Abstract
The median survival for patients with locally advanced pancreatic cancer treated with gemcitabine and radiation is approximately one year. To develop improved treatment, we have combined a Chk1/2 targeted agent, AZD7762, currently in Phase I clinical trials, with gemcitabine and ionizing radiation in preclinical pancreatic tumor models. We found that in vitro AZD7762 alone or in combination with gemcitabine significantly sensitized MiaPaCa-2 cells to radiation. AZD7762 inhibited Chk1 autophosphorylation (S296 Chk1), stabilized Cdc25A, and increased ATR/ATM-mediated Chk1 phosphorylation (S345 Chk1). Radiosensitization by AZD7762 was associated with abrogation of the G2 checkpoint as well as with inhibition of Rad51 focus formation, inhibition of homologous recombination repair, and persistent γ-H2AX expression. AZD7762 was also a radiation sensitizer in multiple tumor xenograft models. In both MiaPaCa-2- and patient- derived xenografts, AZD7762 significantly prolonged the median time required for tumor volume doubling in response to gemcitabine and radiation. Together, our findings suggest that G2 checkpoint abrogation and homologous recombination repair inhibition both contribute to sensitization by Chk1 inhibition. Furthermore, they support the clinical use of AZD7762 in combination with gemcitabine and radiation for patients with locally advanced pancreatic cancer.
Keywords: pancreatic cancer, Chk1, radiosensitization, gemcitabine, homologous recombination repair
Introduction
Pancreatic cancer remains among the least curable cancers, with an overall 5-year survival for all patients of approximately 5% (1). Gemcitabine (2’,2’-difluoro-2’-deoxycytidine; dFdCyd) is the standard chemotherapy for pancreatic cancer, and the combination of radiation with gemcitabine has been shown superior to gemcitabine alone for locally advanced disease (2, 3). Thus we have sought to improve therapy for locally advanced pancreatic cancer by combining additional agents with gemcitabine and radiation (4, 5).
Gemcitabine requires phosphorylation in order to produce its active diphosphorylated (dFdCDP) and triphosphorylated (dFdCTP) metabolites, dFdCDP inhibits ribonucleotide reductase which leads to depletion of deoxynucleotide triphosphate pools while dFdCTP competes with endogenous dCTP resulting in misincorporation of dFdCTP into DNA. Together these activities result in replication stress and inhibition of DNA synthesis and the activation of checkpoint kinase 1 (Chk1) (see below). As a central mediator of the cellular response to DNA damage, activation of Chk1 in response to DNA damage results in cell cycle arrest (6, 7) as well as promotion of HRR (homologous recombination repair), a process promoted by the binding of the recombinase, Rad51, to sites of DNA double strand breaks (8). Based on data demonstrating that Chk1 is an effective target for sensitization to chemo- and radio- therapy (9–11), small molecule Chk1 inhibitors have been developed for clinical use, principally with the idea that they would be used to enhance killing of tumor cells by cytotoxic drugs or by radiation (12–16). The first Chk1 inhibitor to be tested extensively in humans was UCN-01 (7-hydroxystaurosporine) (17–19). Because UCN-01 is a non-selective Chk1 inhibitor with poor protein binding properties in vivo, several other Chk1 antagonists are in development for clinical use, and three of them (SCH900776, AZD7762 and PF-00477736) are currently in Phase-I clinical trials in combination with gemcitabine or irinotecan, with others due to follow (20–22).
In our previous studies we demonstrated that gemcitabine activates Chk1 (23, 24) and that inhibition of Chk1 promotes premature mitotic entry and cytotoxicity in response to gemcitabine (25). In addition, Chk1 inhibition leads to impaired Rad51 focus formation, a key step in HRR and a prolonged DNA damage response in pancreatic cancer cells treated with gemcitabine (26). The goal of the present study was to determine whether the Chk1/2 inhibitor, AZD7762 sensitizes pancreatic cancer cells to radiation as well as gemcitabine-radiation. When we found that AZD7762 sensitized to radiation both in the presence and absence of gemcitabine in our in vitro pancreatic cancer model, we then went on to determine the mechanism(s) of sensitization. We hypothesized that inhibition of both cell cycle checkpoints and HRR was involved in AZD7762-mediated radiosensitization. To begin to test this hypothesis we determined whether AZD7762 interfered with cell cycle checkpoint activation in BrdU pulse-chase experiments and HRR-mediated DNA repair by Rad51 focus formation and an HRR activity assay. Finally, we tested the efficacy of AZD7762 as a radiation sensitizer in vivo in both cell line- and patient- derived pancreatic tumor xenograft models.
Materials and methods
Cell culture and drug solutions
MiaPaCa-2 cells were obtained from American Type Culture Collection and grown in DMEM supplemented with 10% fetal bovine serum (Invitrogen) and 2 mmol/L L-glutamine (Sigma). Experiments were conducted on exponentially growing cells. Cells were tested for mycoplasma once every 3 months. Gemcitabine (Eli Lilly) was dissolved in PBS. AZD7762 was dissolved in DMSO or 11.3% 2-hydroxypropyl-β-cyclodextrin (Sigma), 0.9% sterile saline for in vitro or in vivo purposes, respectively. Clonogenic survival assays were conducted as previously described (5, 27, 28). Non-specific, Chk1, and Chk2 siRNA were purchased from Dharmacon and used as previously described (23).
Flow cytometry
For γ-H2AX analysis, samples were processed as previously described (29). For BrdU pulse-chase experiments, samples were pulsed with 30 µM BrdU for 15 minutes, washed with medium containing 10 µM thymidine, irradiated, then processed and analyzed as previously described (30) using anti-BrdU (Pharmingen) and FITC-conjugated anti-mouse (Sigma Biochemicals) antibodies. Samples were analyzed on a FACScan flow cytometer (Becton Dickinsson) with FlowJo software (Tree Star).
Homologous recombination repair
MiaPaCa-2 cells were transfected with the pDR-GFP plasmid (31) using SuperFect transfection reagent (Qiagen) according to the manufacturer’s protocol. Clones containing the DR-GFP reporter integrated chromosomally were isolated following puromycin selection. To measure repair of a DNA double strand break, cells were infected with the adenovirus, AdNGUS24i expressing the I-SceI enzyme. I-SceI-induced homologous recombination was measured as the percentage of GFP positive cells 48 hours later by flow cytometry.
Immunoblotting
Cell pellets or pulverized frozen tumors were lysed and immunoblotted as previously described (5). Proteins were detected with Chk1 (S345), Chk1 (S296), Chk1, Chk2 (T68), GAPDH (Cell Signaling), Chk2 (Millipore), Cdc25A (Santa Cruz), or β-actin (Calbiochem) antibodies.
Immunofluorescence
Cells cultured on coverslips were treated as illustrated in Fig. 1A. At times 26 and 30 hours cells were fixed and processed as previously described (26). Samples were imaged with an Olympus FV500 confocal microscope (Olympus America) with a 60x objective. For quantitation of Rad51 foci, at least 100 cells from each of three independent experiments were visually scored for each condition. Cells with ≥ 5 Rad51 foci were scored as positive and compared for statistical analyses. Foci positive cells were binned as having 5 – 9 or 10 or more Rad51 foci.
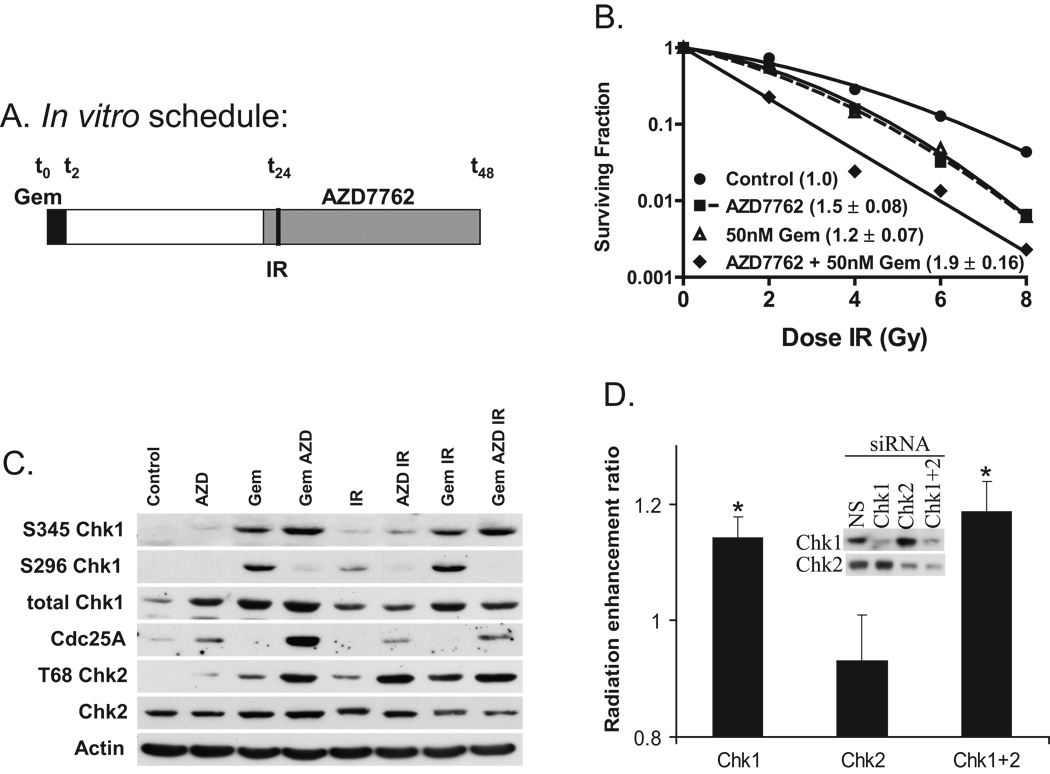
Figure 1. Radiosensitization in response to Chk1 inhibition and gemcitabine.
A, Schedule of treatments. B, MiaPaCa-2 cells were treated with gemcitabine (Gem; 50 nM), AZD7762 (AZD; 100 nM), and radiation (IR; 0–8 Gy) as illustrated (A). The plating efficiency of the untreated control cells was 41 %. C, MiaPaCa-2 cells were treated as illustrated (A) except with 7.5 Gy. Cells were collected for immunoblotting at t = 26 hours. D, Alternatively, cells were treated with Chk1 and/or Chk2 siRNA, irradiated 48 hours post-transfection, and then processed for clonogenic survival. The radiation enhancement ratios were normalized to the mean inactivation dose of the non-specific siRNA (NS) treated samples. Data are from a single experiment representative of 3 independent experiments (B, C) or the mean radiation enhancement ratio of 4 independent experiments ± standard error (B inset, D). For B, error bars are contained within the points. Statistically significant differences are indicated (*, P < 0.05).
Immunohistochemistry
Harvested tumors were fixed in 10% neutral buffered formalin for 24 hours, then embedded in paraffin blocks and sectioned at 5 microns onto slides. Histopathology was conducted using Hematoxylin and Eosin staining and immunohistochemistry using Chk1 (Ser345) (Cell Signaling) antibody, biotinylated rabbit secondary antibody, SA-HRP complex, and DAB chromogen kit (Ventana). Positive rodent control slides showed strong nuclear staining and negative control slides (Rabbit IgG) showed levels of non-specific staining, if any. Tumors were microscopically evaluated with a 20× objective to assess morphological changes and results were reported by a pathologist. Slide images were produced by Aperio Imagescope.
Irradiation
Irradiations were carried out using a Philips RT250 (Kimtron Medical) at a dose rate of approximately 2 Gy/min in the UMCC Experimental Irradiation Core. Dosimetry was carried out using an ionization chamber connected to an electrometer system that is directly traceable to a National Institute of Standards and Technology calibration. For tumor irradiation, animals were anesthetized with isoflurane and positioned such that the apex of each flank tumor was at the center of a 2.4-cm aperture in the secondary collimator, with the rest of the mouse shielded from radiation.
Tumor growth studies
Animals were handled according to a protocol approved by the University of Michigan Committee for Use and Care of Animals. MiaPaCa-2 cells or patient derived pancreatic tumor cells (5×106 or 1×106, respectively) were suspended in a 1 : 1 mixture of 10% FBS/RPMI : Matrigel (BD Biosciences) and injected subcutaneously into the flanks of athymic nude or Nod-scid mice, respectively. Samples of human pancreatic adenocarcinomas were handled as described previously (32). Treatment was initiated when the average tumor volume reached 100 mm3. For tumor growth delay studies, the tumor size was measured 2 times/week. Tumor volume (TV) was calculated according to the equation: TV = π/6 (ab2), where a and b are the longer and shorter dimensions of the tumor, respectively. Measurements were made until day 120 or until the tumor volume increased by approximately a factor of ten.
Statistical analysis
For in vitro radiation enhancement, drug cytotoxicity, and Rad51 foci, statistically significant differences were determined by one-way ANOVA with the Newman-Keuls’ post-comparison test in GraphPad PRISM version 3 (Graph-Pad Software). Additivity was defined by the difference in the area-under-the-curve between the control and gemcitabine-AZD7762 being not significantly different from the sum of the differences between the control and gemcitabine or AZD7762 alone using a two-way ANOVA model with an interaction term (33). For γ-H2AX, data were analyzed using ANOVA (PROC MIXED in SAS). Estimates of means, differences between means, and statistical significance were all derived from the ANOVA model. For in vivo tumor growth, tumor volume doubling was determined for each xenograft by identifying the earliest day on which it was at least twice as large as on the first day of treatment. A cubic smoothing spline (SMOOTH.SPLINE function in R) was used to obtain the exact time of doubling, and the Kaplan-Meier method was used to analyze the doubling times derived from the smoothed growth curves. Log rank test (PROC LIFETEST in SAS) was used for comparisons between any two treatment groups.
Results
AZD7762 radiosensitizes pancreatic cancer cells through inhibition of Chk1
To begin to determine if the Chk1/2 inhibitor, AZD7762 is a radiation sensitizer we treated MiaPaCa-2 pancreatic cancer cells with non-cytotoxic concentrations of gemcitabine and AZD7762 according to the schedule illustrated in Fig. 1A and then assessed radiation survival by a clonogenic assay. We found that AZD7762 alone significantly sensitized MiaPaCa-2 cells to radiation, producing a RER (radiation enhancement ratio) of 1.5 ± 0.08 (P < 0.05; Fig. 1B, Suppl. Table 1). The combination of AZD7762 with gemcitabine further enhanced radiosensitization beyond that observed with gemcitabine alone (RER 1.9 ± 0.16 and 1.2 ± 0.07, respectively; P < 0.05). AZD7762 and gemcitabine produced additive effects on radiosensitization over a range of gemcitabine concentrations (10 – 50 nM) and under conditions which produced minimal to substantial cytotoxicity (0.9 ± 0.06 to 0.2 ± 0.07; Suppl. Table 1). The cytotoxicity produced by AZD7762 in combination with 50 nM gemcitabine (0.2 ± 0.07) was significantly greater (P < 0.05) than that caused by the same concentration of gemcitabine (1.0 ± 0.04) or AZD7762 (0.9 ± 0.05) alone, which is consistent with our previous data demonstrating chemosensitization by Chk1 inhibition (25). We obtained similar data in MPanc96 cells where AZD7762 produced sensitization to radiation (RER 1.4 ± 0.15) and gemcitabine-radiation (RER Gem 1.2 ± 0.16 versus Gem-AZD7762 1.5 ± 0.20) (Suppl. Fig. 1).
To confirm that AZD7762 inhibits Chk1/2 in our models, we analyzed Chk1 and Chk2 signaling. As anticipated, we observed that Chk1 autophosphorylation (S296 Chk1) was inhibited and that Cdc25A was stabilized by AZD7762 in response to gemcitabine, radiation, or gemcitabine-radiation (34) (Fig. 1C). Taken together these results demonstrate that AZD7762 inhibits Chk1. ATR- and ATM-mediated phosphorylation of Chk1 (S345 Chk1) and Chk2 (T68 Chk2) were increased by the addition of AZD7762 to gemcitabine and/or radiation, likely a consequence of the increased level of DNA damage present under these treatment conditions (Fig. 3). To address the relative contributions of inhibition of Chk1 or Chk2 by AZD7762 to radiosensitization, we utilized siRNA to selectively deplete Chk1 or Chk2 from MiaPaCa-2 cells. Relative to non-specific siRNA treated cells, the Chk1 depleted cells were sensitized to radiation similarly while the Chk2 depleted cells were not (Fig. 1D). Depletion of Chk2 did not increase the sensitization produced by depletion of Chk1. These data are consistent with our previous observation that Chk1 but not Chk2 siRNA sensitizes pancreatic cancer cells to gemcitabine (25) and suggest that radiosensitization by AZD7762 is mediated by Chk1 inhibition.
Figure 3. The effects of AZD7762 on Rad51, HRR, and γ-H2AX.
A, MiaPaCa-2 cells were treated as illustrated (Fig. 1A) and fixed for immunofluorescence at 30 hours. Cells were stained with DAPI (blue) and for Rad51 (green). B, Rad51 foci were analyzed at t = 26 and 30 hours. Data are the mean ± standard error of 3 independent experiments. C, MiaPaCa-2-DR-GFP cells were treated as illustrated and at t = 48 hours, the percentage of GFP positive cells was measured by flow cytometry. Data are expressed as the mean percentage of GFP positive cells ± standard error of n = 6 independent experiments. D, γ-H2AX was assessed by flow cytometry in MiaPaCa-2 cells treated with radiation (7.5 Gy) at t = 24 hours, according to the schedule illustrated in Fig. 1A, and collected at various time points following radiation (t = 24 – 48 hours). Data are expressed as the percentage of cells staining positive for γ-H2AX and are the mean ± standard error of n = 4 – 6 independent experiments. B–D, Statistical significance (P < 0.05) is indicated versus Control*, Gem‡, IRπ, Gem IR†.
Gemcitabine- and radiation-induced cell cycle checkpoints are abrogated by AZD7762
To determine whether AZD7762 would modulate Chk1-mediated cell cycle checkpoints, we labeled S-phase cells with BrdU and followed the progression of the cells through the cell cycle over time (t = 26 – 40 hours). This permitted the observation of effects which were more difficult to distinguish by single parameter flow cytometry (Suppl. Fig. 2). Treatment with AZD7762 alone resulted in a more rapid progression from S-phase into G2/M, and subsequently G1, relative to the untreated control cells (Fig. 2; Suppl. Table 2). As anticipated, a non-cytotoxic concentration of gemcitabine resulted in temporary S-phase arrest as evidenced by a narrow S-phase distribution (t = 26 h) and delayed re-entry into the subsequent S-phase (t = 40 h). The addition of AZD7762 to gemcitabine resulted in a more rapid transit of cells from S-phase to G1 and subsequently into a second round of S-phase. Radiation induced a G2 checkpoint, evidenced by G2/M accumulation at 40 hours that was overcome by AZD7762. Finally, the addition of AZD7762 to gemcitabine-radiation resulted in a more rapid transition from G2/M to G1. In response to radiation and gemcitabine-radiation, AZD7762 specifically abrogated the G2 checkpoint as evidenced by an increase in the percentage of phosphorylated histone H3 positive cells (Suppl. Table 3). Together these results support the conclusion that AZD7762 accelerates progression through S-phase and abrogates the G2 checkpoint in response to gemcitabine and radiation treatments, likely via inhibition of Chk1.
Figure 2. The effects of AZD7762 on progression from S-phase in response to gemcitabine and radiation.
MiaPaCa-2 cells were treated as illustrated in Fig. 1A with the exception that cells were pulsed with BrdU immediately prior to radiation (t = 24 h; 7.5 Gy). At times 26, 30, and 40 hours, cells were collected for flow cytometry. Untreated MiaPaCa-2 cells had a 20 hour cell doubling time. Data in the histograms represent only the BrdU positive cells from a single experiment with the percentages of cells in G1, S, and G2/M illustrated. Data are representative of 3 independent experiments.
AZD7762 inhibits homologous recombination repair leading to increased DNA damage
To further explore the mechanisms of radiosensitization by AZD7762, we investigated the effects of AZD7762 on Rad51 and homologous recombination repair. In response to gemcitabine and/or radiation, Rad51 formed discrete nuclear foci at the 30-hour time point (Fig. 3B). The addition of AZD7762 significantly inhibited the appearance of Rad51 foci in response to gemcitabine or radiation alone, as well as in response to the combination of gemcitabine and radiation. In order to distinguish whether AZD7762 was attenuating formation versus promoting dissociation of Rad51 foci, we selected two time points for analysis. We found that in response to gemcitabine and/or radiation, Rad51 foci assembly mainly occurred between 26 and 30 hours. The finding that Rad51 foci failed to assemble in the presence of AZD7762 suggests that AZD7762 acts to inhibit Rad51 focus formation, rather than promote Rad51 focus dissociation.
To specifically measure whether AZD7762 inhibits HRR, we employed the DR-GFP reporter assay which measures homology directed repair of an I-SceI endonuclease-induced DNA double strand break in an integrated GFP reporter gene (31). Consistent with the ability of AZD7762 to inhibit Rad51 focus formation, AZD7762 significantly inhibited HRR as evidenced by a decrease in the percentage of GFP positive cells (Fig. 3C). In the presence of gemcitabine, radiation, or gemcitabine-radiation, AZD7762 also produced significant inhibition of HRR activity. As anticipated, neither gemcitabine nor radiation led to an increase in HRR activity, as this model only measures repair of I-SceI endonuclease-induced DNA double strand breaks. We next assessed the presence of unrepaired DNA damage by conducting quantitative flow cytometric studies of γ-H2AX staining. As anticipated, radiation or gemcitabine-radiation produced a γ-H2AX signal as early as 30 minutes post-irradiation (t = 24.5 hours) that was resolved to basal levels by 16 hours post-irradiation (t = 40 hours) (Fig. 3D). The addition of AZD7762 to radiation resulted in a significant prolongation of γ-H2AX signaling for up to 24 hours post-irradiation (t = 48 hours; 33 ± 5%) compared to radiation alone (10 ± 3%; P < 0.001) (Fig. 3D). Although gemcitabine alone produced Rad51 foci (t = 30 hours), it did not produce a significant increase in γ-H2AX staining, which is likely attributable to the differences in the sensitivity of these two assays. Importantly, treatment with AZD7762 and gemcitabine caused maximal γ-H2AX signaling which persisted through out the course of this study (t = 48 hours; 56 ± 5%). Together, these results demonstrate that AZD7762 inhibits HRR, likely through inhibition of Rad51, in response to gemcitabine and radiation, ultimately resulting in the persistence of unrepaired DNA damage.
Pancreatic tumor xenografts are sensitized to gemcitabine and radiation by AZD7762
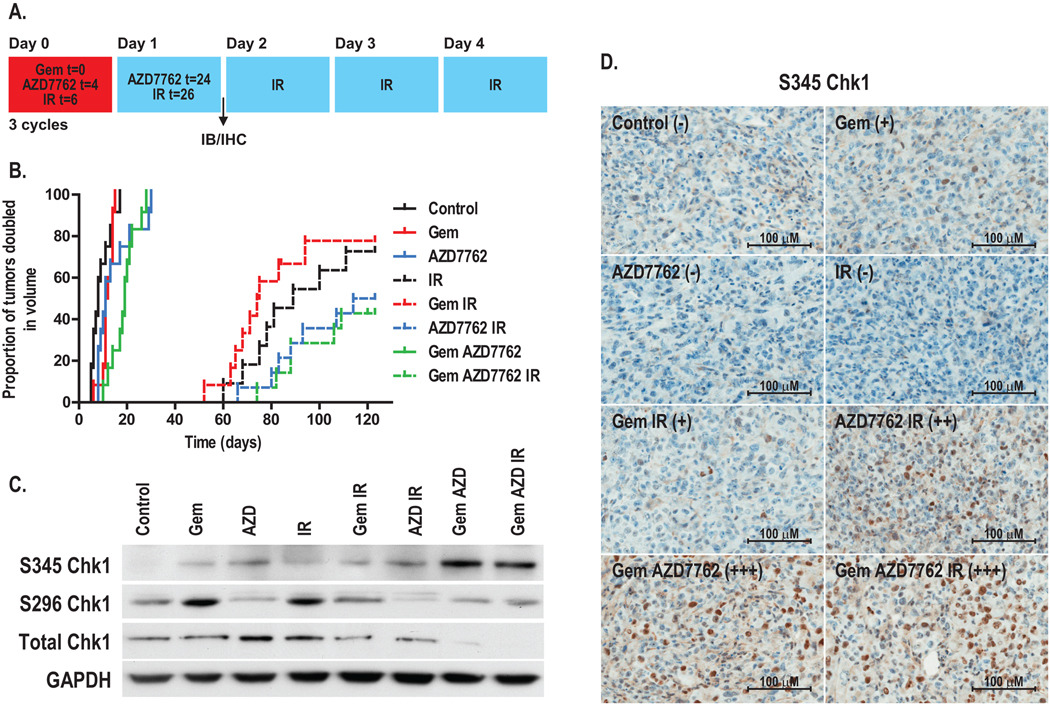
Based on the efficacy of AZD7762 as a sensitizer in vitro, we hypothesized that AZD7762 would be an effective sensitizer in pancreatic tumor models. We began by testing the effects of AZD7762 on the growth of MiaPaCa-2-derived subcutaneous xenografts in response to gemcitabine and radiation. Tumor-bearing mice were treated with gemcitabine, radiation, and AZD7762 as illustrated (Fig. 4A). AZD7762 alone produced a significant growth delay (Δ = 3 days compared to control; P = 0.04) (Fig. 4B and Table 1). More importantly, the combinations of AZD7762 with gemcitabine or gemcitabine-radiation significantly prolonged the time required for tumor volume doubling relative to gemcitabine alone (Δ= 8 days; P < 0.001) or gemcitabine-radiation (Δ > 35 days; P = 0.01). Although there was a trend for AZD7762 to sensitize tumors to radiation (Δ > 25 days), this difference did not reach statistical significance. Therapy with AZD7762, gemcitabine, and radiation was tolerable as the average weight loss for any of the treatment groups in this study was less than 10%.
Figure 4. The effects of AZD7762 on MiaPaCa-2 xenografts in response to gemcitabine and radiation.
A, Schedule of treatments. B, Athymic nude mice bearing bilateral, subcutaneous MiaPaCa-2-derived xenografts were treated with gemcitabine (90 mg/kg), AZD7762 (20 mg/kg), and/or radiation (2 Gy/fraction) for 3 cycles as illustrated (A). Data are expressed as the proportion of tumors doubled in volume. Each treatment group contained 6 – 7 animals (12 – 14 tumors). For immunoblotting (C) or immunohistochemistry (D), tumors were harvested on day 1. Immunoblots are from a single experiment representative of 3 independent experiments. For S345 Chk1 immunohistochemistry, the average score obtained from 3 – 4 tumor specimens is illustrated where ‘ − ’ indicates no staining and ‘+++’, maximal staining.
Table 1.
Tumor doubling time (days)
| Treatment | MiaPaCa-2 | Patient-F | Patient-J |
|---|---|---|---|
| Control | 8 (5,13) | 10.0 (7,10) | 6.5 (6,9) |
| Gem | 12 (10,14) | 33.0* (21,37) | 23.5* (21,26) |
| AZD7762 | 11* (8,21) | 25.5* (20,30) | 26.5* (17,28) |
| IR | 89* (68,nd) | 15.0* (9,18) | 32.5* (16,42) |
| Gem IR | 75* (63,94) | 49.0*π (32,54) | 49.0*π (45,51) |
| AZD7762 IR | >114* (83,nd) | 44.0*π (39,53) | 44.5*π (43,47) |
| Gem AZD7762 | 19*‡ (12,22) | 46.5*‡ (42,52) | 41.0*‡ (37,42) |
| Gem AZD7762 IR | >109*† (88,nd) | 56.0*π (47,62) | 60.5*†π (56,67) |
Data are the median time until tumor volume doubling. The upper and lower limits are indicated in parentheses and nd, where the upper limit could not be determined.
Statistical differences in the time until tumor volume doubling were determined by the log-rank test and are indicated where P<0.05 is indicated versus Control*, Gem‡, Gem IR†, or IRπ.
To confirm Chk1 inhibition by AZD7762 in vivo, we analyzed Chk1/2 signaling in tumors on treatment day one (Fig. 4A). Consistent with our in vitro findings S296 Chk1 was inhibited by AZD7762 in the presence of gemcitabine, radiation, and gemcitabine-radiation (Fig. 4C, Suppl. Fig. 3). Also consistent with our in vitro data, was a trend for S345 Chk1 to be increased in response to any of the treatments; the most prominent increase in S345 Chk1 occurred following treatment with gemcitabine plus AZD7762 (both in the presence and absence of radiation) (Fig. 4C–D; Suppl. Fig. 3). Increased phosphorylation of Chk1 (S345), which targets Chk1 for ubiquitin-mediated proteosomal degradation (35), was paralleled by a loss of total Chk1 protein that is consistent with previous data demonstrating Chk1 degradation in response to cytotoxic doses of gemcitabine and Chk1 inhibitor in MiaPaCa-2 cells (26). Although the in vitro studies (Fig. 1C) presented in this current work did not show Chk1 degradation in response to gemcitabine and AZD7762, it is likely that this difference is due to the non-cytotoxic dose of gemcitabine used in this study.
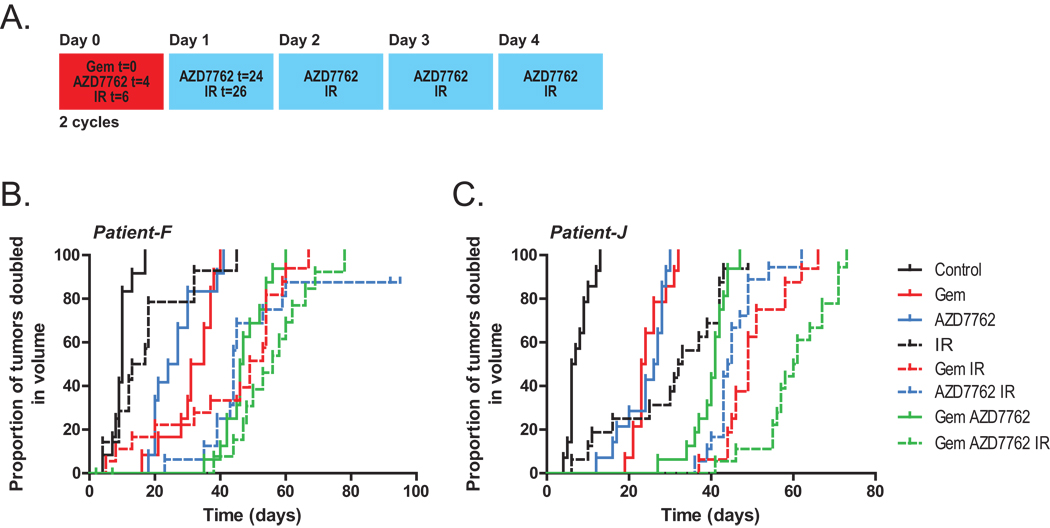
We then wished to determine if AZD7762 could sensitize patient-derived pancreatic tumor xenografts. Pancreatic tumor specimens were obtained from two different patients (designated patient-F and patient-J) at the time of surgical resection, then established, expanded, and implanted into mice for therapeutic studies. In an effort to improve the sensitizing properties of AZD7762 and reduce the effects of radiation alone relative to that observed in the MiaPaCa-2 xenografts (Fig. 4B), we treated mice with AZD7762 five times weekly (versus two times for MiaPaCa-2) and with a total of 18 Gy radiation (versus 30 Gy for MiaPaCa-2) as illustrated (Fig. 5A). For both of the patient-tumor xenografts, treatment with the single agents, gemcitabine, AZD7762, or radiation produced significant effects on tumor growth (Fig. 5B–C, Table 1). Notably, the addition of AZD7762 to radiation resulted in a significantly prolonged time until tumor volume doubling relative to radiation alone (Δ = 29 days; P < 0.001 and 12 days; P < 0.001 in patient-F and patient-J xenografts, respectively). Furthermore, the combination of AZD7762 with gemcitabine or gemcitabine-radiation delayed the tumor volume doubling time relative to gemcitabine (Δ = 14 days; P < 0.001 and 18 days; P < 0.001 in patient-F and patient-J xenografts, respectively) as well as gemcitabine-radiation (Δ = 7 days; P = 0.07 and 12 days; P = 0.001, respectively). Overall these results demonstrate that AZD7762 sensitizes to gemcitabine and radiation in multiple pancreatic cancer model systems.
Figure 5. The effects of AZD7762 on patient-derived xenografts in response to gemcitabine and radiation.
Nod-scid mice bearing bilateral, subcutaneous xenografts derived from two different patient pancreatic tumors (patients ‘F’ and ‘J’) (B and C, respectively) were treated with gemcitabine (90 mg/kg), AZD7762 (20 mg/kg), and/or radiation (1.8 Gy/fraction) for 2 cycles as illustrated (A). Data are expressed as the proportion of tumors doubled in volume. Each treatment group contained 6 – 10 mice (12 – 20 tumors).
Discussion
In this study we have shown that Chk1/2 inhibition by AZD7762 enhances radiation sensitivity and gemcitabine-mediated radiosensitization in pancreatic cancer cells and xenografts. Radiosensitization by AZD7762 is associated with abrogation of the radiation-induced G2 checkpoint as well as inhibition of HRR. Inhibition of these two processes by AZD7762 results in increased DNA damage, evidenced by increased ATR-mediated Chk1 phosphorylation (S345 Chk1) and persistent γ-H2AX expression. These data support the clinical investigation of Chk1 inhibitors, specifically AZD7762, in combination with gemcitabine-radiation in patients with locally advanced pancreatic cancer. Furthermore, these data suggest that S345 Chk1 and γ-H2AX might be useful markers for predicting AZD7762 activity in clinical trials.
While this is the first study demonstrating radiosensitization by a Chk1 inhibitor in clinical development (22), other Chk1-targeted agents are radiosensitizers. Chir-124, a novel Chk1 inhibitor in preclinical development radiosensitized all HCT116 models but to a greater extent in HCT116 p21−/− cells (14). The Chk1 inhibitor, CEP-3891, although discontinued for clinical development, radiosensitized U2-OS cells (36). Furthermore, the non-selective Chk1 inhibitor, UCN-01 induced radiosensitization that was dependent on the presence of mutant p53 (17). These studies have associated radiosensitization induced by Chk1 inhibitors with abrogation of the radiation-induced G2 checkpoint. Our work now demonstrates that inhibition of Rad51 and HRR is an additional mechanism of sensitization by Chk1 inhibitors in pancreatic cancer models.
Our findings suggest that Chk1 inhibitors may have at least two mechanisms by which they selectively sensitize tumor cells compared to normal cells. Substantial literature supports the model that normal cells (e.g., cells with normal p53 function) should respond to stress by halting at the G1 checkpoint (via p53 induction), and thus be unaffected by loss of the Chk1-mediated S or G2 checkpoints. Conversely, tumor cells which harbor p53 mutations should rely exclusively on Chk1/2-mediated pathways for cell cycle arrest in response to stress. This model is supported by the findings that Chk1 inhibition preferentially sensitizes HCT116 p53−/− cells (versus HCT p53+/+) to gemcitabine (13) and radiation (10) as well as HCT116 p53−/− tumors to 5-fluorouracil (37). In addition to p53 however, our model would predict that tumors which overexpress Rad51, such as pancreatic (38), would rely more heavily on HRR and thus be more sensitive to Chk1 inhibition than their normal cell counterparts. Rad51 overexpression results in increased HRR as well as resistance to radiation (39). Since p53 is mutated and Rad51 is overexpressed in more than half of all pancreatic carcinomas (40, 41), both of these may offer a therapeutic window for selective sensitization of tumor cells to gemcitabine/radiation by Chk1 inhibitors. Thus, it remains possible that p53 wild type tumors may still be sensitized through HRR inhibition, and it may be premature to restrict Chk1 inhibitor use to p53 mutant tumors.
While this study demonstrates that both inhibition of the cell cycle checkpoint and HRR are associated with radiosensitization by AZD7762, the relative importance of these effects remains to be determined. HRR plays an important role in radiation-induced DSB repair in S- and G2- phase cells (42, 43), and HRR-deficiency results in radiosensitization relative to matched HRR-proficient cell types (42, 44, 45). Furthermore, the requirement of HRR inhibition in radiosensitization by Chk1 inhibitors is demonstrated by a lack of radiosensitization by checkpoint inhibition in HRR-incompetent cells (44). HRR inhibition by AZD7762 would render gemcitabine-treated cells extremely sensitive to radiation, since gemcitabine arrests cells in S-phase (23, 46) where HRR plays a predominant role. It will be important in future studies to establish a causative link between HRR inhibition and radiosensitization by Chk1 inhibitors.
Because AZD7762 is an inhibitor of both Chk1 and Chk2 (IC50 5 and < 10nM, respectively), our studies can not exclude the possibility that Chk2 inhibition is involved in AZD7762-mediated radiosensitization. The ability of AZD7762 to inhibit Chk2 activity is suggested by the reversal of the radiation-induced Chk2 mobility shift (Fig. 1C)(47). However, several lines of evidence suggest that inhibition of Chk1 and not Chk2 produces sensitization. We found that depletion of Chk1 but not Chk2 with siRNA produced radiosensitization and furthermore, depletion of Chk2 did not increase the radiosensitization caused by Chk1 depletion. In addition, the Chk1 inhibitors, PD-321852 and PF-00477736 (the latter of which is 100-fold more selective for Chk1 than Chk2) have demonstrated in vitro radio- and chemo-sensitizing properties comparable to AZD7762 (16, 26, 48). Finally multiple studies utilizing Chk2 siRNA have demonstrated a lack of effect of Chk2 inhibition on sensitization to radiation or gemcitabine (9–11, 25). Taken together these results suggest that sensitization by AZD7762 is mediated by inhibition of Chk1.
Our finding that AZD7762 in combination with gemcitabine and radiation produced a significant delay in the growth of pancreatic tumor xenografts with tolerable toxicity supports the development of clinical trials in patients with locally advanced disease. In addition, we have found that AZD7762 is a chemosensitizer to gemcitabine (Parsels, manuscript in preparation) (13), suggesting that AZD7762 may also play an important role in improving both adjuvant therapy and the treatment of metastatic disease. It will be important to define the optimal schedule of administration of AZD7762, gemcitabine, and radiation as well as to identify biomarkers of AZD7762 activity in easily attainable surrogate tissues for future clinical trials.
Supplementary Material
Acknowledgements
We thank Drs. Frank Graham and Philip Ng (McMaster University) for the AdNGUS24i adenovirus.
Grant support:
This work was funded by NIH Grant R01CA78554, Cancer Center Core Grant P30 CA046592, and AstraZeneca.
Abbreviations
- AZD or AZD7762
AstraZeneca Drug 7762
- Chk1
checkpoint kinase 1
- HRR
homologous recombination repair
- IR
ionizing radiation
- RER
radiation enhancement ratio
Footnotes
Disclosure of potential conflicts of interest:
L. H.-G., D.M., and S.D.Z. are employees of AstraZeneca.
References
- 1.Ries LAG, Melbert DKM, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 2.Loehrer PJ, Powell ME, Cardenes HR, et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201. J Clin Oncol. 2008 May;20(suppl):4506. ASCO Meeting Abstracts. [Google Scholar]
- 3.McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19(22):4202–4208. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 4.Morgan MA, Meirovitz A, Davis MA, Kollar LE, Hassan MC, Lawrence TS. Radiotherapy combined with gemcitabine and oxaliplatin in pancreatic cancer cells. Translational Oncology. 2008;1(1):36–43. doi: 10.1593/tlo.07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan MA, Parsels LA, Kollar LE, Normolle DP, Maybaum J, Lawrence TS. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008;14(16):5142–5149. doi: 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q, Guntuku S, Cui XS, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14(12):1448–1459. [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao H, Watkins JL, Piwnica-Worms H. Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci U S A. 2002;99(23):14795–14800. doi: 10.1073/pnas.182557299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorensen CS, Hansen LT, Dziegielewski J, et al. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol. 2005;7(2):195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- 9.Karnitz LM, Flatten KS, Wagner JM, et al. Gemcitabine-induced activation of checkpoint signaling pathways that affect tumor cell survival. Mol Pharmacol. 2005;68(6):1636–1644. doi: 10.1124/mol.105.012716. [DOI] [PubMed] [Google Scholar]
- 10.Carrassa L, Broggini M, Erba E, Damia G. Chk1, but not Chk2, is involved in the cellular response to DNA damaging agents: differential activity in cells expressing or not p53. Cell Cycle. 2004;3(9):1177–1181. [PubMed] [Google Scholar]
- 11.Hu B, Zhou XY, Wang X, Zeng ZC, Iliakis G, Wang Y. The radioresistance to killing of A1-5 cells derives from activation of the Chk1 pathway. J Biol Chem. 2001;276(21):17693–17698. doi: 10.1074/jbc.M009340200. [DOI] [PubMed] [Google Scholar]
- 12.Janetka JW, Ashwell S, Zabludoff S, Lyne P. Inhibitors of checkpoint kinases: from discovery to the clinic. Curr Opin Drug Discov Devel. 2007;10(4):473–486. [PubMed] [Google Scholar]
- 13.Zabludoff SD, Deng C, Grondine MR, et al. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol Cancer Ther. 2008;7(9):2955–2966. doi: 10.1158/1535-7163.MCT-08-0492. [DOI] [PubMed] [Google Scholar]
- 14.Tao Y, Leteur C, Yang C, et al. Radiosensitization by Chir-124, a selective CHK1 inhibitor: Effects of p53 and cell cycle checkpoints. Cell Cycle. 2009;8(8) doi: 10.4161/cc.8.8.8203. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DJ, Yakes FM, Chen J, et al. Pharmacological Abrogation of S-Phase Checkpoint Enhances the Anti-Tumor Activity of Gemcitabine In Vivo. Cell Cycle. 2007;6(1) doi: 10.4161/cc.6.1.3699. [DOI] [PubMed] [Google Scholar]
- 16.Blasina A, Hallin J, Chen E, et al. Breaching the DNA damage checkpoint via PF-00477736, a novel small-molecule inhibitor of checkpoint kinase 1. Mol Cancer Ther. 2008;7(8):2394–2404. doi: 10.1158/1535-7163.MCT-07-2391. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Fan S, Eastman A, Worland PJ, Sausville EA, O'Connor PM. UCN-01: a potent abrogator of G2 checkpoint function in cancer cells with disrupted p53. J Natl Cancer Inst. 1996;88(14):956–965. doi: 10.1093/jnci/88.14.956. [DOI] [PubMed] [Google Scholar]
- 18.Kortmansky J, Shah MA, Kaubisch A, et al. Phase I trial of the cyclin-dependent kinase inhibitor and protein kinase C inhibitor 7-hydroxystaurosporine in combination with Fluorouracil in patients with advanced solid tumors. J Clin Oncol. 2005;23(9):1875–1884. doi: 10.1200/JCO.2005.03.116. [DOI] [PubMed] [Google Scholar]
- 19.Fuse E, Kuwabara T, Sparreboom A, Sausville EA, Figg WD. Review of UCN-01 development: a lesson in the importance of clinical pharmacology. J Clin Pharmacol. 2005;45(4):394–403. doi: 10.1177/0091270005274549. [DOI] [PubMed] [Google Scholar]
- 20.Parry D, Shanhan F, Davis N, et al. Targeting the replication checkpoint with a potent and selective CHK1 inhibitor. Proceedings of the 100th Annual Meeting of the American Association for Cancer Research. 2009;50 Abstract number 2490. [Google Scholar]
- 21.Ashwell S, Zabludoff S. DNA damage detection and repair pathways--recent advances with inhibitors of checkpoint kinases in cancer therapy. Clin Cancer Res. 2008;14(13):4032–4037. doi: 10.1158/1078-0432.CCR-07-5138. [DOI] [PubMed] [Google Scholar]
- 22.Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer. 2008;98(3):523–528. doi: 10.1038/sj.bjc.6604208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan MA, Parsels LA, Parsels JD, Mesiwala AK, Maybaum J, Lawrence TS. Role of checkpoint kinase 1 in preventing premature mitosis in response to gemcitabine. Cancer Res. 2005;65(15):6835–6842. doi: 10.1158/0008-5472.CAN-04-2246. [DOI] [PubMed] [Google Scholar]
- 24.Arlander SJ, Eapen AK, Vroman BT, McDonald RJ, Toft DO, Karnitz LM. Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J Biol Chem. 2003;278(52):52572–52577. doi: 10.1074/jbc.M309054200. [DOI] [PubMed] [Google Scholar]
- 25.Morgan MA, Parsels LA, Parsels JD, Lawrence TS, Maybaum J. The relationship of premature mitosis to cytotoxicity in response to checkpoint abrogation and antimetabolite treatment. Cell Cycle. 2006;5(17):1983–1988. doi: 10.4161/cc.5.17.3184. [DOI] [PubMed] [Google Scholar]
- 26.Parsels LA, Morgan MA, Tanska DM, et al. Gemcitabine sensitization by checkpoint kinase 1 inhibition correlates with inhibition of a Rad51 DNA damage response in pancreatic cancer cells. Mol Cancer Ther. 2009;8(1):45–54. doi: 10.1158/1535-7163.MCT-08-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence TS. Ouabain sensitizes tumor cells but not normal cells to radiation. Int J Radiat Oncol Biol Phys. 1988;15(4):953–958. doi: 10.1016/0360-3016(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 28.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99(1):73–84. [PubMed] [Google Scholar]
- 29.Huang X, Halicka HD, Darzynkiewicz Z. Detection of histone H2AX phosphorylation on Ser-139 as an indicator of DNA damage (DNA double-strand breaks) Curr Protoc Cytom. 2004;Chapter 7(Unit 7):27. doi: 10.1002/0471142956.cy0727s30. [DOI] [PubMed] [Google Scholar]
- 30.Hoy CA, Seamer LC, Schimke RT. Thermal denaturation of DNA for immunochemical staining of incorporated bromodeoxyuridine (BrdUrd): critical factors that affect the amount of fluorescence and the shape of BrdUrd/DNA histogram. Cytometry. 1989;10(6):718–725. doi: 10.1002/cyto.990100608. [DOI] [PubMed] [Google Scholar]
- 31.Pierce AJ, Johnson RD, Thompson LH. Jasin M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999;13(20):2633–2638. doi: 10.1101/gad.13.20.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 33.Lindstrom MJ, Kunugi KA, Kinsella TJ. Global comparison of radiation and chemotherapy dose-response curves with a test for interaction. Radiat Res. 1993;135(2):269–277. [PubMed] [Google Scholar]
- 34.Mailand N, Falck J, Lukas C, et al. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288(5470):1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- 35.Zhang YW, Otterness DM, Chiang GG, et al. Genotoxic stress targets human Chk1 for degradation by the ubiquitin-proteasome pathway. Mol Cell. 2005;19(5):607–618. doi: 10.1016/j.molcel.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Syljuasen RG, Sorensen CS, Nylandsted J, Lukas C, Lukas J, Bartek J. Inhibition of Chk1 by CEP-3891 accelerates mitotic nuclear fragmentation in response to ionizing Radiation. Cancer Res. 2004;64(24):9035–9040. doi: 10.1158/0008-5472.CAN-04-2434. [DOI] [PubMed] [Google Scholar]
- 37.Ganzinelli M, Carrassa L, Crippa F, Tavecchio M, Broggini M, Damia G. Checkpoint kinase 1 down-regulation by an inducible small interfering RNA expression system sensitized in vivo tumors to treatment with 5-fluorouracil. Clin Cancer Res. 2008;14(16):5131–5141. doi: 10.1158/1078-0432.CCR-08-0304. [DOI] [PubMed] [Google Scholar]
- 38.Han H, Bearss DJ, Browne LW, Calaluce R, Nagle RB, Von Hoff DD. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 2002;62(10):2890–2896. [PubMed] [Google Scholar]
- 39.Vispe S, Cazaux C, Lesca C, Defais M. Overexpression of Rad51 protein stimulates homologous recombination and increases resistance of mammalian cells to ionizing radiation. Nucleic Acids Res. 1998;26(12):2859–2864. doi: 10.1093/nar/26.12.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20(2):211–226. doi: 10.1016/j.bpg.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Maacke H, Jost K, Opitz S, et al. DNA repair and recombination factor Rad51 is over-expressed in human pancreatic adenocarcinoma. Oncogene. 2000;19(23):2791–2795. doi: 10.1038/sj.onc.1203578. [DOI] [PubMed] [Google Scholar]
- 42.Frankenberg-Schwager M, Gebauer A, Koppe C, Wolf H, Pralle E, Frankenberg D. Single-strand annealing, conservative homologous recombination, nonhomologous DNA end joining, and the cell cycle-dependent repair of DNA double-strand breaks induced by sparsely or densely ionizing radiation. Radiat Res. 2009;171(3):265–273. doi: 10.1667/RR0784.1. [DOI] [PubMed] [Google Scholar]
- 43.Mladenov EV, Kalev PS, Anachkova BB. The complexity of double-strand break ends is a factor in the repair pathway choice. Radiat Res. 2009;171:397–404. doi: 10.1667/RR1487.1. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Wang X, Iliakis G, Wang Y. Caffeine could not efficiently sensitize homologous recombination repair-deficient cells to ionizing radiation-induced killing. Radiat Res. 2003;159(3):420–425. doi: 10.1667/0033-7587(2003)159[0420:ccnesh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 45.Wachters FM, van Putten JW, Maring JG, Zdzienicka MZ, Groen HJ, Kampinga HH. Selective targeting of homologous DNA recombination repair by gemcitabine. Int J Radiat Oncol Biol Phys. 2003;57(2):553–562. doi: 10.1016/s0360-3016(03)00503-0. [DOI] [PubMed] [Google Scholar]
- 46.Lawrence TS, Chang EY, Hahn TM, Hertel LW, Shewach DS. Radiosensitization of pancreatic cancer cells by 2',2'-difluoro-2'-deoxycytidine. Int J Radiat Oncol Biol Phys. 1996;34(4):867–872. doi: 10.1016/0360-3016(95)02134-5. [DOI] [PubMed] [Google Scholar]
- 47.Ahn JY, Schwarz JK, Piwnica-Worms H, Canman CE. Threonine 68 phosphorylation by ataxia telangiectasia mutated is required for efficient activation of Chk2 in response to ionizing radiation. Cancer Res. 2000;60(21):5934–5936. [PubMed] [Google Scholar]
- 48.Morgan MA, Parsels LA, Maybaum J, Lawrence TS. Improving gemcitabine-mediated radiosensitization using molecularly targeted therapy: a review. Clin Cancer Res. 2008;14(21):6744–6750. doi: 10.1158/1078-0432.CCR-08-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.