Abstract
Vascular lesions in the brain are common with advancing age; however, the independent and cumulative contributions of postmortem vascular lesions to antemortem cognitive status are not well established. We examined association of six vascular lesions (large infarcts, lacunar infarcts, leukoencephalopathy, microinfarcts, cribriform changes, and cerebral amyloid angiopathy) with antemortem diagnoses of dementia, Alzheimer’s disease (AD), and vascular dementia (VaD) in 190 older adults from an autopsy series. We also developed a summary score based on three macroscopic vascular lesions: large infarcts (0, 1, and≥2), lacunar infarcts (0, 1, and≥2), and leukoencephalopathy (none, mild, and moderate-to-severe). Sixty-eight percent of cases had vascular lesions. Only leukoencephalopathy was associated with dementia (odds ratio (OR) 3.5, 95% CI 1.0–12.4), and only large infarcts were associated with VaD (OR 4.3, 95% CI 1.2–15.4). The vascular score was associated with dementia (OR 1.6, 95% CI 1.2–2.3), AD (OR 1.5, 95% CI 1.0–2.1) and VaD (OR 2.0, 95% CI 1.4–3.0). Leukoencephalopathy, large infarcts, and higher vascular burden is associated with the clinical expression of dementia and subtypes.
Keywords: Vascular, Pathology, Dementia, Stroke
1. Introduction
Cardiovascular and cerebrovascular diseases are important contributors to cognitive impairment and dementia in older adults. While cardiovascular diseases are closely linked to the clinical syndrome of vascular dementia (VaD), recent studies have suggested that vascular risk factors and vascular pathology may independently contribute to the risk of Alzheimer’s disease (AD) (Launer, 2002). Vascular pathology of varying types and severity is detected in up to two-thirds of the brains of older adults in population-based autopsy series (Fernando and Ince, 2004; Petrovitch et al., 2005; White et al., 2002;Yoshitake et al., 1995). These lesions may result from multiple vascular mechanisms including vascular occlusions, hemorrhages and hypoperfusion (Hachinski et al., 2006). The pathological and etiological heterogeneity in VaD is reflected in the poor sensitivity and specificity of current clinical criteria for VaD (Chui et al., 2000). Presence of vascular lesions on imaging studies and in postmortem brain examinations is used to validate the clinical diagnosis of VaD. On the other hand, studies on the clinical significance of vascular lesions identified at autopsy are limited (Knopman et al., 2003; Vinters et al., 2000; White et al., 2002; Yoshitake et al., 1995).
While cognitive correlates of cortical infarcts and lacunes have been reported in demented and non-demented older adults, the contributions of other types of vascular lesions such as cerebral amyloid angiopathy, leukoencephalopathy and microinfarcts to cognitive impairment states are less well known. Moreover, mixed pathologies are common in brains of older adults (Barker et al., 2002; Nagy et al., 1997; Snowdon et al., 1997; White et al., 2002), and may influence clinical presentations (Schneider et al., 2007). Some vascular pathology such as microinfarcts may not detectable during life using conventional neuroimaging techniques. To examine the clinical relevance of increasing vascular burden on clinical expression of dementia during life we also developed a summary vascular score based on the presence and number of three macroscopic lesions (large infarctions, lacunar infarctions, and leukoencephalopathy) that have neuroimaging correlates during life.
We examined the contribution of postmortem vascular pathology to antemortem cognitive status in an autopsy sample of 190 older adults who participated in longitudinal aging studies in the Bronx during life. Our aims were twofold. First, we assessed the independent contributions of six vascular lesions (large infarcts, lacunar infarcts, leukoencephalopathy, microinfarcts, cribriform changes, and cerebral amyloid angiopathy) to antemortem clinical dementia syndromes (dementia overall, AD, and VaD) accounting for other neurodegenerative pathologies. Second, we investigated the cumulative effect of macroscopic vascular lesions with neuroimaging correlates during life on the antemortem clinical diagnosis of dementia using a novel vascular score. Identifying specific pathological vascular lesions associated with dementia and its subtypes may help refine current clinical and pathological criteria for dementia syndromes, improve diagnostic procedures, and lead to novel interventions.
2. Methods
2.1. Study population
The autopsy series reported in this study included 190 brains of participants in longitudinal aging studies in the Bronx during life. Fifty-four subjects were enrolled in the Bronx Aging Study, 99 in the Albert Einstein College of Medicine Teaching Nursing Home study, 14 in the Einstein Aging Study (EAS), and 23 were community volunteers. Methods of recruitment, subject examination, and determination of cognitive status in these cohorts have been described previously (Crystal et al., 1993, 2000; Katzman et al., 1989; Verghese et al., 1999). In brief, the Bronx Aging Study recruited a volunteer cohort of non-demented elderly individuals over age 75, between 1980 and 1983. The Teaching Nursing Home study (1984–1991) recruited both community residing and institutionalized elderly individuals with and without dementia at baseline. In 1992, the Bronx Aging and the Teaching Nursing Home studies were incorporated in to the EAS. EAS participants were systematically recruited from population lists of Medicare eligible recipients (≥70 years of age) living in the community in Bronx County. Exclusion criteria included presence of severe visual or hearing impairments that would interfere with completion of neuropsychological tests. Subjects previously diagnosed with idiopathic Parkinson’s disease or dementias by their private physicians were excluded from the Bronx Aging Study, but not from the Teaching Nursing Home study or EAS. Written informed consent for clinical evaluation as well as for participation in the autopsy program was obtained from all subjects and surrogate decision makers at enrollment. The local institutional review board approved the study and autopsy protocols.
2.2. Dementia and cognitive assessment
Subjects in all three studies received clinical and neuropsychological evaluations at enrollment and at 12–18-month interval visits as previously described. An informant, usually a family member, accompanied most non-demented subjects and all demented subjects, and was interviewed or contacted to confirm details of history. Subjects with prevalent dementia at study enrollment in the TNH study were examined at their nursing homes if they were unable to come to the clinical research center. Clinical evaluations at both sites were done by board-certified neurologists with expertise in geriatric neurology. Research assistants administered a standardized neuropsychological battery validated for use in normal aging populations. The Blessed-information-memory-concentration test (BIMC) was used to screen for general cognitive status. This test has a high test–retest reliability (0.86) and correlates well with Alzheimer pathology (Grober et al., 1999; Thal et al., 1986).
Dementia was diagnosed after reviewing all available clinical and neuropsychological information at consensus case conferences attended by the study neurologists, licensed neuropsychologist, and a study social worker. Dementia was diagnosed using the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition criteria and the Fourth edition criteria after 1994 (Association, 1987, 1994). Subjects diagnosed with dementia were subtyped using the National Institutes of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria for probable or possibleAD(McKhann et al., 1984), and the State of California Alzheimer’s Disease Diagnostic and Treatment Centers criteria for probable, possible or mixed VaD (Chui et al., 1992). Neuroimaging studies were used to help distinguish “probable” AD or “probable” VaD. We have previously reported good clinicopathological agreement between the clinical diagnoses of AD, VaD, and Lewy body dementia with pathological findings (Crystal et al., 2000; Verghese et al., 1999).
2.3. Neuropathology
A single experienced neuropathologist, blinded to clinical status, provided neuropathologic evaluations. Brains were removed within 24 h of death. One hemisphere (usually the right) was frozen and the other half fixed in 10% formalin for histological examination. Prior to freezing the circle of Willis was dissected for study. Sampling for histologic studies included six neocortical areas (midfrontal cortex—Brodmann area (BA) (BA8/46), superior temporal gyrus (BA38), inferior parietal lobule (BA39/40), motor cortex in the watershed area (BA4/1,2,3), anterior cingulate gyrus (BA 24) and calcarine cortex (BA17/18)), two levels of the hippocampus (anterior and posterior), the basal forebrain with amygdala and lentiform nucleus, basal ganglia with nucleus accumbens, thalamus with subthalamic nucleus, midbrain at the level of the third nerve, rostral pons, mid-medulla with hypoglossal nucleus and inferior olivary nucleus, cerebellar vermis and cerebellar cortex with dentate nucleus. Additional sections were taken of macroscopic lesions (e.g., infarcts, hemorrhages, or mass lesions) for histologic characterization. Select sections from the opposite hemisphere were taken before freezing if abnormalities, including vascular lesions, were detected on visual inspection.
Senile plaques (SP) and neurofibrillary tangles (NFT) were counted with thioflavin-S fluorescent microscopy in five cortical areas, four sectors of the hippocampus and two regions of the amygdala as previously described (Barker et al., 2002; Dickson et al., 1994; Grober et al., 1999). For SP the counts were made at 100× magnification, while NFT were counted at 400× magnification. NFT were also counted in the basal nucleus of Meynert. Cerebral amyloid angiopathy (CAA) was assessed in parenchymal and leptomeningeal vessels with thioflavin-S fluorescent microscopy in five cortical areas (Fig. 1f). All cases were assigned a Braak NFT stage (Braak and Braak, 1991), based upon the distribution of NFT on thioflavin-S sections. In all cases the medial temporal lobe was immunostained for phospho-tau to detect argyrophilic grains and to verify the Braak NFT stage (Grober et al., 1999).A section of the amygdala was screened for Lewy bodies with α-synuclein immunohistochemistry. If Lewy bodies were detected on routine histology in any of the vulnerable brainstem nuclei or in the α-synuclein immunostained section of amygdala, additional cortical and basal forebrain sections were immunostained to determine the Lewy body type. Prior to widespread application of α-synuclein immunohistochemistry, cases were studied with double immunohistochemistry for tau and ubiquitin (Barker et al., 2002). Depending upon the histologic findings, additional immunohistochemical studies were performed to characterize the pathology using antibodies to ubiquitin, glial fibrillary acidic protein, isotype-specific forms of tau and β-amyloid. In cases with white matter pathology, the nature of this pathology was confirmed with histochemical methods for myelin (Luxol fast blue) and axons (Bielschowsky or Bodian silver stains).
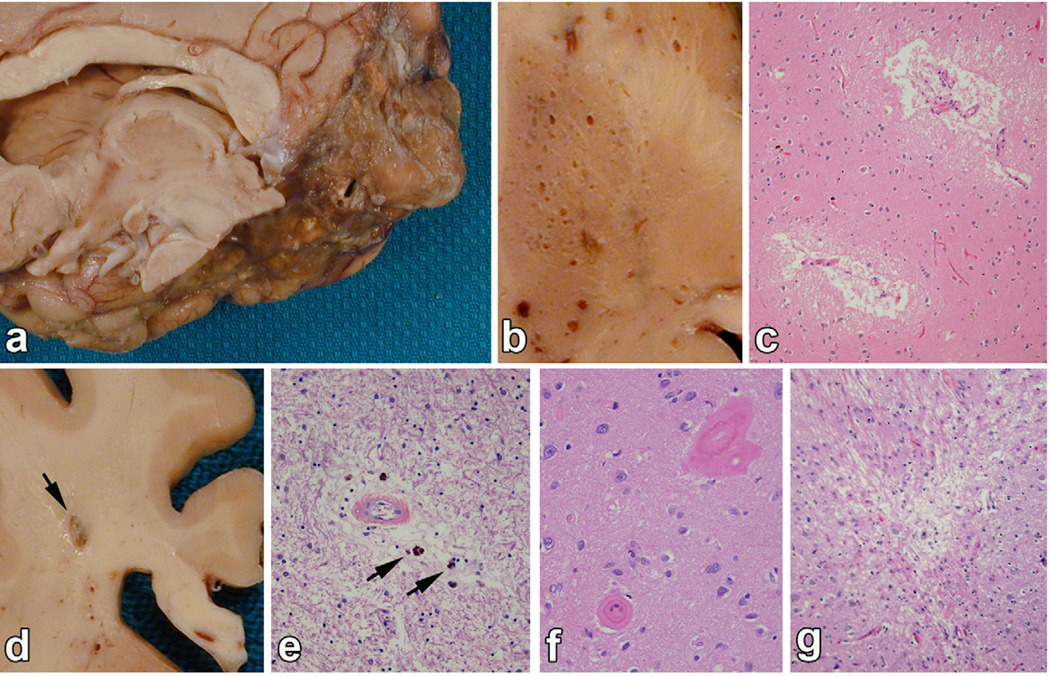
Fig. 1.
Examples of vascular lesions. (a) Ischemic large infarct: a massive area of encephalomalacia affecting the inferior temporal and medial occipital lobe in the distribution of the posterior cerebral artery. (b) Cribriform change is noted in the putamen as dilated perivascular spaces, but not associated with infarcts or ischemic pathology on microscopic examination. (c) and (d) Lacunar infarcts are cystic infarcts 1-cm or less in diameter, such as this lesion in the periventricular white matter (arrow), which has softening and discoloration in surrounding white matter. (e) Leukoencephalopathy is characterized by rarefaction of white matter, dilated perivascular spaces and often perivascular pigment-laden macrophages (arrows). (f) CAA is noted by glassy thickening of vessel wall. (g) Micro-infarcts are small foci of collapsed gliotic tissue or partially cystic tissue necrosis visible only with the microscope.
2.4. Pathological vascular lesions
The six types of vascular lesions identified included ischemic vascular lesions (large infarcts and lacunar infarcts), leukoencephalopathy, microinfarcts, cribriform changes, and CAA. Ischemic vascular lesions were identified by macroscopic examination of 0.5-cm thick coronal sections of cerebral hemispheres and transverse sections of brainstem and cerebellum. Circumscribed areas of softening (encephalomalacia), whether cystic or partially cystic, with maximum diameter greater than one centimeter were classified as large infarct (Fig. 1a). Large infarcts were usually associated with atherosclerotic large vessel disease and in most cases, segmental stenosis or less often recanalized occlusions were detected in named arteries at the base of the brain. Lacunar infarcts were defined as cystic lesions less than one centimeter in diameter (Fig. 1d). Lacunar infarcts, which correlate with the presence of small vessel arteriosclerotic vascular disease, were most often found in basal ganglia, thalamus, pontine base, and periventricular white matter. In a few cases, the lacunes were associated with hemosiderin and were likely related to old hemorrhages. For the purpose of this analysis, this type of lesion was included in the category of lacunar infarcts. Leukoencephalopathy was assessed based on the presence of rarefaction of the white matter, dilation of perivascular spaces in the white matter and the presence of reactive astrocytes and less often macrophages (Fig. 1e). White matter pathology was identified on macroscopic exam and selected areas were sampled for the microscopic exam. Results of leukoencephalopathy were reported as limited to periventricular caps (mild, n = 13), involving the centrum semiovale (moderate, n = 19), or extensively involving periventricular caps and the centrum semiovale (severe, n = 13).
Microinfarcts were defined as ischemic lesions found on microscopic exam and not visible to the naked eye (Fig. 1g). They were most often detected in sections of the motor cortex which was taken from watershed of the anterior and middle cerebral arteries. Cribriform changes were enlarged perivascular spaces often associated with perivascular ischemic gliosis, but not frank infarcts or hemorrhages (Fig. 1b and c). They were evaluated in the basal ganglia and thalamus. Dilated perivascular spaces in the white matter were not included in this category, but were taken into account in assessing overall white matter pathology. Acute and subacute infarcts, lacunes, microinfarcts or hemorrhages near the time of death were noted, but not included in the vascular scores or analyses. The decision to exclude was based upon the histological appearance of the lesions, and presence of acute neuronal necrosis and neutrophils.
2.5. Vascular score
To understand the clinical relevance of our findings as well as to study the effect of cumulative vascular burden irrespective of type of vascular lesion, we developed a summary pathological vascular lesion score based on the presence and number of three types of macroscopic vascular lesions (large infarct, lacunar infarct, and leukoencephalopathy) that are associated with neuroimaging correlates during life. Details of the score are presented in Table 1. Each type of lesion was counted in each subject, and we adjusted for number of large infarcts, number of lacunar infarcts and severity of leukoencephalopathy. We studied the vascular lesion score (Table 1) both as continuous (range 0–6) as well as categorical variables (no vascular lesion, any one vascular lesion, two vascular lesions, and three or more lesions) to look for a possible threshold effect of vascular burden on cognitive impairment.
Table 1.
Determination of the macroscopic vascular lesion score.
| Component | Vascular lesion score points |
|---|---|
| Large infarct | |
| 2 or more | 2 |
| 1 | 1 |
| None | 0 |
| Lacunes | |
| 2 or more | 2 |
| 1 | 1 |
| None | 0 |
| Leukoencephalopathy | |
| Moderate-severe | 2 |
| Mild | 1 |
| None | 0 |
| Total score | 6 |
2.6. Data analysis
The association of six individual types of vascular lesions (large infarcts, lacunar infarcts, leukoencephalopathy, microinfarcts, cribriform changes, and cerebral amyloid angiopathy) to antemortem diagnosis of dementia, AD and VaD was examined using multiple logistic regression adjusted for potential confounders (McCullagh PaN, 1989). Model 1 was adjusted for age at death, gender, and clinico-pathological interval. A term for enrollment group was also entered in this model to account for possible cohort effects. Mixed neurodegenerative and vascular lesions are commonly present in brains of older individuals. Therefore, Model 2 was adjusted for other individual vascular lesions, Alzheimer pathology (Braak NFT stage), and presence of cortical Lewy bodies, to ascertain the independent effect of each vascular lesion after accounting for additional neurodegenerative pathology. Number of large infarcts, number of lacunes and severity of leukoencephalopathy were examined separately. We also conducted analyses using the categorical and continuous vascular lesion score as the predictor. Results are reported as odds ratios with 95% confidence intervals (CI).
Clinical evidence of vascular disease (history, examination, or imaging findings) may influence dementia diagnosis by biasing clinicians to subtype dementia cases as VaD. To test associations of vascular lesion types with an outcome that was not influenced by clinical evidence of vascular disease, we repeated all our analyses with Blessed test scores as the outcome. We dichotomized Blessed scores (>4) to define cognitive impairment based on clinical pathological associations for this test reported in our previous studies (Crystal et al., 1993, 2000; Dickson et al., 1992). In this sample, 72% of subjects were defined as cognitively impaired at their last clinical assessment using this Blessed cut score.
3. Results
3.1. Study population
Among the 190 autopsy cases, mean age at death was 84.4±9.6 years and 65% were female. There were 131 individuals (69%) who met clinical criteria for dementia at their last clinical evaluation including 84 cases of probable and possible AD, 28 cases of VaD (including 17 with mixed Alzheimer-vascular dementia (Chui et al., 1992)), nine cases of Lewy body dementia, three cases of frontotemporal dementia, four cases of dementia of undetermined etiology, and one case each of multisystem atrophy, Parkinsonian dementia, and alcohol related dementia. The median interval between the last clinical evaluation and death was 13 months (interquartile range 6–27 months). Table 2 shows the baseline characteristics of the autopsy sample.
Table 2.
Clinical characteristics based on the macroscopic lesion scorea.
| Overall n = 190 | 0b | 1 | 2 | 3 or more | p-Valuec | |
|---|---|---|---|---|---|---|
| Demographicsd | ||||||
| Age, mean (S.D.), years | 84.4 (9.6) | 82.3 (10.4) | 87.8 (4.7) | 84.3 (8.3) | 88.5 (9.2) | 0.003 |
| Female (%) | 65.3 | 58.8 | 69.2 | 76.7 | 71.9 | 0.2 |
| Clinicopathologic interval, monthe | 13 (6–27) | 11.5 (5–26) | 11 (6–16) | 17.5 (9–31) | 14 (6.5–29) | 0.7 |
| APOE e4 allele (%) | 14.7 | 16.7 | 3.9 | 13.3 | 18.8 | 0.4f |
| Clinical diagnosis at last evaluation (%) | ||||||
| Non-demented (n = 59) | 31.1 | 40.2 | 30.8 | 23.3 | 9.4 | 0.001 |
| AD (n = 84) | 44.2 | 47.1 | 38.5 | 40.0 | 43.8 | |
| VaD (n = 28) | 14.7 | 3.9 | 19.2 | 30.0 | 31.3 | |
| Other (n = 19) | 10.0 | 8.8 | 11.5 | 6.7 | 15.6 | 0.1 |
| Blessed score (n = 184)e | 15 (4–32) | 9 (3–32) | 14.5 (4–26) | 16 (6–32) | 21 (12–32) | 0.1 |
| Pathological lesions (%) | ||||||
| Large infarcts | ||||||
| 1 | 9.0 | – | 26.9 | 3.3 | 28.1 | – |
| ≥2 | 10.0 | – | – | 26.7 | 34.4 | – |
| Lacunes | ||||||
| 1 | 11.1 | – | 53.9 | 6.7 | 15.6 | – |
| ≥2 | 14.2 | – | – | 23.3 | 62.5 | – |
| Leukoencephalopathy | ||||||
| Mild | 6.8 | – | 19.2 | 3.3 | 21.9 | – |
| Mod-severe | 16.8 | – | – | 43.3 | 59.4 | – |
| Microinfarcts | 15.8 | 9.8 | 11.5 | 30.0 | 25.0 | 0.02c |
| Cribriform changes | 15.8 | 3.9 | 26.9 | 16.7 | 43.8 | <0.001c |
| CAA | 27.9 | 27.5 | 23.1 | 46.7 | 15.6 | 0.05c |
| Braak NFT stage, mean (S.D.) | 3.4 (1.8) | 3.3 (2.0) | 3.0 (1.7) | 3.8 (1.8) | 3.5 (1.7) | 0.5 |
| Cortical Lewy bodies (%) | 12.6 | 9.8 | 11.5 | 20.0 | 15.6 | 0.5 |
Macroscopic vascular lesions score ranging 0–6 as outlined in Table 4.
Defined as absence of any macroscopic vascular lesions such as large infarcts, lacunar infarct, or leukoencephalopathy.
Using ANOVA for continuous variables, and Chi-square test for categorical variables.
Mean (±S.D.) unless otherwise specified.
Median, 25–75% quantiles.
APOE missing in 87 cases.
3.2. Neuropathology
There were 129 (68%) individuals with at least one of the six pathological vascular lesions of interest, including large infarcts (n = 36), lacunar infarcts (n = 48), leukoen-cephalopathy (n = 45), microinfarcts (n = 30), cribriform changes (n = 30), and cerebral amyloid angiopathy (n = 53). Neuropathological diagnoses in these 190 cases (assigned independent of clinical diagnosis) included: AD (n = 55), mixed AD with VaD (n = 28), mixed AD and diffuse Lewy body disease (n = 14), vascular dementia (n = 28), diffuse Lewy body disease (n = 7), pathological aging (n = 22), argyrophillic grain dementia (n = 5), frontotemporal dementia (n = 4), corticobasal ganglionic degeneration (n = 2), and one case each of progressive supranuclear palsy, adult polyglucosan disease and Cruetzfeld Jakob disease (Cairns et al., 2007; Dickson, 1996, 1997; Dickson et al., 1995; Fujishiro et al., 2008; Gray et al., 1988; Jicha et al., 2006; Liberski and Ironside, 2004; Lippa et al., 2007; McKeith et al., 2005). Twenty-two cases were diagnosed normal without significant neurodegenerative or vascular pathology.
Number of large infarcts and lacunes are shown in Table 2. The frequency of cortical Lewy bodies and Braak staging did not differ between the different vascular lesion score categories. Compared to subjects with clinical AD, individuals diagnosed with VaD were older (88.5 years vs. 83.3 years), and had a significantly higher proportion of large infarcts (43% vs. 14%).
3.3. Vascular lesions
Table 3 shows that leukoencephalopathy was associated with antemortem diagnosis of both AD and dementia. In the model with adjustments for other vascular and neurodegenerative pathology, leukoencephalopathy overall (OR 3.5, 95% CI 1.0–12.4) and moderate to severe leukoencephalopathy (OR 5.4, 95% CI 1.0–30.2) were associated with clinical diagnosis of dementia, but not with a clinical diagnosis of AD. Large infarcts, lacunar infarcts, and leukoencephalopathy were associated with antemortem diagnosis of VaD when entered in the model individually (Table 3). However, only presence of large infarcts (OR 4.3, 95% CI 1.2–15.4) was significant in the final model. Excluding cases clinically diagnosed with mixed dementia did not materially change the association between large infarcts and vascular dementia (data not shown).
Table 3.
Relationships of macroscopic vascular lesions to clinical diagnoses of dementia, AD or VaD.
| Vascular lesions | Any dementiaa, no. of cases/total = 31/190 | Alzheimer’s diseaseb, no. of cases/total = 84/143 | Vascular dementiac, no. of cases/total = 28/87 | |||
|---|---|---|---|---|---|---|
| Model 1d OR (95% CI) | Model 2e OR (95% CI) | Model 1d OR (95% CI) | Model 2e OR (95% CI) | Model 1d OR (95% CI) | Model 2e OR (95% CI) | |
| Macroscopic | ||||||
| Large infarcts (all) | 1.8 (0.7–4.4) | 2.1 (0.7–6.5) | 1.1 (0.4–2.9) | 1.5 (0.4–4.9) | 4.1 (1.4–12.1)** | 4.3 (1.2–15.4)** |
| None | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 | 2.0 (0.6–7.3) | 2.2 (0.4–10.6) | 1.0 (0.2–4.5) | 1.3 (0.2–7.7) | 4.4 (1.0–19.8)** | 4.6 (0.8–27.0) |
| ≥2 | 1.6 (0.5–5.1) | 2.1 (0.5–8.7) | 1.1 (0.3–4.0) | 1.6 (0.3–7.3) | 3.8 (1.0–14.6)** | 4.0 (0.8–19.8) |
| Lacunes (all) | 2.1 (0.9–4.7) | 1.7 (0.6–4.6) | 1.5 (0.6–3.5) | 1.4 (0.5–3.9) | 3.0 (1.1–8.7)** | 2.4 (0.7–8.4) |
| None | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 | 1.5 (0.5–4.2) | 1.4 (0.4–5.0) | 1.1 (0.4–3.7) | 1.2 (0.3–4.6) | 1.5 (0.3–6.6) | 1.7 (0.3–9.4) |
| ≥2 | 3.0 (0.9–9.3) | 2.1 (0.5–8.3) | 2.0 (0.6–6.7) | 1.6 (0.4–6.9) | 5.4 (1.4–21.0)** | 3.3 (0.7–16.3) |
| Leukoencephalopathy (all) | 5.3 (1.9–14.7)** | 3.5 (1.0–12.4)** | 4.3 (1.5–12.7)*** | 3.7 (0.9–14.0) | 7.2 (2.2–23.9)** | 3.6 (0.9–14.9) |
| None | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Mild | 2.0 (0.5–7.7) | 2.2 (0.4–11.8) | 2.3 (0.6–9.5) | 3.6 (0.6–21.2) | 1.0 (0.1–10.9) | 0.8 (0.1–11.5) |
| Mod-severe | 10.6 (2.3–48.2)*** | 5.4 (1.0–30.2)** | 7.4 (1.5–35.3)** | 4.2 (0.7–25.6) | 16.6 (3.3–85.0)*** | 5.9 (1.0–36.4) |
Comparing overall demented to non-demented.
Comparing AD to non-demented excluding VaD and other dementias.
Comparing VaD to non-demented excluding AD and other dementias.
Adjusted for age at death, sex, recruitment group, interval from last evaluation to death in months.
Additionally adjusted for other vascular lesions, Braak NFT stage and cortical Lewy bodies.
p value < 0.05.
p value < 0.001.
Of the microscopic vascular lesions examined (Table 4), only cerebral amyloid angiopathy was associated with dementia and subtypes, but the associations were not significant when adjusted for additional covariates in the second model.
Table 4.
Relationships of microscopic lesions to clinical diagnoses of dementia, AD or VaD.
| Any dementiaa, no. of cases/total = 131/190 | Alzheimer’s diseaseb, no. of cases/total = 84/143 | Vascular dementiac, no. of cases/total = 28/87 | ||||
|---|---|---|---|---|---|---|
| Model 1d OR (95% CI) | Model 2e OR (95% CI) | Model 1d OR (95% CI) | Model 2e OR (95% CI) | Model 1d OR (95% CI) | Model 2e OR (95% CI) | |
| Microscopic vascular | ||||||
| Microinfarcts | 0.8 (0.3–2.0) | 0.6 (0.2–1.9) | 0.6 (0.2–1.7) | 0.5 (0.1–1.7) | 1.7 (0.6–5.0) | 1.4 (0.3–5.7) |
| Cribriform changes | 1.9 (0.7–5.0) | 1.7 (0.5–6.2) | 1.1 (0.4–3.3) | 1.1 (0.3–4.5) | 3.0 (0.9–10.1) | 1.9 (0.4–8.9) |
| Cerebral amyloid angiopathy | 3.6 (1.5–8.6)** | 1.2 (0.4–3.7) | 4.0 (1.6–10.0)** | 1.2 (0.4–3.9) | 3.6 (1.2–11.1)** | 1.5 (0.4–5.8) |
| Neurodegenerative | ||||||
| Braak Staging | 2.1 (1.6–2.7)*** | 2.1 (1.6–2.9)*** | 2.3 (1.7–3.0)*** | 2.3 (1.7–3.2)*** | 1.8 (1.3–2.5)*** | 1.8 (1.2–2.7)** |
| Cortical LB | 1.9 (0.9–4.1) | 1.9 (0.8–4.2) | 1.9 (0.9–4.0) | 1.8 (0.8–4.1) | 2.0 (0.9–4.5) | 2.2 (0.9–5.0) |
Comparing overall demented to non-demented.
Comparing AD to non-demented excluding VaD and other dementias.
Comparing VaD to non-demented excluding AD and other dementias.
Adjusted for age at death, sex, recruitment group, interval from last evaluation to death in months.
Additionally adjusted for other vascular lesions, Braak NFT stage and cortical Lewy bodies.
p value < 0.05.
p value < 0.001.
3.4. Vascular score
Examined as a continuous variable (Table 5), a one-point increase on the vascular lesion score was significantly associated with antemortem diagnosis of dementia (OR 1.6, 95% CI 1.2–2.3), AD (OR 1.5, 95% CI 1.0–2.1), and VaD (OR 2.0, 95% CI 1.4–3.0) in the fully adjusted models. A score of three or higher was associated with clinical diagnoses of both dementia overall and VaD compared to subjects without vascular lesions. A score of three or more was also associated with antemortem cognitive impairment defined using a cutscore of greater than four on the Blessed test (OR 6.5, 95% CI 1.5–28.9).
Table 5.
Relationship of macroscopic vascular lesion scorea to clinical dementia, AD and VaD.
| Any dementiab | Alzheimer’s diseasec | Vascular dementiad | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) Model 1e | OR (95% CI) Model 2f | OR (95% CI) Model 1e | OR (95% CI) Model 2f | OR (95% CI) Model 1e | OR (95% CI) Model 2f | |
| Severity (0–6) (all)g | 1.6 (1.2–2.2)*** | 1.6 (1.2–2.3)** | 1.4 (1.0–2.0)** | 1.5 (1.0–2.1)** | 2.1 (1.5–3.1)*** | 2.0 (1.4–3.0)*** |
| 0 (n = 102) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1 (n = 26) | 2.4 (0.9–6.5) | 3.6 (1.1–12.2) | 1.6 (0.5–4.7) | 2.7 (0.7–10.2) | 6.9 (1.4–32.6)** | 8.5 (1.6–45.9)** |
| 2 (n = 30) | 2.6 (0.97–7.1) | 2.5 (0.7–8.4) | 1.5 (0.5–4.5) | 1.4 (0.4–5.5) | 13.6 (3.2–58.1)*** | 10.1 (2.0–50.8)** |
| 3 or more (n = 32) | 8.4 (2.3–31.1)*** | 9.1 (2.1–40.0)** | 4.7 (1.2–18.3)** | 5.6 (1.2–26.3)** | 33.1 (6.0–182.9)*** | 27.9 (4.4–175.8)*** |
Macroscopic vascular lesions score ranging 0–6 as outlined in Table 1.
Comparing overall demented to non-demented.
Comparing AD to non-demented excluding VaD and other dementias.
Comparing VaD to non-demented excluding AD and other dementias.
Adjusted for age at death, sex, recruitment group, interval from last evaluation to death in months.
Additionally adjusted for other vascular lesions, Braak NFT stage and cortical Lewy bodies.
Using continuous variable for vascular lesion score.
p value < 0.05.
p value < 0.001.
3.5. Sensitivity analyses
Large infarcts but not lacunar infarcts were more prevalent in brain autopsies of subjects diagnosed with VaD than those with AD (42.9% vs. 14.3%). To account for the possibility that presence of clinical strokes may have influenced diagnosis of VaD during life, we conducted additional analyses excluding large infarcts from the vascular lesion score. Presence of two or more vascular lesions (excluding large infarcts) was still associated with antemortem diagnosis of VaD (OR 5.9, 95% CI 1.4–24.1), suggesting an effect of increasing vascular pathology even in the absence of large infarcts on the clinical expression of dementia.
All our analyses were adjusted for the clinicopathological interval. We examined the association of vascular lesion scores and clinical outcomes in 92 subjects (49%) with autopsies done 12 months or less after their last clinical assessment to account for possible bias due to long clinicopathological interval. Among these 92 cases, two or more vascular lesions were still associated with an antemortem diagnosis of dementia (OR 7.8, 95% CI 1.00–62.5).
4. Discussion
In this well characterized autopsy sample of 190 elderly individuals only presence of leukoencephalopathy, among the six types of pathological vascular lesions examined, was associated with an antemortem diagnosis of dementia and AD after accounting for other vascular and neurodegenerative pathologies. As the severity of leukoencephalopathy increased so did the risk of all causes of dementia, AD, and VaD. This dose-response relationship strengthens our findings. Previous studies that have examined the contributions of leukoencephalopathy to antemortem cognitive status have shown mixed results with some (Englund et al., 1988; Garcia and Brown, 1992) but not all studies (Tatemichi, 1990) showing a significant association.
Most elderly individuals coming to autopsy have vascular lesions (Fernando and Ince, 2004; Kalaria et al., 2004; Knopman et al., 2003; Riekse et al., 2004; Schneider et al., 2004; White et al., 2002). One or more of the six types of vascular lesions were seen in 68% of our sample, comparable to other community-based samples (Neuropathology_group, 2001; Schneider et al., 2007). Previous studies have focused mostly on ischemic infarctions in autopsied brains. Macroscopic vascular lesions have been linked to clinical dementia (Chui et al., 2006; Petrovitch et al., 2005; Schneider et al., 2004; Snowdon et al., 1997) as well as with severity of cognitive impairment (Heyman et al., 1998; Schneider et al., 2004). However, like one previous study (Neuropathology_group, 2001), we did not find an association of large infarcts and lacunar infarcts with a clinical diagnosis of dementia. Large infarcts were associated with antemortem clinical diagnosis of VaD. In a prior analysis of this autopsy sample, lacunar infarcts, but not leukoencephalopathy were associated with dementia (Crystal et al., 1993). However, we now have a four-fold larger sample, and controlled for other previously unaccounted covariates in our multivariate analyses. In our effort to understand the independent effect of specific vascular lesions, we controlled analysis of each vascular lesion for the presence of others. If vascular disease has a common pathophysiological substrate this may represent over-adjustment. To address this issue we developed a vascular score which summarized three types of macroscopic pathology rather than adjusting for it. The success of the vascular risk score supports this approach.
Our results suggest that accumulating macroscopic vascular pathology, regardless of type, contributes to the diagnosis of dementia, and does so even in presence of other neurodegenerative pathology. The association between increasing vascular score and antemortem AD, supports vascular contributions to this clinical dementia subtype (Beach et al., 2007; Honig et al., 2005; Roher et al., 2004). Another community based clinicopathological study with comparable age range and with analyses adjusted for other co-existing pathological processes did not find an association between dementia and individual types of vascular pathology, whereas presence of multiple vascular pathologies was associated with dementia (Neuropathology_group, 2001). While our observations may be driven by the presence of large infarcts in the vascular score, our subgroup analyses excluding infarcts from the vascular score did not materially change findings. Our results, which look at lesions in aggregate in our sample, do not exclude the possibility that a single strategically located vascular lesion may result in dementia in individual patients. Silent thalamic infarcts detected on MRI were associated with increased rate of memory decline in the Rotterdam Study (Vermeer et al., 2003).
In an autopsy series restricted to older Japanese-American men, multiple large infarcts were associated with antemortem dementia, and this association was nearly equal in magnitude to that seen with Alzheimer lesions (Petrovitch et al., 2005). The Religious Order Study reported that large and lacunar cerebral infarctions were associated with a twofold increased risk for dementia (Schneider et al., 2003, 2004). Another study reported a borderline association of subcortical vascular burden with antemortem cognitive status when adjusted for Alzheimer pathology. However, the investigators used a composite vascular score restricted to cystic infarcts, lacunar infarcts, and microinfarcts (Chui et al., 2006). Unlike some studies, we did not find a significant association of microinfarctions with dementia subtypes (Kovari et al., 2007). Strict comparisons with previous studies may be limited by differing study populations, smaller samples in previous studies, limited set of confounders considered in analyses, various definitions of vascular disease burden, differences in clinical diagnostic procedures and pathological techniques.
The antemortem correlates of our vascular lesions should be further explored using techniques such as neuroimaging. Epidemiological studies have reported an association between periventricular white matter lesions on imaging studies and cognitive decline (Prins et al., 2005; Vermeer et al., 2003), mirroring our findings with respect to leukoencephalopathy. Radiographic white matter lesions have been correlated with neuropathological findings such as axonal loss, myelin rarefaction, small lacunes, cerebral amyloid angiopathy, and perivascular demyelination and gliosis (Merino and Hachinski, 2000). Although cerebral amyloid angiopathy was strongly associated with dementia in the initial model, we did not incorporate it in the vascular score for two reasons. Firstly, cerebral amyloid angiopathy represents vessel pathology while infarcts and leukoencephalopathy are parenchymal, and we did not want to mix those two pathologies in the score. Secondly, is not yet possible to verify or quantify cerebral amyloid angiopathy pathology during lifetime. As resolution of imaging techniques improves, correlations between MRI and microscopic vascular lesions should be re-examined.
Some possible limitations of our study merit discussion. The median interval between the last clinical examination and death was 13 months. Hence, it is possible that some individuals may have experienced cognitive decline after their last assessment. We controlled all analyses for clinicopathological interval. The significant associations were unchanged in our subgroup analysis restricted to subjects evaluated within a year of death. In common with other autopsy studies selection bias is the norm rather than the exception. We accounted for possible cohort effects in our analyses since our source populations included community volunteers, institutionalized participants, and population-based randomly recruited individuals. Presence or absence of clinical strokes or infarcts on imaging studies is used to assign dementia subtypes, raising issues of diagnostic circularity. This limitation does not apply to our overall clinical dementia diagnosis, which is assigned prior to subtyping cases as AD or VaD. We conducted a number of sensitivity analyses to address this issue. The results were not materially different when we excluded pathological large infarcts that may have been recognized during life or when cognitive impairment was defined independent of the dementia subtyping process by using cut scores on the Blessed test. The vascular lesions were known to the pathologist at the time of assigning neuropathological diagnoses. Hence, correlation of vascular scores with neuropathological diagnoses is limited by diagnostic circularity. While our dementia diagnostic procedures have been reported to correlate highly with pathological findings, clinical diagnostic misclassification may have reduced the strength of some of the observations. Our results should be confirmed in other independent autopsy samples. Lastly, neuropathologic evaluations were performed on one hemisphere in many cases. Moreover, identification of vascular pathology was based on gross brain sections. This is likely to have resulted in under estimation of the association between vascular lesions and clinical diagnosis of dementia. Utilization of techniques such as myelin staining of brain sections will improve quantification of white matter pathology. However, these techniques may not necessarily have clinical or imaging correlates.
The strengths of our study include the detailed assessment of clinical and neuropathologic data by experienced examiners, validated diagnostic procedures, and the inclusion of a wide range of pathological lesions associated with dementia.
In summary, our study shows that individual types of vascular lesions as well as increasing vascular burden are associated with antemortem diagnosis of clinical dementia. The individual types of lesions as well as the score described can be used to improve definitions of vascular dementia as well as dementia assessment procedures.
Acknowledgments
Funding support: The Einstein Aging Study is funded by the National Institute on Aging (grant AG03949). Dr. Verghese is funded by the National Institute on Aging (RO1 AG025119).
Footnotes
Conflict of interest
Authors have no conflicts of interest to declare.
References
- Association AP. Diagnostic and Statistical Manual of Mental Disorders, 3rd revised ed. Washington, DC: Psychiatric Association; 1987. [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: Psychiatric Association; 1994. [Google Scholar]
- Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, Waters C, Jimison P, Shepherd E, Sevush S, Graff-Radford N, Newland D, Todd M, Miller B, Gold M, Heilman K, Doty L, Goodman I, Robinson B, Pearl G, Dickson D, Duara R. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis. Assoc. Disord. 2002;16:203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Beach TG, Wilson JR, Sue LI, Newell A, Poston M, Cisneros R, Pandya Y, Esh C, Connor DJ, Sabbagh M, Walker DG, Roher AE. Circle of Willis atherosclerosis: association with Alzheimer's disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol. 2007;113:13–21. doi: 10.1007/s00401-006-0136-y. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl.) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, III, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the consortium for frontotemporal lobar degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s disease diagnostic and treatment centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- Chui HC, Mack W, Jackson JE, Mungas D, Reed BR, Tinklenberg J, Chang FL, Skinner K, Tasaki C, Jagust WJ. Clinical criteria for the diagnosis of vascular dementia: a multicenter study of comparability and interrater reliability. Arch. Neurol. 2000;57:191–196. doi: 10.1001/archneur.57.2.191. [DOI] [PubMed] [Google Scholar]
- Chui HC, Zarow C, Mack WJ, Ellis WG, Zheng L, Jagust WJ, Mungas D, Reed BR, Kramer JH, Decarli CC, Weiner MW, Vinters HV. Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann. Neurol. 2006;60:677–687. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal HA, Dickson DW, Sliwinski MJ, Lipton RB, Grober E, Marks-Nelson H, Antis P. Pathological markers associated with normal aging and dementia in the elderly. Ann. Neurol. 1993;34:566–573. doi: 10.1002/ana.410340410. [DOI] [PubMed] [Google Scholar]
- Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch. Neurol. 2000;57:713–719. doi: 10.1001/archneur.57.5.713. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol. Aging. 1992;13:179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or =80 years of age) humans. Acta Neuropathol. (Berl.) 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Crystal HA, Bevona C, Honer W, Vincent I, Davies P. Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol. Aging. 1995;16:285–298. doi: 10.1016/0197-4580(95)00013-5. (discussion 298–304) [DOI] [PubMed] [Google Scholar]
- Dickson DW. Senile cerebral amyloidosis (pathological aging) and cognitive status predictions: a neuropathology perspective. Neurobiol. Aging. 1996;17:936–937. doi: 10.1016/s0197-4580(96)00173-x. (discussion 945–937) [DOI] [PubMed] [Google Scholar]
- Dickson DW. Neuropathological diagnosis of Alzheimer’s disease: a perspective from longitudinal clinicopathological studies. Neurobiol. Aging. 1997;18:S21–S26. doi: 10.1016/s0197-4580(97)00065-1. [DOI] [PubMed] [Google Scholar]
- Englund E, Brun A, Alling C. White matter changes in dementia of Alzheimer’s type. Biochemical and neuropathological correlates. Brain. 1988;111(Pt 6):1425–1439. doi: 10.1093/brain/111.6.1425. [DOI] [PubMed] [Google Scholar]
- Fernando MS, Ince PG. Vascular pathologies and cognition in a population-based cohort of elderly people. J. Neurol. Sci. 2004;226:13–17. doi: 10.1016/j.jns.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Fujishiro H, Ferman TJ, Boeve BF, Smith GE, Graff-Radford NR, Uitti RJ, Wszolek ZK, Knopman DS, Petersen RC, Parisi JE, Dickson DW. Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J. Neuropathol. Exp. Neurol. 2008;67:649–656. doi: 10.1097/NEN.0b013e31817d7a1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Brown GG. Vascular dementia: neuropathologic alterations and metabolic brain changes. J. Neurol. Sci. 1992;109:121–131. doi: 10.1016/0022-510x(92)90158-h. [DOI] [PubMed] [Google Scholar]
- Gray F, Gherardi R, Marshall A, Janota I, Poirier J. Adult polyglucosan body disease (APBD) J. Neuropathol. Exp. Neurol. 1988;47:459–474. doi: 10.1097/00005072-198807000-00007. [DOI] [PubMed] [Google Scholar]
- Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, Lipton RB. Memory and mental status correlates of modified Braak staging. Neurobiol. Aging. 1999;20:573–579. doi: 10.1016/s0197-4580(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- Heyman A, Fillenbaum GG, Welsh-Bohmer KA, Gearing M, Mirra SS, Mohs RC, Peterson BL, Pieper CF. Cerebral infarcts in patients with autopsy-proven Alzheimer’s disease: CERAD. Part XVIII. Consortium to establish a registry for Alzheimer’s disease. Neurology. 1998;51:159–162. doi: 10.1212/wnl.51.1.159. [DOI] [PubMed] [Google Scholar]
- Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer’s coordinating center. Neurology. 2005;64:494–500. doi: 10.1212/01.WNL.0000150886.50187.30. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Petersen RC, Knopman DS, Boeve BF, Smith GE, Geda YE, Johnson KA, Cha R, Delucia MW, Braak H, Dickson DW, Parisi JE. Argyrophilic grain disease in demented subjects presenting initially with amnestic mild cognitive impairment. J. Neuropathol. Exp. Neurol. 2006;65:602–609. doi: 10.1097/01.jnen.0000225312.11858.57. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T. Towards defining the neuropathological substrates of vascular dementia. J. Neurol. Sci. 2004;226:75–80. doi: 10.1016/j.jns.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Katzman R, Aronson M, Fuld P, Kawas C, Brown T, Morgenstern H, Frishman W, Gidez L, Eder H, Ooi WL. Development of dementing illnesses in an 80-year-old volunteer cohort. Ann. Neurol. 1989;25:317–324. doi: 10.1002/ana.410250402. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Parisi JE, Boeve BF, Cha RH, Apaydin H, Salviati A, Edland SD, Rocca WA. Vascular dementia in a population-based autopsy study. Arch. Neurol. 2003;60:569–575. doi: 10.1001/archneur.60.4.569. [DOI] [PubMed] [Google Scholar]
- Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Bouras C, Giannakopoulos P. Cortical microinfarcts and demyelination affect cognition in cases at high risk for dementia. Neurology. 2007;68:927–931. doi: 10.1212/01.wnl.0000257094.10655.9a. [DOI] [PubMed] [Google Scholar]
- Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res. Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- Liberski PP, Ironside JW. An outline of the neuropathology of transmissible spongiform encephalopathies (prion diseases) Folia Neuropathol. 2004;42 Suppl. B:39–58. [PubMed] [Google Scholar]
- Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, Brooks DJ, Dickson DW, Dubois B, Emre M, Fahn S, Farmer JM, Galasko D, Galvin JE, Goetz CG, Growdon JH, Gwinn-Hardy KA, Hardy J, Heutink P, Iwatsubo T, Kosaka K, Lee VM, Leverenz JB, Masliah E, McKeith IG, Nussbaum RL, Olanow CW, Ravina BM, Singleton AB, Tanner CM, Trojanowski JQ, Wszolek ZK. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- McCullagh PaN JA. Generalized linear models. Chapman and Hall. 1989 [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Merino JG, Hachinski V. Leukoaraiosis: reifying rarefaction. Arch. Neurol. 2000;57:925–926. doi: 10.1001/archneur.57.7.925. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Joachim C, Litchfield S, Barnetson L, Smith AD. The effects of additional pathology on the cognitive deficit in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1997;56:165–170. doi: 10.1097/00005072-199702000-00007. [DOI] [PubMed] [Google Scholar]
- Neuropathology group. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, Ross GW, Steinhorn SC, Abbott RD, Markesbery W, Davis D, Nelson J, Hardman J, Masaki K, Vogt MR, Launer L, White LR. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann. Neurol. 2005;57:98–103. doi: 10.1002/ana.20318. [DOI] [PubMed] [Google Scholar]
- Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, Hofman A, Breteler MM. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- Riekse RG, Leverenz JB, McCormick W, Bowen JD, Teri L, Nochlin D, Simpson K, Eugenio C, Larson EB, Tsuang D. Effect of vascular lesions on cognition in Alzheimer’s disease: a community-based study. J. Am. Geriatr. Soc. 2004;52:1442–1448. doi: 10.1111/j.1532-5415.2004.52405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Esh C, Rahman A, Kokjohn TA, Beach TG. Atherosclerosis of cerebral arteries in Alzheimer disease. Stroke. 2004;35:2623–2627. doi: 10.1161/01.STR.0000143317.70478.b3. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennett DA. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- Tatemichi TK. How acute brain failure becomes chronic: a view of the mechanisms of dementia related to stroke. Neurology. 1990;40:1652–1659. doi: 10.1212/wnl.40.11.1652. [DOI] [PubMed] [Google Scholar]
- Thal LJ, Grundman M, Golden R. Alzheimer’s disease: a correlational analysis of the Blessed Information-Memory-Concentration Test and the Mini-Mental State Exam. Neurology. 1986;36:262–264. doi: 10.1212/wnl.36.2.262. [DOI] [PubMed] [Google Scholar]
- Verghese J, Crystal HA, Dickson DW, Lipton RB. Validity of clinical criteria for the diagnosis of dementia with Lewy bodies. Neurology. 1999;53:1974–1982. doi: 10.1212/wnl.53.9.1974. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N. Engl. J. Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- Vinters HV, Ellis WG, Zarow C, Zaias BW, Jagust WJ, Mack WJ, Chui HC. Neuropathologic substrates of ischemic vascular dementia. J. Neuropathol. Exp. Neurol. 2000;59:931–945. doi: 10.1093/jnen/59.11.931. [DOI] [PubMed] [Google Scholar]
- White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann. NY Acad. Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, Ohmori S, Nomiyama K, Kawano H, Ueda K. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]