Abstract
Estrogen administration can alter experimental stroke outcomes. Soy as a source of phytoestrogens may therefore modulate responses in “estrogen-sensitive” stroke models, thus potentially confounding results. We evaluated the effects of dietary soy on acute infarct volumes in a pilot study using a rat focal stroke model. We hypothesized that ovariectomized (OVX) rats fed a soy-rich diet would have smaller acute infarct volumes than rats fed a soy-free diet. OVX rats were randomly assigned to a soy-free (n=6) or a soy-rich (n=6) diet for 4 weeks and weighed weekly. Following the dietary trial, rats underwent 2 hours of middle cerebral artery occlusion (MCAO). Mean arterial blood pressure, rectal and temporalis muscle temperatures, arterial blood gases, and blood glucose were recorded peri-ischemia. Rats were euthanized 22 hours following 2 hours of MCAO. Brains were stained with 2,3,5-triphenyl tetrazolium chloride for acute infarct volume analysis. Uterine weight and histology were also evaluated as additional internal estrogen-sensitive controls. Rats on the soy-free diet had greater gains in body weight (259±6% baseline body weight) than rats on the soy-rich diet (238±4% baseline body weight). No differences were seen in uterine weight and histology, peri-ischemic physiological parameters, and infarct volumes between the treatment groups. Results of this pilot study suggest that the dietary soy level tested may not alter acute infarct volumes in ischemic female rat brain. More studies addressing the potential confounding effects of dietary soy in “estrogen-sensitive” stroke models are needed if investigators are to make informed choices regarding diets used in experimental stroke research.
Keywords: Cerebral ischemia, phytoestrogen, soy, stroke, Husbandry, Nutrition, Animal model, Comparative medicine, Rodents
Exogenous estrogen administration has been s hown to alter experimental outcomes in rodent stroke models.1 Structurally and functionally similar to estrogen, phytoestrogens exhibit estrogenic and antiestrogenic effects and are found in high abundance in most soy containing foods.2–3 The main classes of phytoestrogens are isoflavonoids, which includes isoflavones and coumestans, and lignans. Commercial laboratory rodent diets commonly use soymeal as their protein source which contains predominantly the isoflavones genistein and daidzein in levels ranging from 100 to 600 µg/g.4–8 Because several widely used rodent stroke models are estrogen-sensitive,1 an important laboratory animal husbandry factor like dietary soy as a major phytoestrogen source could modulate physiological and behavioral responses similar to estrogen in these models, thus potentially confounding experimental outcomes.
Several studies have demonstrated effects of phytoestrogens on uninjured rodent brain. For example, neurobehavioral studies have shown a direct link between dietary phytoestrogen consumption, high plasma isoflavone levels, and alterations in learning and memory.9 Morphological, biochemical and physiological brain changes have been demonstrated as well. Dietary phytoestrogen manipulations can alter the weight of the sexually dimorphic nucleus of the preoptic area of the brain,10 cause a decrease in brain calcium-binding proteins,11 and produce anxiolytic effects.12 These diet-induced changes in brain could therefore have profound implications in rodent stroke models.
Very few studies have addressed the potential confounding effects of soy-derived dietary phytoestrogens in a rodent stroke model whose outcomes are influenced by estrogen. Based on the paucity of information about the effects of dietary phytoestrogens in normal and injured rodent brain, researchers can only speculate about the possible consequences of dietary soy from laboratory rodent diets in estrogen-sensitive rodent stroke models. Extrapolation from existing rodent studies outside of neuroscience is difficult due to a number of complexities, including differences concerning in vitro and in vivo estrogenic potency, interactions with other binding proteins, dose-response variability, processing techniques, and environmental influences.8, 13–14 Studies evaluating the effects of dietary soy as a source of phytoestrogens in rodent stroke models are clearly needed if primary investigators are to make informed choices regarding rodent diets used in ischemic brain research.
In this pilot study, we have chosen to evaluate the potential confounding effects of dietary soy in an established model of acute transient focal cerebral ischemia in the ovariectomized (OVX) female rat. Infarct volume, a commonly used outcome in this acute model, has previously been shown to be reduced by exogenous estrogen administration in male and OVX female rodents.1, 15–16 We hypothesized that dietary soy will alter infarct volume acutely in OVX rats and thus could be a potential confounder when evaluating acute infarct volumes in our experimental stroke model. We therefore determined if dietary soy reduces acute infarct volumes by evaluating the effects of soy-free versus soy-rich rodent diets in OVX rats. Body weight, uterine weight and histology are also influenced by estrogen in female rodents and served as additional estrogen-sensitive controls independent of acute infarct volume outcomes in brain.
MATERIALS AND METHODS
Animals And Husbandry
Twenty-three age matched (4 to 7 weeks old) female Wistar rats (Hsd:WI, Harlan Sprague Dawley, Madison, WI, USA) were used. According to vendor health monitoring reports, rats were negative for the following viral pathogens: Hantaan virus, Kilham’s rat virus, lymphocytic choriomeningitis virus, mouse adenovirus types 1 and 2, pneumonia virus of mice, rat minute virus, rat parvovirus, rat Theiler virus, respiratory enteric virus III, Sendai virus, sialodacryoadenitis virus, and Toolan’s H-1 parvovirus. Animals were also negative for the following bacterial, mycoplasmal, and fungal pathogens: CAR bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutscheri, dermatophytes, Helicobacter bilis, Helicobacter hepaticus, Helicobacter spp, Mycoplasma pulmonis, Pasteurella pneumotropica, Pneumocystis carinii, Pseudomonoas aeruginosa, Salmonella spp, Staphylococcus aureus, Streptobacillus moniliformis, Streptococcus pneumonia, and Streptococcus zooepidemicu Additionally, animals were free of ectoparasites, endoparasites, enteric protozoan, and Encephalitozoon cuniculi.
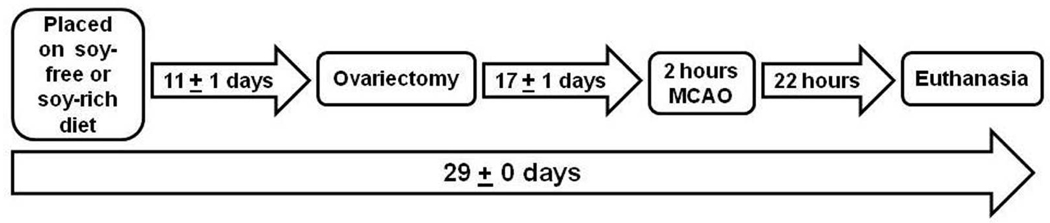
All aspects of the study were approved by the Oregon Health and Science University Institutional Animal Care and Use Committee and were performed in accordance with institutional policy and the National Institutes of Health guidelines governing the humane treatment of vertebrate animals. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International. Rats were pair-housed in static polycarbonate isolator cages (area, 840 cm2; height, 20 cm) on ALPHA-dri™ bedding (Shepherd Specialty Papers, Chicago, IL, USA) in a temperature- (20.0 to 22.2°C) and humidity- (30% to 70%) controlled room on a 12:12-h light:dark cycle. All animals were allowed to acclimatize to the vivarium for 11 ± 1 days before undergoing ovariectomy (Figure 1). Rats had ad libitum access to assigned experimental diets and ultra-violet filtered water. Animals were weighed upon arrival and weekly thereafter.
Figure 1.
Schematic overview of the experimental time line relative to initiation of experimental diets, ovariectomy, experimental stroke (middle cerebral artery occlusion [MCAO]), and euthanasia.
Experimental Diets
Diets used in this study were TD.05087 and TD.96155 (Harlan TekLad, Madison, WI). The TD.05087 diet includes 25.8% soybean meal, for a final total daidzein and genistein level of 500 ppm or mg/kg. This value, as well as value ranges cited from other references and laboratories in this paper, refers to the sum of genistein and daidzein expressed as aglycone or “free isoflavone.” A review of the literature demonstrates commercial soymeal diets have daidzein and genistein levels ranging from 100 to 600 ppm.4–8 While post-production analysis was not performed, the soybean meal used in this lot was tested at 1940 ppm genistein and daidzein before production. The TD.96155 diet which is a minimal phytoestrogen diet (no soybean meal) was chosen as the soy-free diet. While this diet was not analyzed post-production for daidzein and genistein, the typical background level is less than 20 ppm. The same lot and batch number of each diet was used throughout the study. The formulation and nutrient analysis of the two experimental diets are summarized in Table 1.
Table 1.
Composition of the experimental diets.
| Soy-Free Diet (TD.96155) |
Soy-Rich Diet (TD.05087) |
|
|---|---|---|
| Total (g/kg [kcal/g]) | 1000 [3.3] | 1000 [3.4] |
| Nutrient Composition (% by weight [% kcal]) | ||
| Protein | 19.1 [23.3] | 20.0 [23.2] |
| Carbohydrate | 50.4 [61.4] | 53.5 [62.3] |
| Fat | 5.6 [15.3] | 5.5 [14.4] |
| Formula (g/kg) | ||
| Wheat | 380 | 40 |
| Corn | 354.39 | 269.45 |
| Corn gluten Meal (60%) | 50 | |
| Soybean Meal | 258 | |
| Casein | 106.5 | 57 |
| Maltodextrin | 86.11 | |
| Corn Starch | 86.11 | |
| Sucrose | 86.1 | |
| Dicalcium Phosphate | 12.5 | 14.5 |
| Corn Oil | 35.5 | 42.22 |
| Cellulose | 14 | 16 |
| Calcium Carbonate | 15 | 12.5 |
| Sodium Chloride | 1 | 1 |
| Mineral mix, Ca-P deficient | 13.4 | 13.4 |
| Vitamin Mix AIN-93-VX | 14 | 14 |
| Choline Chloride | 2.0 | 1.5 |
| DL-Methionine | 1.2 | 2.1 |
| Ethoxyquin | 0.01 | 0.01 |
| L-Lysine | 0.5 | |
| Amino Acid Profile (g/kg) | ||
| Lys | 10.3 | 12.9 |
| Met | 5.8 | 5.8 |
| Cys | 2.3 | 2.6 |
| Arg | 8.4 | 12.8 |
| Phe | 10.1 | 10.6 |
| Tyr | 7.7 | 8.7 |
| His | 5.1 | 5.3 |
| Ile | 9.6 | 10.3 |
| Leu | 20.4 | 17.5 |
| Thr | 7.7 | 8.4 |
| Trp | 1.9 | 2.7 |
| Val | 11.4 | 11.4 |
| Mineral Composition (%) | ||
| Ca | 0.86 | 0.86 |
| P | 0.56 | 0.59 |
| Na | 0.16 | 0.17 |
| K | 1.00 | 0.69 |
Rats were randomized to receive either soy-free (TD.96155, n=11) or soy-rich (TD.05087, n=12) rodent diets. Animals were placed on their assigned diet upon arrival at the study facility and remained on these diets throughout the study. Figure 1 shows the experimental time line relative to initiation of experimental diets, ovariectomy, experimental stroke (middle cerebral artery occlusion [MCAO]), and euthanasia.
Ovariectomy
OVX rats were used to ensure relative uniformity of the animals’ endogenous hormonal background and to minimize interference from endogenous estrogen during most of the dietary trial and experimental stroke studies. OVX rats were used instead of males as our laboratories have ongoing studies evaluating the effects of estrogen and hormone replacement therapy in females on stroke outcomes. Ovariectomy was performed on young female rats (5 to 8 weeks) 17 ± 1 days before experimental stroke (Figure 1). Surgery was performed aseptically by a single surgeon blinded to dietary treatment group. Surgical anesthesia was induced via chamber induction with 4–5% halothane and maintained with 1–2% halothane via face-mask in oxygen-enriched air. A 1 cm paracostal incision approximately 0.5 cm caudal to the last rib was made through the skin and then through the lateral body wall on one side of the animal after the intended surgical site was clipped free of hair and the skin prepared with betadine. The ovarian artery and vein were ligated and the ovary removed. The lateral body wall incision was sutured, and the skin closed with skin staples. The same surgical approach was then repeated on the opposite side. Rats were recovered and returned to animal quarters. Skin staples were removed 7 to 10 days following ovariectomy.
Analgesics were not given after ovariectomy as the use of nonsteroidal anti-inflammatory drugs and opioids preceding acute experimental surgical stroke models can potentially confound outcomes.17 However, all postoperative animals were scored based on previously described pain/distress indices18–19 two to three times daily post-operatively until skin staples were removed. Pain scores which fell within established ranges for moderate to severe pain mandated immediate veterinary consultation or euthanasia.18
Reversible Focal Cerebral Ischemia
Surgery was performed aseptically by a single surgeon blinded to dietary treatment group. Rats were anesthetized with halothane (induction 4%–5%, maintenance 1.25% to 1.5%) delivered via mask in oxygen-enriched air. Temporary femoral arterial cannulation was done for continuous monitoring of mean arterial blood pressure (MABP) and measurement of peri-ischemic arterial blood gases and plasma glucose. Rectal and temporalis muscle temperatures were continuously monitored using temperature thermistors (Mon-a-therm, model 6510; Mallinckrodt Medical, Inc., St. Louis, MO, USA) and maintained with warming blankets and heat lamps. In each rat, parietal cortical perfusion within the middle cerebral artery (MCA) territory was monitored by laser Doppler flowmetry (LDF) signal (model DRT4, Moor Instruments LTD, Oxford, England) obtained by probe placement 6 mm lateral and 2 mm posterior to bregma.15–16 The effectiveness of vascular occlusion was determined by sustained reduction in LDF signal while restoration of blood flow following release of vascular occlusion was assessed by increases in LDF signal towards baseline levels.
Reversible focal cerebral ischemia was induced by middle cerebral artery occlusion (MCAO) via the intraluminal suture technique.20 The right common and external carotid arteries were exposed and ligated. The right occipital artery was cauterized, and then the pterygopalatine artery was ligated. An incision was made in the common carotid artery distal to ligation and an occluding filament (4-0 monofilament nylon surgical suture with heat-rounded tip) advanced into the internal carotid artery to the MCA origin, a point at which LDF signal abruptly decreases, indicating low cortical flow. 15–16 LDF signal was measured during ischemia over 15 to 30 minute intervals and for the first 15 minutes following termination of MCAO to confirm cortical reperfusion when the occluding filament was removed. To ensure relative uniformity of the ischemic insult, animals were excluded if mean ischemic LDF signal was greater than 50% of baseline LDF signal. Catheters and probes were removed after the final LDF measurement was made 15 minutes after termination of MCAO. Incisions were then closed, and the animals recovered from anesthesia.
Pain-relieving drugs were not given after MCAO surgery as the use of nonsteroidal anti-inflammatory drugs and opioids in acute experimental surgical stroke models can potentially confound outcomes.17 However, all postoperative animals were evaluated for pain and distress according to a previously published behavioral scoring system based on subjective and objective measures for pain evaluation in the rat MCAO surgical model.18 Pain scores which fell within established ranges for moderate to severe pain mandated immediate veterinary consultation or euthanasia.18
After 22 hours of recovery from MCAO, animals were deeply anesthetized with halothane (4%–5%), and blood samples for serum hormone (17β-estradiol, progesterone) measurements were obtained by cardiac puncture before euthanasia via decapitation. Following euthanasia, brains and uteri were harvested for acute infarct volume and weight determinations subsequent to fixation, respectively.
Infarct Volume Determinations
Brains were sliced into 2 mm thick coronal sections (seven slices total) using a brain matrix (Kent Scientific Corporation, Torrington, CA, USA). Coronal sections were placed in a 1% 2,3,5-triphenyl tetrazolium chloride (TTC; Sigma, St. Louis, MO, USA) and incubated at 37°C for 30 minutes.21 Stained sections were then fixed in 10% neutral buffered formalin. Viable brain areas were stained dark red due to reduction of TTC by mitochondrial enzymes, whereas infarcted areas were unstained and appeared white. The anterior and posterior sides of each stained coronal section were digitally photographed. Regional areas (cortex, striatum, hemisphere) within each cerebral hemisphere and infarct areas within each region were measured on the anterior and posterior sides of each coronal section using digital image analysis (SigmaScan Pro; SPSS Science, Chicago, IL, USA). Regional and infarct volumes for each individual coronal slice were then determined by averaging the anterior and posterior areas and then multiplying the average area by the thickness of the coronal section. Regional and infarct volumes were then integrated across all coronal sections and expressed as mm3, or indirectly as a percent of the contralateral structure (cortex, striatum, and hemisphere) as a partial correction for edema.22
Uterine Weight and Histology
In each rat, the uterus was cut just above the junction of the uterine body with the cervix and at the junction of the uterine horns with the ovaries. Each uterus was then removed, trimmed free of fat and mesentery, blotted to remove excess fluid, and weighed. Uterine to body weight ratios were calculated and presented as % terminal body weight (uterine weight/body weight × 100). Afterwards, 10% neutral-buffered formalin was injected into the uterine lumen until the uterus was visibly extended. Uteri were then placed in 10% neutral-buffered formalin for further fixation and eventual histological examination. Tissue was dehydrated, embedded in paraffin, sectioned at 4 to 5 microns, and stained with hematoxylin and eosin. Uterine histopathological analyses were performed by a single individual blinded to dietary treatment group.
Serum Hormone Measurements
Serum 17β-estradiol and progesterone were measured in duplicate with commercial radioimmunoassay kits (Diagnostic Products Corp, Los Angeles, CA, USA). 17β-estradiol was measured as it is the primary estrogen observed in rodents and in women. Lower limit of detection for the 17β-estradiol radioimmunoassay is 10 pg/mL. Samples with 17β-estradiol levels <10 pg/ml were not used in calculating mean estradiol levels per treatment group.
Statistical Analysis
Values expressed as mean ± SEM. Using data from previous stroke studies with estrogen, we performed a pre-test power analysis (desired power = 0.8, number of groups = 2, α = 0.05, minimum detectable difference in means = 15, expected standard deviation of residuals = 8) to determine the minimum number of OVX rats needed to observe preliminary trends and differences in infarct volume. Based on these analyses, six OVX rats per experimental group was recommended for the proposed pilot study to examine the potential confounding effects of dietary soy on acute infarct volume outcomes. Baseline body weight was subjected to one-way analysis of variance (ANOVA) with post-hoc Newman-Keuls test. Weekly body weight (g, % baseline body weight) was subjected to two-way ANOVA with post-hoc Newman-Keuls test. Differences in mean ischemic LDF (% baseline), infarction volume (mm3, % contralateral structure), serum hormone levels (17β-estradiol, progesterone), and uterine weight (mg, % terminal body weight) between dietary groups were determined by one-way analysis ANOVA with post-hoc Newman-Keuls. Physiological variables (blood gases, blood glucose, MABP, rectal and temporalis muscle temperatures) and LDF signal were subjected to two-way ANOVA with post-hoc Newman-Keuls test. Statistical significance was p<0.05. Statistical analyses were done using SigmaStat Statistical Software, Version 3.1 (SPSS, Inc.; Chicago, IL, USA).
RESULTS
Exclusions Relative To Experimental Focal Cerebral Ischemia
In the soy-free diet group (n=11), 4 animals were excluded due to intra-operative death (n=2, 18%), death during the post-MCAO recovery period (n=1, 9%), or tearing of the right common carotid artery during surgical manipulations (n=1, 9%). In the soy-rich diet group (n=12), 2 animals were excluded due to death during the post-MCAO recovery period. Therefore, surgical mortality rates were 36% (n=4) in the soy-free diet group, 17% (n=2) in the soy-rich diet group, and 26% (n=6) overall. Our mortality rates fall within the range of values reported by our laboratories and collaborators using a comparable 2 hour rat model of transient MCAO with 22 hours of recovery from MCAO.23–27
One animal (9%) met the mean ischemic LDF exclusion criteria of greater than 50% of baseline LDF in the soy-free diet group. In the soy-rich diet group, 4 animals (33%) were euthanized due to failure to achieve MCAO based on LDF monitoring (n=2, 17%), maintain MCAO based on LDF monitoring (n=1, 8%), or met the mean ischemic LDF exclusion criteria of greater than 50% of baseline LDF (n=1, 8%). Soy-free (n=5, 45%) and soy-rich (n=6, 50%) diet groups therefore had similar total numbers and percentages of rats that died or were excluded based on LDF monitoring and exclusion criteria. Consequently, only 6 rats per dietary group were included in the final data analysis.
Dietary Effects on Serum Hormone Levels
There were 3 samples from the soy-free diet group versus no samples from the soy-rich diet group that were below <10 pg/ml for serum 17β-estradiol levels. Although not statistically significant (p=0.093), serum 17β-estradiol levels were generally lower in the soy-free diet group (17±2 pg/ml, n=3) compared with the soy-rich diet group (23±2 pg/ml, n=4) (Table 2). There were no significant differences (p=0.719) in serum progesterone levels between the soy-free (12±1 ng/mL, n=6) and soy-rich (11±3 ng/mL, n=4) diet groups (Table 2).
Table 2.
Plasma 17β-estradiol and progesterone levels at 22 hours following 2 hours of middle cerebral artery occlusion in ovariectomized Wistar rats fed soy-free (n=6) or soy-rich (n=6) diets for 4 weeks before experimental transient focal cerebral ischemia. Individual animal and experimental group values are given. Experimental group data shown as mean ± SEM.
| Diet Group | 17β-Estradiol (pg/mL) |
Progesterone (ng/mL) |
|---|---|---|
| Soy-Free Diet | ||
| Rat #1 | 17 | 10 |
| Rat #2 | 19 | 12 |
| Rat #3 | <10 | 13 |
| Rat #4 | <10 | 10 |
| Rat #5 | <10 | 14 |
| Rat #6 | 14 | 15 |
| Soy-Free Diet | 17 ± 2 (n=3) | 12 ± 1 (n=6) |
| Soy-Rich Diet | ||
| Rat #1 | Not determined | Not determined |
| Rat #2 | 20 | 12 |
| Rat #3 | 23 | 5 |
| Rat #4 | 21 | 17 |
| Rat #5 | 28 | 12 |
| Rat #6 | Not determined | Not determined |
| Soy-Rich Diet | 23 ± 2 (n=4) | 11 ± 3 (n=4) |
Dietary Effects On Body Weight And Uterus
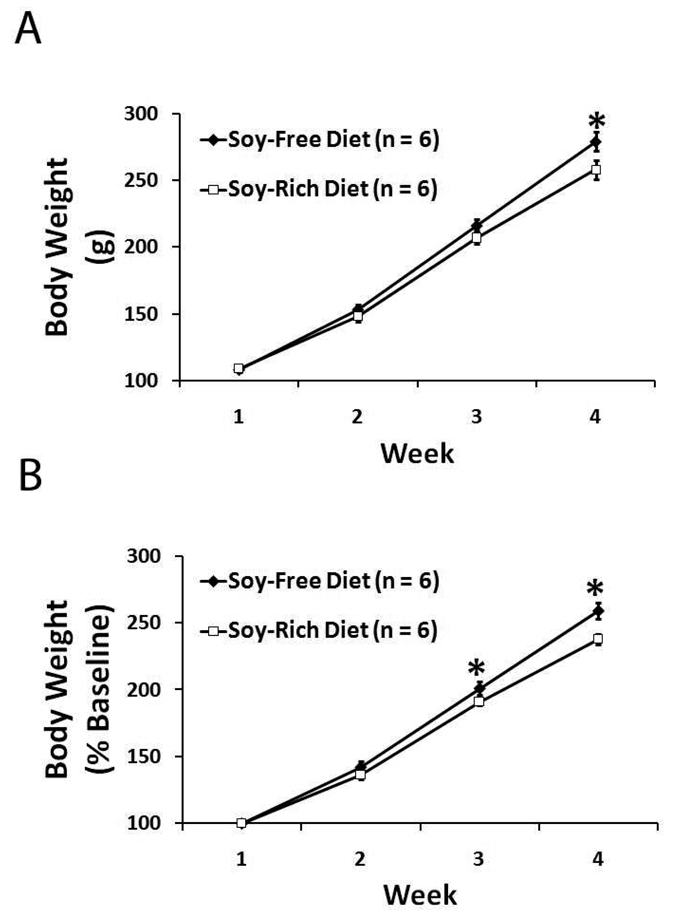
There were no significant differences (p=0.731) in baseline body weights for soy-free (108±2 g, n=6) and soy-rich (109±2 g, n=6) diet groups (Figure 2A). All animals had significant gains in weekly body weight throughout the course of the study regardless of assigned dietary group (Figure 2). The soy-free diet group (279 ± 7 g) was only observed to be significantly heavier than the soy-rich diet group (258 ± 7 g) during the fourth and final week of the study (Figure 2A). However, significant differences in weekly body weight as a percentage of baseline values were observed during the third and fourth weeks of the study (Figure 2B), with the soy-free diet group (week 3, 201 ± 5%; week 4, 259 ± 6%) having greater gains in body weight than the soy-rich diet group (week 3, 191 ± 3%; week 4, 238 ± 4%).
Figure 2.
Weekly body weights expressed as A) absolute weight in g and as B) % baseline body weight in ovariectomized female Wistar rats fed either a soy-free or a soy-rich diet before experimental transient focal cerebral ischemia. Values are mean ± SEM. *P<0.05.
There were no significant differences (p=0.440, p=0.867) in uterine weight (mg, percentage of terminal body weight) for soy-free (168±5 mg, 0.061±0.003%; n=6) and soy-rich (159±10 mg, 0.062±0.003%, n=6) diet groups. Regardless of dietary treatment group, histological evaluation of multiple sections from each animal demonstrated similar findings of a narrowed uterine lumen and limited endometrial gland development (data not shown).
Dietary Effects In Ischemic Brain
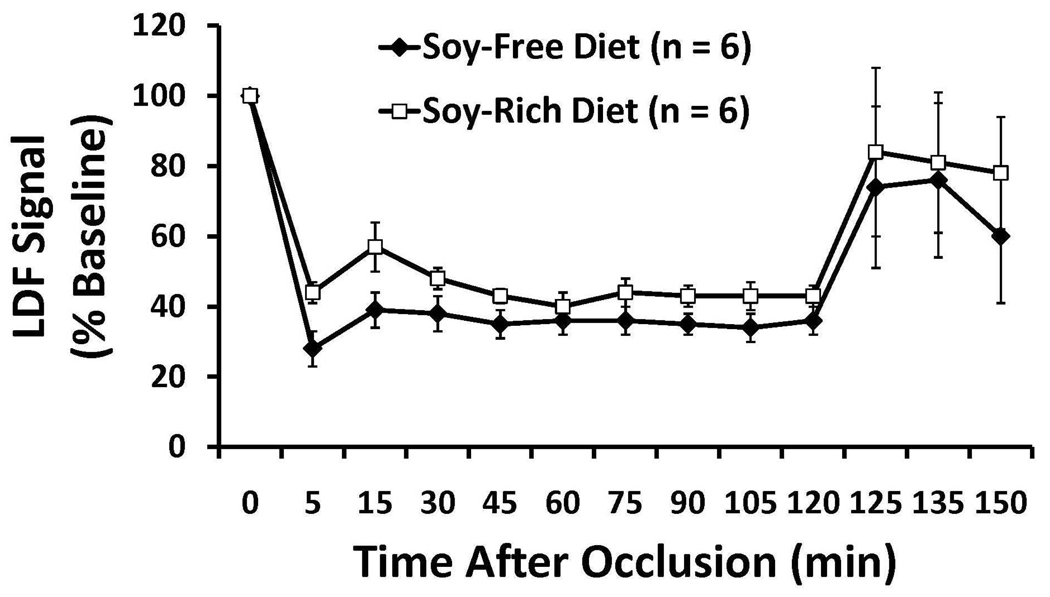
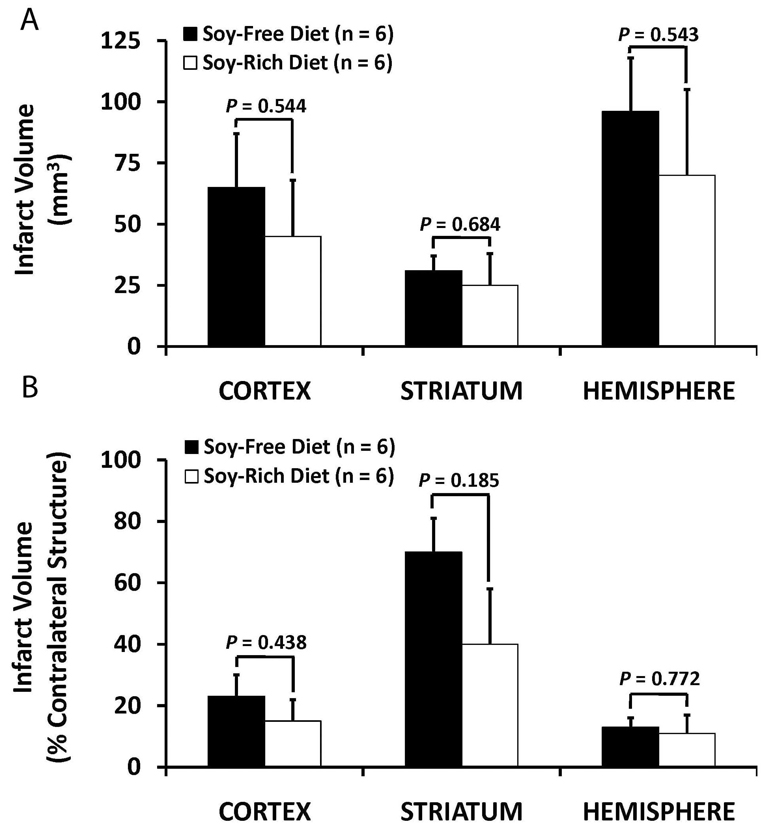
Baseline, intra-ischemic, and early post-MCAO MABP, blood gases, and glucose were comparable between diet treatment groups (Table 3). Peri-ischemic rectal and temporalis muscle temperatures were not significantly different between diet groups (Table 3). Mean ischemic LDF (% baseline signal) in OVX rats fed the soy-free (36 ± 3%) or soy-rich (45 ± 2%) diets was significantly different (p=0.032). However, there were no differences in reduction and maintenance of residual intra-ischemic LDF signal as well as in increase of residual post-MCAO LDF signal (expressed as a percent of baseline signal) between diet groups (Figure 3). No significant differences in regional and total infarct volumes (lesion size - mm3, % contralateral structure) were observed between the soy-free (Cortex, 65± 22 mm3, 23±7%; Striatum, 31±6 mm3, 70±11%; Hemisphere, 96±22 mm3, 13±3%) and soy-rich diet groups (Cortex, 45±23 mm3, 15±7%; Striatum, 25±13 mm3, 40±18%; Hemisphere, 70±35 mm3, 11±6%,) (Figure 4).
Table 3.
Physiological parameters before, during, and after 2 hours of middle cerebral artery occlusion (MCAO) in ovariectomized Wistar rats fed soy-free (n=6) or soy-rich (n=6) diets for 4 weeks before experimental transient focal cerebral ischemia. Measurements were made at 5 min before MCAO (baseline), 1 hour of MCAO, and 15 minutes after termination of MCAO. Data are mean ± SEM.
| Diet Group | pH | PaCO2 (mm Hg) |
PaO2 (mm Hg) |
Glucose (mg/dL) |
MABP (mm Hg) |
Rectal Temperature (°C) |
Temporalis Muscle Temperature (°C) |
|---|---|---|---|---|---|---|---|
| Soy-Free Diet | |||||||
| 5 min before MCAO (Baseline) | 7.44±0.01 | 41±2 | 150±20 | 130±9 | 111±7 | 37.7±0.2 | 37.2±0.2 |
| (n=5) | (n=5) | (n=5) | (n=4) | (n=6) | (n=6) | (n=6) | |
| 1 hour of MCAO | 7.45±0.01 | 36±3 | 141±20 | 111±9 | 115±7 | 37.2±0.3 | 37.1±0.2 |
| (n=5) | (n=5) | (n=5) | (n=5) | (n=6) | (n=5) | (n=6) | |
| 15 min after termination of MCAO | 7.44±0.01 | 35±3 | 152±15 | 106±9 | 113±5 | 37.1±0.2 | 36.8±0.2 |
| (n=6) | (n=6) | (n=6) | (n=6) | (n=5) | (n=5) | (n=5) | |
| Soy-Rich Diet | |||||||
| 5 min before MCAO (Baseline) | 7.43±0.01 | 41±3 | 144±21 | 110±10 | 108±4 | 37.7±0.1 | 36.6±0.0 |
| (n=5) | (n=5) | (n=5) | (n=6) | (n=6) | (n=6) | (n=6) | |
| 1 h MCAO | 7.45±0.03 | 37±2 | 152±20 | 110±8 | 115±4 | 37.7±0.1 | 37.2±0.2 |
| (n=4) | (n=4) | (n=4) | (n=5) | (n=6) | (n=6) | (n=6) | |
| 15 min after termination of MCAO | 7.43±0.02 | 34±1 | 160±18 | 98±9 | 111±3 | 37.5±0.2 | 36.9±0.1 |
| (n=5) | (n=5) | (n=5) | (n=5) | (n=6) | (n=6) | (n=6) | |
Figure 3.
Cerebral cortical microvascular perfusion as determined by laser Doppler flow (LDF) signal during 2 hours of middle cerebral artery occlusion (MCAO) and the first 15 minutes following recovery from MCAO. LDF was measured over the ipsilateral parietal cortex and expressed as a percentage of baseline signal in ovariectomize female Wistar rats fed either a soy-free or a soy-rich diet before MCAO. There were no differences in reduction and maintenance of residual intra-ischemic LDF signal as well as in increase of residual post-ischemic LDF signal between diet groups. Values are mean ± SEM.
Figure 4.
Cortical, striatal, and total hemispheric infarction volumes as determined by 2,3,5-triphenyl tetrazolium chloride staining (expressed as A) mm3 and indirectly as B) % contralateral structure as a correction for edema) in ovariectomized female Wistar rats fed either a soy-free or a soy-rich diet before 2 hours of middle cerebral artery occlusion followed by 22 hours of recovery from MCAO. There were no differences in regional and total infarction volumes between treatment groups. Values are mean ± SEM.
DISCUSSION
This pilot study demonstrates 3 findings. First, the presence of dietary soy at the level tested may have contributed to the attenuated weight gain in OVX rats. Second, there were no differences in uterine weights and histology in OVX rats between the soy-free and soy-rich diet groups. Last, acute infarct volumes 22 hours following MCAO were similar regardless of the presence or absence of dietary soy. Our preliminary results suggest that dietary soy at the level tested may not alter acute infarct volumes in ischemic female rat brain in our experimental rat focal stroke model.
Previous rodent stroke studies have focused on individual phytoestrogens28,33 rather than on dietary sources of phytoestrogens present in laboratory rodent chows. To our knowledge, this is the first study to evaluate the effects of dietary soy on acute outcomes in OVX rats following 2 h of transient focal cerebral ischemia. We found that dietary soy at the levels tested did not appear to alter acute infarct volumes. Other rat studies with dietary phytoestrogens have evaluated acute outcomes in males and OVX females after 90 minutes of transient focal cerebral ischemia34–35 or in OVX females following 24 hours of permanent focal cerebral ischemia.36 In contrast to our findings, these studies using diets high in isoflavones demonstrated varying degrees of reduced infarct volumes in ischemic brain.34–36 Age, gender, dietary trial length, total isoflavone content of the diets, other dietary components and duration of ischemia could explain some of the differences in our findings relative to the effects of individual dietary phytoestrogens.
In this pilot study, serum 17β-estradiol levels were generally lower in the soy-free diet group than in the soy-rich diet group while serum progesterone levels were similar. These results would suggest that ovariectomy reduced progesterone levels comparably but that the presence of dietary soy at the level tested may have increased estradiol levels. In humans, dietary phytoestrogens have variable effects on estradiol levels, with lower doses increasing and higher doses reducing estradiol levels.37 Based primarily on in vitro assays, phytoestrogens can potentially alter estradiol biosynthesis and metabolism through modulation of steroidogenic enzyme activity and expression, thereby altering serum estradiol levels.38 However, phytoestrogen effects on estrogen steroidogenesis are not as well characterized in vivo.39 Another mechanism by which soy-based dietary phytoestrogens could promote increased estradiol levels is through stimulation of gastrointestinal deconjugation of estrone, thus leading to its peripheral reabsorption and conversion to estradiol.40 However, the small changes in estradiol levels promoted by the soy-rich diet in our study were not sufficient to alter acute infarct volume outcomes.
Estrogen is known to attenuate body weight gains in rats.36, 41 In our study, OVX rats on the soy-rich diet had lower gains in body weight compared to animals on the soy-free diet. This is in agreement with other studies demonstrating that modest to high levels of dietary phytoestrogens can decrease body weight gains in rats.10, 12, 42–43 However, these studies did not assess circulating estrogen levels as was done in this study. Therefore, the small differences in circulating estrogen levels observed between dietary groups in this study may have been a contributing factor to the differences in body weight gain seen between the two dietary groups.
Differences in body weight gain could also be due to differences between the two diets in other nutritional factors levels of protein, carbohydrates, fat, and metabolic energy. Although the protein, carbohydrate, and fat content of both diets and the total kcal/g and g/kg were similar (Table 1), the formulation of the soy-free diet did require use of larger amounts of wheat, corn, corn gluten meal, and casein to attain adequate protein levels which could have affected diet palatability and protein digestibility. Variable levels of trypsin inhibitor activity and biologically active dietary factors such as digestible amino acid levels, carbohydrate profile, and nonstarch polysaccharides between the diets might have contributed as well to diverging body weight gains between soy-free and soy-rich dietary groups.
Finally, while we did not measure food intake in our study, differences in food intake could potentially account for the differences in body weight gains observed between the two dietary groups. However, other studies have reported either no difference34 or higher12, 43 food intake in animals fed diets containing dietary soy or phytoestrogens compared to animals fed a soy- or phytoestrogen-free diet. Regardless of the possible reasons discussed above for the differences in body weight gains between the dietary groups, these differences in body weight changes between the two dietary groups did not appear to influence acute infarct volume outcomes.
Although estrogen can increase uterine size and activity in the classical rat uterotrophic assay,44 uterine responses to dietary phytoestrogens have been more variable depending on type, source, amount and food consumption rates.7, 39, 45 Previous studies suggest that modest to low levels of dietary phytoestrogens (350 µg total genistein equivalents/g diet or less) do not significantly increase baseline uterine weights or reduce uterine responsiveness.7 Therefore, the current recommendation for conducting the rat uterotrophic bioassay is to use diets containing phytoestrogen levels less than 325 to 350 µg total genistein equivalents/g diet, as uterine responsiveness may be compromised at higher levels.7 While the soy-rich diet tested in our study had higher phytoestrogen levels than the recommended limit for the rat uterotrophic bioassay, we did not observe any differences in uterine weight and activity as compared to the soy-free diet group. However, this recommendation is limited to immature females and does not include adult OVX females.7 For control adult OVX animals, rat uterotrophic assay guidelines suggest that mean blotted uterine weights greater than 115 mg should be questioned.7 In this study, mean blotted uterine weights in the soy-free diet group were greater than 115 mg. This suggests that control values may be anomalous for reasons unrelated to dietary phytoestrogens. Dietary factors other than phytoestrogen levels have been shown to affect uterine weights7, 46–48 and may have been a contributing factor in the soy-free diet group.
Studies on cerebral vessels and cerebral blood flow (CBF) would suggest that phytoestrogens may act as relaxants and vasodilators.49–51 Although systemic hemodynamic parameters did not vary between the dietary groups in our study, we cannot rule out the possibility that there may have been some differences in cerebral hemodynamic status between the dietary groups. Although we did not observe any differences in the pattern of the peri-ischemic LDF response in parietal cortex or at the individual time points evaluated during and after ischemia between the dietary groups, we did see a significantly higher mean ischemic parietal cortical LDF signal for the soy-rich diet group compared with the soy-free diet group. This is in contract to other studies which have reported no differences in cortical LDF between rats fed soy-based and isoflavone-free diets in transient (90 minutes) and permanent (24 hours) focal stroke models.34–36 However, LDF signal measures only relative changes in cortical perfusion rather than absolute CBF in cortex and other brain regions. Therefore, LDF may not be sensitive enough to determine if soy-based dietary phytoestrogens have an effect on peri-ischemic preservation of regional CBF in ischemic brain and may partly explain the differences between our study and others regarding cortical LDF. Future studies using more quantitative measures of regional CBF will need to be done to determine if soy-based dietary phytoestrogens have sufficient cerebrovascular effects in ischemic brain to potentially confound acute experimental outcomes relative to infarct volumes.
Although our group sizes are small, we felt that the pattern of our initial findings on body weight gain, serum 17β-estradiol levels, uterine response as well as acute infarct volume outcomes in OVX rats raised important issues relative to dietary choices in laboratory animals that would be of immediate interest to the laboratory animal and scientific communities. Using the data from this pilot study, we have performed a post-test power analysis. Based on these analyses, 17 to 21 rats and 7 to 36 rats would be needed in this model to detect significant differences in mm3 and % of volume of contralateral structure, respectively, in cortex (n=21 for mm3, n=13 for %), striatum (n=17 for mm3, n=7 for %), and hemisphere (n=20 for mm3, n=36 for %). Future studies from our laboratory will expand upon this initial report by assessing the impact of the presence or absence of soy-based dietary phytoestrogens in OVX rats treated with either vehicle or a known protective estrogen dose on acute infarct volume outcomes.
In summary, dietary soy at the level tested did not appear to alter acute infarct volumes in OVX rat brain 22 hours following transient MCAO in this initial pilot study but preliminary results suggest that more studies with larger groups are needed as well as using estrogen administration as a positive control. In addition, the impact of soy-derived dietary phytoestrogens still remains to be examined on functional outcomes and at more chronic end-points as well as in other estrogen-sensitive brain injury models. Lastly, more detailed pathology will need to be done to further characterize damage at the cellular level and to evaluate effects of dietary soy on specific cell types. Future studies evaluating different levels, types, and sources of dietary phytoestrogens in estrogen-sensitive stroke and other brain injury models are needed if laboratory animal clinicians, animal facility managers, and investigators are to make informed choices regarding diets in these models.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Oregon Health and Science University’s Center of Women’s Health, Portland, OR, USA. Rats and experimental diets were provided by Harlan Teklad. The authors would like to thank Barbara Mickelson, Dave Johnson, Terry Burns-Heffner, and Chuck Benton at Harlan Teklad in Madison, WI for assistance with diet selection and analysis.
REFERENCES
- 1.Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. ILAR J. 2004;45:147–159. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- 2.Patisaul HB, Dindo M, Whitten PL, Young LJ. Soy isoflavone supplements antagonize reproductive behavior and estrogen receptor α- and β-dependent gene expression in the brain. Endocrinology. 2001;142:2946–2952. doi: 10.1210/endo.142.7.8241. [DOI] [PubMed] [Google Scholar]
- 3.Thigpen JE, Setchell KD, Saunders HE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disrupter research and testing studies. ILAR J. 2004;45:401–416. doi: 10.1093/ilar.45.4.401. [DOI] [PubMed] [Google Scholar]
- 4.Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- 5.Degen GH, Janning P, Diel P, Bolt HM. Estrogenic isoflavones in rodent diets. Toxicol Lett. 2002;128:145–157. doi: 10.1016/s0378-4274(02)00009-7. [DOI] [PubMed] [Google Scholar]
- 6.Hartley DE, Edwards JE, Spiller CE, et al. The soya isoflavone content of rat diet can increase anxiety and stress hormone release in the male rat. Psychopharmacology (Berl) 2003;167:46–53. doi: 10.1007/s00213-002-1369-7. [DOI] [PubMed] [Google Scholar]
- 7.Owens W, Ashby J, Odum J, Onyon L. The OECD program to validate the rat uterotrophic bioassay. Phase 2: dietary phytoestrogen analyses. Environ Health Perspect. 2003;111:1559–1567. doi: 10.1289/ehp.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thigpen JE, Setchell KD, Ahlmark KB, et al. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab Anim Sci. 1999;49:530–536. 1999. [PubMed] [Google Scholar]
- 9.Lephart ED, West TW, Weber KS, et al. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. 2002. [DOI] [PubMed] [Google Scholar]
- 10.Lund TD, Rhees RW, Setchell KD, Lephart ED. Altered sexually dimorphic nucleus of the preoptic area (SDN-POA) volume in adult Long-Evans rats by dietary soy phytoestrogens. Brain Res. 2001;914:92–99. doi: 10.1016/s0006-8993(01)02779-2. [DOI] [PubMed] [Google Scholar]
- 11.Lephart ED, Thompson JM, Setchell K, Adlercreutz H, Weber KS. Phytoestrogens decrease brain calcium-binding proteins but do not alter hypothalamic androgen metabolizing enzymes in adult male rats. Brain Res. 2000;589:123–131. doi: 10.1016/s0006-8993(00)01968-5. [DOI] [PubMed] [Google Scholar]
- 12.Lund TD, Lephart ED. Dietary soy phytoestrogens produce anxiolytic effects in the elevated plus-maze. Brain Res. 2001;913:180–184. doi: 10.1016/s0006-8993(01)02793-7. [DOI] [PubMed] [Google Scholar]
- 13.Thigpen JE, Setchell KD, Padilla-Banks E, et al. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats. Environ Health Perspect. 2007;115:1717–1726. doi: 10.1289/ehp.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Bittner GD. Effects of some dietary phytoestrogens in animal studies: review of a confusing landscape. Lab Animal. 2002;31:43–48. doi: 10.1038/5000192. [DOI] [PubMed] [Google Scholar]
- 15.Rusa R, Alkayed NJ, Crain BJ, et al. 17β-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- 16.Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in males. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 17.Graham SM, McCullough LD, Murphy SJ. Animal models of ischemic stroke: balancing experimental aims and animal care. Comp Med. 2004;54:486–496. [PubMed] [Google Scholar]
- 18.Kirsch JH, Klaus JA, Blizzard KK, Hurn PD, Murphy SJ. Pain evaluation and response to buprenorphine in rats subjected to sham middle cerebral artery occlusion. Contemp Top Lab Anim Sci. 2002;41:9–14. [PubMed] [Google Scholar]
- 19.Morton DB, Griffiths PHM. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet Rec. 1985;116:431–436. doi: 10.1136/vr.116.16.431. [DOI] [PubMed] [Google Scholar]
- 20.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 21.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyl tetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 22.Swanson RA, Morton MT, Tsai-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 23.Chen TY, Goyagi T, Toung TJK, et al. Prolonged opportunity for ischemic neuroprotection with selective κ-opioid receptor agonist in rats. Stroke. 2004;35:1180–1185. doi: 10.1161/01.STR.0000125011.93188.c6. [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Toung TJK, Hurn PD, Koehler RC, Bhardwaj A. Ischemic neuroprotection with selective κ-opioid receptor agonist is gender specific. Stroke. 2005;36:1557–1561. doi: 10.1161/01.STR.0000169928.76321.3d. [DOI] [PubMed] [Google Scholar]
- 25.Dziennis S, Yang D, Cheng J, Anderson KA, Alkayed NJ, Hurn PD. Developmental exposure to polychlorinated biphenyls influences stroke outcome in adult rats. Environ Health Perspect. 2008;116:474–480. doi: 10.1289/ehp.10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24:1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Chen TY, Kirsch JR, et al. Kappa-opioid receptor selectivity for ischemic neuroprotection with BRL 52537 in rats. Anesth Analg. 2003;97:1776–1783. doi: 10.1213/01.ANE.0000087800.56290.2E. [DOI] [PubMed] [Google Scholar]
- 28.Hong JT, Ryu SR, Kim HJ, et al. Neuroprotective effect of green tea extract in experimental ischemia-reperfusion brain injury. Brain Res Bull. 2000;53:743–749. doi: 10.1016/s0361-9230(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 29.Hong JT, Ryu SR, Kim HJ, et al. Protective effect of green tea extract on ischemia/reperfusion-induced brain injury in Mongolian gerbils. Brain Res. 2001;888:11–18. doi: 10.1016/s0006-8993(00)02935-8. [DOI] [PubMed] [Google Scholar]
- 30.Inanami O, Watanabe Y, Syuto B, Nakano M, Tsuji M, Kuwabara M. Oral administration of (−)catechin protects against ischemia-reperfusion-induced neuronal death in the gerbil. Free Rad Res. 1998;29:359–365. doi: 10.1080/10715769800300401. [DOI] [PubMed] [Google Scholar]
- 31.Kindy MS. Inhibition of tyrosine phosphorylation prevents delayed neuronal death following cerebral ischemia. J Cereb Blood Flow Metab. 1993;13:372–377. doi: 10.1038/jcbfm.1993.50. [DOI] [PubMed] [Google Scholar]
- 32.Shutenko Z, Henry Y, Pinard E, et al. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem Pharmacol. 1999;57:199–208. doi: 10.1016/s0006-2952(98)00296-2. [DOI] [PubMed] [Google Scholar]
- 33.Trieu VN, Uckun FM. Genistein is neuroprotective in murine models of familial amyotrophic lateral sclerosis and stroke. Biochem Biophys Res Commun. 1999;258:685–688. doi: 10.1006/bbrc.1999.0577. [DOI] [PubMed] [Google Scholar]
- 34.Burguete MC, Torregrosa G, Perez-Asensio FJ, et al. Dietary phytoestrogens improve stroke outcome after transient focal cerebral ischemia in rats. Eur J Neurosci. 2006;23:703–710. doi: 10.1111/j.1460-9568.2006.04599.x. [DOI] [PubMed] [Google Scholar]
- 35.Lovekamp-Swan T, Glendenning M, Schreihofer DA. A high soy diet reduces programmed cell death and enhances bcl-xL expression in experimental stroke. Neurosci. 2007;148:644–652. doi: 10.1016/j.neuroscience.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreihofer DA, Do KD, Schreihofer AM. High-soy diet decreases infarct size after permanent middle cerebral artery occlusion in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R103–R108. doi: 10.1152/ajpregu.00642.2004. [DOI] [PubMed] [Google Scholar]
- 37.Patisaul HB, Whitten PL. Dietary phytoestrogens. In: Naz RK, editor. Endocrine Disruptors: Effects on Male and Female Reproductive Systems. Boca Raton: CRC Press; 1999. pp. 89–122. [Google Scholar]
- 38.Whitehead SA, Rice S. Endocrine-disrupting chemicals as modulators of sex steroid synthesis. Best Pract Res Clin Endocrinol Metab. 2006;20:45–61. doi: 10.1016/j.beem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Whitten PL, Patisaul HB. Cross-species and interassay comparisons of phytoestrogen action. Environ Health Perspect. 2001;109 suppl:5–20. doi: 10.1289/ehp.01109s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison RM, Phillippi PP, Swan KF, Henson MC. Effect of genistein on steroid hormone production in the pregnant rhesus monkey. Proc Soc Exp Biol Med. 1999;222:78–84. doi: 10.1111/j.1525-1373.1999.09998.x. [DOI] [PubMed] [Google Scholar]
- 41.Anderson WR, Simpkins JW, Brewster ME, Bodor N. Effects of a brain-enhanced chemical delivery system for estradiol on body weight and serum hormones in middle-aged male rats. Endocr Res. 1998;14:131–148. doi: 10.3109/07435808809032982. [DOI] [PubMed] [Google Scholar]
- 42.Lephart ED, Porter JP, Lund TD, et al. Dietary isoflavones alter regulatory behaviors, metabolic hormones and neuroendocrine function in Long-Evans male rats. Nutr Metab (Lond) 2004;1:16–29. doi: 10.1186/1743-7075-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber KS, Setchell KD, Stocco DM, Lephart ED. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5α-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J Endocrinol. 2001;170:591–599. doi: 10.1677/joe.0.1700591. [DOI] [PubMed] [Google Scholar]
- 44.Sahlin L, Elger W, Akerberg S, et al. Effects of estradiol and estradiol sulfamate on the uterus of ovariectomized or ovariectomize and hypophysectomized rats. J Steroid Biochem Molec Biol. 2000;74:99–107. doi: 10.1016/s0960-0760(00)00091-1. [DOI] [PubMed] [Google Scholar]
- 45.Thigpen JE, Haseman JK, Saunders H, Locklear J, Caviness G, Grant M, Forsythe D. Dietary factors affecting uterine weights of immature CD-1 mice used in uterotrophic bioassays. Cancer Detect Prev. 2002;26:381–393. doi: 10.1016/s0361-090x(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 46.Ashby J, Tinwell H, Odum J. Uterotrophic activity of a “phytoestrogen-free” diet. Environ Health Perspect. 1999;108:A12–A13. doi: 10.1289/ehp.108-a12c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Odum J, Tinwell H, Jones K, et al. Effect of rodent diets on the sexual development of the rat. Toxicol Sci. 2001;61:115–127. doi: 10.1093/toxsci/61.1.115. [DOI] [PubMed] [Google Scholar]
- 48.Thigpen JE, Lebetkin EH, Dawes ML, Richter CB, Crawford D. The mouse bioassay for the detection of estrogenic activity in rodent diets. III. Stimulation of uterine weight by dextrose, sucrose and c orn starch. Lab Anim Sci. 1987;37:606–609. [PubMed] [Google Scholar]
- 49.Jackman KA, Woodman OL, Chrissobolis S, Sobey CG. Vasorelaxant and antioxidant activity of the isoflavone metabolite equol in carotid and cerebral arteries. Brain Res. 2007;1141:99–107. doi: 10.1016/j.brainres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 50.Sobey CG, Weiler JM, Boujaoude M, Woodman OL. Effect of short-term phytoestrogen treatment in male rats on nitric oxide-mediated responses of carotid and cerebral arteries: comparison with 17β-estradiol. J Pharmacol Exp Ther. 2004;310:135–140. doi: 10.1124/jpet.103.063255. [DOI] [PubMed] [Google Scholar]
- 51.Torregrosa G, Burguete MC, Perez-Asensio FJ, Salom JB, Gil JV, Alborch E. Pharmacological profile of phytoestrogens in cerebral vessels: in vitro study with rabbit basilar artery. Eur J Pharmacol. 2003;482:227–234. doi: 10.1016/j.ejphar.2003.09.026. [DOI] [PubMed] [Google Scholar]