Abstract
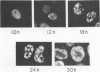
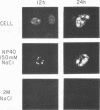
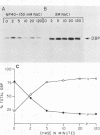
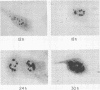
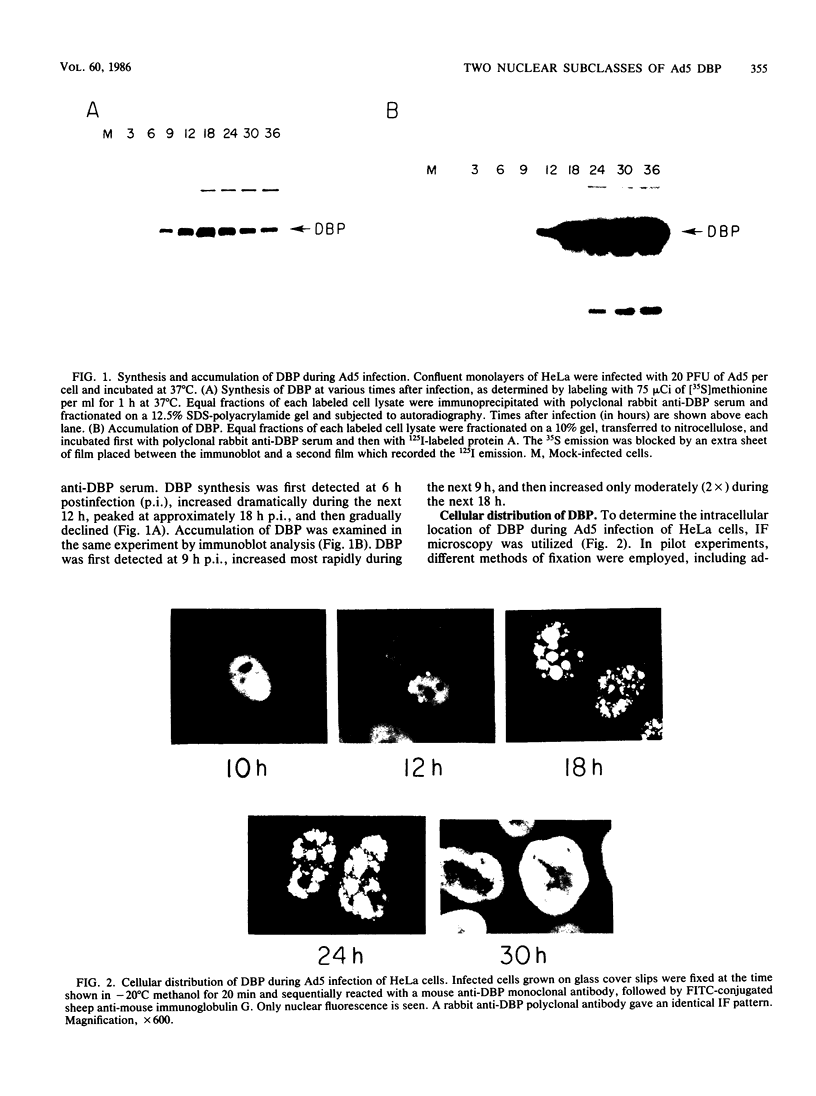
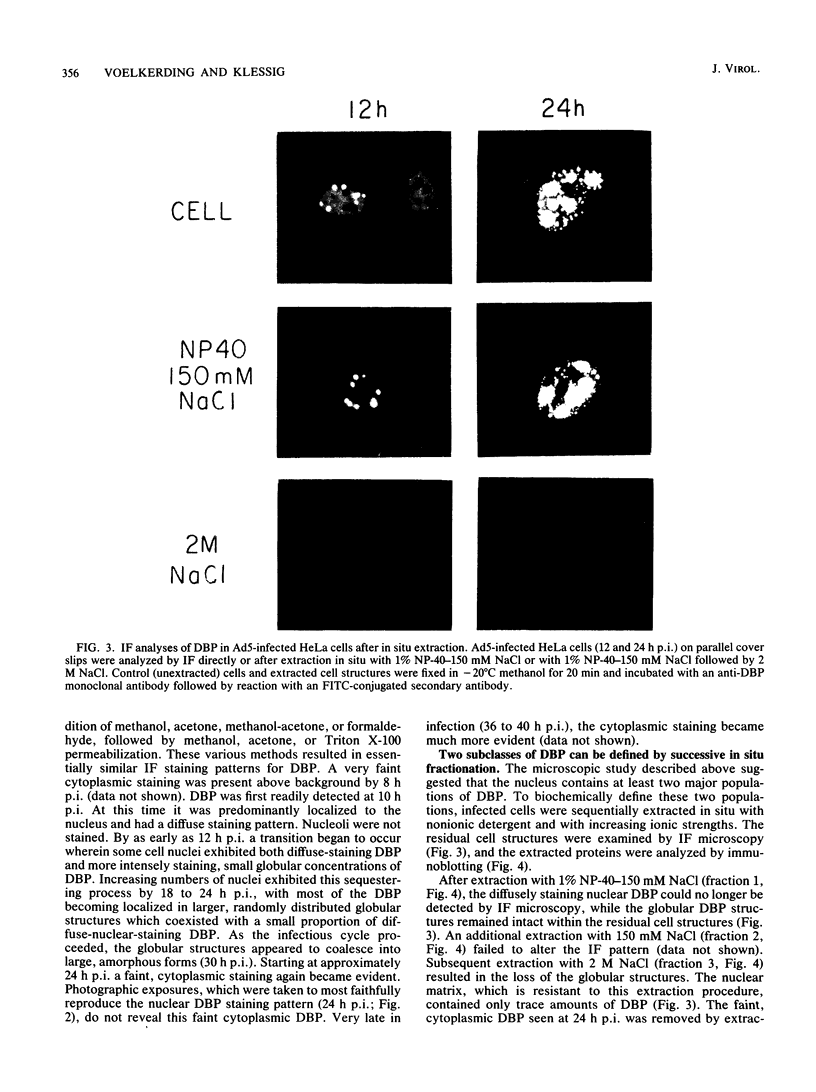
The synthesis, accumulation, and subcellular distribution of the adenovirus serotype 5 DNA-binding protein (DBP) has been examined during the infectious cycle in HeLa cells. With the onset of viral DNA replication and entry into the late phase, two nuclear subclasses of DBP are distinguishable by immunofluorescence microscopy and can be separately isolated by in situ cell fractionation. The first subclass, represented by diffuse-staining DBP, is released by the addition of 1% Nonidet P-40-150 mM NaCl. The second subclass of DBP, which is sequestered into intranuclear globular structures, requires a high ionic strength (2 M NaCl) for extraction and appears to be associated with centers of active viral DNA replication. This association is based on the observations that: DBP within the globules and viral DNA, as detected by in situ hybridization, form identical structures that colocalize within the nuclei of infected cells, the formation of DBP globular structures requires the onset and continuation of viral DNA replication, and once formed, the globular structures can be perturbed by modulating viral DNA synthesis.
Full text
PDF

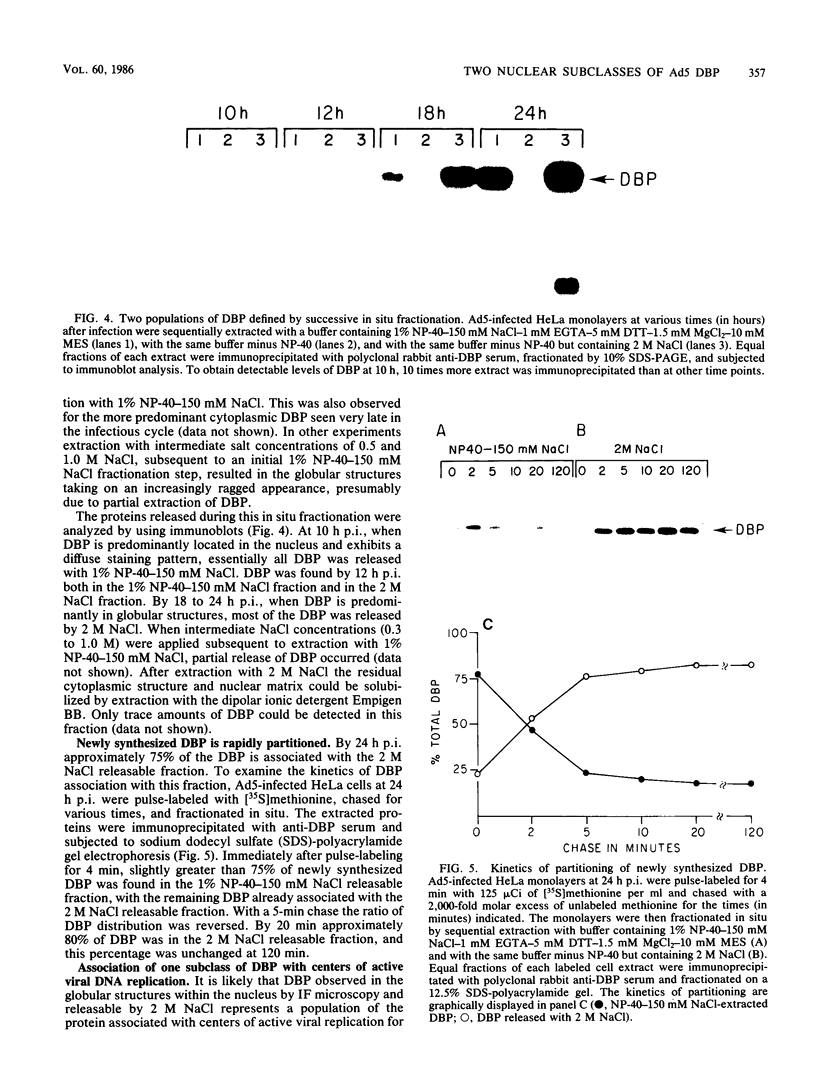
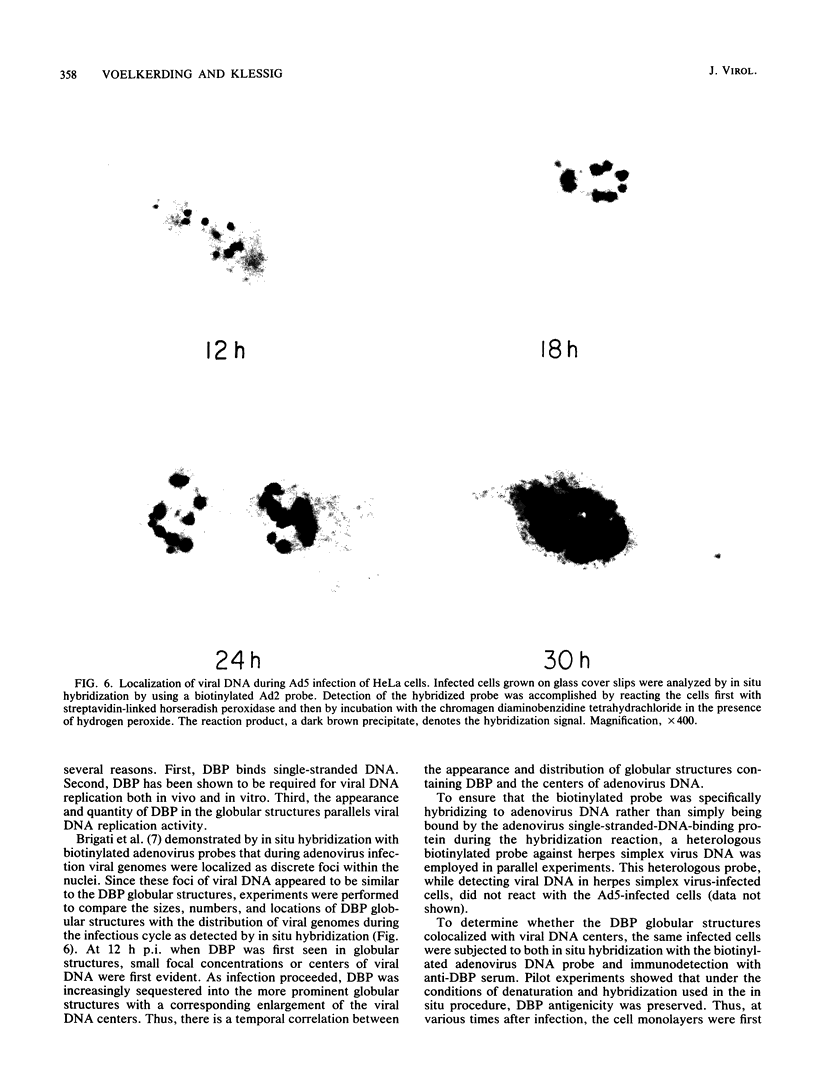
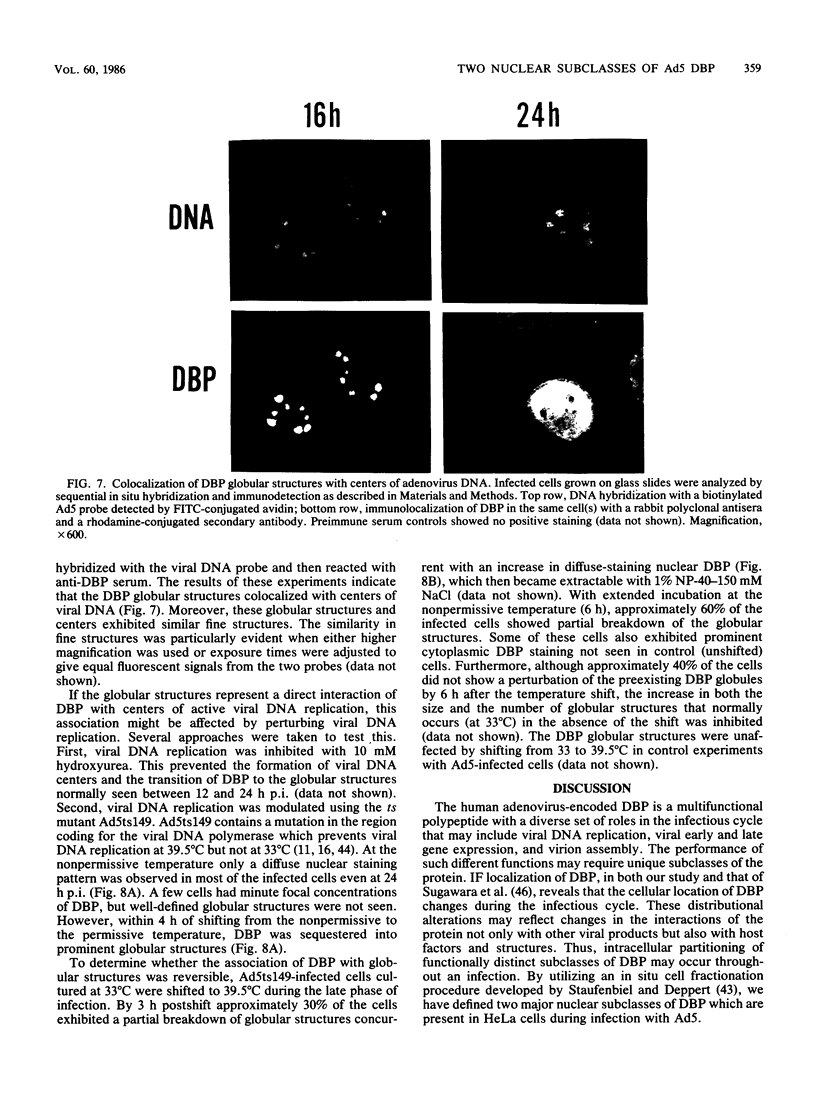
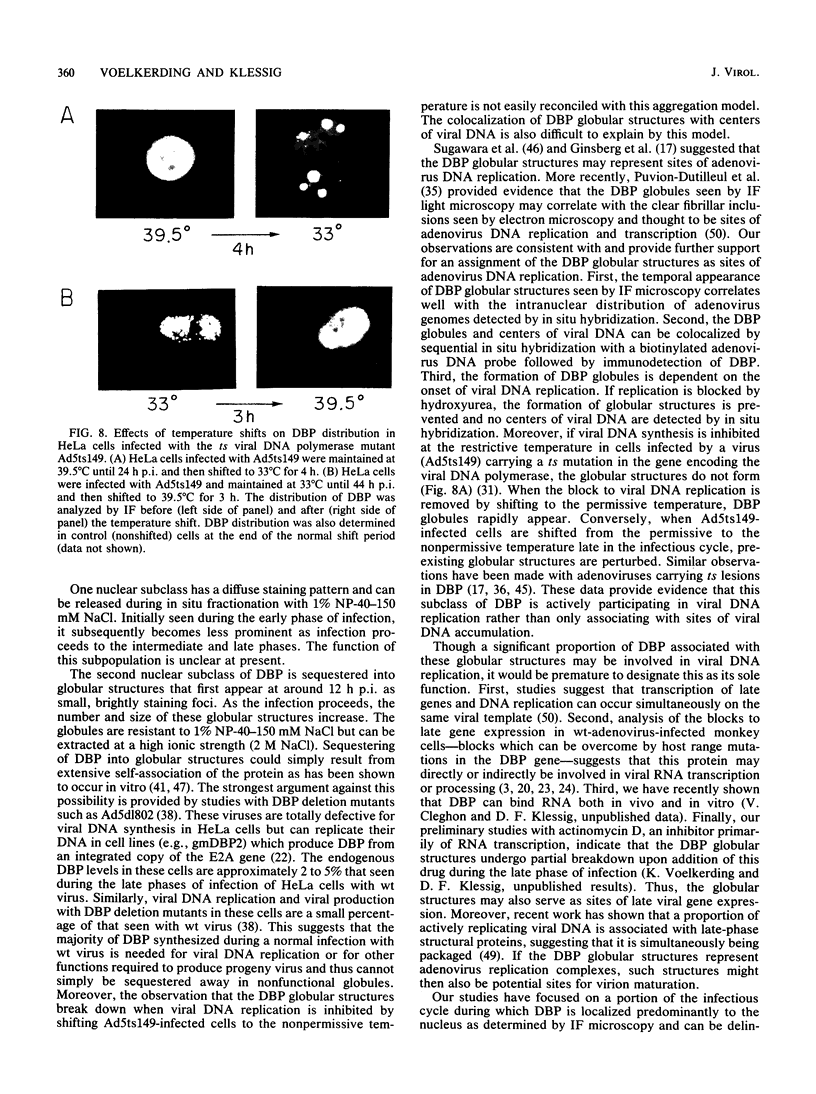


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Hardy M. M., Dunn J. J., Klessig D. F. Independent, spontaneous mutants of adenovirus type 2-simian virus 40 hybrid Ad2+ND3 that grow efficiently in monkey cells possess indentical mutations in the adenovirus type 2 DNA-binding protein gene. J Virol. 1983 Oct;48(1):31–39. doi: 10.1128/jvi.48.1.31-39.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. P., Klessig D. F. Altered mRNA splicing in monkey cells abortively infected with human adenovirus may be responsible for inefficient synthesis of the virion fiber polypeptide. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4023–4027. doi: 10.1073/pnas.81.13.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. P., Klessig D. F. Posttranscriptional block to synthesis of a human adenovirus capsid protein in abortively infected monkey cells. J Mol Appl Genet. 1983;2(1):31–43. [PubMed] [Google Scholar]
- Ariga H., Klein H., Levine A. J., Horwitz M. S. A cleavage product of the adenovirus DNA binding protein is active in DNA replication in vitro. Virology. 1980 Feb;101(1):307–310. doi: 10.1016/0042-6822(80)90510-3. [DOI] [PubMed] [Google Scholar]
- Axelrod N. Phosphoproteins of adenovirus 2. Virology. 1978 Jun 15;87(2):366–383. doi: 10.1016/0042-6822(78)90141-1. [DOI] [PubMed] [Google Scholar]
- Babich A., Nevins J. R. The stability of early adenovirus mRNA is controlled by the viral 72 kd DNA-binding protein. Cell. 1981 Nov;26(3 Pt 1):371–379. doi: 10.1016/0092-8674(81)90206-3. [DOI] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Brough D. E., Rice S. A., Sell S., Klessig D. F. Restricted changes in the adenovirus DNA-binding protein that lead to extended host range or temperature-sensitive phenotypes. J Virol. 1985 Jul;55(1):206–212. doi: 10.1128/jvi.55.1.206-212.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. H., Blanton R. A. Autoregulation of adenovirus type 5 early gene expression II. Effect of temperature-sensitive early mutations on virus RNA accumulation. J Virol. 1978 Nov;28(2):450–456. doi: 10.1128/jvi.28.2.450-456.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. H., Blanton R. A. Possible role of the 72,000 dalton DNA-binding protein in regulation of adenovirus type 5 early gene expression. J Virol. 1978 Feb;25(2):664–674. doi: 10.1128/jvi.25.2.664-674.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. H., Ginsberg H. S. Viral transcription in KB cells infected by temperature-sensitive "early" mutants of adenovirus type 5. J Virol. 1976 Apr;18(1):156–166. doi: 10.1128/jvi.18.1.156-166.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Ostrove J. M., Kelly T. J., Jr Initiation of adenovirus DNA replication: detection of covalent complexes between nucleotide and the 80-kilodalton terminal protein. J Virol. 1982 Jan;41(1):265–270. doi: 10.1128/jvi.41.1.265-270.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Tachibana C. Y., Abrams H. D., Hann S. R. V-myc- and c-myc-encoded proteins are associated with the nuclear matrix. Mol Cell Biol. 1985 Jan;5(1):114–126. doi: 10.1128/mcb.5.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friefeld B. R., Krevolin M. D., Horwitz M. S. Effects of the adenovirus H5ts125 and H5ts107 DNA binding proteins on DNA replication in vitro. Virology. 1983 Jan 30;124(2):380–389. doi: 10.1016/0042-6822(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Ensinger M. J., Kauffman R. S., Mayer A. J., Lundholm U. Cell transformation: a study of regulation with types 5 and 12 adenovirus temperature-sensitive mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):419–426. doi: 10.1101/sqb.1974.039.01.054. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Lundholm U., Linné T. Adenovirus DNA-binding protein in cells infected with wild-type 5 adenovirus and two DNA-minus, temperature-sensitive mutants, H5ts125 and H5ts149. J Virol. 1977 Jul;23(1):142–151. doi: 10.1128/jvi.23.1.142-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Kingston R. E., Sharp P. A. Inhibition of adenovirus early region IV transcription in vitro by a purified viral DNA binding protein. Nature. 1983 Apr 7;302(5908):545–547. doi: 10.1038/302545a0. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Temperature-sensitive replication of H5ts125 adenovirus DNA in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4291–4295. doi: 10.1073/pnas.75.9.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston J. M., Anderson K. P., Klessig D. F. Partial block to transcription of human adenovirus type 2 late genes in abortively infected monkey cells. J Virol. 1985 Nov;56(2):378–385. doi: 10.1128/jvi.56.2.378-385.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H., Maltzman W., Levine A. J. Structure-function relationships of the adenovirus DNA-binding protein. J Biol Chem. 1979 Nov 10;254(21):11051–11060. [PubMed] [Google Scholar]
- Klessig D. F., Brough D. E., Cleghon V. Introduction, stable integration, and controlled expression of a chimeric adenovirus gene whose product is toxic to the recipient human cell. Mol Cell Biol. 1984 Jul;4(7):1354–1362. doi: 10.1128/mcb.4.7.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig D. F., Chow L. T. Incomplete splicing and deficient accumulation of the fiber messenger RNA in monkey cells infected by human adenovirus type 2. J Mol Biol. 1980 May 15;139(2):221–242. doi: 10.1016/0022-2836(80)90306-x. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Grodzicker T. Mutations that allow human Ad2 and Ad5 to express late genes in monkey cells map in the viral gene encoding the 72K DNA binding protein. Cell. 1979 Aug;17(4):957–966. doi: 10.1016/0092-8674(79)90335-0. [DOI] [PubMed] [Google Scholar]
- Klessig D. F., Quinlan M. P. Genetic evidence for separate functional domains on the human adenovirus specified, 72 kd, DNA binding protein. J Mol Appl Genet. 1982;1(4):263–272. [PubMed] [Google Scholar]
- Kruijer W., Nicolas J. C., van Schaik F. M., Sussenbach J. S. Structure and function of DNA binding proteins from revertants of adenovirus type 5 mutants with a temperature-sensitive DNA replication. Virology. 1983 Jan 30;124(2):425–433. doi: 10.1016/0042-6822(83)90358-6. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Van Schaik F. M., Sussenbach J. S. Nucleotide sequence of the gene encoding adenovirus type 2 DNA binding protein. Nucleic Acids Res. 1982 Aug 11;10(15):4493–4500. doi: 10.1093/nar/10.15.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., van Schaik F. M., Sussenbach J. S. Structure and organization of the gene coding for the DNA binding protein of adenovirus type 5. Nucleic Acids Res. 1981 Sep 25;9(18):4439–4457. doi: 10.1093/nar/9.18.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson A., Levine A. J. The isolation and identification of the adenovirus group C tumor antigens. Virology. 1977 Jan;76(1):1–11. doi: 10.1016/0042-6822(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Linné T., Jörnvall H., Philipson L. Purification and characterization of the phosphorylated DNA-binding protein from adenovirus-type-2-infected cells. Eur J Biochem. 1977 Jun 15;76(2):481–490. doi: 10.1111/j.1432-1033.1977.tb11618.x. [DOI] [PubMed] [Google Scholar]
- McPherson R. A., Ginsberg H. S., Rose J. A. Adeno-associated virus helper activity of adenovirus DNA binding protein. J Virol. 1982 Nov;44(2):666–673. doi: 10.1128/jvi.44.2.666-673.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Winkler J. J. Regulation of early adenovirus transcription: a protein product of early region 2 specifically represses region 4 transcription. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1893–1897. doi: 10.1073/pnas.77.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas J. C., Sarnow P., Girard M., Levine A. J. Host range temperature-conditional mutants in the adenovirus DNA binding protein are defective in the assembly of infectious virus. Virology. 1983 Apr 15;126(1):228–239. doi: 10.1016/0042-6822(83)90474-9. [DOI] [PubMed] [Google Scholar]
- Puvion-Dutilleul F., Pédron J., Cajean-Feroldi C. Identification of intranuclear structures containing the 72K DNA-binding protein of human adenovirus type 5. Eur J Cell Biol. 1984 Jul;34(2):313–322. [PubMed] [Google Scholar]
- Reich N. C., Sarnow P., Duprey E., Levine A. J. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology. 1983 Jul 30;128(2):480–484. doi: 10.1016/0042-6822(83)90274-x. [DOI] [PubMed] [Google Scholar]
- Rice S. A., Klessig D. F. Isolation and analysis of adenovirus type 5 mutants containing deletions in the gene encoding the DNA-binding protein. J Virol. 1985 Dec;56(3):767–778. doi: 10.1128/jvi.56.3.767-778.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Klessig D. F. The function(s) provided by the adenovirus-specified, DNA-binding protein required for viral late gene expression is independent of the role of the protein in viral DNA replication. J Virol. 1984 Jan;49(1):35–49. doi: 10.1128/jvi.49.1.35-49.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Westphal H. A cascade of adenovirus early functions is required for expression of adeno-associated virus. Cell. 1981 Nov;27(1 Pt 2):133–141. doi: 10.1016/0092-8674(81)90367-6. [DOI] [PubMed] [Google Scholar]
- Rossini M. The role of adenovirus early region 1A in the regulation of early regions 2A and 1B expression. Virology. 1983 Nov;131(1):49–58. doi: 10.1016/0042-6822(83)90532-9. [DOI] [PubMed] [Google Scholar]
- Schechter N. M., Davies W., Anderson C. W. Adenovirus coded deoxyribonucleic acid binding protein. Isolation, physical properties, and effects of proteolytic digestion. Biochemistry. 1980 Jun 10;19(12):2802–2810. doi: 10.1021/bi00553a041. [DOI] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Different structural systems of the nucleus are targets for SV40 large T antigen. Cell. 1983 May;33(1):173–181. doi: 10.1016/0092-8674(83)90346-x. [DOI] [PubMed] [Google Scholar]
- Staufenbiel M., Deppert W. Preparation of nuclear matrices from cultured cells: subfractionation of nuclei in situ. J Cell Biol. 1984 May;98(5):1886–1894. doi: 10.1083/jcb.98.5.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Tamanoi F., Mathews M. B. Purification of an adenovirus-coded DNA polymerase that is required for initiation of DNA replication. Cell. 1982 Dec;31(3 Pt 2):613–623. doi: 10.1016/0092-8674(82)90317-8. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., White E., Grodzicker T. Independent mutations in Ad2ts111 cause degradation of cellular DNA and defective viral DNA replication. J Virol. 1984 May;50(2):598–605. doi: 10.1128/jvi.50.2.598-605.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara K., Gilead Z., Wold W. S., Green M. Immunofluorescence study of the adenovirus type 2 single-stranded DNA binding protein in infected and transformed cells. J Virol. 1977 May;22(2):527–539. doi: 10.1128/jvi.22.2.527-539.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliet P. C., Sussenbach J. S. An adenovirus type 5 gene function required for initiation of viral DNA replication. Virology. 1975 Oct;67(2):415–426. doi: 10.1016/0042-6822(75)90443-2. [DOI] [PubMed] [Google Scholar]
- Weber J. M., Déry C. V., Mirza M. A., Horvath J. Adenovirus DNA synthesis is coupled to virus assembly. Virology. 1985 Jan 30;140(2):351–359. doi: 10.1016/0042-6822(85)90371-x. [DOI] [PubMed] [Google Scholar]
- Wolgemuth D. J., Hsu M. T. Visualization of nascent RNA transcripts and simultaneous transcription and replication in viral nucleoprotein complexes from adenovirus 2-infected HeLa cells. J Mol Biol. 1981 Apr 5;147(2):247–268. doi: 10.1016/0022-2836(81)90440-x. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Keegstra W., Jansz H. S. Complex formation between the adenovirus type 5 DNA-binding protein and single-stranded DNA. Eur J Biochem. 1978 May 16;86(2):389–398. doi: 10.1111/j.1432-1033.1978.tb12321.x. [DOI] [PubMed] [Google Scholar]