Abstract
Bone morphogenetic protein (BMP) 1 is a vertebrate metalloproteinase of the astacin family. BMP1 plays a key role in regulating the formation of the extracellular matrix (ECM), particularly by processing the C-propeptide of fibrillar procollagens. BMP1 also promotes BMP signaling by releasing BMP signaling molecules from complexes with the BMP-antagonist chordin. As a result of BMP1′s dual role in both ECM formation and BMP signaling, we hypothesized that BMP1 could play a role in ovarian physiology. Using the sheep ovary as a model system, we showed that BMP1 was expressed in the ovary throughout early fetal stages to adulthood. Furthermore, in adult ovaries, BMP1 was expressed along with chordin, BMP4, and twisted gastrulation, which together form an extracellular regulatory complex for BMP signaling. Within ovine ovaries, immunohistochemical localization demonstrated that BMP1 was present in granulosa cells at all stages of follicular development, from primordial to large antral follicles, and that the levels of BMP1 were not affected by the final follicle selection mechanism. In cultured granulosa cells, BMP1 expression was not affected by gonadotropins, but BMP4 and activin A had opposing effects on the levels of BMP1 mRNA. BMP1 appeared to be secreted into the follicular fluid of antral follicles, where it is able to exert procollagen C-proteinase and chordinase activities. Interestingly, BMP1 activity in follicular fluid decreased with follicular growth.
Keywords: BMP1, follicle, follicular development, granulosa cells, ovary
Enzymatically active bone morphogenetic protein 1 (BMP1) is produced by granulosa cells and is present in the follicular fluid of ovine ovarian follicles.
INTRODUCTION
Bone morphogenetic protein (BMP) 1 is the vertebrate prototype of the tolloid family of metalloproteases. The BMPs were initially identified as a complex of proteins capable of inducing ectopic bone formation [1]. Notably, the BMP1 gene also codes for a second, longer protein named mammalian tolloid (mTLD) [2]. Each of these proteins contains a protease domain, common to the astacin family of metalloproteases, near the N-terminus [3]. This protease domain is followed by a regulatory region containing epidermal growth factor-like motifs, which are thought to bind calcium ions [4, 5], and “CUB” domains [6], which are thought to mediate protein-protein interactions [7]. Furthermore, a third alternatively spliced variant encodes a protein in which a novel histidine-rich region replaces the BMP1-specific C-terminal domain (denoted BMP1/His) [2]. BMP1 differs from the other BMPs in that it is a metalloproteinase rather than a transforming growth factor beta (TGFB)-like protein. Through cleavage of the BMP-antagonist chordin, BMP1 is thought to promote BMP signaling (and, thereby, bone formation) by releasing the TGFB-like morphogens BMP2 and BMP4 from extracellular latent complexes with chordin [8, 9]. BMP1 and mTLD also remove the C-propeptides from procollagens I–III [10, 11], an essential step in the formation of collagen fibrils and, hence, of all fibrous extracellular matrices (ECMs). The tolloid family of metalloproteinases further promotes ECM formation in numerous ways, including the processing of the minor collagens V and XI [12–14] and the proteolytic activation of lysyl oxidase [15], which is required for the formation of covalent cross-links in both collagen fibrils and in elastic fibers [16].
Regarding the biological actions described above (i.e., BMP ligand bioavailability and ECM remodeling), we hypothesized that BMP1 could play a role in ovarian physiology. BMP2 and BMP4 have been implicated in the autocrine/paracrine regulation of ovarian function in mammals and are thought to modulate folliculogenesis and ovulation rate [17–20]. For example, these BMP factors regulate granulosa cell differentiation and delay the luteinization process [21]. Coordinated release of BMP2 and BMP4 from chordin, by the chordinase activity of BMP1, could therefore regulate BMP signaling in the ovary. Through its procollagen C-proteinase (PCP) activity, BMP1 could also regulate the formation of ECM during ovarian folliculogenesis. The importance of the ECM for follicular development is exemplified by the observation that both the growth and atresia of ovine ovarian follicles is associated with dramatic changes in the composition of the collagenous ECM in which the theca and granulosa cells are embedded [22–24].
Using the ovine species as a model, the present study aimed to 1) identify BMP1 mRNA and protein in ovarian tissues, 2) study the regulation of BMP1 transcripts by important modulators of folliculogenesis (i.e., gonadotropins, insulin-like growth factor 1 [IGF1], and TGFB family members), and 3) assay the PCP and chordinase activities of BMP1 in ovarian follicular fluids.
MATERIALS AND METHODS
Hormones and Reagents
Flurogestone acetate sponges used to synchronize estrous cycles were obtained from Intervet. Porcine follicle-stimulating hormone (pFSH) from pituitary extracts (pFSH activity = 1.15-fold the activity of National Institutes of Health [NIH] pFSH-P1) (used for animal injections) and ovine luteinizing hormone (oLH activity = 1.38-fold the activity of NIH oLH-S21) were obtained from Dr. Y. Combarnous (INRA, Nouzilly, France). Purified ovine FSH-20 (oFSH; lot no. AFP-7028D; 4453 IU/mg; FSH activity = 175-fold the activity of oFSH-S1), used in cell culture, was a gift from the National Institute of Diabetes and Digestive and Kidney Diseases (National Hormone Pituitary Program, Bethesda, MD). Recombinant human BMP1, BMP4, and activin A were obtained from R&D Systems Europe. IGF1 was obtained from Ciba-Geigy. Recombinant TGFB1, rabbit polyclonal anti-BMP1, mouse monoclonal anti-Myc 9E10, and McCoy5A culture medium were obtained from Sigma-Aldrich.
Animals
The estrous cycles of all adult ewes in the present study were synchronized with intravaginal sponges impregnated with fluorogestone acetate (40 mg) for 14 days. This treatment mimics a luteal phase, with LH surge and ovulation occurring approximately 40 and 60 h, respectively, after sponge removal. All procedures were approved by the Agricultural and Scientific Research agencies (approval no. C37‐175‐2) and were conducted in accordance with the Guidelines for Care and Use of Agricultural Animals in Agricultural Research and Teaching.
In a first set of in vivo experiments, Ile-de-France ewes were synchronized and killed at three time points after sponge removal: 36 h (group 1), 48 h (group 2), and 66 h (group 3). Before killing, blood samples were collected from the jugular vein at 2-h intervals: between 30 and 36 h for group 1, between 32 and 48 h for group 2, and between 34 and 66 h for group 3. Plasma was recovered from these samples and assayed to quantify serum levels of LH [25]. At slaughtering, ovaries were collected individually for immediate follicle dissection (to obtain granulosa cells and follicular fluid) or were frozen in cryoprotectant (Tissue-Tek, Sakura Finetek) for immunohistochemistry. At the same time, numerous tissues were collected, frozen in liquid nitrogen, and stored at −80°C. After LH assays, samples were postclassified as “follicular” (follicular phase, before the LH surge) or “preovulatory” (after LH surge, before ovulation). Animals killed during the LH surge or after ovulation were not considered in the present study.
In a second set of in vivo experiments to assess fetal expression of BMP1, cyclic Préalpes ewes were synchronized and inseminated after sponge removal. Pregnant female tracts at different developmental stages were collected at slaughter and rapidly dissected to extract fetuses. Fetuses were collected at different stages from Day 25 to Day 127 of gestation. The gonads of newborn animals were collected at slaughtering. All gonads were first detached from the mesonephros (except from Day 25 to Day 34), immediately frozen in liquid nitrogen, and then stored at −80°C.
In a third set of in vitro cell culture experiments, Romanov ewes were synchronized for 14 days and further stimulated 10 days after sponge removal by intramuscular injections of 6 and 5 IU of pFSH administered 24 and 12 h, respectively, before slaughter. Ovaries were collected at slaughtering for immediate follicle dissection, recovery of granulosa cells, and in vitro cultures.
Immunohistochemistry
Frozen ovaries from group 1 (36 h after sponge removal) were serially sectioned at a thickness of 20 μm with a cryostat. Sections were fixed for 10 min in PBS containing 4% paraformaldehyde. After two 5-min washes with 0.1% saponin in PBS, sections were incubated at 4°C for 30 min with PBS containing 0.1% saponin and 0.3% H2O2 to remove endogenous peroxidase activity. After three 5-min washes in 0.1% saponin in PBS, sections were incubated at 4°C overnight with rabbit polyclonal BMP1 antibody (B5058; Sigma) diluted 1:1000 in PBS containing 0.1% saponin and 0.1% bovine serum albumin. After three 5-min washes in PBS/0.1% saponin, sections were incubated for 4 h at room temperature with the donkey anti-rabbit peroxidase-conjugated antibody (Jackson ImmunoResearch Laboratories, Interchim) diluted 1:1000 in PBS containing 0.1% saponin and 0.1% bovine serum albumin. After three 5-min washes with 0.1% saponin in PBS, sections were incubated with PBS solution containing diaminobenzidine and H202 for 2 min at room temperature and then counterstained with hematoxylin. Negative controls were performed in which the primary antibody was replaced with rabbit IgG.
Isolation of Granulosa Cells
For in vivo experiments, all visible follicles larger than 1 mm in diameter were rapidly dissected from the ovaries of Ile-de-France ewes. After dissection, follicle diameter was measured, and each follicle was classified according to size, animal, and status: small antral immature follicles (diameter, 1–3 mm) and large antral follicles (diameter, >6 mm) were classified in the “follicular” group, and preovulatory follicles (diameter, >6 mm) were classified into the “preovulatory” group. Subsequently, follicular fluid was recovered individually and stored at −20°C for downstream BMP1 protease activity assays. Granulosa cells were separated from the theca layer by gentle scraping, as previously described [26]. A small aliquot of the cells was smeared onto a glass slide and stained with Feulgen to assess the degree of follicle atresia [27]. Once the atresia status had been determined, granulosa cells from healthy small antral follicles from the same animal were pooled to obtain sufficient quantities of RNA after extraction. The granulosa cells from healthy large antral and preovulatory follicles were treated individually.
For each independent in vitro culture experiment, ovaries from four Romanov ewes were dissected to recover granulosa cells from small antral follicles (diameter, 1–3 mm) or large antral follicles (diameter, ≥6 mm), as previously described [28]. Granulosa cell suspensions were seeded at 2 × 106 viable cells/well in six-well plates and cultured for 24 h at 37°C with 5% CO2 in McCoy5a medium supplemented with 3% fetal ovine serum. The cell culture medium was removed at 24 h, and the plated cells were washed twice with PBS. Cells were grown for 48 h in serum-free condition with or without the addition of exogenous factors (50 ng/ml of BMP4, 5 ng/ml of TGFB1, 50 ng/ml of activin A, 10 ng/ml of IGF1, 50 ng/ml of oFSH, or 50 ng/ml of oLH), either alone or in combination. Cells were then trypsinized, washed with PBS, pelleted by centrifugation (1300 × g), and stored at −20°C for subsequent RNA extraction. Each combination of treatments was tested in at least four independent culture experiments.
RNA Extraction and Reverse Transcription
Total RNA from adult tissues or granulosa cell pellets was isolated using Nucleospin RNA L or Nucleospin RNA II kits, respectively, according to the manufacturer's protocol (Macherey-Nagel) and diluted at 0.2 μg/μl in RNase-free water. RNA (1 μg) was then reverse transcribed [29]. Total RNA from fetal gonads was extracted from each sample using RNA-plus solution (MP Biomedicals). RNA (5 μg) was reverse transcribed as previously described [30]. All RNA samples were DNase-treated to avoid genomic DNA contamination. The quantitative yield of the purification and the quality of the RNA were determined using a NanoDrop ND1000 (Labtech) and by electrophoresis to ensure equal purity and the absence of RNA degradation in all samples.
Polymerase Chain Reaction
Standard PCR was used initially to investigate gene expression in multiple tissues and in fetal gonads. Two microliters of each reverse transcription reaction were amplified in a 50-μl PCR reaction using 0.5 U of Taq polymerase (Takara or Sigma-Aldrich) for 28–30 cycles, with 30–45 sec annealing at 55–58°C (depending on the primers), with 200 μM of each dNTP and 150 nM of each primer. PCR reactions run without cDNA (water blank) on the one hand and with total RNA (non-reverse transcribed) to check for absence of genomic DNA contamination on the other served as negative controls. Primer sequences for each gene studied (Table 1) were designed in different exons (except for BMP4 and TWSG1). BMP1 primers were designed to amplify part of the region common to all RNA splice variants. The RPL19 or GAPDH genes were used as cDNA quantity controls.
TABLE 1.
PCR primer sequences used in this study.
Real-time PCR was performed to compare differential gene expression in granulosa cells using the iCycler iQ Multicolor Detection System (Bio-Rad), as already described [29]. A melting curve was constructed for each primer pair to verify the presence of one gene-specific peak and the absence of primer dimers. This was further confirmed by agarose gel electrophoresis of the amplicon. For each primer pair, efficiency curves were generated using serial dilutions of granulosa cell cDNA in abscissa and the corresponding cycle threshold (Ct) in ordinate. The slope of the log-linear phase reflects the amplification efficiency (E) derived from the formula E = e(−1/slope). Amplification efficiency was found to be EBMP1 = 1.86 and ERPL19 = 1.95. To quantify relative gene expression, the Ct of BMP1 amplification was compared with that of the internal reference gene RPL19, according to the ratio R = [EL19CtL19/EtargetCt BMP1]. For each sample, real-time PCR was performed in duplicate.
PCP Activity Assay
Neat follicular fluid samples (3 μl) were assayed for PCP activity using human 14C-labeled type I procollagen substrate (0.6 μg), as previously described [31, 32]. Digests were performed in procollagen assay buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 50 mM CaCl2, 0.01% Brij 35, and 0.02% NaN3) containing ethylenediaminetetra-acetic acid (EDTA)-free Protease Inhibitor Cocktail (Roche Applied Science) in a final volume of 20 μl. Negative controls contained 10 mM EDTA to inhibit metalloproteinase activity. Positive controls contained 500 ng of recombinant human BMP1 (R&D Systems) in place of follicular fluid. Digests were incubated at 37°C overnight and stopped by the addition of SDS loading buffer with reducing agent. Samples were analyzed by SDS PAGE (7% separating, 3.5% stacking), fixed twice for 20 min each time in methanol (10% v/v) with acetic acid (10% v/v), and then dried under vacuum. The gels were exposed to a BAS-MS phosphorimaging plate (Fujifilm), which was processed using an FLA3000 PhosphorImager (Fujifilm). Quantification was performed as previously described [33], with the percentage of labeled procollagen cleavage expressed as the mean ± SD. The data were analyzed by one-way ANOVA.
Chordinase Activity Assay
Individual follicular fluid samples from small, large, and preovulatory follicles were pooled, and each sample group was assayed for chordinase activity. Follicular fluid pools (3 μl) were incubated with 30 μl of conditioned cell culture medium containing c-Myc-tagged chordin [34] in the presence of EDTA-free Protease Inhibitor Cocktail. Negative controls contained 10 mM EDTA to inhibit metalloproteinase activity. Positive controls contained 500 ng of recombinant human BMP1 (R&D Systems) in place of follicular fluid. Digests were made up to a final volume of 50 μl with assay buffer (50 mM Tris HCl [pH 7.4], 150 mM NaCl, 50 mM CaCl2, 0.01% Brij 35, and 0.02% NaN3) and incubated at 37°C overnight. The reactions were stopped by the addition of SDS loading buffer with reducing agent and resolved by electrophoresis on a 10% SDS-Prosieve gel (Cambrex Bio Science Wokingham Ltd., Wokingham, UK). Chordin digestion products were detected by Western blotting with the mouse monoclonal 9E10 antibody (Roche Applied Science), anti-mouse horseradish peroxidase-conjugated secondary antibody (Pierce/ThermoScientific), and Supersignal West Femto Maximum Sensitivity Substrate (Pierce/ThermoScientific).
Data Analysis
All experimental data are presented as the mean ± SEM, except where indicated. Data were analyzed using the Student t-test or by one-way ANOVA followed by either Tukey HSD or Newman-Keuls multiple-comparison test for comparisons between two or several means, respectively. For all analyses, differences with P > 0.05 were considered to be not significant.
RESULTS
Spatiotemporal Expression of BMP1 in Sheep Ovaries
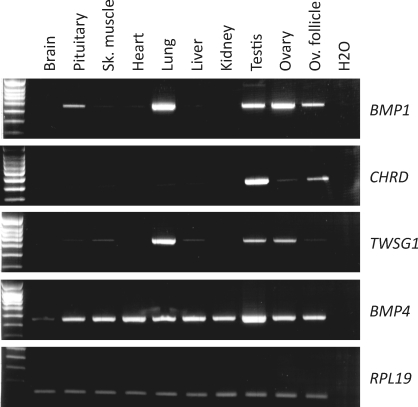
BMP1 mRNA expression was assayed by RT-PCR in whole ovaries and ovarian follicles as well as in other ovine tissues (Fig. 1). BMP1 appeared to be preferentially expressed in the ovary and ovarian follicles as well as in the pituitary, lung, and testis. The expression of chordin (CHRD), twisted gastrulation (TWSG1), and BMP4 mRNA also was tested. Interestingly, CHRD exhibited a preferential expression in gonads, with an apparently higher level in testis than in ovary. TWSG1 was expressed in the gonads of both sexes, with a preferential expression in lung compared to the other tissues tested. In contrast, BMP4 was ubiquitously expressed.
FIG. 1.
Expression of BMP1, CHRD, TWSG1, and BMP4 mRNAs in adult sheep tissues. Total mRNA was extracted from the tissues indicated and reverse transcribed before PCR analysis. Amplification of the RPL19 gene amplification was used as a cDNA quality and quantity control.
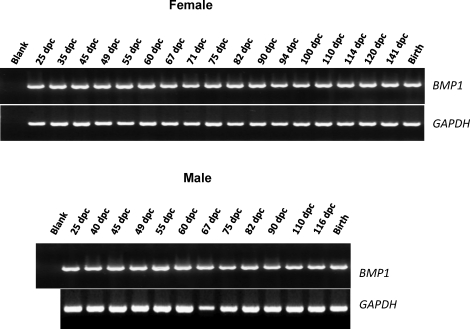
Expression of BMP1 was assayed at multiple stages of ovarian development by RT-PCR. RNA was extracted from gonads of sheep between 25 and 141 days postcoitum and at the time of birth. As shown in Figure 2, BMP1 PCR products were present in the ovary at all stages of development, with no significant variation in mRNA levels. The same pattern of expression was observed throughout testis development.
FIG. 2.
BMP1 mRNA expression in developing sheep gonads. Total RNA was extracted from sheep gonads obtained during embryogenesis (between 25 and 141 days postcoitum) or at birth and reverse transcribed before PCR analysis. The GAPDH gene was used as a cDNA quality control.
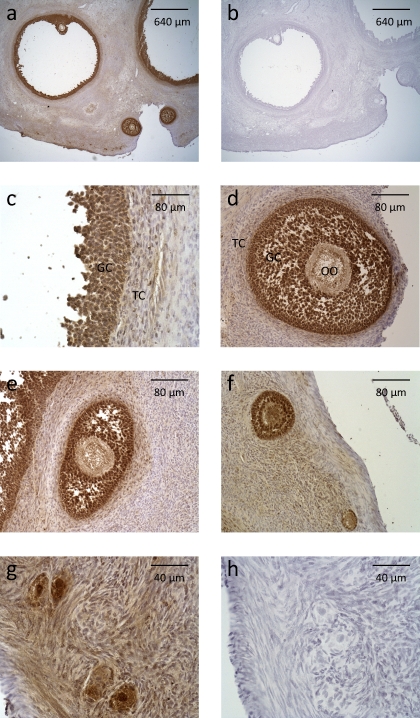
Immunohistochemistry was used to localize BMP1 within the adult ovine ovary. Using an antibody to the prodomain of BMP1, BMP1 was produced predominantly by cells in the granulosa cell layer of all categories of follicle, from large antral to primordial follicle stages (Fig. 3). A faint staining also could be observed in oocytes and theca cells.
FIG. 3.
Immunostaining for BMP1 in the sheep ovary. Photomicrographs of antral (a and c), preantral (d), secondary (e and f), primary (f), and primordial (g) follicles are shown. The photomicrographs shown in a and c–g correspond to immunostaining with anti-BMP1 rabbit polyclonal antibody. The photomicrographs shown in b and h correspond to immunostaining with control rabbit IgG. GC, granulosa cell; OO, oocyte; TC, theca cell.
Regulation of BMP1 Expression in Granulosa Cells
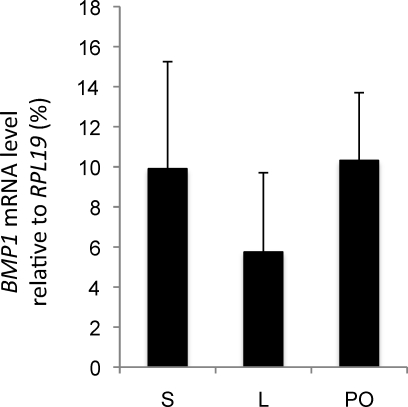
Within the ovary, BMP1 appeared to be expressed mainly by granulosa cells. To determine whether BMP1 mRNA expression changes during the final maturation of antral follicles in vivo, real-time PCR analysis was performed on RNA from granulosa cells of small (diameter, 1–3 mm) and large (diameter, >6 mm) antral follicles recovered during the follicular phase of the estrous cycle and from preovulatory large follicles recovered after the endogenous LH surge. No difference was found in the level of BMP1 mRNA in granulosa cells from small, large, or preovulatory follicles (Fig. 4).
FIG. 4.
In vivo regulation of BMP1 mRNA expression during terminal follicular maturation. Total RNA extracted from granulosa cells isolated from small (S; n = 5), large (L; n = 3), and preovulatory (PO; n = 4) ovine follicles was reverse transcribed and analyzed by real-time PCR. Data (mean ± SEM) represent the percentage of expression of BMP1 mRNA relative to the RPL19 reference gene.
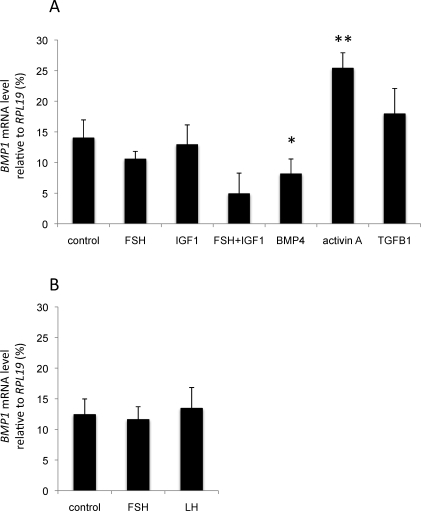
The effect of well-known regulators of folliculogenesis on BMP1 mRNA expression in granulosa cells in vitro was then tested. Granulosa cells, isolated from either small or large follicles during the follicular phase, were cultivated in the presence or absence of gonadotropins (FSH and LH), IGF1, or TGFB family members (BMP4, activin A, and TGFB1). BMP1 mRNA levels were then determined using real-time PCR (Fig. 5). In granulosa cells from small follicles, both BMP4 and activin A were potent regulators of BMP1 expression, in that BMP1 mRNA levels were inhibited by BMP4 (P < 0.05), whereas BMP1 expression was stimulated by activin A (P < 0.01) (Fig. 5A). Neither FSH nor IGF1 or TGFB1 alone were able to modulate BMP1 expression. However, FSH and IGF1 in combination showed a tendency to inhibit the expression of BMP1 mRNA (P = 0.09). In granulosa cells from large follicles, neither FSH nor LH was found to regulate BMP1 mRNA expression (Fig. 5B).
FIG. 5.
In vitro regulation of BMP1 mRNA expression by the main regulators of folliculogenesis. Granulosa cells from small (A) or large (B) ovine follicles were cultured for 24 h in the presence of fetal ovine serum and then grown for 48 h in serum-free medium in the absence (control) or presence of FSH (50 ng/ml), LH (50 ng/ml), IGF1 (10 ng/ml), BMP4 (50 ng/ml), activin A (50 ng/ml), or TGFB1 (5 ng/ml). At the end of the culture period, mRNA was extracted and reverse transcribed before quantitative real-time PCR analysis. Data are presented as the percentage of BMP1 mRNA levels (mean ± SEM, n = 5) relative to the RPL19 reference gene. *P < 0.05 or **P < 0.01 for each treatment vs. control.
BMP1 Biological Activity in Follicular Fluids
Because BMP1 protein was detected in granulosa cells by immunohistochemistry, we hypothesized that biologically active BMP1 could be present in the follicular fluid of antral follicles. Therefore, follicular fluids from small, large, and preovulatory ovarian follicles (collected from Ile-de-France ewes) were tested for specific proteolytic activity using PCP [11] and chordinase [8, 35] activity assays.
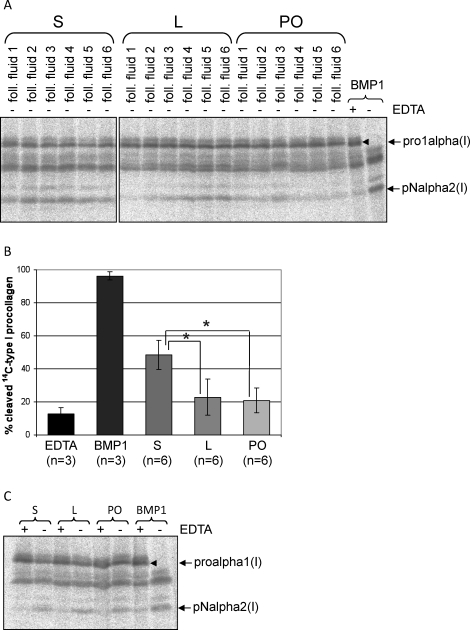
For the PCP assay, follicular fluids were incubated with 14C-labeled type I procollagen overnight, and the resulting digests were analyzed by SDS-PAGE. Procollagen was converted to pN collagen by recombinant BMP1 and also by the follicular fluid samples (Fig. 6A). The proalpha2 and pNalpha1 chains are not well resolved, but the proalpha1 and pNalpha2 (proalpha2 digestion product) chains are readily distinguished by their slower and faster migration speeds, respectively. The calculated percentage of cleavage was significantly higher in fluids from small follicles as compared to follicular fluid from large or preovulatory follicles (Fig. 6B). Of note, EDTA treatment does not result in complete loss of the pNalpha2 chain, because the 14C-labeled type I procollagen preparation used in the assay originally contained small amounts of contaminating pNcollagen chains. To confirm the absence of any contaminating protease activity in the PCP assay, a series of procollagen assays were carried out in which 14C-labeled type I procollagen was incubated with and without BMP1 and/or EDTA, in triplicate. Student t-test showed no significant difference (P = 0.66) between 14C-labeled type I procollagen incubated with both BMP1 and EDTA and incubated with neither BMP1 nor EDTA (data not shown). PCP activity in pooled follicular fluid samples was readily abolished by EDTA (Fig. 6C), confirming the presence of a specific and active metalloproteinase within follicular fluids.
FIG. 6.
Evidence of procollagen C-proteinase activity in sheep ovarian follicular fluids. Neat follicular fluid samples were incubated with 14C-labeled type I procollagen overnight at 37°C, as described in Materials and Methods. The digests were separated by SDS-PAGE, and the radiolabeled collagen chains were detected using a PhosphorImager. Recombinant BMP1 was used as a positive control in which almost all of the procollagen was converted to pN collagen, a normal intermediate in the conversion of procollagen to collagen in which the N-propeptides, but not the C-propeptides, are retained. A) SDS-PAGE gel (7%) of [14C]procollagen digests. Procollagen C-proteinase (PCP) activity is readily detected by the appearance of the pNalpha2 collagen chain. Follicular fluids from small (S), large (L), and preovulatory (PO) follicles were assayed for PCP activity. B) Graph representing the results of densitometric quantification of the extent of procollagen cleavage (mean ± SD) following overnight incubation with recombinant BMP1 (BMP1), recombinant BMP1 with EDTA (EDTA), or follicular fluids from S, L, or PO follicles. *P < 0.01 by one-way ANOVA and Tukey HSD. C) PCP activity in follicular fluid samples is inhibited by EDTA. Pools of follicular fluid samples from small or large follicles were incubated with 14C-labeled type I procollagen in the presence or absence of EDTA. Addition of EDTA inhibited the metalloproteinase-dependent conversion of procollagen to pN collagen.
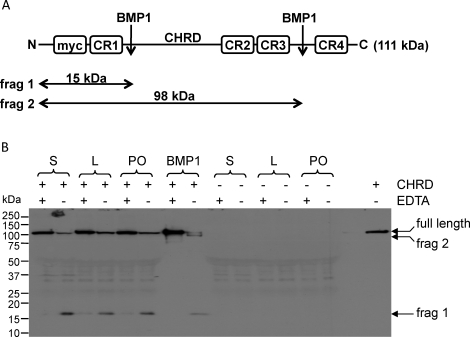
Follicular fluids from small, large, and preovulatory follicles were incubated with c-Myc-tagged human chordin, and the cleavage products were detected by Western blotting (Fig. 7). Cleavage of chordin was evaluated by loss of the full-length c-Myc-tagged chordin and the appearance of a partially digested, 98-kDa fragment lacking the C-terminus as well as a smaller, 15-kDa partial fragment; both containing the N-terminal c-Myc tag (Fig. 7A). As expected, human recombinant BMP1 was able to cleave chordin to give these two fragments, and this cleavage was abolished by EDTA treatment. Follicular fluids also cleaved chordin (Fig. 7B), but interestingly, only the 15-kDa fragment was generated. The chordinase activity detected in follicular fluids was inhibited by EDTA treatment. Based on the intensity of the 15-kDa band, the chordinase activity appeared to be higher in follicular fluid from small follicles as compared to that from large antral and preovulatory follicles, as observed for PCP activity. Altogether, these results show a readily detectable, BMP1-like activity in follicular fluids from ovine follicles.
FIG. 7.
Evidence of chordinase activity in sheep ovarian follicular fluids. A) Schematic depicting the domain structure of N-terminal c-Myc-tagged chordin. B) c-Myc-tagged chordin from the cell culture medium of transfected 293EBNA cells was incubated with follicular fluid samples or with recombinant BMP1, in the presence or absence of EDTA, overnight at 37°C. Control incubations were also performed using unconditioned cell culture medium in place of Myc-tagged chordin to identify bands resulting from the nonspecific binding of 9E10 to untagged proteins on the Western blot. Samples were separated by electrophoresis, and the chordin cleavage products were detected by Western blotting using the anti-Myc monoclonal antibody 9E10. Chordinase activity was inhibited in samples treated with EDTA. In follicular fluid samples, chordinase activity could be detected a reduction in the amount of full-length chordin and by the appearance of an approximately 17-kDa chordin cleavage product, corresponding to fragment 1. Both fragment 1 and fragment 2 were produced by recombinant BMP1 (see Discussion). Chordinase activity appears to be slightly higher in follicular fluid samples from small follicles.
DISCUSSION
Numerous groups of proteinases are involved in diverse aspects of ovarian function in mammals; these include matrix metalloproteinases [36], plasminogen activator/plasmin [37], disintegrin and metalloproteinase domain with thrombospondin motif proteins (ADAMTS) [38], cathepsin L [39], and pregnancy-associated plasma protein A, pappalysin 1 (PAPPA) [40]. These proteinases have been implicated in ECM remodeling and in the modulation of growth factor activity in the ovary [41, 42]. It has been hypothesized that BMP1 could function in ovarian development by cooperating with procollagen N-proteinases (namely, ADAMTS2, ADAMTS3, and ADAMTS14) to promote the deposition and maturation of collagen fibrils [42]. However, until now, BMP1 had not been identified or localized within ovarian tissues.
The present data show that BMP1 is expressed in ovine ovarian tissues throughout ovarian development, from fetal stages to adulthood. The BMP1 mRNA expression levels were not significantly different when comparing small (diameter, 1–3 mm) and large (diameter, ≥6 mm) antral follicles before and after the LH surge, and this correlated with the immunostaining pattern obtained using the BMP1 antibody. These results are also consistent with the observation that neither FSH, LH, nor IGF1 regulated BMP1 expression in vitro despite being known to potentiate the maturation of granulosa cells.
Little is known about the regulation of the BMP1 gene expression, but BMP1 is up-regulated by TGFB1 in fibrogenic cells and keratinocytes [43, 44]. In our granulosa cell cultures, TGFB1 showed only a slight tendency to increase BMP1 expression, but activin A had a highly significant effect. This may indicate a crucial role for the SMAD2/3 pathway in regulating BMP1 expression, which is induced preferentially by activin A in granulosa cells rather than by TGFB1, as in fibroblasts or keratinocytes. In the present study, BMP4 inhibited BMP1 RNA accumulation in granulosa cells. Because BMP1 can regulate the bioavailability of BMP4 by cleaving chordin [9] and of TGFB1 by cleaving latent TGFB-binding protein [45], BMP1 is now found at the center of a putative feedback loop that orchestrates TGFB superfamily ligand signaling in the ovary. BMP1 could therefore result in coordinated signaling through both the SMAD2/3 and SMAD1/5/8 signaling pathways, which are used by TGFB/activin and BMP4, respectively [46].
The antibody used to immunolocalize BMP1 was raised against a sequence from the prodomain of BMP1 and, hence, recognizes latent, but not active, BMP1. The immunostaining pattern therefore likely represents the intracellular, newly synthesized zymogen, because BMP1 is activated just before secretion [47], and this conclusion is supported by the predominantly cellular staining pattern obtained in the tissue sections. Interestingly, the BMP1 prodomain itself directly binds BMP2 and BMP4 and modulates processes regulated by BMP signaling [48]. The lower levels of immunoreactivity observed between cells in ovarian tissues could represent the extracellular free prodomain of BMP1 alone or the prodomain complexed to BMP2 or BMP4. Therefore, the identification of both the proform as well as enzymatically active BMP1 in the present study has both direct (via prodomain binding) and indirect (via chordinase cleavage) implications for the regulation and modulation of BMP signaling in the ovary.
In mature ovaries, granulosa cells were the main sites of BMP1 synthesis, as determined by immunohistochemistry, implying that BMP1 is activated and secreted directly into the antrum from the granulosa cell layer. BMP1 could not be detected in follicular fluids by immunohistochemistry, because only the tissue portions of the follicle and the ovary were retained during tissue processing. However, PCP and chordinase enzymatic activities were present in follicular fluid. Only BMP1 (but not mTLD) and the related tolloid-like metalloproteinase, tolloid-like 1 (TLL1), have been shown to possess chordinase activity [8, 9] as well as PCP activity. Hence, although we have demonstrated BMP1 mRNA and protein expression, the enzymatic activities detected within follicular fluids could be attributed, either in part or entirely, to TLL1.
The BMP1-like enzymatic activity present in follicular fluid appeared to decrease during the final phase of follicular growth, during the maturation from small to large antral follicles. This decrease in activity could be the result of a decrease in protein concentration, although no such differences were detected by Western blotting (data not shown), which confirms the result obtained at the RNA level in vivo and in vitro. As an alternative explanation, BMP1 could become denatured over time and lose its activity. The stability of BMP1 could be regulated by posttranslational glycosylation, which could affect its susceptibility to subsequent denaturation [49]. BMP1 activity is regulated by both enhancers and inhibitors of enzyme activity. For example, PCP enhancers can promote the PCP activity of BMP1 and TLL1, but they do not enhance chordinase activity [35, 50]. Hence, it is unlikely that PCP enhancers regulate BMP1 activity in follicular fluids, because an increased chordinase activity, as well as increased PCP activity, was observed in fluid from small follicles. However, it has recently been shown that both the PCP and chordinase activities of BMP1 can be enhanced by fibronectin [51]. Furthermore, fibronectin is present in follicular fluids [23]; therefore, increased levels or bioavailability of fibronectin in small follicles could act to enhance BMP1 activity. BMP1 can be irreversibly inhibited by alpha-2-macroglobulin (A2M) [52], a plasma protein that is present in follicular fluids. A2M has been shown to inhibit matrix metalloproteinases involved in ECM remodeling around the time of ovulation [53] so that increased A2M levels in large follicles could act to reduce BMP1 activity. Secreted frizzled-related protein (Sfrp/sizzled), an inhibitor of the Wnt signaling pathway, has been reported to act as a BMP1 inhibitor in Xenopus sp. and zebrafish [54, 55], whereas SFRP2 is a PCP enhancer in mice [56]. Little is known about SFRP levels during ovarian follicular maturation, but they could act to regulate intrafollicular BMP1 activities.
Cleavage of chordin by follicular fluids produced fragment 1 (the 15-kDa N-terminal Myc-tagged fragment) in preference to fragment 2 (the 98-kDa N-terminal Myc-tagged fragment), whereas incubation with recombinant BMP1 produced both fragments, in accordance with published data [8, 34, 35, 50, 57]. The concentration of recombinant BMP1 used was sufficient to convert an approximately equimolar amount of procollagen to pNcollagen, and it is unlikely that the amount of BMP1 present in the chordinase assay was insufficient to completely cleave chordin. Therefore, this could indicate that proteolysis at one chordin cleavage site could inhibit the second cut, either by an unfavorable conformational change in the substrate or because of negative feedback inhibition by the shorter cleavage product. Enhancer molecules present in follicular fluid, such as fibronectin [51], could favor the cleavage reaction proceeding to completion. Alternatively, twisted gastrulation (TWSG1), which has been shown to facilitate cleavage of chordin at a distinct site between CR1 and CR2 [57], could indirectly result in further chordin cleavage to produce the 15-kDa fragment. In support of this notion, TWSG1 was expressed in ovarian follicles (Fig. 1) along with BMP1 and CHRD. Additionally, it is conceivable that cleavage between CR3 and CR4 is directly inhibited by an unidentified protein present in follicular fluid, resulting in preferential cleavage at the site located just C-terminal to the CR1 domain, or that the protein composition of follicular fluids prevents the enzymatic denaturation of BMP1 during the assay.
During terminal development of ovarian follicles in sheep, as in other mammals, granulosa cells lose their proliferative activity, differentiate into estradiol-secreting cells, and finally, luteinize into progesterone-secreting cells in response to the LH preovulatory surge [58]. These transitions in granulosa cell activity are under the control of endocrine and paracrine factors, such as pituitary gonadotropins and ovarian growth factors, but also are regulated by ECM components [59]. Ovine granulosa cells are surrounded by fibronectin, laminin, collagen types I and IV, and heparan sulfate proteoglycans [23]. Type I collagen levels in particular are increased in the granulosa cell layer during terminal follicular growth, and these collagens are particularly important in follicles around 3 mm in diameter [23]. Therefore, as well as regulating the BMP signaling pathway and contributing to the assembly and maturation of collagenous ECM in the theca cell layers and collagenous stroma, BMP1 could participate in type I collagen fibril deposition in the granulosa cell layer—as attested by the PCP activity detected in the follicular fluid of small ovarian follicles. Furthermore, the decline in PCP activity in large follicles at the end of follicular growth and after the LH surge could make way for collagenases, such as matrix metalloproteinases, or for other proteases implicated in follicle rupture during ovulation [42].
In conclusion, the present data show the expression of BMP1 in the ovary at both the mRNA and protein levels, as well as a BMP1-like protease activity in follicular fluids. Taken together, these observations suggest a new physiological role for BMP1 metalloproteinases in the ovary. Further investigations are needed to determine whether BMP1 exerts this function mainly through modulating the action of BMP/TGFB molecules, by contributing to the deposition of ECM, or by a concerted action through both pathways.
Acknowledgments
The authors thank the “ruminant” team of the Experimental Unit UEPAO for animal management and Catherine Taragnat for participation in the experimental design. The authors also thank Joël Fontaine for LH assays in ovine plasma.
Footnotes
1Supported in part by the French “Région Centre” and by a Wellcome Trust Program Grant to K.E.K.
These authors contributed equally to this work.
REFERENCES
- Wozney J, Rosen V, Celeste A, Mitsock L, Whitters M, Kriz R, Hewick R, Wang E.Novel regulators of bone formation: molecular clones and activities. Science 1988; 242: 1528–1534. [DOI] [PubMed] [Google Scholar]
- Takahara K, Lyons GE, Greenspan DS.Bone morphogenetic protein-1 and a mammalian tolloid homologue (mTld) are encoded by alternatively spliced transcripts which are differentially expressed in some tissues. J Biol Chem 1994; 269: 32572–32578. [PubMed] [Google Scholar]
- Dumermuth E, Sterchi E, Jiang W, Wolz R, Bond J, Flannery A, Beynon R.The astacin family of metalloendopeptidases. J Biol Chem 1991; 266: 21381–21385. [PubMed] [Google Scholar]
- Davis CG.The many faces of epidermal growth factor repeats. New Biol 1990; 2: 410–419. [PubMed] [Google Scholar]
- Handford PA, Mayhew M, Baron M, Winship PR, Campbell ID, Brownlee GG.Key residues involved in calcium-binding motifs in EGF-like domains. Nature 1991; 351: 164–167. [DOI] [PubMed] [Google Scholar]
- Bork P, Beckmann G.The CUB domain: a widespread module in developmentally regulated proteins. J Mol Biol 1993; 231: 539–545. [DOI] [PubMed] [Google Scholar]
- Tosi M, Duponchel C, Meo T, Julier C.Complete cDNA sequence of human complement Cls and close physical linkage of the homologous genes Cls and Clr. Biochemistry 1987; 26: 8516–8524. [DOI] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KW, Greenspan DS.Mammalian BMP-1/tolloid-related metalloproteinases, including novel family member mammalian tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol 1999; 213: 283–300. [DOI] [PubMed] [Google Scholar]
- Pappano WN, Steiglitz BM, Scott IC, Keene DR, Greenspan DS.Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/tolloid-like metalloproteinases. Mol Cell Biol 2003; 23: 4428–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Sieron AL, Fertala A, Hojima Y, Arnold WV, Prockop DJ.The C-proteinase that processes procollagens to fibrillar collagens is identical to the protein previously identified as bone morphogenic protein-1. Proc Natl Acad Sci U S A 1996; 93: 5127–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS.Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science 1996; 271: 360–362. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Steiglitz BM, Greenspan DS.Bone morphogenetic protein-1 processes the NH2-terminal propeptide, and a furin-like proprotein convertase processes the COOH-terminal propeptide of pro-alpha1(V) collagen. J Biol Chem 1998; 273: 27511–27517. [DOI] [PubMed] [Google Scholar]
- Unsold C, Pappano WN, Imamura Y, Steiglitz BM, Greenspan DS.Biosynthetic processing of the pro-alpha 1(V)2pro-alpha 2(V) collagen heterotrimer by bone morphogenetic protein-1 and furin-like proprotein convertases. J Biol Chem 2002; 277: 5596–5602. [DOI] [PubMed] [Google Scholar]
- Medeck RJ, Sosa S, Morris N, Oxford JT.BMP-1-mediated proteolytic processing of alternatively spliced isoforms of collagen type XI. Biochem J 2003; 376: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzel MI, Scott IC, Babakhanlou-Chase H, Palamakumbura AH, Pappano WN, Hong HH, Greenspan DS, Trackman PC.Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J Biol Chem 2001; 276: 22537–22543. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Trackman PC.Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol 1991; 5: 206–210. [DOI] [PubMed] [Google Scholar]
- Monget P, Fabre S, Mulsant P, Lecerf F, Elsen J-M, Mazerbourg S, Pisselet C, Monniaux D.Regulation of ovarian folliculogenesis by IGF and BMP system in domestic animals. Domest Anim Endocrinol 2002; 23: 139–154. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF.The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 2004; 25: 72–101. [DOI] [PubMed] [Google Scholar]
- Fabre S, Pierre A, Mulsant P, Bodin L, Di Pasquale E, Persani L, Monget P, Monniaux D.Regulation of ovulation rate in mammals: contribution of sheep genetic models. Reprod Biol Endocrinol 2006; 4: e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight PG, Glister C.TGF-{beta} superfamily members and ovarian follicle development. Reproduction 2006; 132: 191–206. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C.Local roles of TGF-beta superfamily members in the control of ovarian follicle development. Anim Reprod Sci 2003; 78: 165–183. [DOI] [PubMed] [Google Scholar]
- Huet C, Monget P, Pisselet C, Hennequet C, Locatelli A, Monniaux D.Chronology of events accompanying follicular atresia in hypophysectomized ewes. Changes in levels of steroidogenic enzymes, connexin 43, insulin-like growth factor II/mannose 6 phosphate receptor, extracellular matrix components, and matrix metalloproteinases. Biol Reprod 1998; 58: 175–185. [DOI] [PubMed] [Google Scholar]
- Huet C, Monget P, Pisselet C, Monniaux D.Changes in extracellular matrix components and steroidogenic enzymes during growth and atresia of antral ovarian follicles in the sheep. Biol Reprod 1997; 56: 1025–1034. [DOI] [PubMed] [Google Scholar]
- Berkholtz CB, Lai BE, Woodruff TK, Shea LD.Distribution of extracellular matrix proteins type I collagen, type IV collagen, fibronectin, and laminin in mouse folliculogenesis. Histochem Cell Biol 2006; 126: 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure M-O, Nicol L, Fabre S, Fontaine J, Mohoric N, McNeilly A, Taragnat C.BMP-4 inhibits follicle-stimulating hormone secretion in ewe pituitary. J Endocrinol 2005; 186: 109–121. [DOI] [PubMed] [Google Scholar]
- Monniaux D, Pisselet C.Control of proliferation and differentiation of ovine granulosa cells by insulin-like growth factor-I and follicle-stimulating hormone in vitro. Biol Reprod 1992; 46: 109–119. [DOI] [PubMed] [Google Scholar]
- Monniaux D.Short-term effects of FSH in vitro on granulosa cells of individual sheep follicles. J Reprod Fertil 1987; 79: 505–515. [DOI] [PubMed] [Google Scholar]
- Le Bellego F, Pisselet C, Huet C, Monget P, Monniaux D.Laminin-alpha6beta1 integrin interaction enhances survival and proliferation and modulates steroidogenesis of ovine granulosa cells. J Endocrinol 2002; 172: 45–59. [DOI] [PubMed] [Google Scholar]
- Monniaux D, Clemente Nd, Touze J-L, Belville C, Rico C, Bontoux M, Picard J-Y, Fabre S.Intrafollicular steroids and anti-mullerian hormone during normal and cystic ovarian follicular development in the cow. Biol Reprod 2008; 79: 387–396. [DOI] [PubMed] [Google Scholar]
- Mandon-Pepin B, Oustry-Vaiman A, Vigier B, Piumi F, Cribiu E, Cotinot C.Expression profiles and chromosomal localization of genes controlling meiosis and follicular development in the sheep ovary. Biol Reprod 2003; 68: 985–995. [DOI] [PubMed] [Google Scholar]
- Hojima Y, van der Rest M, Prockop DJ.Type I procollagen carboxyl-terminal proteinase from chick embryo tendons. Purification and characterization. J Biol Chem 1985; 260: 15996–16003. [PubMed] [Google Scholar]
- Garrigue-Antar L, Barker C, Kadler KE.Identification of amino acid residues in bone morphogenetic protein-1 important for procollagen C-proteinase activity. J Biol Chem 2001; 276: 26237–26242. [DOI] [PubMed] [Google Scholar]
- Hartigan N, Garrigue-Antar L, Kadler KE.Bone morphogenetic protein-1 (BMP-1). Identification of the minimal domain structure for procollagen C-proteinase activity. J Biol Chem 2003; 278: 18045–18049. [DOI] [PubMed] [Google Scholar]
- Garrigue-Antar L, Francois V, Kadler KE.Deletion of epidermal growth factor-like domains converts mammalian tolloid into a chordinase and effective procollagen C-proteinase. J Biol Chem 2004; 279: 49835–49841. [DOI] [PubMed] [Google Scholar]
- Petropoulou V, Garrigue-Antar L, Kadler KE.Identification of the minimal domain structure of bone morphogenetic protein-1 (BMP-1) for chordinase activity: chordinase activity is not enhanced by procollagen C-proteinase enhancer-1 (PCPE-1). J Biol Chem 2005; 280: 22616–22623. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Osteen KG.The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24: 428–465. [DOI] [PubMed] [Google Scholar]
- Ny A, Leonardsson G, Hagglund A-C, Hagglof P, Ploplis VA, Carmeliet P, Ny T.Ovulation in plasminogen-deficient mice. Endocrinology 1999; 140: 5030–5035. [DOI] [PubMed] [Google Scholar]
- Shozu M, Minami N, Yokoyama H, Inoue M, Kurihara H, Matsushima K, Kuno K.ADAMTS-1 is involved in normal follicular development, ovulatory process and organization of the medullary vascular network in the ovary. J Mol Endocrinol 2005; 35: 343–355. [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS.Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A 2000; 97: 4689–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt HB, Conover CA.Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res 2007; 17: 10–18. [DOI] [PubMed] [Google Scholar]
- Curry TE, Smith MF.Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med 2006; 24: 228–241. [DOI] [PubMed] [Google Scholar]
- Ohnishi J, Ohnishi E, Shibuya H, Takahashi T.Functions for proteinases in the ovulatory process. Biochim Biophys Acta 2005; 1751: 95–109. [DOI] [PubMed] [Google Scholar]
- Lee S, Solow-Cordero DE, Kessler E, Takahara K, Greenspan DS.Transforming growth factor-beta regulation of bone morphogenetic protein-1/procollagen C-proteinase and related proteins in fibrogenic cells and keratinocytes. J Biol Chem 1997; 272: 19059–19066. [DOI] [PubMed] [Google Scholar]
- Bock O, Hoftmann J, Theophile K, Hussein K, Wiese B, Schlue J, Kreipe H.Bone morphogenetic proteins are overexpressed in the bone marrow of primary myelofibrosis and are apparently induced by fibrogenic cytokines. Am J Pathol 2008; 172: 951–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge G, Greenspan DS.BMP1 controls TGF{beta}1 activation via cleavage of latent TGF{beta}-binding protein. J Cell Biol 2006; 175: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D.Smad transcription factors. Genes Dev 2005; 19: 2783–2810. [DOI] [PubMed] [Google Scholar]
- Leighton M, Kadler KE.Paired basic/furin-like proprotein convertase cleavage of pro-BMP-1 in the trans-Golgi network. J Biol Chem 2003; 278: 18478–18484. [DOI] [PubMed] [Google Scholar]
- Jasuja R, Ge G, Voss NG, Lyman-Gingerich J, Branam AM, Pelegri FJ, Greenspan DS.Bone morphogenetic protein 1 prodomain specifically binds and regulates signaling by bone morphogenetic proteins 2 and 4. J Biol Chem 2007; 282: 9053–9062. [DOI] [PubMed] [Google Scholar]
- Garrigue-Antar L, Hartigan N, Kadler KE.Post-translational modification of bone morphogenetic protein-1 is required for secretion and stability of the protein. J Biol Chem 2002; 277: 43327–43334. [DOI] [PubMed] [Google Scholar]
- Moali C, Font B, Ruggiero F, Eichenberger D, Rousselle P, Francois V, Oldberg A, Bruckner-Tuderman L, Hulmes DJS.Substrate-specific modulation of a multisubstrate proteinase: C-terminal processing of fibrillar procollagens is the only BMP1-dependent activity to be enhanced by PCPE-1. J Biol Chem 2005; 280: 24188–24194. [DOI] [PubMed] [Google Scholar]
- Huang G, Zhang Y, Kim B, Ge G, Annis DS, Mosher DF, Greenspan DS.Fibronectin binds and enhances the activity of bone morphogenetic protein 1. J Biol Chem 2009; 284: 25879–25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ge G, Greenspan DS.Inhibition of bone morphogenetic protein 1 by native and altered forms of {alpha}2-macroglobulin. J Biol Chem 2006; 281: 39096–39104. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Mann JS, Estes RS, Jones PB.{alpha}2-Macroglobulin and tissue inhibitor of metalloproteinases: collagenase inhibitors in human preovulatory ovaries. Endocrinology 1990; 127: 63–68. [DOI] [PubMed] [Google Scholar]
- Lee HX, Ambrosio AL, Reversade B, De Robertis EM.Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell 2006; 124: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka O, Shimizu T, Yabe T, Nojima H, Bae Y-K, Hashimoto H, Hibi M.Sizzled controls dorso-ventral polarity by repressing cleavage of the chordin protein. Nat Cell Biol 2006; 8: 329–338. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge G, Grieskamp T, Yamada C, Liu T-C, Huang G, Basson CT, Kispert A, Greenspan DS, et al. Secreted frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol 2009; 11: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott IC, Blitz IL, Pappano WN, Maas SA, Cho KWY, Greenspan DS.Homologues of Twisted gastrulation are extracellular cofactors in antagonism of BMP signaling. Nature 2001; 410: 475–478. [DOI] [PubMed] [Google Scholar]
- Monniaux D, Huet C, Besnard N, Clement F, Bosc M, Pisselet C, Monget P, Mariana JC.Follicular growth and ovarian dynamics in mammals. J Reprod Fertil Suppl 1997; 51: 3–23. [PubMed] [Google Scholar]
- Huet C, Pisselet C, Mandon-Pepin B, Monget P, Monniaux D.Extracellular matrix regulates ovine granulosa cell survival, proliferation and steroidogenesis: relationships between cell shape and function. J Endocrinol 2001; 169: 347–360. [DOI] [PubMed] [Google Scholar]