Abstract
This paper describes a method for a single-step, site-specific conjugation of bioactive peptides to proteins that exploits the monitoring advantages provided by the unique UV signature absorbance of a bis-arylhydrazone. The utility of this method is demonstrated by the conjugation of a decapeptide molecular adjuvant, YSFKDMP(MeL)aR (EP67), to two test proteins, ovalbumin (OVA) and bovine serum albumin (BSA), and to proteins expressed on intact influenza virons and fungal arthroconidia (spores) of Coccidioides. Conjugation is accomplished with a version of EP67 in which its N-terminus is modified with succinimidyl-4-benzoylhydrazino-nicotinamide (S4BHyNic) (peptide 7), thus enabling conjugation to these large entities via formation of amide bonds with surface-exposed amino groups. The presence of the strongly absorbing bis-arylhydrazone S4BHyNic (ε354 nm = 29,000 Lmol−1cm−1) allows for determination of EP67-to-protein molar substitution ratios (MSR), which are in good agreement with the MSRs determined by amino acid analysis. Conjugation to OVA does not compromise the ability of EP67 to engage C5a receptor bearing antigen presenting cells (APC) as measured by the EP67-mediated release of interleukin-6 (IL-6) from APCs. Mice immunized with the resulting OVA-EP67 vaccine conjugate produce high serum titers of OVA-specific IgG antibodies relative to OVA alone. Also, the conjugation of EP67 does not affect the surface integrity of influenza virons or the biological viability of Coccidioides spores. This method of conjugating bioactive peptides to proteins and other large biological entities may represent a convenient and effective way generating various bio-conjugates for use in mechanistic studies or novel therapeutic entities such as EP67-containing vaccines.
INTRODUCTION
Conjugation of bioactive peptides to proteins with retention of biological activity requires a method of chemoselective ligation that does not interfere with or obscure the essential topochemical features that impart biological activity to the peptide. Typically, this is achieved by first modifying the protein with a functional group that possesses reactive specificity for a complementary functional group introduced on a specific site on the peptide.
An innovative example of such chemoselective ligation is the formation of a bis-arylhydrazone linkage between a protein modified with an aromatic aldehyde (4-formyl benzoic acid) and a peptide modified with a hydrazinonicotinamide (HyNic) moiety (1,2). This method has the advantage that the resulting bis-arylhydrazone conjugate possesses a strong signature absorbance at 354 nm (ε = 29,000 Lmol−1cm−1), which can be used to follow the course of the conjugation reaction and to determine the final peptide-to-protein molar substitution ratio (MSR). However, this conjugation method requires multiple steps, the first being modification of the protein with an excess of the OSu ester of 4-formylbenzoic (S4FB) at high pH (7.5–8.0). Unreacted S4FB is removed via dialysis and the high pH buffer is replaced with low pH buffer (4.5–6.0) for the formation of the bis-arylhydrazone linkage with the HyNic-modified peptide. Unreacted peptide is removed via dialysis and the low pH reaction buffer replaced with a more physiologically compatible buffer. The principal encumbrance of this multi-step conjugation is that it presents opportunities for inadvertent loss of protein at each modification, conjugation, and buffer exchange step. Another drawback is that not all 4SFB moieties on the protein will react with the HyNic-containing peptide leaving behind 4SFB-modified sites that could unnecessarily alter certain biological attributes of the protein such as immunogenicity.
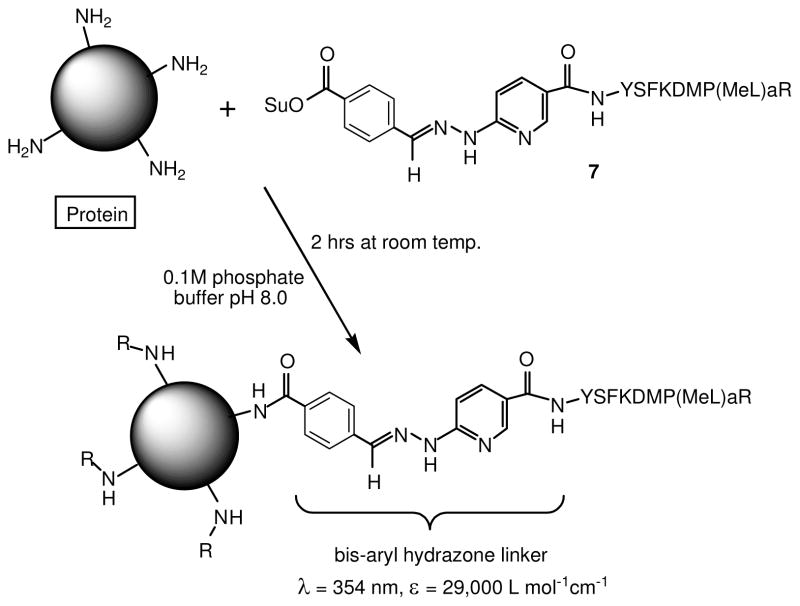
In this paper, we describe a method of conjugating bioactive peptides to proteins that retains the chemoselective and monitoring advantages of the bis-arylhydrazone conjugation, but is accomplished in a single step. This is achieved by conjugating the intact protein with a peptide modified with a self-contained bis-arylhydrazone in the form of succinimidyl-4-benzoylhydrazino-nicotinamide (S4BHyNic). Conjugation occurs efficiently in a single step through the formation of stable amide bonds via the OSu ester on the peptide. This single-step method does not require prior modification of the protein and retains the same monitoring advantages provided by the bis-arylhydrazone in the multi-step conjugation method described above.
The utility of this single-step process is demonstrated by the conjugation of our molecular adjuvant EP67 (Tyr-Ser-Phe-Lys-Asp-Met-Pro-N-methylLeu-D-Ala-Arg or YSFKDMP(MeL)aR) to two test proteins ovalbumin (OVA) and bovine serum albumin (BSA) as well as with intact influenza virons and live Coccidioides spores. EP67 is a conformationally-biased, response-selective agonist of human C5a and operates as a molecular adjuvant by targeting covalently attached antigens (Ag) to and stimulating the Ag processing and presentation capacity of antigen presenting cells (APC) via interaction with C5a receptors (C5aR) expressed on the surface of these cells (3–6).
Conjugation of EP67 to these larger molecular and biological entities is accomplished by modifying the N-terminus of EP67 with S4BHyNic, which in the presence of the selected proteins, virons, and live fungal spores, forms stable amide linkages with ε-amino groups of Lys residues on the exposed proteins. This single-step conjugation method exploits the bis-aryhydrazone signature absorbance as a convenient method of following the course of the conjugation reaction and determination of the peptide-to-protein MSR. A key feature of this method is that it allows conjugation at a specific site on EP67, the N-terminus, which leaves the biologically important C-terminal region of EP67 free to interact withC5aRs expressed on APCs.
The results presented in this paper are discussed against the backdrop of a new generation of EP67-containing vaccines in which whole intact proteins and biological entities such as viruses and live attenuated fungal spores can be used as target Ags. This not only expands the repertoire of Ags that can be used in such vaccines, but enables the EP67-activated APCs to process and present multiple epitopes within the protein(s) in the context of MHC class I and II.
EXPERIMENTAL PROCEDURES
Reagents and General Methods
Chemicals and reagents for peptide synthesis were purchased from Sigma-Aldrich (St. Louis, MO), AAPPTEC (Louisville, KY), Creosalus (Louisville, KY), and AnaSpec (San Jose, CA). All reagents were used as supplied by the manufacturer without further purification. UV-inactivated influenza virus (A/Puerto Rico/8/34) was obtained from Charles River Laboratory. Conjugation of EP67 to live spores of Coccidioides posadasii, which has been designated as a select agent by the Centers for Disease Control and Prevention (http://www.selectagents.gov/agentToxinlist.htm), was performed in a certified biological safety level 3 (BSL3) laboratory at the University of Texas at San Antonio.
For protein conjugate analyses, single-wavelength absorbance measurements were obtained with a Shimadzu BioSpec-1601 spectrometer in 1.5 ml quartz cuvettes (1 cm path length). Scanning spectra for proteins, viral, and fungal conjugates were obtained using a Molecular Devices Spectra Max M2 spectrometer in 1.5 ml quartz cuvettes (1 cm path length) against a water standard.
Mice
Female C57BL/6 mice (2–3 months old) were purchased from Harlan-Sprague Dawley and housed in the Sidney Kimmel Cancer Center (SKCC) vivarium under specific pathogen free conditions. All animal experiments were approved by the SKCC Institutional Animal Care and Use Committee (IACUC) and performed in compliance with the institute’s guidelines.
Synthesis
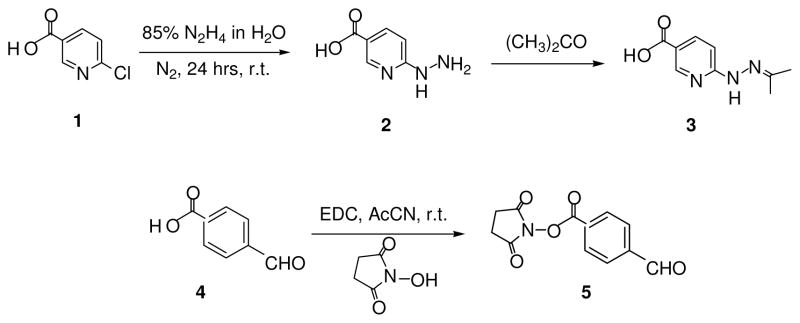
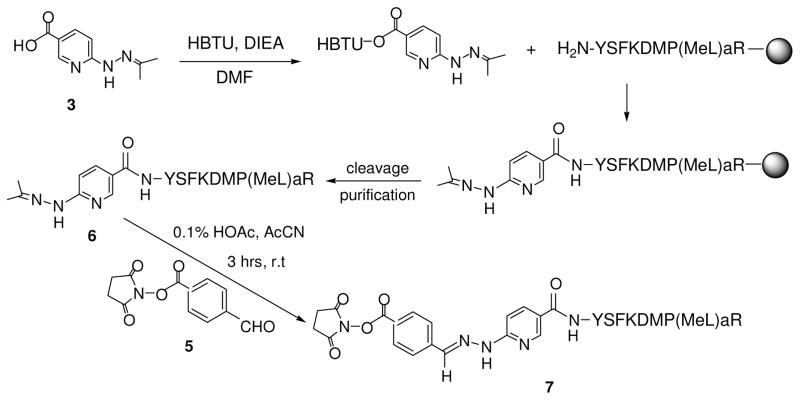
Compounds 3 and 5 were synthesized by the reactions shown in Scheme 1 and the bis-arylhydrazone-containing peptide, S4BHyNic-YSFKDMP(MeL)aR (7), as shown in Scheme 2.
Scheme 1.
Scheme 2.
6-Hydrazinonicotinic acid acetone hydrazone (3)
6-chloronicotinic acid (1) (2.1g, 13.3 mmol) was dissolved in 10 ml of 85% hydrazine in water and stirred overnight under a N2 atmosphere to generate the hydrazine 2 (7). The reaction solution was placed on ice and cooled for several minutes and the hydrazone (3) precipitated by the slow addition of acetone (ca. 150 ml) with stirring. The precipitate was collected by filtration and washed three times with dichloromethane (DCM) to remove the acetone hydrazone impurity. Yield 81%, mp 260–262 °C, 1H-NMR (500 MHz, DMSO-d6) δ1.94 (s, 3H), 1.97 (s, 3H), 7.07 (d, J = 8.8 Hz, 1H), 7.99 (d, J = 8.8 Hz, 1H), 8.60 (s, 1H), 9.89 (s, 1H) 12.55 (brs, 1H); 13C-NMR (125.7 MHz, DMSO-d6) δ17.36, 25.41, 105.62, 116.81, 138.82, 149.96, 150.49, 160.33, 166.76; C9H11N3O2, Mcalc = 193, MH+ = 194.08.
Succinimidyl 4-formylbenzoate (5)
4-formyl benzoic acid (4) (2.0g, 13.3 mmol) was dissolved in acetonitrile (CH 3CN) (ca. 50 ml) along with 1.1 equivalent each of N-hydroxy-succinimide and N-(3-dimethylamino-propyl)-N′-ethylcarbodiimide HCl (EDC) and stirred overnight at room temperature. CH3CN was removed in vacuo and replaced by DCM (ca. 100 ml) and washed three times with water. The solution was dried over MgSO4 and DCM removed in vacuo to yield 5 as a white powder. Yield 91%, mp 173–175 °C, 1H-NMR (500 MHz, CDCl3) δ2.94 (s,4H), 8.03 (d, J = 7.8 Hz, 2H), 8.31 (d, J = 7.8 Hz, 2H), 10.14 (s, 1H); 13C-NMR (125.7 MHz, CDCl3) δ 25.64, 129.70, 129.92, 131.15, 140.28, 161.02, 168.95, 191.18.
EP67and HyNic-EP67 (6)
EP67 was synthesized by standard solid phase methods (AAPPTEC Apex 396 synthesizer) on a pre-loaded Arg Wang resin using the Fmoc (9- fluorenylmethoxy-carbonyl) method of orthogonal synthesis with HBTU [2-(1H-benzotriazol-1-yl)-1,1,3,3-tetra-methyluronium hexafluorophospate]-activated esters of the amino acids. HyNic-EP67 (6) was generated by coupling the HBTU-activated ester of 5 to the Fmoc-deprotected and exposed N-terminus of EP67 while still attached to the Wang resin. The course of this HyNic coupling reaction was monitored by the loss of the free amine using the ninhydrin reaction.
EP67 was cleaved from the resin and side-chain protecting groups removed by stirring the peptide-resin for 1.5 hrs at room temperature in a cleavage cocktail made of TFA (87.5%), phenol (5%), water (5%), and triisopropylsilane (2.5%). NyNic-EP67 was cleaved under identical conditions but with a cocktail made of TFA (92.5%), water (2.5%), triisopropylsilance (2.5%), and acetone (2.5%), which was added in order to generate the acetone hydrazone 6.
EP67 and HyNic-EP67 were precipitated by the addition of cold ether (ca. 50 ml) to the cleavage cocktails and were collected by centrifugation. Peptides were purified by analytical and preparative reverse-phase HPLC on C18-bonded silica columns with a running buffer of 0.1% TFA (Solvent A) and 60% CH3CN in 0.1% TFA (Solvent B) as the eluant. Peptides were characterized by confirmation of molecular mass using MALDI mass spectrometry. EP67: M calc = 1240, MH+ = 1241.3; HyNic-EP67: Mcalc = 1604, MH+ = 1605.2.
Succinimidyl-4-benzoylhydrazinonicotinamide-EP67 (S4B-HyNic-EP67) (7)
HyNic-EP67 (6) (20 mg, 0.015 mmol), 1.1 equivalent of S4FB (5), and 1.0 μl of anisole were dissolved in 15 ml of 0.1% acetic acid and CH3CN (70:30) and stirred at room temperature for 3 hours. The solution was frozen and lyophilized to yield 7 as a dry yellow powder, which was purified using the HPLC conditions described above and characterized by MALDI mass spectrometry, Mcalc = 1603, MH+ = 1604.4.
Conjugation of EP67 to Proteins
Conjugation of 7 to proteins was accomplished via the reaction shown in Scheme 3. BSA and OVA (ca. 5 mg) were dissolved in 1.0 ml of 0.1M phosphate buffer pH 8.0. To these solutions was added 100 μl of a stock solution of 7 dissolved in dimethylsulfoxide (DMSO) such that at least a10-fold molar excess of 7 was introduced. The protein-peptide reaction solutions were mixed at room temperature for 2 hours, after which unreacted peptide removed and buffer replaced with water by centrifugation dialysis (MW cutoff 10K).
Scheme 3.
Conjugation of EP67 to Influenza
Conjugation of 7 to UV-inactivated influenza was performed under identical conditions with the 10-fold molar excess of 7 estimated from the known total protein content and composition of the virus. Unreacted peptide was removed and buffer replaced with water by centrifugation dialysis (MW cutoff 100K).
Conjugation of EP67 to Fungal Spores
Conjugation of 7 to live spores of C. posadasii isolated from a genetically-engineered, attenuated vaccine strain (8) was performed by a modification of the above method. Spores were harvested from Petri plate cultures of C. posadasii grown on GYE medium (1% glucose, 0.5% yeast extract, 1.5% agar) after 4 weeks of incubation at 30°C. The plates were washed and spores suspended in 0.1 M phosphate buffer, pH 8.0. The concentration of viable spores in the buffer suspension was calculated by hemocytometer counts compared to serial dilutions of the suspension to determine plate counts of colony-forming units grown on GYE medium. A stock solution of 7 dissolved in DMSO was prepared as above. Aliquots of the spore suspension, each containing 1.0 × 107 viable cells, were mixed with 7 at different concentrations (10 μg to 1,000 μg) in a final volume of 1.0 ml of phosphate buffer pH 8.0. The spore suspensions were incubated for 2 hours at room temperature with mild agitation and then washed twice with PBS to remove unreacted 7 and resuspended in 1.0 ml of PBS.
Determination of Molar Substitution Ratios (MSR)
At the end of the 2-hour conjugation reaction of 7 with OVA or BSA, 20 μl of the reaction solution was diluted with water to 1.0 ml in a quartz cuvette (1 cm path length). The concentration of the protein-7 conjugate was determined by measuring the absorbance (A) of the bis-arylhydrazone linker at 354 nm and using Beer’s law to determine final concentration: A = εlc, where ε = 29,000 Lmol−1cm−1,l = cuvette path length, and c = concentration in mol/L. MSR was determined by dividing the concentration of the bis- arylhydrazone-containing protein-peptide conjugate by the known concentration of the protein used in the conjugation reaction. MWs for OVA and BSA used in these calculations were 45,000 and 66,400, respectively.
MSR also was determined by amino acid compositional analysis, which was performed by the Protein Structure Core Facility at the University of Nebraska Medical Center using a Hitachi L-8800A amino acid analyzer. Conjugate samples were hydrolyzed for 20 hours at 110° C in 6N HCl vapor under an argon atmosphere with 1% phenol and 0.5 % sodium sulfite in the hydrolysis mix. After hydrolysis, the samples were dissolved in 200 μl of 0.02N HCl and 50 μl was injected automatically onto the amino acid analyzer. Norleucine was added to a measured amount of sample as an internal standard. Chromatographic runs were monitored at 570 and 440 nm. In data analysis, a correction was made to the amount of the internal standard, thus minimizing dilutional errors.
Post-Conjugation Determination of EP67 Biologic Activity
Prior to assaying for biological activity in vitro or in vivo, the OVA-EP67 conjugates were depleted of any potentially interfering endotoxin by utilizing an AffinityPak Detoxi-Gel endotoxin removal kit (ThermoScientific, Rockford, IL) according to the manufacturer’s instructions. Retention of biologic activity of EP67 after its conjugation to OVA was assessed by measurement of the EP67-medated release of interleukin-6 (IL-6) from C5aR-bearing APCs derived from the spleens of C57BL/6 mice. Spleen cells were cultured for 48 hours in the presence of EP67 or OVA-EP67, supernatants collected, and assayed for the presence IL-6 by a sandwich ELISA as described previously (9).
Immunologic activity of the OVA-EP67 conjugate vaccine was assessed by measuring the titers of OVA-specific antibodies (Ab) in the sera of C57BL/6 mice immunized with the OVA-EP67 construct. Mice were immunized by intraperitoneal injection of 100 μg (100 μL) of OVA-EP67 on day 0 and bled on day 21. Anti-OVA Ab titers (total IgG) were measured by a direct ELISA as previously described (10).
Electron Microscopy
Viral particles were negatively stained with a mixture of uranyl acetate and methyl cellulose (25 centipoises, Sigma M-6385) in water at a final concentration of 1.3 % each for 10 minutes at room temperature. Images were obtained with a Morgagni 268D electron microscope equipped with a MegaView III digital camera at 100 kV.
Viability and Immunofluorescence
Serial dilutions of known number of spores incubated with different initial concentrations of 7 as described above, or spores incubated in buffer alone, were plated on GYE medium and the numbers of CFUs were determined. The percent spore viability was calculated as CFUs of EP67-conjugated spores/CFUs of non-conjugated spores X 100.
For immunofluorescence studies, the EP67-conjugated spores (reacted with 1,000 μg of 7/ml in phosphate buffer) and non-conjugated spores incubated as described above. The cells were subsequently fixed with 1% paraformaldehyde for 15 minutes at room temperature, washed with PBS, blocked with10% fetal bovine serum at 4oC overnight, and then incubated with rabbit anti-EP67 Ab followed by fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG. The cells were examined with a Leica DM6000 fluorescence microscope equipped with a DFC 300FX digital camera.
RESULTS
EP67 Conjugates to OVA and BSA
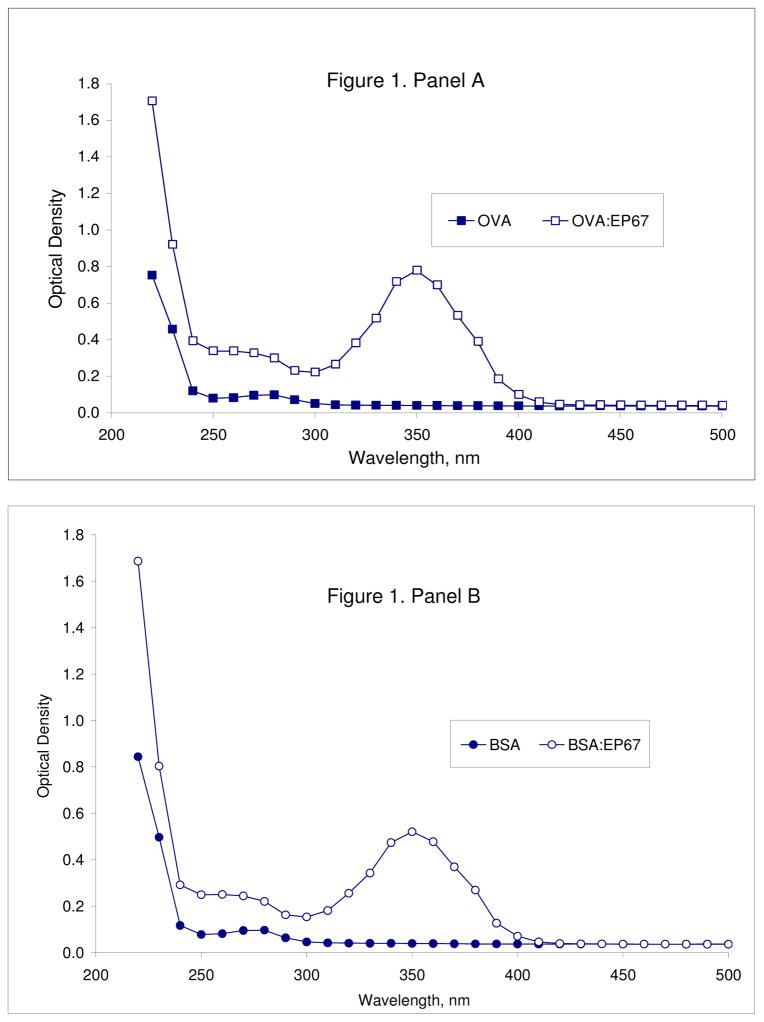
A 10-fold molar excess of S4BHyNic-modified EP67 (7) effectively conjugates to the proteins OVA (Figure 1, Panel A) and BSA (Figure 1, Panel B) in 0.1M phosphate buffer pH 8.0 after a 2-hour reaction as indicated by the increased absorbance at 354 nm from the bis-arylhydrazone on the peptide. MSRs determined by Beer’s law are in good agreement with those determined by amino acid analysis of the conjugates (Table 1). Neither total absorbance at 354 nm nor MSR (as determined by Beer’s law) were changed significantly by reaction with a 20-fold molar excess of 7 or increased reaction time up to 16 hours (data not shown). Conjugation of 7 to protein appears to go to completion since eluents obtained from dialysis of the reaction mixtures were free of 7 as indicated by a lack of ninhydrin-detectable amino groups from the available Lys in EP67.
Figure 1.
Panel A. Absorbance spectra of 4.3 mg OVA and OVA reacted in the presence of a 20-fold molar excess of 7 in 1.0 ml of phosphate buffer pH 8.0 for 2 hours at room temperature. Spectra were generated from 20 μl of the solutions (after dialysis) diluted to 1.0 ml with water. Panel B. Absorbance spectra of 4.0 mg BSA and BSA reacted in the presence of a 20-fold molar excess of 7 in 1.0 ml of phosphate buffer pH 8.0 for 2 hours at room temperature. Spectra were generated from 20 μl of the solutions (after dialysis) diluted to 1.0 ml with water.
Table 1.
MSR Determination of OVA-EP67 and BSA-EP67 Conjugates
Average of 3 measurements
Average of 2 measurements
EP67 Conjugates to Intact Influenza
An absorbance peak at 354 nm is observed after reaction of 7 with intact UV-inactivated influenza for 2 hours in 0.1M phosphate buffer pH 8.0 (Figure 2). Electron micrographs of the influenza virus are shown in Figure 3 prior to (Panel A) and after conjugation of 7 (Panel B). A comparison of these electron micrographs shows that the conjugation process does not appear to alter overall viral structure.
Figure 2.
Absorbance spectra of intact, UV-inactivated influenza virons equivalent to 1.7 mg of protein in the presence of an approximate 20-fold molar excess of 7 in 1.5 ml phosphate buffer pH 8.0 for 3 hours at room temperature. The spectrum was generated from 50 μl of the reaction solution (after dialysis) diluted to 1.0 ml with water.
Figure 3.
Scanning electron micrograph of intact, UV-inactivated influenza virons before (Panel A) and after (Panel B) conjugation with 7.
EP67 Conjugates to Live Spores of Coccidioides
Figure 4 shows an absorbance peak at 354 nm after reaction of 107 viable spores of C. posadasii with 1000 μg/ml of 7 in 0.1 M phosphate buffer pH 8.0 for 2 hours at room temperature, stored at 4 oC for 24 hours, and followed by incubation at room temperature for an additional period of 4 hours. The absorbance peak at 354 nm increased with higher concentrations of 7 added to equal numbers of spores of C. posadasii with a maximum being obtained at 1000 μg/ml of 7. Determination of the CFUs of spore suspensions incubated with different concentrations of 7 revealed no statistically significant difference between the viability of EP67-conjugated and non-conjugated cells (Figure 5, Student’s t-test, P<0.05).
Figure 4.
Absorbance spectra of arthroconidia (107 viable cells) incubated with 7(1000 μg/ml) in 0.1 M phosphate buffer pH 8.0 for 2 hours at room temperature (closed circles) and arthroconidia (107 viable cells) only (open circles).
Figure 5.
Arthroconidia of C. posadasii (isolate C735) grown on GYE plate cultures for 4 weeks at 30° C, harvested in PBS (ca. 107 cells), incubated for 2 hours with and without 1000 μg/ml of 7 at room temperature, and assessed for viability.
Immunofluorescence microscopy of EP67-conjugated spores (Figure 6, Panel A) and EP67-conjugated spores incubated with primary rabbit anti-EP67 antiserum followed by secondary FITC-labeled goat anti-rabbit IgG clearly show the presence of EP67 on the surface of the live fungal cells (Figure 6, Panel B). The spores were universally labeled, although they displayed variable levels of fluorescence intensity. Spores that were not conjugated with EP67, but were incubated with both the primary and secondary FITC-conjugated Ab, showed an absence of the fluorescent label (Figure 6, Panels C and D).
Figure 6.
Arthroconidia of C. posadasii (isolate C735) grown on GYE plate cultures for 4 weeks at 30° C. Panel A: Cells (ca. 107) incubated with 7 (1000 μg/ml) for 2 hours at room temperature in 0.1 M phosphate buffer pH 8.0, washed with PBS, fixed with 1% paraformaldehyde, washed with PBS, and blocked with 10% FBS overnight at 4°C. Panel B: Cells from Panel A incubated with rabbit anti-EP67 followed by incubation with FITC-labeled goat anti-rabbit IgG and examined by fluorescence microscopy. Control cells (Panels C and D) were not incubated with 7, but were otherwise prepared and examined as in Panels A and B.
S4B-HyNic-EP67 (7) is not Self-Reactive
Conjugation of 7 to proteins is accomplished via amide bond formation between the activated OSu ester of 7 with ε-amino groups on the side-chains of Lys residues on the protein surface. Because a potentially reactive ε-amino group is also available from the lone Lys residue in EP67, the possibilities of self-reactivity cannot be ignored. Thus, to determine whether the OSu-activated ester of the bis-arylhydrozone moiety of one EP67 peptide can react with the free ε-amino group on the Lys side-chain of another EP67, 2.1 mg of 7 was dissolved in 1 ml of 0.1 M phosphate buffer pH 8.0 and mixed at room temperature for 2.5 hours. The concentration of the ε-amino group contributed by EP67 was determined with a quantitative ninhydrin reaction as previously described (11). The concentration of free ε–NH2 after 5 minutes was 1.13 × 10−6 M and 1.10 × 10−6 M after 2.5 hours, indicating negligible self-reactivity under the conditions used in the conjugation reactions described above.
Conjugation of EP67 to OVA does not Interfere with EP67 Activity
Conjugation of the small decapeptide, EP67, to a whole protein raises the possibilities that the bulk introduced by the protein may sterically hinder the biologically important C-terminal region of EP67 from interacting with C5aRs on APCs. To assess for this possibility, OVA-EP67 constructs were evaluated for the EP67-mediated release of IL-6 from C5aR-bearing splenic APCs obtained from C57BL/6 mice. The OVA-EP67 conjugate effectively engages C5aR-bearing APCs to induce the release of IL-6 relative to OVA alone (Table 2).
Table 2.
EP67-Induced IL-6 Production
| Activator | μg/ml | IL-6 (pg/ml ± S.D.) |
|---|---|---|
| None | 0 | 125 ± 7 |
| OVA-EP67 | 500 | 420 ± 11 |
| OVA | 500 | 200 ± 3 |
To further demonstrate that EP67 activity is unaffected by conjugation to a large protein, C57BL/6 mice were immunized once with either OVA alone or with an OVA-EP67 conjugate vaccine and the post-injection Ab titers were measure 21 days later by direct ELISA. As shown in Table 3, mice Mice immunized with OVA-EP67 showed a significantly greater anti-OVA Ab response than mice immunized with OVA alone. These results indicate that the EP67 moiety of these conjugates retains biological activity in vivo.
Table 3.
EP67-Induced Enhancement of Anti-OVA IgG Responses in C57BL/6 Mice
1:1,000 dilution
p < 0.05, using a single-tailed t-Test
DISCUSSION
This paper describes a convenient method for the single-step conjugation of bioactive peptides to proteins, viruses, and live fungal cells via formation of stable amide bonds with a peptide modified at a specific site with S4BHyNic. The presence of the bis-arylhydrazone moiety in the conjugation linkage provides a unique UV signature for monitoring/verifying the conjugation reaction and determining the final peptide-to-protein molar substitution ratio. An important benefit of this method is that it enables conjugation via a site chosen on the peptide that will not interfere with topochemical features required for biological activity. This is readily accomplished by first modifying a specific amino group on the peptide with the HBTU-activated ester of HyNic (3) while attached to the solid phase resin. The HyNic-modified peptide is cleaved from the resin, purified, and then used in the solution-phase formation of the bis-arylhydrazone by reaction with OSu4FB (5). The resulting S4BHyNic-modified peptide (7) now can be conjugated to intact proteins in a single step via formation of amide linkages with ε-amino groups on the protein surface.
An important advantage of this single-step method is that it does not require the prior modification of the protein in order to achieve the chemoselective ligation with the peptide. Thus, there are fewer chances for the inadvertent and irreversible modification of important biologic or immunologic features on the protein. Also, since conjugation is accomplished in a single step, there are fewer chances of protein loss.
The applicability of this single-step chemoselective ligation is demonstrated by the successful conjugation of the immunologically active decapeptide EP67 to proteins, influenza virons, and Coccidioides spores. EP67 is a conformationally-biased, response-selective agonist of complement component C5a. The unique conformational features biased in EP67 are well accommodated by C5aRs expressed on APCs, but not on inflammatory neutrophils (3–5). Thus, EP67 has the ability to induce C5a-like immune stimulatory responses via engagement of C5aR-bearing APCs at the expense of C5a-like inflammatory responses via engagement of C5aR-bearing neutrophils.
We have used EP67 (YSFKDMP(MeL)aR) and an earlier sister analogue EP54 (YSFKPMP-LaR) as molecular adjuvants in peptide-based vaccines made by the covalent attachment of well-characterized peptide epitopes to their N-termini. The EP67/EP54 moiety targets the attached epitope to and simultaneously activates C5aR-bearing APCs such that the Ag processing and presentation capacity of the APC is enhanced (6). Immunization with these EP67/EP54-containing vaccines in the absence of added adjuvants results in robust Ag-specific humoral and/or cell-mediated immune outcomes with no discernable inflammatory side effects (10,12–16). However, these adjuvant activities of EP67/EP54 are abrogated if the C-terminal carboxyl group is blocked (10,12), which underscores the necessity of chemoselective ligation via the N-terminus of EP67 if proteins are to be used as the target Ags in protein-EP67 conjugate vaccine constructs.
The ability to effectively conjugate EP67 to proteins is significant, because until now such EP67/EP54-containing vaccines have been restricted to the use of well-characterized peptide epitopes as the target Ags. This was because the only effective way of covalently attaching the epitope to the N-terminus of EP67 was by the solid-phase synthesis of the entire eptiope-EP67 peptide. While such EP67/EP54-containing vaccines are easy to generate, their effectiveness is dependant upon an intimate knowledge of the specific immunogenic epitope(s) within a particular disease-associated protein. Typically, this information is difficult to come by and, consequently, has imposed severe limitations the broad applicability of EP67/EP54-containing vaccines.
The results presented in this paper show that EP67 retains its adjuvant activities following single-step conjugation to large immunogenic entities. The ability to conjugate EP67 to intact proteins, viruses, or live fungal spores greatly expands the repertoire of Ags that can now be used in such molecular adjuvant-containing vaccines. The entire immunogenic entity can now be used as the target Ag with all potential immunoreactive epitopes contained therein available for processing and presentation by the EP67-activated APCs.
Results presented in this study indicate that the bulk contributed by the protein (OVA) does not interfere with the ability of EP67 to engage C5aR-bearing APCs. It is not know whether this will hold true for all possible EP67-containing vaccine conjugates. However, should the bulk contributed by other large target Ag entites interfere with the ability of EP67 to engage C5aR-bearing APCs, it is a straight forward synthetic process to introduce a variety of spacers between the bis-arylhydrazone moiety and EP67 to more effectively expose the C-terminal region of EP67 for C5aR interactions.
Also encouraging was the observation of negligible self-reactivity between S4BHyNic- modified EP67 peptides. This can be explained by a general inaccessibility of the ε-amino group on the Lys side-chain of one EP67 to the reactive S4HyNic moiety on the N-terminus of another. Such inaccessibility may be due to the conformational constraints imposed on the EP67 backbone and a resulting topographic masking of this side-chain along with the likelihood of a salt bridge between the side-chain carboxyl moiety of the adjacent Asp residue (3,5).
Finally, it is worth noting that the immunogenicity of the target Ag does not appear to be affected by the conjugation of EP67. This is supported by the robust humoral response seen in mice vaccinated with OVA-EP67. Serum titers of anti-OVA Abs were significantly higher in mice immunized with OVA-EP67 than mice immunized with OVA alone. Immune outcomes of the influenza virus-EP67 vaccine and the live attenuated fungal spore-EP67 vaccine are currently being evaluated in our laboratories.
Acknowledgments
This project was supported in part by NIH grants AI065712 and AG031496 (JP), AI071118 (GTC), and pilot project funding form the School of Allied Health Professions at the University of Nebraska Medical Center. We would like to thank Halina Witkiewicz and Elisabeth Stein of the Sidney Kimmel Cancer Center for their expert assistance in electron microscopy and cytokine-specific ELISA analysis, respectively. We also acknowledge the assistance of the Protein Structure Core Laboratory at the University of Nebraska Medical Center for mass spectrometry and amino acid analyses.
References
- 1.Schwartz DA, Abrams MJ, Hauser MM, Gaul FE, Larsen SK, Rauh D, Zubieta JA. Preparation of hydrazino-modified proteins and their use for the synthesis of technietium-99m protein conjugates. Bioconjugate Chem. 1991;2:333–338. doi: 10.1021/bc00011a007. [DOI] [PubMed] [Google Scholar]
- 2.Miranda-Olvera AD, Ferro-Flores G, Pedraza-Lopez M, Arteaga de Murphy C, De Leon-Rodriguez LM. Synthesis of oxytocin HYNIC derivatives as potential diagnostic agents for breast cancer. Bioconjugate Chem. 2007;18:1560–1567. doi: 10.1021/bc070047a. [DOI] [PubMed] [Google Scholar]
- 3.Taylor SM, Sherman SA, Kirnarsky L, Sanderson SD. Development of response-selective agonists of human C5a anaphylatoxin: conformational, biological, and therapeutic considerations. Curr Med Chem. 2001;8:675–684. doi: 10.2174/0929867013373156. [DOI] [PubMed] [Google Scholar]
- 4.Short AJ, Paczkowski NJ, Vogen SM, Sanderson SD, Taylor SM. Response-selective C5a agonists: differential effects on neutropenia and hypertension in the rat. Br J Pharmacol. 1999;128:511–514. doi: 10.1038/sj.bjp.0702847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogen SM, Paczkowski NJ, Kirnarsky L, Short A, Whitmore JB, Sherman SA, Taylor SM, Sanderson SD. Differential activities of decapeptide agonists of human C5a: the conformational effects of backbone N-methylation. Int Immunopharmacol. 2001;1:2151–2162. doi: 10.1016/s1567-5769(01)00141-2. [DOI] [PubMed] [Google Scholar]
- 6.Hegde GV, Meyers-Clark E, Joshi SS, Sanderson SD. A conformationally-biased, response-selective agonist of C5a acts as a molecular adjuvant by modulating antigen processing and presentation activities of human dendritic cells. Int Immunopharmacol. 2008;8:819–827. doi: 10.1016/j.intimp.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Bao T, Bai Y, Chang Y, Chen S, Li Z. Technetium-99m-labeling and synthesis of thydmidine analogs: potential candidates for tumor imaging. Bioorg Med Chem Lett. 2007;17:3440–3444. doi: 10.1016/j.bmcl.2007.03.086. [DOI] [PubMed] [Google Scholar]
- 8.Xue J, Chen X, Selby D, Hung C-Y, Yu J-J, Cole GT. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun. 2009;77 doi: 10.1128/IAI.00459-09. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan EL, Sanderson SD, Scholz W, Noonan DJ, Weigle WO, Hugli TE. Identification and characterization of the effector region within human C5a responsible for stimulation of IL-6 synthesis. J Immunol. 1992;148:3937–3942. [PubMed] [Google Scholar]
- 10.Tempero RM, Hollingsworth MA, Burdick MD, Finch AM, Taylor SM, Vogen SM, Morgan EL, Sanderson SD. Molecular adjuvant effects of a conformationaly biased agonist of human C5a anaphylatoxin. J Immunol. 1997;158:1377–1382. [PubMed] [Google Scholar]
- 11.Sarin VK, Kent SBH, Tam JP, Merrifield RB. Quantitative monitoring of solid phase peptide synthesis by the ninhydrin reaction. Anal Biochem. 1981;117:147–157. doi: 10.1016/0003-2697(81)90704-1. [DOI] [PubMed] [Google Scholar]
- 12.Ulrich JT, Cieplak W, Paczkowski NJ, Taylor SM, Sanderson SD. Induction of an antigen-specific CTL response by a conformationally biased agonist of human C5a anaphylatoxin as a molecular adjuvant. J Immunol. 2000;164:5492–5498. doi: 10.4049/jimmunol.164.10.5492. [DOI] [PubMed] [Google Scholar]
- 13.Buchner R, Vogen SM, Sanderson SD, Ye RD, Morgan EL. Anti-human kappa opioid receptor antibodies: characterization of site-directed neutralizing antibodies specific for a peptide κR(33–52) derived from the predicted amino-terminal region of the human kappa receptor. J Immunol. 1997;158:1670–1680. [PubMed] [Google Scholar]
- 14.Sanderson SD, Cheruku SR, Padmanilayam MP, Vennerstrom JL, Thiele GM, Palmatier MI, Bevins RA. Immunization to nicotine with a peptide-based vaccine composed of a conformationally biased agonist of C5a as a molecular adjuvant. Int Immunopharmacol. 2003;3:137–146. doi: 10.1016/s1567-5769(02)00260-6. [DOI] [PubMed] [Google Scholar]
- 15.Floreani AA, Gunselman SJ, Heires AJ, Hauke RJ, Tarantolo S, Jackson JD. Novel C5a agonist-based dendritic cell vaccine in a murine model of melanoma. Cell Cycle. 2007;6:2835–2839. doi: 10.4161/cc.6.22.4899. [DOI] [PubMed] [Google Scholar]
- 16.Duryee MJ, Bevins RA, Reichel RM, Murray JE, Dong T, Thiele GM, Sanderson SD. Immune responses to methamphetamine by active immunization with peptide-based, molecular adjuvant-containing vaccines. Vaccine. 2009;27:2981–2988. doi: 10.1016/j.vaccine.2009.02.105. [DOI] [PubMed] [Google Scholar]