Abstract
Prior work showed that neurons in the lateral, dorsal, and perifornical regions of the tuberal and mammillary levels of the hypothalamus participate in the control of breathing. The same areas also contain large numbers of neurons that produce either orexins (hypocretins) or melanin concentrating hormone (MCH). These peptides have been implicated in regulating energy balance and physiological changes that occur in transitions between sleep and wakefulness, amongst other functions. The goal of this study was to determine if hypothalamic neurons involved in respiratory control, which were identified in cats by the retrograde transneuronal transport of rabies virus from the diaphragm, were immunopositive for either orexin-A or MCH. In animals with limited rabies infection of the hypothalamus (< 10 infected cells/section), where the neurons with the most direct influences on diaphragm motoneurons were presumably labeled, a large fraction (28–75%) of the infected hypothalamic neurons contained orexin-A. In the same cases, 6–33% of rabies-infected hypothalamic cells contained MCH. However, in animals with more extensive infection, where rabies had presumably passed transneuronally through more synapses, the fraction of infected cells that contained orexin-A was lower. The findings from these experiments thus support the notion that hypothalamic influences on breathing are substantially mediated through orexins or MCH.
Keywords: rabies virus, transneuronal tracing, respiration, homeostasis
1. Introduction
Two peptides with extensive overlap in their amino acid sequences are localized to neurons in the lateral, dorsal and perifornical hypothalamic regions of mammalian species (Abrahamson and Moore, 2001; de Lecea et al., 1998; Peyron et al., 1998; Zhang et al., 2001). These peptides were initially named hypocretins by one group due to their structural similarity to the gastrointestinal hormone secretin (de Lecea et al., 1998), and orexins by another because they were implicated in regulating appetite (Sakurai et al., 1998). In cats, the two orexin peptides (orexin-A and orexin-B) are co-expressed in the same hypothalamic neurons (Zhang et al., 2002). Orexinergic neurons project widely throughout the nervous system (Peyron et al., 1998), although the heaviest concentrations of terminations in felines are present in the nucleus raphe dorsalis, the laterodorsal tegmental nucleus, and the locus coeruleus (Zhang et al., 2004). This pattern of connections is consistent with a now well-established role of the orexins in modulating alterations in physiological activity that occur between sleep and wakefulness (Bourgin et al., 2000; Chemelli et al., 1999; Gerashchenko et al., 2001; Lin et al., 1999; Taheri et al., 2002; Willie et al., 2001; Xi et al., 2002; Zeitzer et al., 2003). The peptides have also been shown to participate in generating arousal, addiction, goal-oriented behaviors, and energy homeostasis, as discussed in a number of recent reviews (Aston-Jones et al., 2009; de Lecea et al., 2002; Martynska et al., 2005; Siegel, 2004; Sutcliffe and de Lecea, 2000; Sutcliffe and de Lecea, 2002; Taylor and Samson, 2003; Winsky-Sommerer et al., 2003; Zhang et al., 2006).
It has also long been appreciated that lesions of the lateral hypothalamic areas containing orexinergic neurons depress the rate and depth of respiration (Redgate and Gellhorn, 1958). Lateral ventricular administration of orexin elicits increases in both tidal volume and respiratory frequency (Zhang et al., 2005). In addition, knockout mice lacking orexin have attenuated hypercapnic respiratory responses while awake, but not while asleep (Deng et al., 2007; Nakamura et al., 2007), and lack the capacity for respiratory long-term facilitation following hypoxic stimuli (Terada et al., 2008). Orexinergic neurons apparently influence breathing via connections with multiple brainstem regions involved in respiratory control. Orexin receptors are present on neurons in the pre-Bötzinger region of the ventral respiratory group and the phrenic motor nucleus, and microperfusion of orexin at either site produces a dose-dependent increase in diaphragm electromyographic activity (Young et al., 2005). The firing rate of neurons in the retrotrapezoid nucleus (a central chemosensory area) is also modulated by orexinergic inputs (Dias et al., 2009; Fortuna et al., 2009). Stimulation of the perifornical hypothalamic region increases the activity of retrotrapezoid nucleus neurons (Fortuna et al., 2009), and blockade of orexin receptors in this region inhibits the ventilatory response to hypercapnia during wakefulness, but less so during sleep (Dias et al., 2009).
Further evidence that orexinergic neurons participate in the control of breathing comes from our experiments where the transneuronal tracer rabies virus was injected into the diaphragm of cats (Lois et al., 2009). The hypothalamic neurons infected following the multisynaptic passage of virus from diaphragm motoneurons were located in the perifornical region, where orexinergic cells are concentrated. A caveat is that neurons that produce other peptides, particularly melanin-concentrating hormone (MCH) (Abrahamson and Moore, 2001; Bayer et al., 2002; Bittencourt et al., 1992; Nahon et al., 1989; Torterolo et al., 2006), are also found in the same region. Although MCH and the orexins are present in separate populations of neurons (Bayer et al., 2002; Broberger et al., 1998; Torterolo et al., 2006), these cells may have synaptic interconnections (Bayer et al., 2002; Guan et al., 2002; Torterolo et al., 2006). It is thus not surprising that MCH-containing neurons have been attributed similar physiological roles as orexinergic cells, including regulation of sleep/wake cycles and energy homeostasis (Georgescu et al., 2005; Goutagny et al., 2005; Modirrousta et al., 2005; Shimada et al., 1998; Tritos and Maratos-Flier, 1999; Verret et al., 2003). Nonetheless, no prior studies have directly implicated MCH-containing neurons in the regulation of breathing.
The goal of the present study was to estimate the fraction of hypothalamic neurons that provide polysynaptic inputs to phrenic motoneurons and contain either orexins or MCH. As in our prior studies (Lois et al., 2009; Rice et al., 2009), neurons that polysynaptically regulate breathing were identified by the transneuronal transport of rabies virus from the diaphragm. Dual-labeling immunohistochemistry was performed to identify rabies-infected cells in the hypothalamus that were immunopositive for either orexin-A or MCH. We tested the hypothesis that a majority of hypothalamic neurons that regulate diaphragm activity are orexinergic.
2. Results
Orexin-A immunopositive neurons were present in the lateral, dorsal and perifornical hypothalamus, and were concentrated in the tuberal and tuberomamillary regions. Orexin-A immunopositive cells were present in each hypothalamic section; the median number of labeled cells/section was 159. When the analysis was limited to sections through the caudal half of the hypothalamus, where orexinergic neurons were most heavily clustered, 267 ± 41 (median of 206) cells/section contained the peptide. The same hypothalamic areas also contained comparable numbers of neurons that were immunopositive for MCH. As such, our observations of the locations of hypothalamic neurons containing orexin-A and MCH were similar to those previously documented in felines (Torterolo et al., 2006; Zhang et al., 2001). Micrographs of neurons that were immunopositive for orexin-A and MCH are illustrated in Figs. 1 and 2, respectively. Plots of the locations of neurons containing orexin-A and MCH in representative sections from two animals (C37 and C39) are shown in Fig. 3.
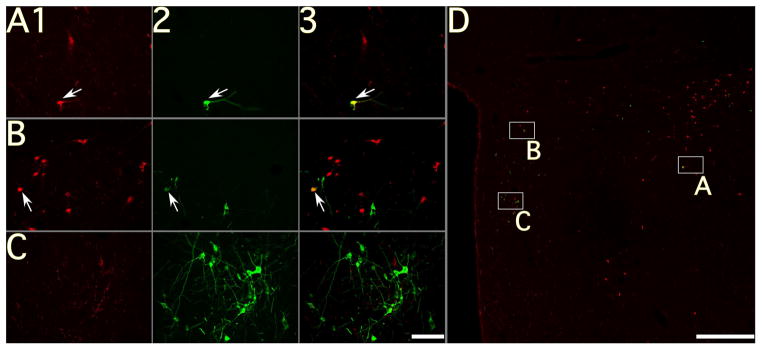
Figure 1.
Photomicrographs of neurons in a section through the tuberal region of the hypothalamus of animal C37; the section was processed using immunofluorescence to dual-localize orexin-A and rabies virus. A–C: photomicrographs taken using a 20X objective; column 1 shows orexin-A immunopositivity, column 2 shows rabies immunopositivity, and column 3 shows the combined immunopositivity for both antigens. Arrows denote examples of double-labeled neurons; note that no double-labeled cells are present in C. D: a montage of photomicrographs taken using a 4X objective, showing immunopositivity to both rabies and orexin-A. Boxes in panel D show the locations of the neurons depicted at higher magnification in A–C. Calibration bars represent 100 μA in A–C and 1 mm in D.
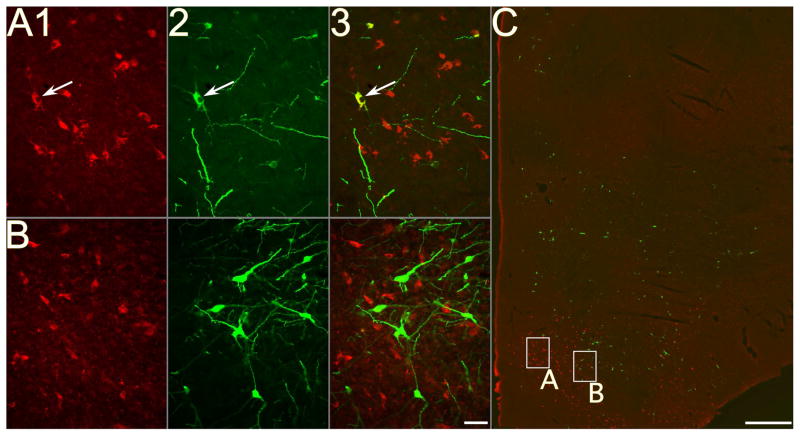
Figure 2.
Photomicrographs of neurons in a section through the tuberal region of the hypothalamus of animal C37; the section was processed using immunofluorescence to dual-localize MCH and rabies virus. A–B: photomicrographs taken using a 20X objective; column 1 shows MCH immunopositivity, column 2 shows rabies immunopositivity, and column 3 shows the combined immunopositivity for both antigens. Arrows denote examples of double-labeled neurons; note that no double-labeled cells are present in B. C: a montage of photomicrographs taken using a 4X objective, showing immunopositivity to both rabies and MCH. Boxes in panel C show the locations of the neurons depicted at higher magnification in A–B. Calibration bars represent 50 μA in AB and 1 mm in C.
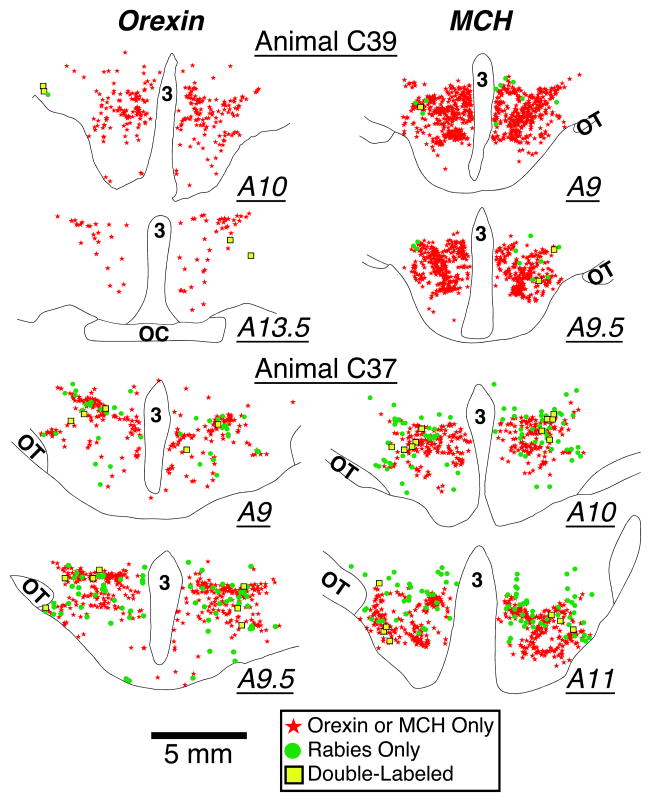
Figure 3.
Drawings of representative sections from an animal with early-stage rabies infection of the hypothalamus (C39, top panel) and an animal with more advanced hypothalamic infection (C37, bottom panel). Rabies-immunopositive neurons are depicted by green circles; neurons immunopositive for either orexin-A (left column) or melanin concentrating hormone (right column) are indicated by red stars; neurons that were immunopositive for both rabies and either orexin-A or MCH are shown by yellow squares. The location of each section relative to stereotaxic zero (A0) is indicated. Abbreviations: 3, third ventricle; OC, optic chiasm; OT, optic tract.
Cells infected by the transneuronal transport of rabies virus from the diaphragm were present in the same hypothalamic areas containing neurons that were immunopositive for orexin-A and MCH, as indicated in the micrographs in Figs. 1–2 and the plots of neuronal locations provided in Fig. 3. The distribution of rabies-infected neurons in the brain and spinal cord of four of the animals (C36, C37, C39, C51) was described in detail in previous publications (Lois et al., 2009; Rice et al., 2009). These studies also reported a variety of controls that have been performed to demonstrate that this pattern of rabies infectivity is due to transport of virus from the diaphragm. Tissue from two additional animals (C93 and C94) was added to this study to increase the number of cases with infected neurons in the diencephalon following rabies injections into the diaphragm. Table 1 indicates the number of rabies-infected neurons in hypothalamic sections from each animal. Only a few infected hypothalamic neurons were present in each brain section of animals C39, C51, and C94, but such cells were somewhat more prevalent in animal C93. Nonetheless, the locations of infected neurons in the brainstem and spinal cord of both animals C93 and C94 were similar to those previously described for animals C39 and C51 (Lois et al., 2009). Hypothalamic infection never appeared until a variety of brainstem and midbrain areas contained substantial numbers of rabies-immunopositive neurons, suggesting that cells in the hypothalamus are mainly linked to phrenic motoneurons through multisynaptic pathways (Lois et al., 2009). Hypothalamic infection was much more extensive in animals C36 and C37 than the other cases, as indicated in Table 1. Furthermore, additional brain regions contained infected neurons in these cats than cases C39, C51, C93, and C94, including multiple areas of cerebral cortex and the substantia nigra (Lois et al., 2009; Rice et al., 2009). Rabies virus presumably passed transneuronally through more synapses in animals C36 and C37 than the others, such that it is likely that rabies-immunopositive hypothalamic neurons in these two cases had more indirect connections with phrenic motoneurons than in animals C39, C51, C93, or C94.
Table 1.
Expression of orexin-A and melanin concentrating hormone (MCH) immunoreactivity by hypothalamic neurons that were infected by rabies injections into the diaphragm. The data included indicate the following: the amount of rabies virus injected across multiple sites of the left diaphragm, the survival times following rabies virus injections, the average (± one standard error of the mean) of rabies-infected hypothalamic neurons in each section, the range and median number (in parentheses) of infected hypothalamic cells in each section, the percentage of the infected cells that were immunopositive for orexin-A, and the percentage of the infected cells that were immunopositive for MCH.
| Animal | Virus Amount Injected (μl) | Survival Time (hr) | Average # Rabies- Infected Cells/Section | Range (Median #) Rabies- Infected Cells/Section | % Rabies- Infected Neurons that were Orexin-+ | % Rabies- Infected Neurons that were MCH-+ |
|---|---|---|---|---|---|---|
| C39 | 200 | 96 | 6.7 ± 1.0 | 1–16 (5) | 75.0% | 11.5% |
| C51 | 200 | 107 | 4.5 ± 0.9 | 1–11 (5) | 27.8% | 6.3% |
| C94 | 300 | 97 | 2.6 ± 0.7 | 0–7 (2) | 31.6% | 33.3% |
| C93 | 300 | 97 | 13.3 ± 3.4 | 3–37 (10) | 18.8% | 10.3% |
| C37 | 300 | 107 | 169.2 ± 29.1 | 41–400 (118) | 10.5% | 5.2% |
| C36 | 200 | 102 | 558.0 ± 62.7 | 253–1167 (605) | 8.0% | —— |
Table 1 additionally shows the fraction of rabies-infected cells in the hypothalamus of each animal that was also immunopositive for orexin-A. Photomicrographs of such neurons are illustrated in Fig. 1. A considerable fraction (28–75%) of neurons in the three animals (C39, C51, and C94) with the most limited hypothalamic infection were dual-labeled for the presence of both rabies and orexin-A. When the analysis was limited to sections through the caudal half of the hypothalamus (where orexinergic neurons were most prevalent), 33, 36, and 86% of the infected cells in these three cases contained the peptide. In animal C94, where rabies immunoreactivity was somewhat more prevalent in the hypothalamus than in the cases described above, 19% of the infected neurons contained orexin-A. However, in the two cats where rabies immunoreactivity was extensive in the diencephalon, the percentage of rabies-infected cells labeled for orexin-A was much lower: only 8 and 11%. As such, there was an inverse relationship between the number of rabies-infected cells observed per section and the fraction of these cells that were immunopositive for orexin (see Table 1), although the limited sample size precluded a statistical demonstration of this trend.
An alternate bin of sections through the diencephalon of the same animals was processed to dual-detect rabies virus and MCH. An exception is animal C36, because insufficient tissue was available from this case to conduct the analysis. Examples of neurons that were immunopositive for both MCH and rabies are illustrated in Fig 2. In most animals, a lower percentage of neurons was dual-labeled for rabies and MCH than for rabies and orexin-A (see Table 1).
3. Discussion
The major finding of this study was that a large fraction (28–75%) of feline hypothalamic neurons that were infected following injection of the transneuronal tracer rabies virus into the diaphragm contained orexin-A, in cases with limited infection of cells in the hypothalamus (<10 neurons/section). In the same animals, 6–33% of rabies-infected hypothalamic cells contained MCH. However, in cases with extensive rabies infection of the diencephalon, a smaller fraction of infected hypothalamic neurons contained orexin-A. The speed at which virus is transported transneuronally to the cell body of a second-order neuron is affected by many factors, including the length of its axon, the density of its synaptic connections with an infected cell, and whether these connections are near the soma or are located on the distal dendrites (Aston-Jones and Card, 2000). As such, determining the number of synapses separating a transneuronally-infected neuron from the injection site is difficult. Nonetheless, it seems likely that in animals where only a limited number of hypothalamic neurons were infected following rabies virus injections into the diaphragm, the infected cells provided relatively direct and/or strong inputs to phrenic motoneurons, and thus were labeled early. A sizeable proportion of the rabies-immunopositive cells in animals with limited hypothalamic infection contained orexin-A, which underscores the conclusions of recent physiological studies suggesting that orexinergic neurons participate in the control of breathing (Deng et al., 2007; Dias et al., 2009; Fortuna et al., 2009; Nakamura et al., 2007; Terada et al., 2008; Young et al., 2005; Zhang et al., 2005).
Although in most cases fewer infected neurons in the hypothalamus expressed MCH than orexin-A, at least a few cells were dual-labeled for MCH and rabies in every cat. It is presently unclear whether the MCH-containing neurons were infected via projections to brainstem regions that participate in the control of breathing, or through their extensive interconnections with orexinergic cells (Bayer et al., 2002; Guan et al., 2002; Torterolo et al., 2006). Nonetheless, the present results raise the prospect that hypothalamic neurons containing MCH participate in some fashion in the control of breathing. Further experiments should thus be conducted to examine this possibility.
Prior studies suggested that the orexins influence breathing through actions at a number of sites in the nervous system, including the retrotrapezoid nucleus, phrenic motor nucleus, and pre-Bötzinger region of the ventral respiratory group (Dias et al., 2009; Fortuna et al., 2009; Young et al., 2005). Such observations are consistent with previous findings that orexinergic neurons project diffusely throughout the nervous system (Peyron et al., 1998; Zhang et al., 2004), but are somewhat at odds with our data showing that hypothalamic neurons infected by rabies virus injections into the diaphragm could not be detected until after extensive labeling was also evident in the periaqueductal gray and additional midbrain regions (Lois et al., 2009; Rice et al., 2009). Others have also speculated that influences of the diencephalon on breathing are mainly mediated through relays in the midbrain (Horn and Waldrop, 1998). It is certainly feasible that several parallel descending pathways from the caudal hypothalamus participate in the control of breathing, including those providing inputs to midbrain neurons as well as medullary regions that contribute to respiratory rhythmogenesis. If hypothalamic neurons make connections with the proximal dendrites and soma of neurons in the midbrain, but the distal dendrites of medullary neurons, the former pathway could provide the most rapid mechanism for the retrograde transmission of rabies virus to the diencephalon (Aston-Jones and Card, 2000). This would explain the delayed infection of orexinergic cells following the injection of rabies virus into the diaphragm, despite the fact that these neurons provide direct inputs to the brainstem respiratory groups. Additional studies, including those combining the injection of retrograde monosynaptic tracers into brainstem areas that regulate respiration along with the detection of orexin-containing neurons, will be required to test this hypothesis.
Several caveats must be considered when interpreting the present data. First, there was some variability in the fraction of hypothalamic neurons that were dual-labeled for the presence of rabies and orexin/MCH, even in the animals with limited infection in the diencephalon. This was undoubtedly partly due to sampling bias, as the number of rabies-infected cells in three of the cases was very low, but also could reflect inter-animal variability. Nonetheless, in all cats with limited diencephalic infection, at least a third of the infected cells in the caudal half of the hypothalamus were orexinergic. Another potential concern is that our methods did not label all the motor pathways that regulate diaphragm activity, despite the fact that we disbursed rabies virus across multiple sites of the crural diaphragm on one side. As noted previously (Lois et al., 2009), our methodology resulted in the infection of the majority of neurons in the brainstem respiratory groups, such that is unlikely that we failed to identify populations of cells that participate in the control of diaphragm contractions. A converse issue is whether rabies virus infected neurons other than those regulating diaphragm activity. Numerous control experiments executed in prior studies argue against this possibility (Lois et al., 2009). A final consideration relates to the observation that the fraction of rabies-infected cells that expressed orexin-A decreased as rabies immunoreactivity became more prevalent. This finding raises the concern that infection of a cell by rabies virus limits its ability to produce non-viral proteins. We have previously provided evidence that this is not the case (Rice et al., 2009), which supports our results that fewer late-infected hypothalamic neurons were orexinergic than early-infected cells. Nonetheless, any possibility that we underestimated the fraction of diencephalon neurons that participate in respiratory control and contained either orexin or MCH would only serve to strengthen our major conclusion: neurons producing these peptides constitute a major population of hypothalamic neurons that influence breathing.
In summary, the present study showed that an appreciable fraction of hypothalamic neurons that regulate diaphragm activity, which were identified by the transneuronal transport of rabies virus, expressed either orexin-A or MCH. In the animals with limited infection of hypothalamic neurons, over a quarter of these cells, and over a third of the neurons in the caudal hypothalamus, were immunopositive for orexin-A. Presumably, since the hypothalamic infection was at an early stage in these cases, the rabies-immunopositive neurons had relatively strong or direct, albeit multisynaptic, connections with phrenic motoneurons. Although a variety of previous studies have shown that the orexins contribute to the control of breathing (Deng et al., 2007; Dias et al., 2009; Fortuna et al., 2009; Nakamura et al., 2007; Terada et al., 2008; Young et al., 2005; Zhang et al., 2005), the present work contributes evidence that hypothalamic influences on respiration are mediated in large part by these peptides, and also suggests that MCH-containing cells play some role in respiratory regulation.
4. Experimental Procedures
All of the procedures used in this study conformed to the National Research Council’s Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh’s Institutional Animal Care and Use Committee. Brainstem tissue obtained from 8 adult cats (Liberty Research, Waverly, NY) was used for the present study. Tissue from two of the animals was employed to determine the optimal dilutions of orexin and MCH antibodies to detect hypothalamic neurons containing these peptides, and also to demonstrate the specificity of the antibodies for the target cells. The other six cats received injections of the N2C strain of rabies virus at a titer of 1 x108 plaque forming units/ml into the left diaphragm, and sections through the hypothalamus were processed for the dual localization of rabies virus and orexin-A or rabies virus and MCH. The histological procedures employed in this study were similar to those used in our prior experiments where rabies virus and an enzyme involved in serotonin synthesis were co-localized in the same neurons (Rice et al., 2009).
The distribution of rabies-infected neurons in the brain and spinal cord of four of the animals (C36, C37, C39, C51) was described in a prior manuscript (Lois et al., 2009). Two additional animals (C93 and C94) received injections of rabies virus for the purpose of this study, to increase the number of cases available for data analysis. The surgical procedures and biosafety precautions employed for the extra animals were identical to those used in our previous experiments (Lois et al., 2009). The amount of rabies virus injected and the post-innoculation survival times in each animal are provided in Table 1. Coronal sections through the diencephalon of 40 μm thickness were collected in six bins of phosphate-Tris-azide (PTA) buffer or cryoprotectant (Watson et al., 1986), and stored at either 4°C (tissue placed in PTA) or −20° C (tissue placed in cryoprotectant). The brainstem and spinal cord were also extracted from the animals, and sectioned as described previously (Lois et al., 2009).
One well of sections of spinal cord segments, the brainstem, and the diencephalon were processed using avidin-biotin immunoperoxidase techniques to detect rabies-infected neurons, as discussed in our earlier study (Lois et al., 2009). This analysis utilized a mouse monoclonal antibody directed against the rabies virus phosphoprotein that resides in the infective nucleocapsid core (M957, (Kelly and Strick, 2000). Dr. Peter Strick provided the latter antibody, and its specificity for detecting rabies virus has previously been described (Lois et al., 2009; Nadin-Davis et al., 2000). Maps of the locations of rabies-infected cells were generated (Lois et al., 2009) and employed to guide subsequent data analyses.
As a first step in the immunofluorescence analysis, we incubated diencephalon sections in different dilutions of rabbit anti-orexin-A, anti-orexin-B and anti-MCH antibodies (Phoenix Pharmaceuticals, Burlingame, CA) to determine the dilution that produced optimal neuronal labeling. These antibodies were previously demonstrated to be selective for the target neurons in cats (Torterolo et al., 2006). Both the orexin-A (1:200) and MCH (1:200) antibodies produced robust labeling of hypothalamic neurons when visualized using CY3, as described below (see Figs. 1 and 2 for examples); however, the orexin-B labeling was much weaker. Since orexin-A and orexin-B are co-expressed by the same neurons (Zhang et al., 2002), we employed the orexin-A antibody in the primary experiment. The orexin-A labeling was blocked by combining 10 μg of orexin-A peptide (Phoenix Pharmaceuticals) in 1 ml of antibody solution. A similar blocking experiment using anti-MCH antibody and MCH peptide also confirmed antibody selectivity.
One bin of diencephalon sections from the six animals that received rabies virus injections into the diaphragm was processed using immunofluorescence techniques to dual-localize rabies and orexin-A, whereas another was employed to co-localize rabies and MCH. Sections were incubated for 2 days at 4°C in a combination of rabbit anti-orexin-A (1:200) or anti-MCH (1:200) antibody and the mouse monoclonal anti-rabies antibody used in the immunoperoxidase analysis (1:30). Subsequently, after rinsing in phosphate-buffered saline, the sections were incubated in a combination of goat anti-rabbit secondary antibody conjugated to CY3 (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA) and goat anti-mouse secondary antibody conjugated to BODIPY-FL (1:300, Molecular Probes, Eugene, OR). On completion of the immunohistochemical processing, the tissue was mounted on gelatin-coated slides, dehydrated, cleared, and coverslipped using Cytoseal 60 (VWR Scientific, West Chester, PA).
Immunofluorescent sections were inspected using an Olympus BX51TRF photomicroscope equipped with a Hamamatsu camera (Hamamatsu Photonics, Hamamatsu, Japan) and a Simple-32 PCI image analysis system (Compix, Lake Oswego, OR). The hypothalamic region of every section at and caudal to the level of the optic chiasm was examined. The areas containing rabies and orexin-A or MCH immunopositive neurons were photographed at both low and high magnification, using epifluorescence in combination with filters that selectively excited CY3 or BODIPY-FL and in double exposures that revealed the cellular localization of both the target peptide and rabies. Great care was taken to ascertain that yellow fluorescence reflected the colocalization of both the BODIPY-FL and CY3 fluorophors and was not attributable to the presence of overlapping cells that each contained one of the fluorophors. Montages of images were assembled using PTGui-Pro photostitching software (New House Internet Services B.V., Netherlands); examples of such montages are shown in Figs. 1D and 2C. Such montages, in conjunction with observations of sections at high magnification, were used to generate plots of the locations of labeled cells, such as those provided in Fig. 3. Data were tabulated and statistical analyses were performed using Prism 5 software (GraphPad Software, San Diego, CA). Pooled data are presented as means ± one SEM.
Acknowledgments
The authors thank Lucy Cotter, Derek Reighard, Abdul Ahmed, Mary Jessell, Allison Waggoner, and Sarah Weber for technical assistance in the completion of these experiments. We additionally thank Dr. Peter Strick of the University of Pittsburgh for providing rabies virus and anti-rabies antibodies. This work was supported by National Institutes of Health (NIH) grants 5R01-DC003732-11 and 3R01-DC003732-11S1, as well as by grant P40-RR-018604 from NIH’s National Center for Research Resources.
Abbreviations
- MCH
Melanin concentrating hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. doi: 10.1016/s0006-8993(00)03015-8. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharm. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Card JP. Use of pseudorabies virus to delineate multisynaptic circuits in brain: opportunities and limitations. J Neurosci Methods. 2000;103:51–61. doi: 10.1016/s0165-0270(00)00295-8. [DOI] [PubMed] [Google Scholar]
- Bayer L, Mairet-Coello G, Risold PY, Griffond B. Orexin/hypocretin neurons: chemical phenotype and possible interactions with melanin-concentrating hormone neurons. Regul Pept. 2002;104:33–39. doi: 10.1016/s0167-0115(01)00320-2. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Bourgin P, Huitron-Resendiz S, Spier AD, Fabre V, Morte B, Criado JR, Sutcliffe JG, Henriksen SJ, de Lecea L. Hypocretin-1 modulates rapid eye movement sleep through activation of locus coeruleus neurons. J Neurosci. 2000;20:7760–7765. doi: 10.1523/JNEUROSCI.20-20-07760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Sutcliffe JG, Fabre V. Hypocretins/orexins as integrators of physiological information: lessons from mutant animals. Neuropeptides. 2002;36:85–95. doi: 10.1054/npep.2002.0892. [DOI] [PubMed] [Google Scholar]
- Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol. 2009;587:2059–2067. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuna MG, Stornetta RL, West GH, Guyenet PG. Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J Physiol. 2009;587:5121–5138. doi: 10.1113/jphysiol.2009.176875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutagny R, Luppi PH, Salvert D, Gervasoni D, Fort P. GABAergic control of hypothalamic melanin-concentrating hormone-containing neurons across the sleep-waking cycle. NeuroReport. 2005;16:1069–1073. doi: 10.1097/00001756-200507130-00008. [DOI] [PubMed] [Google Scholar]
- Guan JL, Uehara K, Lu S, Wang QP, Funahashi H, Sakurai T, Yanagizawa M, Shioda S. Reciprocal synaptic relationships between orexin- and melanin-concentrating hormone-containing neurons in the rat lateral hypothalamus: a novel circuit implicated in feeding regulation. Int J Obes Relat Metab Disord. 2002;26:1523–1532. doi: 10.1038/sj.ijo.0802155. [DOI] [PubMed] [Google Scholar]
- Horn EM, Waldrop TG. Suprapontine control of respiration. Resp Physiol. 1998;114:201–211. doi: 10.1016/s0034-5687(98)00087-5. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods. 2000;103:63–71. doi: 10.1016/s0165-0270(00)00296-x. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol. 2009;106:138–152. doi: 10.1152/japplphysiol.91125.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynska L, Wolinska-Witort E, Chmielowska M, Bik W, Baranowska B. The physiological role of orexins. Neuro Endocrinol Lett. 2005;26:289–292. [PubMed] [Google Scholar]
- Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–2816. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- Nadin-Davis SA, Sheen M, Abdel-Malik M, Elmgren L, Armstrong J, Wandeler AI. A panel of monoclonal antibodies targeting the rabies virus phosphoprotein identifies a highly variable epitope of value for sensitive strain discrimination. J Clin Microbiol. 2000;38:1397–1403. doi: 10.1128/jcm.38.4.1397-1403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahon JL, Presse F, Bittencourt JC, Sawchenko PE, Vale W. The rat melanin-concentrating hormone messenger ribonucleic acid encodes multiple putative neuropeptides coexpressed in the dorsolateral hypothalamus. Endocrinology. 1989;125:2056–2065. doi: 10.1210/endo-125-4-2056. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102:241–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgate ES, Gellhorn E. Respiratory activity and the hypothalamus. Am J Physiol. 1958;193:189–194. doi: 10.1152/ajplegacy.1958.193.1.189. [DOI] [PubMed] [Google Scholar]
- Rice CD, Lois JH, Kerman IA, Yates BJ. Localization of serotoninergic neurons that participate in regulating diaphragm activity in the cat. Brain Res. 2009;1279:71–81. doi: 10.1016/j.brainres.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Siegel JM. Hypocretin (orexin): role in normal behavior and neuropathology. Annu Rev Psychol. 2004;55:125–148. doi: 10.1146/annurev.psych.55.090902.141545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: excitatory neuromodulatory peptides for multiple homeostatic systems, including sleep and feeding. J Neurosci Res. 2000;62:161–168. doi: 10.1002/1097-4547(20001015)62:2<161::AID-JNR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JG, de Lecea L. The hypocretins: setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Samson WK. The other side of the orexins: endocrine and metabolic actions. Am J Physiol Endocrinol Metab. 2003;284:E13–17. doi: 10.1152/ajpendo.00359.2002. [DOI] [PubMed] [Google Scholar]
- Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- Torterolo P, Sampogna S, Morales FR, Chase MH. MCH-containing neurons in the hypothalamus of the cat: searching for a role in the control of sleep and wakefulness. Brain Res. 2006;1119:101–114. doi: 10.1016/j.brainres.2006.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritos NA, Maratos-Flier E. Two important systems in energy homeostasis: melanocortins and melanin-concentrating hormone. Neuropeptides. 1999;33:339–349. doi: 10.1054/npep.1999.0055. [DOI] [PubMed] [Google Scholar]
- Verret L, Goutagny R, Fort P, Cagnon L, Salvert D, Leger L, Boissard R, Salin P, Peyron C, Luppi PH. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, De Lecea L. The role of the hypocretinergic system in the integration of networks that dictate the states of arousal. Drug News Perspect. 2003;16:504–512. doi: 10.1358/dnp.2003.16.8.829349. [DOI] [PubMed] [Google Scholar]
- Xi MC, Fung SJ, Yamuy J, Morales FR, Chase MH. Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat. J Neurophysiol. 2002;87:2880–2888. doi: 10.1152/jn.2002.87.6.2880. [DOI] [PubMed] [Google Scholar]
- Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Sampogna S, Morales FR, Chase MH. Orexin (hypocretin)-like immunoreactivity in the cat hypothalamus: a light and electron microscopic study. Sleep. 2001;24:67–76. doi: 10.1093/sleep/24.1.67. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Sampogna S, Morales FR, Chase MH. Co-localization of hypocretin-1 and hypocretin-2 in the cat hypothalamus and brainstem. Peptides. 2002;23:1479–1483. doi: 10.1016/s0196-9781(02)00084-0. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Sampogna S, Morales FR, Chase MH. Distribution of hypocretin (orexin) immunoreactivity in the feline pons and medulla. Brain Res. 2004;995:205–217. doi: 10.1016/j.brainres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett. 2005;385:131–136. doi: 10.1016/j.neulet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shimoyama M, Fukuda Y, Kuwaki T. Multiple components of the defense response depend on orexin: evidence from orexin knockout mice and orexin neuron-ablated mice. Auton Neurosci. 2006;126–127:139–145. doi: 10.1016/j.autneu.2006.02.021. [DOI] [PubMed] [Google Scholar]