Abstract
Objectives
We aimed to identify the predictors of left atrial (LA) enlargement by multi-detector computed tomography (CT) and determine its association and predictive value for acute coronary syndrome (ACS).
Background
LA enlargement is associated with myocardial ischemia and coronary artery disease (CAD) and is a strong predictor for cardiovascular events. These studies were performed primarily with echocardiography. With the rise of cardiac CT, LA volume can be readily measured.
Methods
In 377 emergency department patients with chest pain, we performed 64-slice CT for coronary artery assessment. We derived LA volumes (LAVmax, LAVmin) and indices (LAVImax, LAVImin) using a threshold-based volumetric method.
Results
Subjects, with cardiac risk factors or CAD by CT, had larger LA (ΔLAVmax 9.1 ml, p=0.004; ΔLAVmin 8.1 ml, p=0.001; ΔLAVImax 3.3 ml/m2, p=0.03; ΔLAVImin 3.4 ml/m2, p=0.006) than controls. Predictors of LA enlargement were related to risk factors for diastolic dysfunction. ACS risk was greater in patients with top quartile LAVmax (odds ratio [OR] 3.4, p=0.02) and LAVmin (OR 4.7, p=0.01) than lowest quartile, but not when indexed. Similarly, the predictive values of LA volumes were incrementally better when added to CT finding of indeterminate stenosis (LAVmax: C statistic 0.62 to 0.70, p=0.046; LAVmin: C statistic 0.65 to 0.73, p=0.008), but not when indexed.
Conclusions
Risk factors related to diastolic dysfunction are independent predictors of LA enlargement. LA enlargement by volumes are associated with a 3–5 fold increase risk for ACS and have incremental value for predicting ACS when added to the CT finding of indeterminate stenosis.
Keywords: left atrium, left atrial volume, left atrial volume index, computed tomography, acute coronary syndrome
1. Introduction
Left atrial (LA) size has been associated with coronary artery disease (CAD) and shown to provide incremental prognostic value for the detection of myocardial ischemia for stress testing. [1–4] It is a powerful independent predictor of cardiovascular events and mortality in both asymptomatic patients and in those with CAD. [5–8] However, these LA assessments were performed using echocardiography, which measured diameters or required geometric shape assumptions for volumetric calculations.
With the increase use of cardiac multi-detector computed tomography (CT) for the noninvasive evaluation of suspected CAD, three-dimensional (3D) visualization of the left atrium is readily available for analysis without additional testing. While the excellent negative predictive value of cardiac CT angiography has been well established for the evaluation of acute chest pain, the positive predictive value has been less ideal. [9–11] Additional data such as LA enlargement may be incrementally beneficial in the evaluation of these patients.
Thus, in this study, we aimed to quantify LA volumes and indices as measured by CT in a large cohort and identify the predictors of LA enlargement. In addition, we sought to determine the association and incremental predictive value of LA enlargement to the cardiovascular event of acute coronary syndrome (ACS) in patients presenting to the emergency department (ED) with a chief complaint of chest pain.
2. Materials and Methods
2.1 Study population
“The Rule Out Myocardial Infarction Using Computer Assisted Tomography” (ROMICAT) trial was a prospective observational cohort study of consecutive adult patients at low-to-intermediate likelihood of acute coronary syndrome who presented to the emergency department of Massachusetts General Hospital with acute chest pain whose initial electrocardiogram (ECG) and biomarkers were inconclusive and were awaiting hospital admission over a cumulative period of 18 months ending May 2007. The details of the study design have been previously reported [12] and notable for the exclusion of patients with atrial fibrillation. All eligible patients who consented underwent ECG gated contrast enhanced 64-slice CT. Patients received standard of care to rule out ACS during index hospitalization, including serial ECGs, biomarkers, cardiac testing (stress test or cardiac catheterization). Our institutional review board approved the study protocol and all patients provided written informed consent.
In this substudy, we excluded patients with a history of severe mitral or aortic valvular disease. A total of 377 patients, whom there was full visualization of the left atrium on the multi-phase reformatted (MPR) dataset of the CT, were included in our analysis. In the analysis of patients with cardiac risk factors versus controls, we excluded subjects with a prior history of hypertension (HTN), diabetes mellitus, hyperlipidemia, prior history of CAD, history of LV dysfunction, any coronary artery plaque as determined by CT, or ACS during index hospitalization for our control group.
2.2 CT Data Acquisition
CT imaging was performed using a standard 64-slice CT coronary angiography (Sensation 64, Siemens Medical Solutions, Forchheim, Germany) protocol that was acquired at end inspiration with a test bolus protocol and included the administration of sublingual nitroglycerin (0.6 mg) and intravenous beta-blocker (metoprolol 5–20 mg) for those with the baseline heart rate >60 beats per minute and no other contraindications. A test bolus protocol was used to determine the optimal timing of contrast injection (20 ml contrast agent followed by 40 ml saline, flow rate of 5 ml/s). Contrast agent (80–100 ml, Iodhexodol 320 g/cm3, Visipaque, General Electrics Healthcare, Princeton, NJ, USA) with 40 ml saline was injected intravenously at a rate of 5 ml/s. CT images were acquired in spiral mode, gantry rotation time of 330 ms, 64 × 0.6 mm slice collimation, tube voltage of 120 kV, maximum effective tube current of 850 mAs, with ECG-correlated tube current modulation used when appropriate. The maximum effective tube current was on during the time interval from 470 ms to 140 ms before the next expected R wave and the tube current was reduced by 80% during the remain portion of the cardiac cycle. Reconstructions were performed using retrospectively ECG-gated half-scan algorithm for a temporal resolution of 165 ms. At this temporal resolution, transaxial images were reconstructed for 10 phases, each at 10% of the RR-interval, for the multi-phase reformatted (MPR) dataset with 1.5 mm slice thickness and 1.5 mm increments for volumetric and functional analyses.
2.3 CT Measurements
Two experienced readers, blinded to the clinical outcome, performed the CT measurements offline using dedicated cardiac workstations. Quantitative LA volumes, which included the left atrial appendage but excluded the pulmonary veins, were obtained at end-systole and end-diastole. We used a highly reproducible threshold-based method for quantifying LA volume three-dimensionally without geometric shape assumptions, as previously validated. [13] Briefly, LA volumes were derived by pure volumetric summation of manually traced regions of interests on sequential axial 1.5 mm thick slices with a threshold window width set at 100–1000 Hounsfeld units using a dedicated semi-automated volumetric software program (Volume Viewer, Leonardo, Siemens Medical Solutions, Forchheim, Germany). The maximum LA volume (LAVmax) was measured from the end-systolic phase just before the mitral valve opening with the largest LA cavity and smallest LV cavity, as determined qualitatively from multiplanar LV short-axis, two-chamber, and four-chamber views. Conversely, the minimum LA volume (LAVmin) was measured from the end-diastolic phase at the mitral valve closure with the smallest LA cavity and largest LV cavity. LAVmax and LAVmin were indexed to body surface area (BSA) as LAVImax and LAVImin, respectively. Quantitative LV measurement of LV ejection fraction (LV EF) was derived from automated software (Vital Images, Minnetonka, Minnesota). The presence of coronary atherosclerotic plaque and indeterminate stenosis were visually classified by two experienced CT readers, as described previously. [12, 14]
2.4 Risk Factor and Outcome Assessment
Cardiovascular risk factors and medical history were assessed at the time of subject’s enrollment based on self-report or obtained from the medical records during the index hospitalization. Body mass index (BMI) was defined as weight (kilograms) divided by the height squared (meters). BSA was calculated using the Dubois formula. [15] Hypertension was defined as systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg or current antihypertensive treatment. Diabetes mellitus was defined as a fasting plasma glucose ≥126 mg/dL or treatment with a hypoglycemic agent. Hyperlipidemia was defined as total cholesterol of ≥200 mg/dl or treatment with a lipid lowering medication. Documented history of CAD included previous myocardial infarction or coronary revascularization. Family history of CAD was defined as having a first-degree female (<65 years) or male (<55 years) relative with a documented history of myocardial infarction (MI) or sudden cardiac death. History of LV dysfunction was obtained from review of prior echocardiography or nuclear imaging reports. Subjects were classified as smokers if they had smoked at least one cigarette per day in the year prior to the study.
An adjudication panel of 2 physicians, who were blinded to CT, reviewed the medical records and determined the diagnosis of ACS during index hospitalization. ACS was defined as either an acute myocardial infarction or unstable angina, according to the AHA/ACC/ESC guidelines. [16] Disagreement was solved by consensus, which included an additional cardiologist.
2.5 Statistical analysis
Descriptive statistics were expressed as mean ± standard deviation (SD) and interquartile ranges [IQR] for continuous variables and as frequency and percentages for nominal variables. The differences in means between groups were determined using Student’s t tests. To evaluate the associations between risk factors and LA volumes and indices, relationships with univariate parameters and LA measurements were assessed using Pearson’s correlation for continuous variables and Student’s t tests for categorical variables. BMI was not included for the index measurements due to its collinearity with BSA. For the multivariable linear regression models, we included all univariate variables that may be associated with LA measurements (p<0.15). We also included gender in the LAVImin multivariable model for face validity, although its relation was not significant in univariate analysis. For analysis of LA measurements and ACS, Student’s t tests were used to compare the difference in mean values. We then dichotomized the LA volumes and indices into the top quartile versus the lowest quartile. We used logistic regression to examine the association of LA measurements for ACS and evaluated the incremental predictive value of the LA measurements for the detection of ACS by comparing the C statistic of nested models using the likelihood ratio test. Baseline model included the CT finding of indeterminate stenosis and subsequent models included this CT finding with the addition of the individual LA measurements. The interobserver variability for LA measurements was determined for 25 randomly selected studies and assessed using intraclass correlation coefficient (ICC). The LA measurements by two independent readers had excellent reproducibility with ICC for LAVmax of 0.998 and for LAVmin of 0.986. A 2-tailed p-value of <0.05 was considered to indicate statistical significance. All analyses were performed using SAS (Version 9.1.3, SAS Institute Inc., Cary, North Carolina) and SPSS (Version 16.0, Chicago, Illinois).
3. Results
3.1 Baseline Characteristics in the ROMICAT cohort
In our cohort of 377 ED patients presenting with chest pain and without atrial fibrillation and significant left-sided valvular heart disease, mean heart rate during CT scan 65 ± 12 beats per minutes with the following CT scanning variables: beta-blockers given in 236 (63%) of patients, sublingual nitroglycerin given in 299 (80%), with ECG tube modulation performed in 167 (46%) of patients. The average age was 53 ± 12 years (range 21 to 89 years), 240 (64%) were men, and LV function was preserved (mean LV EF 67 ± 9%). There were 176 (47%) of patients without any coronary artery plaque by CT. For the cardiovascular event endpoint, 38 (10%) patients had ACS during index hospitalization [30 (79%) unstable angina, 8 (21%) myocardial infarction]. The characteristics of the study group are summarized in Table 1. The distribution of LAVmax, LAVmin, LAVImax, and LAVImin are presented in Table 2.
Table 1.
Demographics of the study group
| Characteristics | Total n=377 |
|---|---|
| Age, yrs | 53.4 ± 12.0 |
| Men | 240 (64%) |
| BSA, m2 | 2.00 ± 0.27 |
| BMI, kg/m2 | 29.2 ± 6.0 |
| Hypertension | 161 (42%) |
| Diabetes mellitus | 44 (12%) |
| Hyperlipidemia | 153 (41%) |
| History of documented CAD | 43 (11%) |
| FH of premature CAD | 94 (25%) |
| History of LV dysfunction | 14 (4%) |
| Smoker | 192 (51%) |
| No CAD* | 176 (46.7%) |
| ACS during index hospitalization | 38 (10%) |
| LV EF, %* | 67.5 ± 9.4 |
BSA denotes body surface area; BMI, body mass index; CAD, coronary artery disease; FH, family history; LV, left ventricular; ACS, acute coronary syndrome; and EF, ejection fraction.
CT measurement.
Table 2.
Left atrial (LA) volumes and indices in the ROMICAT cohort and “Risk Factor” Group versus “Controls”.
| Total | Risk Factor Group | Controls* | Difference | p-value | |
|---|---|---|---|---|---|
| mean ± SD [IQR] | mean ± SD | mean ± SD | mean (95% CI) | ||
| LAVmax (ml) | 97.4 ± 27.3 [77.9, 93.3, 112.9] | 99.7 ± 29.0 | 90.9± 21.4 | 9.1 (3.0, 15.1) | 0.004 |
| LAVmin (ml) | 57.9 ± 21.8 [44.7, 52.3, 66.2] | 60.2 ± 23.9 | 52.1 ± 13.8 | 8.1 (3.3, 13.0) | 0.001 |
| LAVImax (ml/m2) | 49.1 ± 13.2 [39.2, 47.8, 57.4] | 50.0 ± 14.0 | 46.7 ± 10.7 | 3.3 (0.4, 6.3) | 0.03 |
| LAVImin (ml/m2) | 29.1 ± 10.7 [22.9, 26.9, 32.9] | 30.1 ± 11.7 | 26.7 ± 7.0 | 3.4 (1.0, 5.7) | 0.006 |
SD denotes standard deviation; IQR, interquartile range; CI, confidence interval; LAVmax, maximum LA volume; LAVmin, minimum LA volume; LAVImax, maximum LA volume index; and LAVImin, minimum LA volume index.
Excludes patients with any of the following: hypertension, diabetes mellitus, hyperlipidemia, prior history of coronary artery disease, history of left ventricular dysfunction, any coronary artery plaque by CT, and acute coronary syndrome during index hospitalization.
3.2 Comparison of LA measurements in the “Risk Factor” and “Control” groups
In the control group, there were 107 patients included (43 women and 64 men). These patients had no prior history of hypertension, diabetes, hyperlipidemia, history of CAD, and LV dysfunction. They were also determined to have no coronary artery plaque on CT and did not develop ACS during index hospitalization. The remainder of the study group (n=270) were classified as the “risk factor” group and had at least one cardiovascular risk factor, or coronary artery plaque documented on CT. Table 2 summarizes the means and differences in LA volumes and indices between the risk factor and control groups. Overall, the patients with risk factors had significantly larger LA volumes and indices than the controls (all p<0.05). Figure 1 depicts the LA size differences between a patient in the control and one in the risk factor group.
Figure 1.
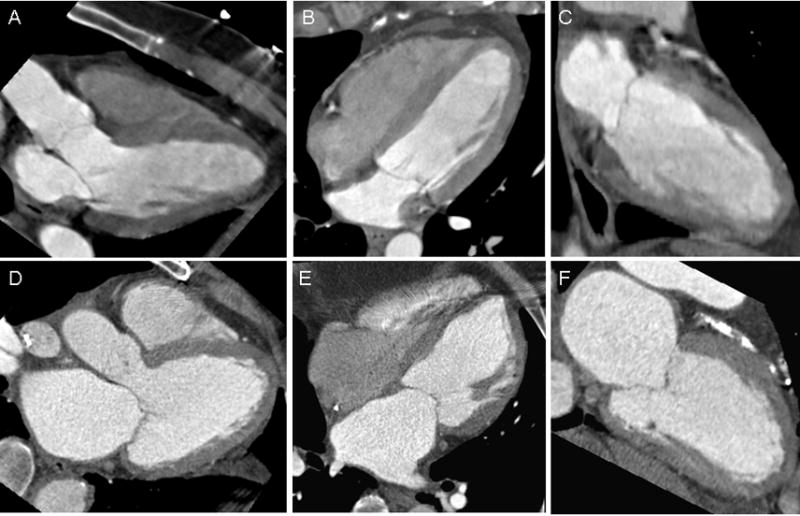
Three-chamber (A), four-chamber (B) and two-chamber (C) views obtained at end-systole of a patient in the control group, without acute coronary syndrome, and in the lowest quartile of LAVmax. Corresponding views (D–F) in a patient in the “risk factor” group, with acute coronary syndrome, and in the top quartile of LAVmax. LAVmax denotes maximal left atrial volume.
3.3 Clinical Predictors of LA volumes
There were no significant differences in LA volumes or indices in those who receive beta-blockers pre-CT scan and those who did not, as well as those who received sublingual nitroglycerin and those who did not (all p=NS). The univariate relationship of cardiovascular risk factors and covariates to LA volumes and indices are shown in Table 3. All unadjusted covariates except for family history of premature CAD and smoking were significantly associated to LA volumes. Both increasing age and BMI had weakly positive correlations to LA volumes. Patients who were men, with hypertension, diabetes, hyperlipidemia, history of CAD, and history of LV dysfunction had larger LA volumes than those who were not (all p<0.05). However, when indexed to BSA, women had slightly larger LAVImax and there was no difference between gender with LAVImin.
Table 3.
Univariate relationship of cardiovascular risk factors and covariates to left atrial (LA) volumes and indices.
| Parameters | LAVmax (ml) | LAVmin (ml) | LAVImax (ml/m2) | LAVImin (ml/m2) | ||||
|---|---|---|---|---|---|---|---|---|
| Continuous | Pearson’s r | p-value | Pearson’s r | p-value | Pearson’s r | p-value | Pearson’s r | p-value |
| Age, yrs | 0.17 | 0.001 | 0.26 | <0.0001 | 0.29 | <0.0001 | 0.35 | <0.0001 |
| BMI, kg/m2 | 0.24 | <0.0001 | 0.16 | 0.002 | - | - | ||
|
| ||||||||
| Categorical | mean ± SD | p-value | mean ± SD | p-value | mean ± SD | p-value | mean ± SD | p-value |
|
| ||||||||
| Men | 100.3±29.4 | 0.003 | 60.7±23.6 | 0.0003 | 47.8±13.8 | 0.02 | 29.0±11.2 | 0.71 |
| Women | 92.2±22.3 | 52.9±17.2 | 51.2±11.9 | 29.4±9.8 | ||||
| Hypertension | 101.3±30.2 | 0.02 | 62.0±25.2 | 0.003 | 50.4±14.7 | 0.12 | 30.8±12.3 | 0.02 |
| No hypertension | 94.6±24.7 | 54.9±18.5 | 48.1±12.0 | 27.9±9.2 | ||||
| Diabetes mellitus | 112.7±35.0 | 0.003 | 72.5±34.6 | 0.003 | 54.0±16.2 | 0.03 | 34.7±16.3 | 0.02 |
| No diabetes mellitus | 95.4±25.5 | 55.9±18.7 | 48.4±12.6 | 28.4±9.5 | ||||
| Hyperlipidemia | 101.4±28.6 | 0.02 | 61.8±25.0 | 0.01 | 51.2±14.4 | 0.01 | 31.2±12.8 | 0.004 |
| No hyperlipidemia | 94.7±26.1 | 55.2±18.9 | 47.6±12.2 | 27.7±8.7 | ||||
| History of CAD | 110.9±36.8 | 0.01 | 73.4±34.5 | 0.002 | 56.0±18.1 | 0.01 | 36.9±16.9 | 0.002 |
| No history of CAD | 95.7±25.4 | 55.9±18.7 | 48.2±12.2 | 28.1±9.2 | ||||
| FH of premature CAD | 98.3±29.1 | 0.72 | 58.8±23.0 | 0.64 | 47.9±13.2 | 0.32 | 28.6±11.0 | 0.63 |
| No FH of CAD | 97.1±26.7 | 57.6±21.4 | 49.5±13.2 | 29.3±10.6 | ||||
| History of LV dysfunction | 130.9±40.9 | 0.01 | 89.9±41.4 | 0.01 | 62.5±20.8 | 0.03 | 42.9±21.0 | 0.03 |
| No LV dysfunction | 96.1±25.8 | 56.5±19.5 | 48.6±12.7 | 28.5±9.7 | ||||
| Smoker | 97.0±28.1 | 0.76 | 57.6±21.9 | 0.82 | 48.8±14.0 | 0.68 | 28.9±10.8 | 0.74 |
| Non-smoker | 97.8±26.5 | 58.1±21.8 | 49.4±12.4 | 29.3±10.5 | ||||
| CAD (by CT) | 102.0±29.9 | 0.0003 | 62.5±23.9 | <0.0001 | 50.8±14.5 | 0.006 | 31.1±12.0 | <0.0001 |
| No CAD | 92.1±23.0 | 52.5±17.8 | 47.1±11.3 | 26.8±8.3 | ||||
In multivariable regression analyses (Table 4), age, gender, BMI, diabetes mellitus, and history of LV dysfunction were independent predictors of the LA measures, though varied slightly depending on the parameter. Consistently, age and history of LV dysfunction were remained significant for all the LA measures, with an estimated 5 ml increase in volumes and 3 ml/m2 in the indices for every decade increase in age. Patients with history of LV dysfunction had the greatest magnitude of effect and were estimated to have 25–29 ml increase in LA volumes and 11–12 ml/m2 in indices over those who were not. In addition, males trended towards having larger LAVmax (β 5.6 ml, 95% confidence interval [CI]: −0.1, 11.3 ml; p=0.05) than females. However, when indexed to BSA, men had smaller LAVImax by 3.7 ml/m2 than women, which was non-significant with LAVImin (p=0.63). For diabetic patients, in addition to a 9 ml increase in LAVmin and 4.3 ml/m2 increase in LAVImin over their non-diabetic counterpart, they trended towards having larger LAVmax (β 8.4 ml, 95% CI: −0.3, 17.0 ml; p=0.06) and LAVImax (β 4.2 ml/m2, 95% CI: −0.01, 8.3 ml/m2; p=0.05) than non-diabetics.
Table 4.
Multivariable clinical predictors of left atrial (LA) volumes and indices.
| Parameters | LAVmax (ml) | LAVmin (ml) | LAVImax (ml/m2) | LAVImin (ml/m2) | ||||
|---|---|---|---|---|---|---|---|---|
| β-estimate [95% CI] | p-value | β-estimate [95% CI] | p-value | β-estimate [95% CI] | p-value | β-estimate [95% CI] | p-value | |
| Age, per 10 years | 4.9 [2.2, 7.5] | 0.0004 | 5.0 [2.9, 7.0] | <0.0001 | 3.1 [1.8, 4.4] | <0.0001 | 3.0 [2.0, 4.0] | <0.0001 |
| Gender, male | - | - | 6.5 [2.1, 10.9] | 0.004 | −3.7 [−6.5, −0.9] | 0.01 | - | - |
| BMI, per 5 kg/m2 | 5.4 [3.1, 7.6] | <0.0001 | 2.7 [1.0, 4.5] | 0.002 | N/A | N/A | ||
| Diabetes mellitus | - | - | 9.0 [2.3, 15.6] | 0.008 | - | - | 4.3 [1.0, 7.5] | 0.01 |
| History of LV dysfunction | 29.4 [13.5, 45.4] | 0.0003 | 25.5 [13.2, 37.8] | <0.0001 | 12.1 [4.3,20.0] | 0.003 | 11.0 [4.9, 17.1] | 0.0005 |
3.4 Association and Predictive Value of LA volumes and Indices for ACS
For the ROMICAT cohort, patients with ACS had significantly larger LA volumes (LAVmax: 108.3±31.0 ml vs 96.2±26.6 ml, p<0.01; LAVmin: 68.9±29.2 ml vs 56.6±20.5 ml, p=0.015). Even after indexing for BSA, the LA size differences persisted with larger LA indices in ACS patients than patients without ACS (LAVImax: 53.7 ± 15.5 ml/m2 vs 48.6 ± 12.9 ml/m2, p=0.02; LAVImin: 34.0 ± 14.4 ml/m2 vs 28.6 ± 10.1 ml/m2, p=0.03).
For its association with ACS, we compared patients in the top quartile of LA volumes and indices to those in the lowest quartile (LAVmax: 112.9 – 213.8 ml versus 47.0 – 77.9 ml; LAVmin: 66.2 – 190.5 ml versus 25.7 – 44.7 ml; LAVImax: 57.4 – 101.3 ml/m2 versus 24.0 –39.2 ml/m2; LAVImin 32.9 – 93.9 ml/m2 versus 13.2 – 22.9 ml/m2). For LAVmax, patients in the top quartile had a 3-fold increase risk for ACS (odds ratio [OR] 3.4, 95% CI: 1.2, 9.7; p=0.02) as compared to lowest quartile. Similarly, for LAVmin, there was a near 5-fold increase risk for ACS (OR 4.7, 95% CI: 1.5, 14.7; p=0.01). However, when indexed to BSA, the odds for having ACS when comparing those in the top quartile to lowest quartile of LAVImax (OR 2.6, 95% CI: 0.9, 7.0; p=0.07) and LAVImin (OR 2.5, 95% CI: 0.99, 6.5; p=0.05) were attenuated and no longer statistically significant.
Table 5 showed the incremental predictive value of LA enlargement for ACS. Using these top versus lowest quartile cutoff values for the LA volumes and indices, there was incremental value for predicting ACS with the addition of the LA volume measures when added to that of positive coronary artery plaque but indeterminate stenosis by CT (LAVmax: C statistic improved from 0.62 to 0.70, p=0.049; LAVmin: C statistic improved from 0.65 to 0.73, p=0.008). While the C statistic increased for the LA indices, these changes were both not statistically significant.
Table 5.
Incremental Predictive Value of LA Volumes and Indices for Predicting Acute Coronary Syndrome.
| Models Predicting ACS | Patients with indeterminate stenosis | −2 Log Likelihood | C statistics | p-value |
|---|---|---|---|---|
| Indeterminate stenosis | 17/180 (9.4%) | 112.758 | 0.62 | 0.049 |
| Indeterminate stenosis + LAVmax | 108.884 | 0.70 | ||
| Indeterminate stenosis | 27/181 (14.9%) | 112.070 | 0.65 | 0.008 |
| Indeterminate stenosis + LAVmin | 105.030 | 0.73 | ||
| Indeterminate stenosis | 19/180 (10.6%) | 119.314 | 0.56 | 0.10 |
| Indeterminate stenosis + LAVImax | 116.609 | 0.63 | ||
| Indeterminate stenosis | 21/180 (11.7%) | 131.180 | 0.56 | 0.09 |
| Indeterminate stenosis + LAVImin | 128.258 | 0.63 |
Abbreviations as in Table 2.
4. Discussion
As cardiac CT becomes more frequently used for the assessment of CAD, additional non-coronary data is available for analysis and includes comprehensive evaluation of the cardiac chambers without the need for an additional test. [17] The LA, in particular, is easily visualized due to contrast-enhancement of the left-sided chambers and can be reliably quantified three-dimensionally during both end-systole and end-diastole. In contrast to echocardiography, CT quantification of LA volumes allows for accurate assessments of true volumes by accounting for the unique shape of the left atrium without the dependency of geometric shape assumptions. [18]
4.1 Modality-specific LA measurements
In this study, we report the LA volumes and indices as quantified by CT in the ROMICAT cohort. The strength of our study is the large number of patients (n=377) where CT was performed and LA measurements analyzed. While reference ranges for normal and abnormal LA volumes have been standardized for echocardiography, there has been no such volumetric reference using contrast-enhanced CT. [19] Stolzmann, et al recently described the normal LA anterior posterior diameter in end-systole using cardiac CT in 120 “normal” patients, but they did not quantify LA volumes and indices. [20] In comparison with previously described cardiac magnetic resonance imaging (CMR) measurements of LA dimensions by Hudsmith et al. of 108 normal healthy volunteers, the CT mean maximal LA volume were similar (LAVmax 91± 21 ml by CT vs 97 ± 27 ml by CMR), with slightly larger minimum LA volume (LAVmin 52 ± 14 ml by CT vs 44 ± 13 ml by CMR) in our “normal” subgroup. [21] These differences in CT and CMR values may be due to our normal cohort being older than the CMR normal volunteers (45 ± 10 years vs 38 ± 12 years, respectively). Most notably, our CT values and those reported by CMR are much larger than those reported by echocardiography. This is likely due to underestimation of the true LA volume by echocardiography[18, 22] and supports the need to establish modality-specific reference ranges.
4.2 Clinical Predictors of LA enlargement
We observed that our “risk factor” group, which had at least one cardiac risk factor and/or presence of CAD, had larger LA volumes and indices than the control group. This additional information may be helpful in risk stratifying patients, since LA dilatation has been shown to predict mortality and cardiovascular events, including atrial fibrillation, heart failure, and stroke. [5–8, 23–26] The left atrium can remodel in atrial fibrillation patients [27] and it is likely that the left atrium also remodels in the setting of chronic ischemic heart disease. To better understand the pathophysiology of LA enlargement, we then examined the relationship of clinical parameters and risk factors to LA size in the ROMICAT cohort. We found positive associations between LA volumes and indices with risk factors related to advancing diastolic dysfunction such as increasing age and BMI, diabetes, and history of LV dysfunction. [28–30] Our findings are in keeps with Pritchett et al. population-based study, where they observed that age, gender, and BMI had explained up to 29% of the variability of LA size in the reference group of 767 patients who were without cardiovascular disease or cardiac dysfunction. [31] Our results confirm others report that LA enlargement is related to aging and progression of diastolic dysfunction due to myocardial stiffness and a less compliant LA. [5, 30, 32, 33] Moreover, there are gender differences in LA volumes, which may be attributable to body size. [31, 34]
4.3 Association of LA Enlargement and Its Predictive Value for ACS
Lastly, we examined the association of CT-based LA volumes and indices to ACS. Since there have been no predefined established cutoff values with CT LA volumes to date, we compared patients in the top quartile versus those in the lowest quartile. We found that patients with the top quartile of LA volumes had a 3–5 fold increase in risk of having ACS over those with the lowest quartile. However, this increase risk was no longer significant if indexed to BSA. Similarly, we found that the LA volumes but not indices were incremental predictive of ACS when added to the CT finding of indeterminate stenosis. While LA size has been suggested to provide incremental value for predicting myocardial ischemia during stress echocardiography [2–4], our results suggest that LA enlargement may possibly be useful in predicting ACS when the CT findings of significant stenosis are inconclusive. This finding that LA volumes but not indices are associated and have incremental predictive value for ACS needs to be validated prospectively.
4.4 Limitations
Several limitations are noteworthy in the interpretation of our study results. Our study consists of emergency room chest pain patients with low- to intermediate- risk for ACS and the generalizability may be limited to this patient population. We do not have echocardiographic or CMR comparisons because these additional tests were not clinically indicated for these patients during their hospitalization. However, these comparisons have been described before. [18, 35] We are unable to grade the severity of LA enlargement since there are currently no established CT values for this assessment, thus, emphasizing the need for modality-specific reference ranges. Given that there are currently no standardized cut-off values for CT volumetric measurements of the left atrium, we arbitrarily used the top versus lowest quartiles in our comparisons for the risk of ACS. We believe this comparison is valid since the lowest quartile group most likely represents patients with normal LA size, while the top quartile is representative of those with at least some extent of LA enlargement. Because this is a subanalysis of ROMICAT, we were limited to the cardiovascular endpoint of ACS. It would be of interest to examine other cardiovascular endpoints, such as long-term mortality, heart failure or stroke. Lastly, the radiation exposure inherent in the acquisition of cardiac CT should preclude its use for the sole purpose of measuring LA size. We were able to perform our analysis because our CT acquisitions were acquired for coronary artery assessment with the use of retrospective gating, providing us with data from both end-systole and end-diastole.
5. Conclusion
Cardiac CT measurements of LA volumes and indices are readily available for analysis from a conventional retrospective gated CT angiography. While CT values are comparable to previously reported CMR ones, they are vastly different and are larger than those reported by echocardiography and thus emphasize the importance of establishing modality-specific reference ranges. Patients with cardiac risk factors and coronary artery plaque by CT have larger LA volumes and indices than controls. We confirm the clinical predictors of LA enlargement, which are driven by age and risk factors of diastolic dysfunction. CT-based LA enlargement by volumes but not indices are associated with a 3–5 fold increase risk for ACS and have incremental value for predicting ACS when added to the CT findings of indeterminate stenosis. To incorporate our findings in a clinical perspective, if LA enlargement is identified, regardless of the imaging modality, consideration for intensifying medical therapy may be warranted to target modifiable risk factors to reduce future cardiovascular events. There may be a role for integrating LA volumes in the management of patients with a CT finding of indeterminate stenosis.
Acknowledgments
Sources of Funding: This work was supported by the NIH R01 HL080053, and in part supported by Siemens Medical Solutions and General Electrics Healthcare. Drs. Rogers and Truong received support from NIH grant T32HL076136 and L30HL093896. Amir A. Mahabadi is supported by a grant from the German National Academic Foundation.
We gratefully acknowledge the enthusiastic support in patient enrollment of the team of faculty, residents, nursing and administrative staff of the Emergency Department Services of the Massachusetts General Hospital. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology. [36]
Footnotes
Disclosure: No conflicts of interest to be disclosed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gardin JM, Iribarren C, Detrano RC, Liu K, Schreiner PJ, Loria CM, et al. Relation of echocardiographic left ventricular mass, geometry and wall stress, and left atrial dimension to coronary calcium in young adults (the CARDIA study) Am J Cardiol. 2005;95:626–629. doi: 10.1016/j.amjcard.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Bangalore S, Yao SS, Chaudhry FA. Role of left atrial size in risk stratification and prognosis of patients undergoing stress echocardiography. J Am Coll Cardiol. 2007;50:1254–1262. doi: 10.1016/j.jacc.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Alsaileek AA, Osranek M, Fatema K, McCully RB, Tsang TS, Seward JB. Predictive value of normal left atrial volume in stress echocardiography. J Am Coll Cardiol. 2006;47:1024–1028. doi: 10.1016/j.jacc.2005.09.069. [DOI] [PubMed] [Google Scholar]
- 4.Ariyarajah V, Kranis M, Apiyasawat S, Spodick DH. Association of myocardial ischemia and coronary angiographic lesions with increased left atrial dimension during exercise tolerance tests among patients without known coronary heart disease. Am J Cardiol. 2007;99:1187–1192. doi: 10.1016/j.amjcard.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS) Am Heart J. 2006;151:412–418. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Laukkanen JA, Kurl S, Eranen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005;165:1788–1793. doi: 10.1001/archinte.165.15.1788. [DOI] [PubMed] [Google Scholar]
- 7.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]
- 8.Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study) Am J Cardiol. 2008;102:70–76. doi: 10.1016/j.amjcard.2008.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leschka S, Alkadhi H, Plass A, Desbiolles L, Grunenfelder J, Marincek B, et al. Accuracy of MSCT coronary angiography with 64-slice technology: first experience. Eur Heart J. 2005;26:1482–1487. doi: 10.1093/eurheartj/ehi261. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein JA, Gallagher MJ, O’Neill WW, Ross MA, O’Neil BJ, Raff GL. A randomized controlled trial of multi-slice coronary computed tomography for evaluation of acute chest pain. J Am Coll Cardiol. 2007;49:863–871. doi: 10.1016/j.jacc.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 11.Hamon M, Biondi-Zoccai GG, Malagutti P, Agostoni P, Morello R, Valgimigli M. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: a meta-analysis. J Am Coll Cardiol. 2006;48:1896–1910. doi: 10.1016/j.jacc.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahabadi AA, Samy B, Seneviratne SK, Toepker MH, Bamberg F, Hoffmann U, et al. Quantitative assessment of left atrial volume by electrocardiographic-gated contrast-enhanced multidetector computed tomography. J Cardiovasc Comput Tomogr. 2009;3:80–87. doi: 10.1016/j.jcct.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bamberg F, Dannemann N, Shapiro MD, Seneviratne SK, Ferencik M, Butler J, et al. Association between cardiovascular risk profiles and the presence and extent of different types of coronary atherosclerotic plaque as detected by multidetector computed tomography. Arterioscler Thromb Vasc Biol. 2008;28:568–574. doi: 10.1161/ATVBAHA.107.155010. [DOI] [PubMed] [Google Scholar]
- 15.DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Archives of Internal Medicine. 1916;17:865–871. [Google Scholar]
- 16.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction--2002: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina) Circulation. 2002;106:1893–1900. doi: 10.1161/01.cir.0000037106.76139.53. [DOI] [PubMed] [Google Scholar]
- 17.Schuijf JD, Bax JJ, Jukema JW, Lamb HJ, Salm LP, de Roos A, et al. Assessment of left ventricular volumes and ejection fraction with 16-slice multi-slice computed tomography; comparison with 2D-echocardiography. Int J Cardiol. 2007;116:201–205. doi: 10.1016/j.ijcard.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 18.Christiaens L, Lequeux B, Ardilouze P, Ragot S, Mergy J, Herpin D, et al. A new method for measurement of left atrial volumes using 64-slice spiral computed tomography: Comparison with two-dimensional echocardiographic techniques. Int J Cardiol. 2009;131:217–224. doi: 10.1016/j.ijcard.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Stolzmann P, Scheffel H, Leschka S, Schertler T, Frauenfelder T, Kaufmann PA, et al. Reference values for quantitative left ventricular and left atrial measurements in cardiac computed tomography. Eur Radiol. 2008;18:1625–1634. doi: 10.1007/s00330-008-0939-4. [DOI] [PubMed] [Google Scholar]
- 21.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S. Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson. 2005;7:775–782. doi: 10.1080/10976640500295516. [DOI] [PubMed] [Google Scholar]
- 22.Vandenberg BF, Weiss RM, Kinzey J, Acker M, Stark CA, Stanford W, et al. Comparison of left atrial volume by two-dimensional echocardiography and cine-computed tomography. Am J Cardiol. 1995;75:754–757. doi: 10.1016/s0002-9149(99)80676-6. [DOI] [PubMed] [Google Scholar]
- 23.Beinart R, Boyko V, Schwammenthal E, Kuperstein R, Sagie A, Hod H, et al. Long-term prognostic significance of left atrial volume in acute myocardial infarction. J Am Coll Cardiol. 2004;44:327–334. doi: 10.1016/j.jacc.2004.03.062. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 25.Dini FL, Cortigiani L, Baldini U, Boni A, Nuti R, Barsotti L, et al. Prognostic value of left atrial enlargement in patients with idiopathic dilated cardiomyopathy and ischemic cardiomyopathy. Am J Cardiol. 2002;89:518–523. doi: 10.1016/s0002-9149(01)02290-1. [DOI] [PubMed] [Google Scholar]
- 26.Sabharwal N, Cemin R, Rajan K, Hickman M, Lahiri A, Senior R. Usefulness of left atrial volume as a predictor of mortality in patients with ischemic cardiomyopathy. Am J Cardiol. 2004;94:760–763. doi: 10.1016/j.amjcard.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 27.Imada M, Funabashi N, Asano M, Uehara M, Ueda M, Komuro I. Anatomical remodeling of left atria in subjects with chronic and paroxysmal atrial fibrillation evaluated by multislice computed tomography. Int J Cardiol. 2007;119:384–388. doi: 10.1016/j.ijcard.2006.07.162. [DOI] [PubMed] [Google Scholar]
- 28.Grandi AM, Zanzi P, Piantanida E, Gaudio G, Bertolini A, Guasti L, et al. Obesity and left ventricular diastolic function: noninvasive study in normotensives and newly diagnosed never-treated hypertensives. Int J Obes Relat Metab Disord. 2000;24:954–958. doi: 10.1038/sj.ijo.0801261. [DOI] [PubMed] [Google Scholar]
- 29.Lester SJ, Tajik AJ, Nishimura RA, Oh JK, Khandheria BK, Seward JB. Unlocking the mysteries of diastolic function: deciphering the Rosetta Stone 10 years later. J Am Coll Cardiol. 2008;51:679–689. doi: 10.1016/j.jacc.2007.09.061. [DOI] [PubMed] [Google Scholar]
- 30.Gottdiener JS, Reda DJ, Williams DW, Materson BJ. Left atrial size in hypertensive men: influence of obesity, race and age. Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol. 1997;29:651–658. doi: 10.1016/s0735-1097(96)00554-2. [DOI] [PubMed] [Google Scholar]
- 31.Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41:1036–1043. doi: 10.1016/s0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 32.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the cardiovascular health study) Am J Cardiol. 2006;97:83–89. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 33.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol. 2005;45:87–92. doi: 10.1016/j.jacc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 34.Knutsen KM, Stugaard M, Michelsen S, Otterstad JE. M-mode echocardiographic findings in apparently healthy, non-athletic Norwegians aged 20–70 years. Influence of age, sex and body surface area. J Intern Med. 1989;225:111–115. doi: 10.1111/j.1365-2796.1989.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 35.Sievers B, Kirchberg S, Addo M, Bakan A, Brandts B, Trappe HJ. Assessment of left atrial volumes in sinus rhythm and atrial fibrillation using the biplane area-length method and cardiovascular magnetic resonance imaging with TrueFISP. J Cardiovasc Magn Reson. 2004;6:855–863. doi: 10.1081/jcmr-200036170. [DOI] [PubMed] [Google Scholar]
- 36.Coats AJ. Ethical authorship and publishing. Int J Cardiol. 2009;131:149–150. doi: 10.1016/j.ijcard.2008.11.048. [DOI] [PubMed] [Google Scholar]


