous cycle length, fertility was not compromised by neonatal genistein treatment, at least at 6 mo. This contrasts with previous reports showing that neonatal genistein injections (50 mg kg−1 day−1) impaired or abolished adult fertility [37]. Although our model shows extensive concurrence with injection studies in terms of some parameters (e.g., uterotrophic effects, estrous cycle abnormalities), there is a clear discrepancy between results obtained with our system and genistein injections with regard to fertility despite comparable doses in these studies. This may indicate that the more physiological oral administration of genistein may not produce some abnormalities seen with injections of the same dose [33], although differing mouse strains in the two studies could have contributed to the discordant results.
Our model provides an additional tool that can be used in conjunction with ongoing human prospective studies [17–19]. This system enables researchers to examine endpoints that are difficult and/or ethically unsound to examine in human populations, or where data will not be obtained from human studies for several decades. For example, data on genistein effects on incidence of reproductive pathologies such as breast and endometrial cancer will not be available from the human studies for at least a half century. Moreover, effects of neonatal soy formula on incidence of autoimmune disorders are unlikely to be detected in present human studies because of low incidence of these diseases and consequent lack of statistical power of these studies to address these questions. Finally, any potential effects on reproductive senescence (i.e., menopause) in soy formula-fed infant females would not be revealed for decades, and at the present rates of consumption, many millions of human infants would have utilized these soy formulas in the interim.
The present system involves administration of the free aglycone form of genistein, whereas the conjugated form (genistin; genistein linked to a sugar moiety) predominates in soy-based foods, including soy formula. However, genistin is readily and quickly hydrolyzed in the gut of both infants and older individuals to the absorbable aglycone form [51]. Hence, genistein is absorbed through the gut even in animals given genistin. Although some rodent studies have shown subtle differences in genistin and genistein effects (possibly resulting from altered pharmacokinetics of the compounds [52]), the biological effects result from circulating free genistein.
In the present study, the genistein dose (50 mg [kg bw] −1 day−1) utilized clearly exceeds the 6–11 mg [kg bw] −1 day−1 of total genistein exposure reported for human infants consuming soy formulas [9]. However, genistein is metabolized more rapidly in mice than in humans [53], and therefore a higher dose was necessary to achieve similar genistein total serum concentrations that more closely resemble those reported in human infants.
In summary, this mouse model of neonatal genistein exposure provides a more physiologically relevant route of administration and produces similar serum genistein concentrations as in soy-fed human infants. Although care should always be taken when extrapolating data obtained with rodent models to humans, our model offers a valuable approach to evaluate short- and long-term effects of neonatal genistein exposure. This model should facilitate determination of the consequences for human infants resulting from high consumption of genistein and other phytoestrogens through soy formula.
Supplementary Material
Acknowledgments
The authors thank Drs. Meagan Mann for technical assistance in development of the soy formula-corn oil emulsion, Juan E. Andrade for HPLC analysis of the genistein suspension in soy formula-corn oil emulsion, and Liz Simon for immunohistochemical data.
Footnotes
1Supported by NIH grant P01 AG24387 (to S.L.S., D.R.D., W.G.H., and P.S.C.). M.A.C. and S.L.N. were supported by NIEHS grant ES07326 during conduct of this research. Work at the University of Illinois was conducted in a facility constructed with support from Research Facilities Improvement Program grant C06 RR16515 from the National Center for Research Resources, NIH.
REFERENCES
- Bhatia J, Greer F.Use of soy protein-based formulas in infant feeding. Pediatrics 2008; 121: 1062–1068. [DOI] [PubMed] [Google Scholar]
- Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, Stallings VA, Drulis JM, Nelson SE, Hanson SA.Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 2001; 286: 807–814. [DOI] [PubMed] [Google Scholar]
- Berger-Achituv S, Shohat T, Romano-Zelekha O, Ophir E, Rachmani S, Malovizky D, Garty BZ.Widespread use of soy-based formula without clinical indications. J Pediatr Gastroenterol Nutr 2005; 41: 660–666. [DOI] [PubMed] [Google Scholar]
- Agostoni C, Axelsson I, Goulet O, Koletzko B, Michaelsen KF, Puntis J, Rieu D, Rigo J, Shamir R, Szajewska H, Turck D.Soy protein infant formulae and follow-on formulae: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr 2006; 42: 352–361. [DOI] [PubMed] [Google Scholar]
- Badger TM, Gilchrist JM, Pivik RT, Andres A, Shankar K, Chen JR, Ronis MJ.The health implications of soy infant formula. Am J Clin Nutr 2009; 89: 1668S–1672S. [DOI] [PubMed] [Google Scholar]
- Lasekan JB, Ostrom KM, Jacobs JR, Blatter MM, Ndife LI, Gooch WM, III, Cho S.Growth of newborn, term infants fed soy formulas for 1 year. Clin Pediatr (Phila) 1999; 38: 563–571. [DOI] [PubMed] [Google Scholar]
- Mendez MA, Anthony MS, Arab L.Soy-based formulae and infant growth and development: a review. J Nutr 2002; 132: 2127–2130. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA.Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998; 139: 4252–4263. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE.Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 1997; 350: 23–27. [DOI] [PubMed] [Google Scholar]
- Setchell KD.Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 1998; 68: 1333S–1346S. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Markkanen H, Watanabe S.Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 1993; 342: 1209–1210. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Bingham S, Setchell KD.Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr 1994; 60: 333–340. [DOI] [PubMed] [Google Scholar]
- Freni-Titulaer LW, Cordero JF, Haddock L, Lebron G, Martinez R, Mills JL.Premature thelarche in Puerto Rico. A search for environmental factors. Am J Dis Child 1986; 140: 1263–1267. [DOI] [PubMed] [Google Scholar]
- Zoppi G, Mantovanelli F, Pittschieler K, Delem A, Teuwen DE.Response to RIT 4237 oral rotavirus vaccine in human milk, adapted-and soy-formula fed infants. Acta Paediatr Scand 1989; 78: 759–762. [DOI] [PubMed] [Google Scholar]
- Cordle CT, Winship TR, Schaller JP, Thomas DJ, Buck RH, Ostrom KM, Jacobs JR, Blatter MM, Cho S, Gooch WM, III, Pickering LK.Immune status of infants fed soy-based formulas with or without added nucleotides for 1 year: part 2: immune cell populations. J Pediatr Gastroenterol Nutr 2002; 34: 145–153. [DOI] [PubMed] [Google Scholar]
- Ostrom KM, Cordle CT, Schaller JP, Winship TR, Thomas DJ, Jacobs JR, Blatter MM, Cho S, Gooch WM, III, Granoff DM, Faden H, Pickering LK.Immune status of infants fed soy-based formulas with or without added nucleotides for 1 year: part 1: vaccine responses, and morbidity. J Pediatr Gastroenterol Nutr 2002; 34: 137–144. [DOI] [PubMed] [Google Scholar]
- Bernbaum JC, Umbach DM, Ragan NB, Ballard JL, Archer JI, Schmidt-Davis H, Rogan WJ.Pilot studies of estrogen-related physical findings in infants. Environ Health Perspect 2008; 116: 416–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, Ye X, Rogan WJ.Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol 2009; 19: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H, Pivik RT, Gilchrist JM, Badger TM.No difference indicated in electroencephalographic power spectral analysis in 3- and 6-mo-old infants fed soy- or milk-based formula. Matern Child Nutr 2008; 4: 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Churchwell MI, Newbold RR, Delclos KB.Lactational transfer of the soy isoflavone, genistein, in Sprague-Dawley rats consuming dietary genistein. Reprod Toxicol 2006; 21: 307–312. [DOI] [PubMed] [Google Scholar]
- Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N.Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod 1990; 43: 478–484. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR.Disruption of the developing female reproductive system by phytoestrogens: genistein as an example. Mol Nutr Food Res 2007; 51: 832–844. [DOI] [PubMed] [Google Scholar]
- Ashby J, Odum J, Tinwell H.Predictive value of the uterotrophic assay for genistein carcinogenicity in the neonatal mouse: relevance to infants consuming soy-based formula. Environ Health Perspect 2001; 109: A568–A570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, Flaws JA, Hansen DK, Hoyer PB, Jeffery EH, Kesner JS, Marty S, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of soy formula. Birth Defects Res B Dev Reprod Toxicol 2006; 77: 280–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, Flaws JA, Hansen DK, Hoyer PB, Jeffery EH, Kesner JS, Marty S, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res B Dev Reprod Toxicol 2006; 77: 485–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaddle NC, Churchwell MI, Doerge DR.High-throughput quantification of soy isoflavones in human and rodent blood using liquid chromatography with electrospray mass spectrometry and tandem mass spectrometry detection. J Chromatogr B Analyt Technol Biomed Life Sci 2002; 777: 139–145. [DOI] [PubMed] [Google Scholar]
- Tomic D, Frech MS, Babus JK, Symonds D, Furth PA, Koos RD, Flaws JA.Effects of ERalpha overexpression on female reproduction in mice. Reprod Toxicol 2007; 23: 317–325. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL.The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol 2007; 80: 84–97. [DOI] [PubMed] [Google Scholar]
- Forsberg JG.The different responses of the female mouse thymus to estrogen after treatment of neonatal, prepubertal, and adult animals. Acta Anat (Basel) 1996; 157: 275–290. [DOI] [PubMed] [Google Scholar]
- Yellayi S, Naaz A, Szewczykowski MA, Sato T, Woods JA, Chang J, Segre M, Allred CD, Helferich WG, Cooke PS.The phytoestrogen genistein induces thymic and immune changes: a human health concern? Proc Natl Acad Sci U S A 2002; 99: 7616–7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern HA, Edery M, Mills KT, Kohrman AF, Mori T, Larson L.Long-term alterations in histology and steroid receptor levels of the genital tract and mammary gland following neonatal exposure of female BALB/cCrgl mice to various doses of diethylstilbestrol. Cancer Res 1987; 47: 4165–4172. [PubMed] [Google Scholar]
- Thigpen JE, Haseman JK, Saunders HE, Setchell KD, Grant MG, Forsythe DB.Dietary phytoestrogens accelerate the time of vaginal opening in immature CD-1 mice. Comp Med 2003; 53: 607–615. [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR.Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod 2005; 73: 798–806. [DOI] [PubMed] [Google Scholar]
- Kouki T, Kishitake M, Okamoto M, Oosuka I, Takebe M, Yamanouchi K.Effects of neonatal treatment with phytoestrogens, genistein and daidzein, on sex difference in female rat brain function: estrous cycle and lordosis. Horm Behav 2003; 44: 140–145. [DOI] [PubMed] [Google Scholar]
- Yellayi S, Teuscher C, Woods JA, Welsh TH, Jr, Tung KS, Nakai M, Rosenfeld CS, Lubahn DB, Cooke PS.Normal development of thymus in male and female mice requires estrogen/estrogen receptor-alpha signaling pathway. Endocrine 2000; 12: 207–213. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Banks EP, Jefferson WN, Newbold RR.Pharmacokinetic analysis in serum of genistein administered subcutaneously to neonatal mice. Cancer Lett 2002; 184: 21–27. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR.Disruption of the female reproductive system by the phytoestrogen genistein. Reprod Toxicol 2007; 23: 308–316. [DOI] [PubMed] [Google Scholar]
- Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, Ono H.Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod Toxicol 2001; 15: 399–411. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Doerge D, Padilla-Banks E, Woodling KA, Kissling GE, Newbold R.Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environ Health Perspect 2009; 117: 1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel SC, vom Saal FS, Welshons WV.Developmental effects of estrogenic chemicals are predicted by an in vitro assay incorporating modification of cell uptake by serum. J Steroid Biochem Mol Biol 1999; 69: 343–357. [DOI] [PubMed] [Google Scholar]
- Milligan SR, Khan O, Nash M.Competitive binding of xenobiotic oestrogens to rat alpha-fetoprotein and to sex steroid binding proteins in human and rainbow trout (Oncorhynchus mykiss) plasma. Gen Comp Endocrinol 1998; 112: 89–95. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR.Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod 2000; 62: 821–830. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Fukazawa Y, Sato T, Suzuki A, Nishimura N, Iguchi T.Effect of estrogen on ontogenic expression of progesterone and estrogen receptors in rat uterus. Zoolog Sci 1996; 13: 143–149. [DOI] [PubMed] [Google Scholar]
- Zung A, Glaser T, Kerem Z, Zadik Z.Breast development in the first 2 years of life: an association with soy-based infant formulas. J Pediatr Gastroenterol Nutr 2008; 46: 191–195. [DOI] [PubMed] [Google Scholar]
- Manivel JC, Dehner LP, Burke B.Ovarian tumorlike structures, biovular follicles, and binucleated oocytes in children: their frequency and possible pathologic significance. Pediatr Pathol 1988; 8: 283–292. [DOI] [PubMed] [Google Scholar]
- Gougeon A.Frequent occurrence of multiovular follicles and multinuclear oocytes in the adult human ovary. Fertil Steril 1981; 35: 417–422. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME.Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 2007; 148: 3580–3590. [DOI] [PubMed] [Google Scholar]
- Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE.Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology 2007; 148: 1968–1976. [DOI] [PubMed] [Google Scholar]
- Guillette LJ, Jr, Moore BC.Environmental contaminants, fertility, and multioocytic follicles: a lesson from wildlife? Semin Reprod Med 2006; 24: 134–141. [DOI] [PubMed] [Google Scholar]
- Delclos KB, Newbold R.NTP toxicity report of reproductive dose range-finding study of genistein (CAS No. 446-72-0) administered in feed to Sprague-Dawley rats. Toxic Rep Ser 2007; 79: 1–C2 [PubMed] [Google Scholar]
- Hoey L, Rowland IR, Lloyd AS, Clarke DB, Wiseman H.Influence of soya-based infant formula consumption on isoflavone and gut microflora metabolite concentrations in urine and on faecal microflora composition and metabolic activity in infants and children. Br J Nutr 2004; 91: 607–616. [DOI] [PubMed] [Google Scholar]
- Allred CD, Ju YH, Allred KF, Chang J, Helferich WG.Dietary genistin stimulates growth of estrogen-dependent breast cancer tumors similar to that observed with genistein. Carcinogenesis 2001; 22: 1667–1673. [DOI] [PubMed] [Google Scholar]
- Whitten PL, Patisaul HB.Cross-species and interassay comparisons of phytoestrogen action. Environ Health Perspect 2001; 109(suppl 1):5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.