Abstract
Alzheimer's disease (AD) can present with non-amnestic clinical syndromes. We investigated whether there is an imaging signature of AD pathology in these atypical subjects. We identified 14 subjects that had pathological AD, a non-amnestic presentation (i.e. atypical AD), and MRI. These subjects were matched to 14 with clinical and pathological AD (i.e. typical AD), 14 with the same non-amnestic presentations with frontotemporal lobar degeneration (FTLD) pathology, and 20 controls. Voxel-based morphometry and region-of-interest (ROI) analysis were used to assess patterns of grey matter loss. Loss was observed in the temporoparietal cortex in both typical and atypical AD, and showed significantly greater loss than FTLD. However, the medial temporal lobes were more severely affected in typical AD and FTLD compared to atypical AD. A ratio of hippocampal and temporoparietal volumes provided excellent discrimination of atypical AD from FTLD subjects. Temporoparietal atrophy may therefore provide a useful marker of the presence of AD pathology even in subjects with atypical clinical presentations, especially in the context of relative sparing of the hippocampus.
Keywords: Alzheimer's disease, pathology, voxel-based morphometry, atypical presentation, frontotemporal lobar degeneration, temporoparietal cortex, hippocampus
Introduction
Alzheimer's disease (AD) is characterized by the deposition of beta-amyloid plaques and neurofibrillary tangles in the brain. The typical distribution and progression of pathology has been described by Braak and Braak (Braak and Braak, 1991), with neurofibrillary tangles starting in the medial temporal lobes and spreading to involve the temporoparietal neocortex. Subjects with AD typically present with the clinical feature of episodic memory impairment in the context of dementia (McKhann et al., 1984), although subjects with AD can present with other, atypical syndromes, in which memory impairment is not the most salient feature. Subjects have been reported with AD pathology that present clinically with an aphasia syndrome (Galton et al., 2000; Alladi et al., 2007; Josephs et al., 2008b; Mesulam et al., 2008), corticobasal syndrome (Alladi et al., 2007; Hu et al., 2009; Shelley et al., 2009), and frontotemporal dementia (Johnson et al., 1999; Alladi et al., 2007; Taylor et al., 2008).
Many subjects with one of these non-amnestic clinical presentations have a non-AD pathological diagnosis, such as frontotemporal lobar degeneration with deposition of the TAR DNA binding protein 43 (FTLD-TDP) (Mackenzie et al., 2009) or corticobasal degeneration (CBD) (Josephs, 2008), making it very difficult to predict which subjects with these atypical syndromes have underlying AD pathology. The ability to be able to predict pathology will be increasingly important when treatments for AD become available. Previous studies have suggested that imaging may provide a useful biomarker of AD pathology. Subjects with aphasia and AD pathology have been shown to have more parietal lobe atrophy than subjects with aphasia and FTLD pathology (Josephs et al., 2008b). Similarly, subjects with corticobasal syndrome with AD pathology showed greater parietal hypoperfusion on SPECT than those with CBD (Hu et al., 2009). The aim of this study was therefore to determine whether there is an imaging signature of AD pathology across a range of atypical AD clinical syndromes.
Methods
Subjects
We identified all subjects from the Mayo Clinic Neuropathological Files that were evaluated and followed by an experienced behavioral neurologist, had a pathological diagnosis of AD, a clinical presentation that was determined not to be typical for AD by an experienced behavioral neurologist, and a volumetric MRI (n=14). These subjects had a non-Alzheimer's dementia clinical diagnosis at the time of MRI and will be referred to as “atypical AD”. Clinical research diagnostic criteria had been prospectively applied in all subjects. Of these 14 subjects, the diagnoses provided by the clinician at the time of MRI were aphasic dementia (Caselli, 1993; Josephs et al., 2008b)(n=6), corticobasal syndrome (CBS) (Boeve et al., 2003) (n=5), and frontotemporal dementia of the behavioral type (bvFTD) (n=3) (Neary et al., 1998). All subjects, except one, maintained the diagnosis rendered at the time of MRI scan up until the last evaluation before death. In one patient initially classified as bvFTD, the diagnosis had changed prior to death to AD (subject 9). Aphasic dementia is a term we have used to define patients in which aphasia is the most prominent component of the syndrome and is present in the context of more widespread cognitive impairment (Caselli, 1993; Josephs et al., 2008b). None of the six aphasic dementia subjects met criteria for progressive non-fluent aphasia or semantic dementia (Neary et al., 1998). Detailed speech and language findings have been previously published for five of these six aphasic dementia subjects (Josephs et al., 2008b). The clinical features at presentation for each of the atypical subjects are shown in Table 1 to emphasize the absence of the typical amnestic presentation associated with AD.
Table 1.
Presenting symptoms in the atypical AD subjects
| Cl # | Clinician's diagnosis at the time of MRI scan | Gender | Age onset | Age death | Disease duration | Most prominent initial symptoms within the first year of onset* |
|---|---|---|---|---|---|---|
| 1 | Aphasic dementia | F | 74 | 86 | 11 | Difficulty with names of children and grandchildren. Also would misplace items. |
| 2 | Aphasic dementia | M | 78 | 88 | 10 | Difficulty finding the correct words |
| 3 | Aphasic dementia | M | 79 | 89 | 10 | Difficulty finding words and expressing himself |
| 4 | Aphasic dementia | F | 76 | 84 | 8 | Difficulty with names of people |
| 5 | Aphasic dementia | M | 54 | 65 | 10 | Difficulty expressing himself and putting words together |
| 6 | Aphasic dementia | F | 57 | 65 | 8 | Difficulty with the sequencing of words |
| 7 | bvFTD | M | 63 | 70 | 7 | Difficulty multitasking, personality change – becoming apathetic, difficulty with directions, and difficulty remember names of family members |
| 8 | bvFTD | F | 56 | 63 | 6 | Personality change, disinhibition, difficulty with problem solving and loss of episodic memory |
| 9 | bvFTD | M | 56 | 61 | 5 | Loss of personal conduct, apathy and poor initiation |
| 10 | CBS | M | 59 | 65 | 6 | Odd behaviors of the left arm |
| 11 | CBS | M | 56 | 65 | 9 | Difficulty with technical skills such as with using familiar tools |
| 12 | CBS | F | 70 | 76 | 6 | Difficulty seeing things in front of her, hand tremor, Parkinsonism, odd left hand posture, and loss of episodic memory |
| 13 | CBS | F | 62 | 71 | 9 | Difficulty using the right hand for skilled movements |
| 14 | CBS | F | 55 | 61 | 6 | Dragging right foot, right foot would turn out with walking and tripping |
CBS = corticobasal syndrome, bvFTD = frontotemporal dementia
Prominent symptoms were those emphasized in the medical records and were determined by the patient, carer and evaluating physician as being the most problematic.
These subjects were then matched by age and gender to two different disease control groups: 1) 14 subjects that had a pathological diagnosis of AD, a typical amnestic presentation and hence a clinical diagnosis of Alzheimer's dementia (McKhann et al., 1984) at the time of MRI and at the time of death, who will be referred to as “typical AD”, and 2) 14 subjects that had the same non-Alzheimer's dementia clinical diagnoses (i.e. 6 with aphasic dementia, 5 with CBS and 3 with bvFTD) but that had a non-AD pathological diagnosis. Similar to the aphasic dementia subjects with AD pathology, none of the aphasic dementia subjects with a non-AD pathology met criteria for PNFA or SD. Detailed speech and language findings have been previously published for five of these six subjects (Josephs et al., 2006). The aphasic dementia and bvFTD subjects all had a pathological diagnosis of FTLD-TDP (Mackenzie et al., 2009) and the CBS subjects all had a pathological diagnosis of CBD (Dickson et al., 2002). This group will hence be referred to as the “FTLD” group. All subjects had been prospectively followed in the Mayo Clinic Alzheimer's Disease Research Center (ADRC) or Alzheimer's Disease Patient Registry (ADPR) during life. Informed consent was obtained from all subjects for participation in the studies, which were approved by the Mayo Institutional Review Board.
A group of 20 age and gender matched cognitively normal controls was also selected. All the healthy control subjects were initially recruited into the ADRC or ADPR, and were selected from the ADRC/ADPR database based purely on age and gender. Controls were identified as individuals who a) were independently functioning community dwellers, b) did not have active neurologic or psychiatric conditions, c) had no cognitive complaints, d) had a normal neurological and neurocognitive examination, and e) were not taking any psychoactive medications in doses that would affect cognition. Subject demographics are shown in Table 2.
Table 2.
Subject demographics
| Typical AD (n=14) |
Atypical AD (n=14) |
FTLD (n=14) |
Controls (n=20) |
P value across all 4 groups | P value across the 3 disease groups | |
|---|---|---|---|---|---|---|
| No. of women (%) | 7 (50%) | 7 (50%) | 9 (64.3%) | 9 (45%) | 0.73 | 0.68 |
| % APOE 4 carriers | 57% | 36% | 36% | 20% | 0.22 | 0.71 |
| Education, yrs. | 14.1 ± 3.2 | 15.4 ± 2.3 | 12.4 ± 2.3 | 14.5 ± 2.5 | 0.04 | 0.05 |
| Age at scan, yrs. | 67.4 ± 12.7 | 67.2 ± 9.5 | 67.9 ± 9.0 | 68.7 ± 8.3 | 0.98 | 0.99 |
| Age at onset, yrs | 64.0 ± 12.9 | 64.0 ± 9.4 | 63.7 ± 8.0 | NA | NA | 0.99 |
| Time from onset-scan, yrs | 3.4 ± 1.5 | 3.2 ± 1.6 | 4.2 ± 2.7 | NA | NA | 0.67 |
| MMSE (/30) | 18.8 ± 6.1 | 22.8 ± 5.5 | 19.2 ± 5.2 | 28.8 ± 1.4 | <0.001 | 0.16 |
| CDR-SB (/18) | 6.2 ± 4.8 | 4.9 ± 2.7 | 6.0 ± 2.2 | 0 ± 0 | <0.001 | 0.62 |
Results are shown as mean ± standard deviation. MMSE = Mini-Mental State Examination; CDR-SB = Clinical Dementia Rating Sum of Boxes.
Pathological assessment
All disease subjects underwent standardized neuropathological examination using the recommended diagnostic protocol for AD (Mirra et al., 1991). Pathological diagnoses were conducted by one of our experienced neuropathologists (JEP or DWD). After removal, the brain was divided into right and left hemibrains. The left hemibrain was fixed in 10% buffered formaldehyde for 7 to 10 days, and then sectioned. Samples were processed in paraffin and stained with hematoxylin and eosin and modified Bielschowsky silver impregnation, and immunostained with antibodies to β-amyloid (clone 6F/3D, 1:10 dilution; Novocastra Vector Labs, Burlingame, CA), tau (clone AT8, 1:1,000 dilution; Endogen, Woburn, MA), alpha-synuclein (clone LB509, 1:200 dilution; Zymed, San Francisco, CA), neurofilament (DAKO clone 2F11, 1:75 dilution; DAKO, Carpinteria, CA), ubiquitin (DAKO polyclonal, 1:100 dilution), and the TAR DNA-binding protein 43 (1:8000; ProteinTech group, Chicago, IL).
In each case Braak staging was performed using Bielschowsky silver stain (Braak and Braak, 1991) and AD was diagnosed based on high probability of AD according to the NIA Reagan criteria (WorkingGroup, 1997). All 28 pathologically confirmed AD cases had a Braak stage of V or VI. The presence of secondary pathologies, including Lewy bodies, vascular pathology and TDP-43 immunoreactivity, were assessed in all cases with AD pathology. Frontotemporal lobar degeneration with TAR DNA binding protein 43 was diagnosed if there was neuronal loss and gliosis in frontal and temporal cortices, as well as ubiquitin and TDP-43 immunoreactive neuronal inclusions (Mackenzie et al., 2009). Subjects were given a pathological diagnosis of CBD if there was cortical neuronal loss and gliosis with balloon neurons and tau-positive lesions including astrocytic plaques, corticobasal bodies, and abundant neuropil threads that were located in cardinal regions that met diagnostic criteria for CBD (Dickson et al., 2002).
MRI acquisition
All subjects underwent a standardized protocol head MRI scan at 1.5T that included a T1-weighted 3-dimensional spoiled gradient echo sequence (22×16.5cm or 24×18.5cm FOV, 25° flip angle, 124 contiguous 1.6mm thick coronal slices).
Voxel-based morphometry
Patterns of cerebral atrophy were assessed using the automated and unbiased technique of voxel-based morphometry (VBM) (Ashburner and Friston, 2000). An optimized method of VBM was applied using both customized templates and prior probability maps, implemented using SPM2 (http://www.fil.ion.ucl.ac.uk/spm). The customized template and priors were generated from all subjects in the study. The processing steps were performed as previously described (Senjem et al., 2005; Whitwell et al., 2007). Briefly, all images were normalized to a customized template. The spatial normalization was optimized by normalizing the grey matter images to the customized grey matter template. Images were segmented using customized prior probability maps, modulated, and smoothed with an 8mm full-width at half-maximum smoothing kernel.
A single subject condition and covariate model was used to compare the smoothed modulated gray matter images between 1) the subjects with typical AD and controls, 2) the subjects with atypical AD and controls, and 3) between the FTLD subjects and controls. Conjunction analyses (Nichols et al., 2005) were then performed in order to investigate regions of grey matter loss that were common to the atypical and typical AD groups, and also regions of loss that were common to the atypical AD and the FTLD group. Direct comparisons were also performed in order to identify differences between the atypical AD group and the other two disease groups. Firstly, comparisons were performed to identify regions that showed greatest loss in the atypical AD group compared to the typical AD group, and compared to the FTLD group. These comparisons were masked by the results from the control versus atypical AD contrast in order to investigate only those regions of the brain that were affected in atypical AD. Second, the reverse comparisons were performed in order to identify regions that showed greatest loss in the typical AD group compared to the atypical AD group, and in the FTLD group compared to the atypical AD group. These comparisons were masked by the control versus typical AD or control versus FTLD contrasts respectively.
In addition, the atypical AD subjects were divided into groups based on their clinical diagnosis at scan (aphasic dementia, CBS, and bvFTD) and patterns of grey matter loss were assessed in each group compared to controls. A conjunction analysis was also performed to identify regions of grey matter loss that were common to all three of the different atypical AD groups.
Age, gender and total intracranial volume (TIV) were included as nuisance variables in all of the statistical analyses. All analyses that involved comparing the three main disease groups (typical AD, atypical AD and FTLD) to controls were assessed at a statistical threshold of p<0.0005 corrected for multiple comparisons using the false discovery rate (FDR) (Genovese et al., 2002). In order to avoid using multiple statistical thresholds the direct comparisons between these groups, and all analyses involving the smaller clinical groups (aphasic dementia, CBS, and bvFTD) were assessed at a more lenient threshold of p<0.001 uncorrected for multiple comparisons. However, if the results of any of these latter comparisons survived a correction for multiple comparisons, it was noted in the text in order to provide a sense of the robustness of the findings.
Atlas-based parcellation
In order to investigate subject-level regional differences in our different disease groups an atlas-based parcellation technique was employed using SPM5 and the automated anatomic labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) to generate grey matter volumes for specific regions-of-interest (ROIs). The ROIs that were analyzed were based on the results of the VBM analysis. Therefore, volumes were calculated for the temporoparietal cortex (consisting of angular gyrus and superior temporal lobe) and hippocampus. We also calculated volumes for the medial parietal lobe (including precuneus and posterior cingulate) since these regions are typically associated with AD pathology, and the lateral (inferior, middle and superior frontal gyri, and rolandic operculum) and medial frontal lobes (superior medial frontal, gyrus rectus, anterior cingulum) which are typically associated with FTLD pathology.
The single-subject brain image (Tzourio-Mazoyer et al., 2002) with atlas labels was normalized to a customized template (Ashburner and Friston, 2005) and edited. Each subject MRI scan was also then spatially normalized to the custom template, and then for each subject, the inverse transformation was applied to the atlas in order to warp the atlas to the subject's native anatomical space. Each subject scan was segmented into grey matter, white matter and cerebrospinal fluid in native space. The segmented grey matter probability map was thresholded at a value of 0 to create a binary grey matter mask, and multiplied by the subject-specific warped atlas, to generate a custom grey matter atlas for each subject, parcellated into the aforementioned ROIs. Grey matter volumes were calculated for each ROI for each subject by multiplying the mean grey matter probability by the total number of voxels within a region-of-interest and by the voxel volume. In addition, TIV was calculated by propagating a template-drawn TIV mask to the subject space as above, and then performing an erosion step to remove border voxels.
Statistics
Statistical analyses were performed utilizing the JMP computer software (JMP Software, version 6.0.0; SAS Institute Inc, Cary, NC) with α set at 0.05. We tested for differences in demographic variables and ROI volume measurements using the Kruskal-Wallis test across all four groups, and also across just the three disease groups. Categorical variables were similarly assessed using the chi-squared test. If significant differences were observed across the three disease groups, pair-wise testing was performed using two-sided Wilcoxon rank-sum test. Therefore only variables that differed across our three groups of interest were analyzed in more detail. This strategy reduces the number of statistical analyses performed and hence reduces the chance of type 1 error (false positives). We performed an area under the receiver operating characteristic curve (AUROC) analysis using a logistic regression model to determine the ability of ROI volumes to differentiate subjects with atypical AD and FTLD. We examined temporoparietal and hippocampal volumes since these showed significant differences across the three disease groups using Kruskal-Wallis testing, and a ratio metric of hippocampal volume over temporoparietal volume. We also performed an AUROC analysis to determine the ability of the temporoparietal volume to differentiate all AD subjects (typical + atypical) and FTLD. We report the AUROC which represents the proportion of times the model will correctly classify the clinical diagnosis of subjects given only the value of the model predictor.
Results
Subject demographics
There were no significant differences across the three disease groups in any demographic feature, including age, gender, and disease severity (Table 2), although there was a significant difference across the disease groups and controls in measures of disease severity.
Pathology
Of the subjects with atypical AD, 29% had a secondary pathology of Lewy body disease (two cases with limbic-only Lewy bodies, and two cases with diffuse Lewy bodies) and only one case had significant vascular pathology with a small right superior temporal gyrus hemorrhage (not present at time of MRI). Only one case had TDP-43 immunoreactivity. Of the typical AD subjects, 21% had a secondary pathology of Lewy body disease (one brainstem-only Lewy bodies, one limbic-only Lewy bodies and one diffuse Lewy bodies) and only one case had significant vascular pathology with a small left superior middle frontal gyrus ischemic infarct (not present at time of MRI). However, 29% of the typical AD subjects had TDP-43 immunoreactivity. There was no difference in the proportion of subjects with Lewy bodies (p=1.00), vascular pathology (p=1.00) or TDP-43 immunoreactivity (p=0.32) across the typical and atypical AD groups.
Voxel-based morphometry
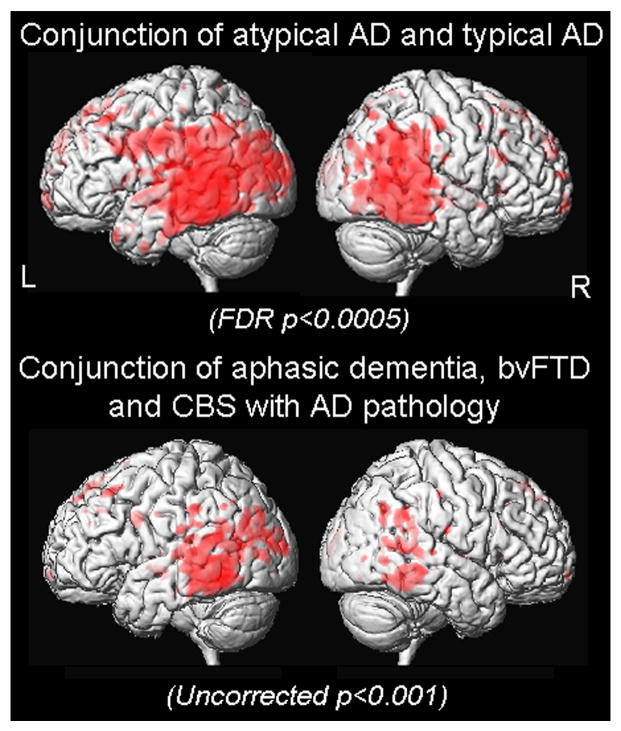
Figure 1 and Table 3 show the results of the comparisons between each disease group and controls. The typical AD group showed grey matter loss predominantly in the medial temporal lobes (including the hippocampi, amygdala and fusiform gyrus) and the posterior temporal and parietal cortices, with some loss also observed in the posterior cingulate and frontal lobes. The atypical AD group showed grey matter loss predominantly in the posterior temporal and parietal cortices, with additional involvement of the left putamen, posterior cingulate and frontal lobes, but relative sparing of the medial temporal lobes. In contrast, the FTLD group showed grey matter loss predominantly in the anterior temporal lobes, including medial and inferior temporal regions, and posterior frontal lobes, with very little involvement of the posterior temporal or parietal cortices. Conjunction analyses highlighted regions of loss that were common between groups. Grey matter loss in the posterior temporal and parietal cortices was common to both the typical and atypical AD groups when compared to controls (Figure 2). No regions were identified that were common to the atypical AD and FTLD groups at the stringent threshold of p<0.0005 (FDR corrected).
Figure 1.
Results of the VBM comparisons of typical AD, atypical AD and FTLD groups with controls. Results are shown on 3D renderings of the brain and representative coronal slices through the customized template.
Table 3.
MNI coordinates for predominant regions of grey matter loss in the typical AD, atypical AD and FTLD groups when compared to controls. Peak voxels in clusters over 500 voxels are shown and labels identified using the AAL atlas. Zo = Z score.
| Region | Typical AD | Atypical AD | FTLD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zo | x | y | z | Zo | x | y | z | Zo | x | y | z | |
| Medial temporal lobe | ||||||||||||
| Left hippocampus | 6.27 | -31 | -12 | -15 | 6.45 | -25 | -9 | -15 | ||||
| Left fusiform gyrus | 6.23 | -24 | -12 | -41 | 6.22 | -25 | -11 | -42 | ||||
| Temporoparietal cortex | ||||||||||||
| Right middle temporal gyrus | 6.43 | 61 | -56 | 6 | 5.65 | 68 | -35 | -7 | ||||
| 6.43 | 57 | -45 | -4 | |||||||||
| Left superior temporal gyrus | 6.37 | -64 | -38 | -1 | 6.47 | -61 | -44 | 14 | ||||
| 6.37 | -64 | -38 | -1 | 6.43 | -61 | -34 | 6 | |||||
| 6.41 | -54 | -30 | 0 | |||||||||
| Right angular gyrus | 6.43 | 61 | -47 | 30 | 5.71 | 61 | -49 | 30 | ||||
| 5.70 | 56 | -62 | 28 | |||||||||
| Frontal lobes | ||||||||||||
| Left middle frontal gyrus | 6.03 | -30 | 22 | 49 | 6.17 | -29 | -1 | 55 | ||||
| 5.62 | -26 | 16 | 53 | |||||||||
| Right middle frontal gyrus | 5.74 | 39 | 17 | 56 | ||||||||
| Medial superior frontal lobe | 5.70 | -7 | 37 | 52 | 6.12 | 0 | 25 | 55 | ||||
| 5.69 | 0 | 43 | 40 | |||||||||
| Left precentral gyrus | 6.42 | -49 | -9 | 35 | 5.66 | -50 | -8 | 34 | ||||
| Left inferior frontal opercula | 5.36 | -39 | 7 | 29 | 5.69 | -38 | 10 | 9 | ||||
| Right inferior frontal opercula | 5.22 | 40 | 8 | 34 | ||||||||
| Right inferior frontal orbital | 5.32 | 37 | -10 | -8 | ||||||||
| Right gyrus rectus | 5.96 | 2 | 11 | -16 | ||||||||
| Other | ||||||||||||
| Left putamen | 6.28 | -27 | -10 | -8 | ||||||||
| Right caudate nucleus | 5.57 | 13 | 25 | 0 | ||||||||
| Left anterior temporal lobe | 7.00 | -23 | 7 | -27 | ||||||||
| Right insula | 5.35 | 38 | 10 | 3 | ||||||||
Figure 2.
Results of the conjunction analyses shown on 3D renderings of the brain. Two conjunction analyses are shown: 1) conjunction of “atypical AD versus controls” and “typical AD versus controls”, and 2) conjunction using the three clinical variants of atypical AD: “aphasic dementia versus controls”, “CBS versus controls”, and “bvFTD versus controls”.
Figure 3 shows the results of direct comparisons between the atypical AD group and the typical AD and FTLD groups. Both the typical AD and FTLD groups had significantly more grey matter loss in the bilateral hippocampi than the atypical AD group. Conversely, the atypical AD group had greater loss in the left putamen than the typical AD group, and greater loss in the temporoparietal cortex than the FTLD group.
Figure 3.
Results of VBM direct comparisons between the typical and atypical AD groups, and between the atypical AD and FTLD groups. The typical AD and FTLD groups showed greater grey matter loss in the hippocampi than the atypical AD group. Conversely, the atypical AD group showed greater loss in the left putamen than the typical AD group, and greater loss in the temporoparietal cortex than the FTLD group.
The patterns of grey matter loss identified in each of the different clinical variants of atypical AD (i.e. aphasic dementia, bvFTD and CBS) when compared to controls are shown in Figure 4. The pattern of loss varied by clinical diagnosis, but grey matter loss was observed in the posterior temporal and parietal cortices in each clinical group compared to controls. This finding was confirmed by the conjunction analysis that showed that grey matter loss in the temporoparietal cortices was common to all three groups (Figure 2). In addition, the aphasic dementia group showed grey matter loss in the anterior temporal lobes (including left hippocampus) and the frontal lobes, the bvFTD group showed loss in bilateral frontal lobes, and the CBS group showed loss in the bilateral posterior frontal lobes and putamen. All these regions remained significant after correction for multiple comparisons using the FDR at p<0.01.
Figure 4.
Regions of grey matter loss in each of the different atypical clinical diagnosis groups (aphasic dementia, corticobasal syndrome and behavioral variant frontotemporal dementia) when compared to controls.
Atlas-based parcellation
Grey matter volumes of the temporoparietal cortex, hippocampus, medial parietal lobe and lateral frontal lobes for each subject are shown in Figure 5. Differences were observed across the typical AD, atypical AD and FTLD groups for the hippocampus (p=0.03) and temporoparietal cortex (p=0.01), but not for the lateral (p=0.36) or medial (p=0.35) frontal lobes. There was only a trend for differences in the medial parietal lobe (p=0.07). Both the typical AD and FTLD group showed smaller hippocampal volumes than the atypical AD group (p=0.03 for both), with no differences observed between the typical AD and FTLD groups (p=0.63). In contrast, the temporoparietal volumes were smaller in the atypical AD group than the FTLD group (p=0.005), but were no different between the atypical and typical AD groups (p=0.16). There was only a trend for smaller temporoparietal volumes in the typical AD group compared to the FTLD group (p=0.09).
Figure 5.
Box-plots with individual data points superimposed showing hippocampal, temporoparietal, medial parietal and lateral frontal volumes for the three disease groups and controls (CN). Clinical diagnoses for subjects in the atypical AD and FTLD groups are highlighted in different colors. P values represent Kruskal-Wallis testing across the typical and atypical AD groups and the FTLD group. Kruskal-Wallis testing across all four groups, including controls, showed differences for all regions (p<0.001). The horizontal lines of the boxes represent the 25th, 50th (median), and 75th percentiles of the distributions. The vertical lines extending from the boxes stop at the most extreme data point within 1.5 inter-quartile ranges of the box.
The ROC plots for discrimination of the atypical AD and FTLD groups using ROI volumes are shown in Figure 6. The temporoparietal cortex volumes provided better discrimination between the atypical AD and FTLD groups than the hippocampal volume. The AUROC (95% confidence interval) for the hippocampus was 0.74 (0.55, 0.94) (p<0.01) and the temporoparietal cortex was 0.81 (0.65, 0.97) (p<0.01). However, discrimination was further improved by accounting for both the hippocampal and temporoparietal volumes in a ratio measure, with an AUROC of 0.93 (0.84, 1.0) (p<0.01). The temporoparietal volume also provided good discrimination between all AD subjects (typical + atypical) and the FTLD subjects with an AUROC of 0.75 (0.59, 0.91) (p<0.01).
Figure 6.
ROC curves showing the ability of hippocampal volume, temporoparietal volume and the ratio of hippocampal to temporoparietal volume to differentiate patients in the atypical AD and FTLD groups.
Discussion
The findings from this study suggest that grey matter loss in the temporoparietal neocortex may be a sensitive and specific marker of AD pathology. Temporoparietal grey matter loss was a feature of AD pathology in subjects with both a typical and atypical clinical presentation. Importantly, however, it was also significantly more affected in atypical AD than in a clinically matched cohort of subjects with FTLD pathology. Volume measurements of the temporoparietal cortex allowed good discrimination between the AD and FTLD groups demonstrating potential clinical utility.
Temporoparietal atrophy is well documented to be a classic, and relatively early, feature in subjects with typical AD (Baron et al., 2001; Chetelat et al., 2002; Frisoni et al., 2005; Whitwell et al., 2007). Temporoparietal atrophy has also been previously associated with aphasia and AD pathology in which subjects were clinically diagnosed with aphasic dementia (Josephs et al., 2008b). The term aphasic dementia has been used at our institution as a diagnosis whenever the clinical presentation was that of a prominent aphasia; however because of more widespread cognitive impairment, such as loss of episodic memory or poor calculations, the patient was not given a diagnosis of primary progressive aphasia. The diagnosis of aphasic dementia predates more recent terminology. As discussed in our previous manuscripts (Josephs et al., 2008b), many of our subjects with aphasic dementia, but not all, would fulfill criteria for the more recently characterized logopenic progressive aphasia syndrome (Gorno-Tempini et al., 2004) which is also characterized by temporoparietal and medial temporal atrophy.
It is less common, however, for subjects presenting with CBS or bvFTD to show underlying AD pathology (Johnson et al., 1999; Alladi et al., 2007; Hu et al., 2009; Shelley et al., 2009), and neither syndrome is typically associated with predominant temporoparietal atrophy. Corticobasal syndrome is characterized by insidious onset and a progressive course of asymmetric cortical dysfunction, for example, ideomotor apraxia, cortical sensory loss or myoclonus, and asymmetric extrapyramidal dysfunction reflected by at least levodopa unresponsive appendicular rigidity or dystonia (Boeve et al., 2003; Litvan et al., 2003). It is typically associated with frontoparietal atrophy (Boxer et al., 2006; Josephs et al., 2008a), although in a recent study we found some suggestion of temporoparietal hypoperfusion on SPECT in a subset of CBS subjects with AD pathology (Hu et al., 2009). Subjects with bvFTD show behavioral and personality changes (Neary et al., 1998) and are typically associated with frontotemporal atrophy (Neary et al., 1998; Rosen et al., 2002; Boccardi et al., 2005). Our findings suggest that the presence of AD pathology is associated with a somewhat different pattern of atrophy than what is typically observed in CBS and bvFTD. Specifically, in bvFTD, the pattern of atrophy involved more parietal lobe atrophy than is typically observed, and in CBS, temporal lobe atrophy was observed in addition to the typical frontoparietal pattern. It therefore appears that a pattern of temporoparietal atrophy may suggest the presence of AD pathology even in subjects presenting with non-amnestic clinical syndromes.
Another important finding from this study was that the atypical AD subjects showed a relative sparing of the hippocampus and medial temporal lobes, which concurs with the fact that episodic memory impairment was not the dominant symptom in these subjects. Conversely, severe involvement of the hippocampus and medial temporal lobes was observed in the typical AD subjects, as has previously been demonstrated (Jack et al., 1992; Fox et al., 1996) and is expected, given the early and dominant episodic memory impairment observed in typical AD (McKhann et al., 1984). Severe involvement of the hippocampus and medial temporal lobe was also found in the FTLD group, driven mainly by the bvFTD and aphasic dementia subjects, which concurs with findings from a previous study that investigated pathologically confirmed FTLD subjects (Barnes et al., 2006). Some involvement of the left hippocampus was observed in the aphasic dementia subjects fitting with previous aphasia studies (Gorno-Tempini et al., 2004; Josephs et al., 2008b), although to a lesser degree than the typical AD and FTLD subjects. Although there was a slight trend for disease severity to be worse in the typical AD and FTLD subjects there was no significant difference across the three groups suggesting that greater medial temporal lobe atrophy is likely not a result of more severe disease. Similarly, there was no difference in the time from onset to scan across groups. The fact that the atypical AD subjects showed more temporoparietal atrophy than the other groups also argues against this confound. The ROC analysis showed that while hippocampal volumes provide good discrimination between the atypical AD and FTLD groups the temporoparietal volume provides a little better discrimination. However, the addition of hippocampal volume to the temporoparietal volume further improves the discrimination over temporoparietal volume alone. Therefore, consideration of both these regions would improve the ability of a clinician to predict underlying pathology. If a subject with a bvFTD, CBS or aphasic dementia clinical syndrome showed temporoparietal atrophy in the context of relative sparing of the hippocampus one could predict the presence of AD pathology.
Atrophy of medial parietal regions, including the posterior cingulate and precuneus, has also been associated with AD (Baron et al., 2001; Jones et al., 2006). While no significant differences were identified across disease groups for the medial parietal lobe in this study, there was a trend for greater involvement in the typical and atypical AD groups compared to the FTLD group. Therefore, although the temporoparietal cortex provides the better marker of AD pathology the medial parietal cortex may also be helpful. Another finding was that the atypical AD subjects were also associated with greater involvement of the putamen than the typical AD subjects. This finding appears to be driven by the presence of putamen volume loss in the CBS subjects which is not unexpected. In fact, we could speculate that parkinsonism in the CBS patients with AD pathology may be associated with atrophy of this region.
The strengths of this study are that all subjects had histological confirmation of diagnosis and that the subject groups were well matched. The sample sizes were also reasonable in the main disease groups given that they are pathologically confirmed, although were smaller for the atypical AD group when split by clinical diagnoses. It should also be noted that these ROC analyses demonstrate group discrimination for our cohort and will need to be validated. Furthermore, the degree of hippocampal atrophy observed in the FTLD group is likely to vary dependent on the specific FTLD pathologies (Whitwell et al., 2005; Josephs et al., 2008a; Whitwell et al., 2009). Additional strengths are that both the VBM and atlas-based parcellation techniques are automated and unbiased. The presence of secondary pathologies was also assessed and we found no differences across the typical and atypical AD groups in either the presence of Lewy Bodies or vascular pathology. The atypical AD subjects had a lower proportion of cases with TDP-43 immunoreactive inclusions than the typical AD subjects, although this difference was not significant. The presence of these pathologies therefore does not appear to be driving the clinical findings in the atypical group. We have previously shown that TDP-43 in AD does not account for a frontotemporal like phenotype (Hu et al., 2007). We have also demonstrated that hippocampal atrophy is a feature of AD in the absence of TDP-43 (Josephs et al., 2008c), showing that the lack of TDP-43 immunoreactivity in the atypical AD subjects could not explain the lack of hippocampal atrophy. The box plot in Figure 5 also shows that the finding of temporoparietal loss in the atypical AD subjects is not being driven by one particular clinical group with small volumes observed in bvFTD, CBS and aphasic dementia subjects. This study therefore suggests that patterns of atrophy on MRI, particularly with reference to the temporoparietal cortex and hippocampus, could prove to be useful in predicting the presence of underlying AD pathology.
Acknowledgments
This study was supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078, The Dana Foundation, NIH grants P50-AG16574, U01-AG06786, R01-AG11378, as well as the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program of the Mayo Foundation, the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation, and the NIH Construction Grant (NIH C06 RR018898).
Footnotes
Disclosure Statement
The authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, Hodges JR. Focal cortical presentations of Alzheimer's disease. Brain. 2007;130:2636–2645. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barnes J, Whitwell JL, Frost C, Josephs KA, Rossor M, Fox NC. Measurements of the amygdala and hippocampus in pathologically confirmed Alzheimer disease and frontotemporal lobar degeneration. Arch Neurol. 2006;63:1434–1439. doi: 10.1001/archneur.63.10.1434. [DOI] [PubMed] [Google Scholar]
- Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Sabattoli F, Laakso MP, Testa C, Rossi R, Beltramello A, Soininen H, Frisoni GB. Frontotemporal dementia as a neural system disease. Neurobiol Aging. 2005;26:37–44. doi: 10.1016/j.neurobiolaging.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54 5:S15–19. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, Weiner MW, Rosen HJ. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63:81–86. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Caselli RJ. Aphasic dementia. Ann Neurol. 1993;33:200–207. doi: 10.1002/ana.410330210. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13:1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS, Tabaton M, Vonsattel JP, Wakabayashi K, Litvan I. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, Rossor MN. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 1996;119(Pt 6):2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Testa C, Sabattoli F, Beltramello A, Soininen H, Laakso MP. Structural correlates of early and late onset Alzheimer's disease: voxel based morphometric study. J Neurol Neurosurg Psychiatry. 2005;76:112–114. doi: 10.1136/jnnp.2003.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Xuereb JH, Hodges JR. Atypical and typical presentations of Alzheimer's disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain. 2000;123(Pt 3):484–498. doi: 10.1093/brain/123.3.484. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WT, Josephs KA, Dickson DW, Knopman DS, Boeve BF, Petersen RC, Parisi JE. Dual pathologies: utility of TAR DNA-binding protein 43 (TDP-43) staining in patients with frontal and temporal lobe abnormalities and Alzheimer's disease. Exp Biology. 2007;21:A21. [Google Scholar]
- Hu WT, Rippon GW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Josephs KA. Alzheimer's disease and corticobasal degeneration presenting as corticobasal syndrome. Mov Disord. 2009 doi: 10.1002/mds.22574. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [DOI] [PubMed] [Google Scholar]
- Jones BF, Barnes J, Uylings HB, Fox NC, Frost C, Witter MP, Scheltens P. Differential regional atrophy of the cingulate gyrus in Alzheimer disease: a volumetric MRI study. Cereb Cortex. 2006;16:1701–1708. doi: 10.1093/cercor/bhj105. [DOI] [PubMed] [Google Scholar]
- Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 2008;64:4–14. doi: 10.1002/ana.21426. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Jack CR., Jr Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008a;29:280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, Dickson DW, Jack CR, Jr, Petersen RC. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, Boeve BF, Graff-Radford NR, Parisi JE, Knopman DS, Dickson DW, Jack CR, Jr, Petersen RC. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008b;70:25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, Rademakers R, Boeve BF, Parisi JE, Smith GE, Ivnik RJ, Petersen RC, Jack CR, Jr, Dickson DW. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008c;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, Quinn N, Sethi KD, Shults C, Wenning GK. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–486. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE, Ince PG, Kamphorst W, Revesz T, Rozemuller AJ, Kumar-Singh S, Akiyama H, Baborie A, Spina S, Dickson DW, Trojanowski JQ, Mann DM. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, Weintraub S, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR., Jr Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26:600–608. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley BP, Hodges JR, Kipps CM, Xuereb JH, Bak TH. Is the pathology of corticobasal syndrome predictable in life. Mov Disord. 2009 doi: 10.1002/mds.22558. [DOI] [PubMed] [Google Scholar]
- Taylor KI, Probst A, Miserez AR, Monsch AU, Tolnay M. Clinical course of neuropathologically confirmed frontal-variant Alzheimer's disease. Nat Clin Pract Neurol. 2008;4:226–232. doi: 10.1038/ncpneuro0746. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski S, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr 3D Maps from Multiple MRI Illustrate Changing Atrophy Patterns as Subjects Progress from MCI to AD. Brain. 2007 doi: 10.1093/brain/awm112. Epub May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Rossor MN, Stevens JM, Revesz T, Holton JL, Al-Sarraj S, Godbolt AK, Fox NC, Warren JD. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol. 2005;62:1402–1408. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Boeve BF, Senjem ML, Baker M, Ivnik RJ, Knopman DS, Wszolek ZK, Petersen RC, Rademakers R, Josephs KA. Atrophy patterns in IVS10+16, IVS10+3, N279K, S305N, P301L, and V337M MAPT mutations. Neurology. 2009;73:1058–1065. doi: 10.1212/WNL.0b013e3181b9c8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WorkingGroup. Consensus recommendation for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for Neuropathologic Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18(Supp1):S1–S2. [PubMed] [Google Scholar]