Abstract
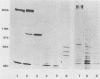
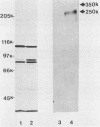
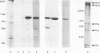
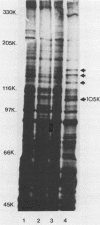
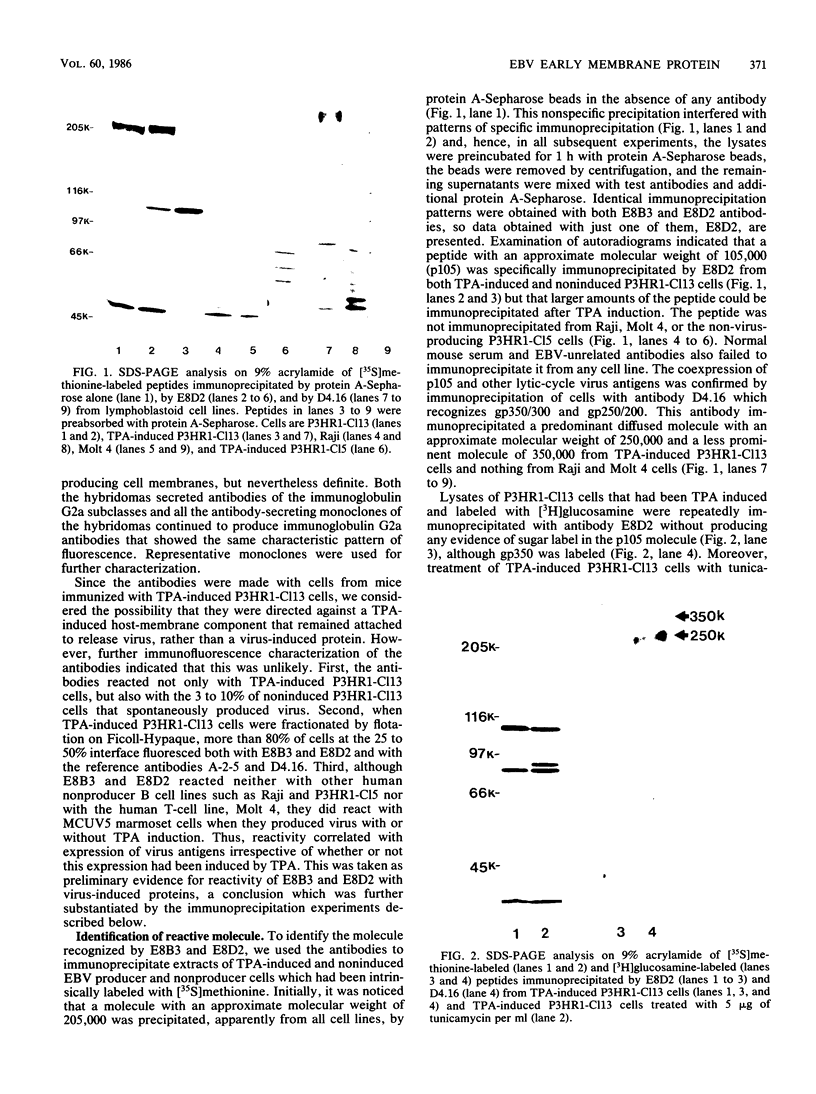
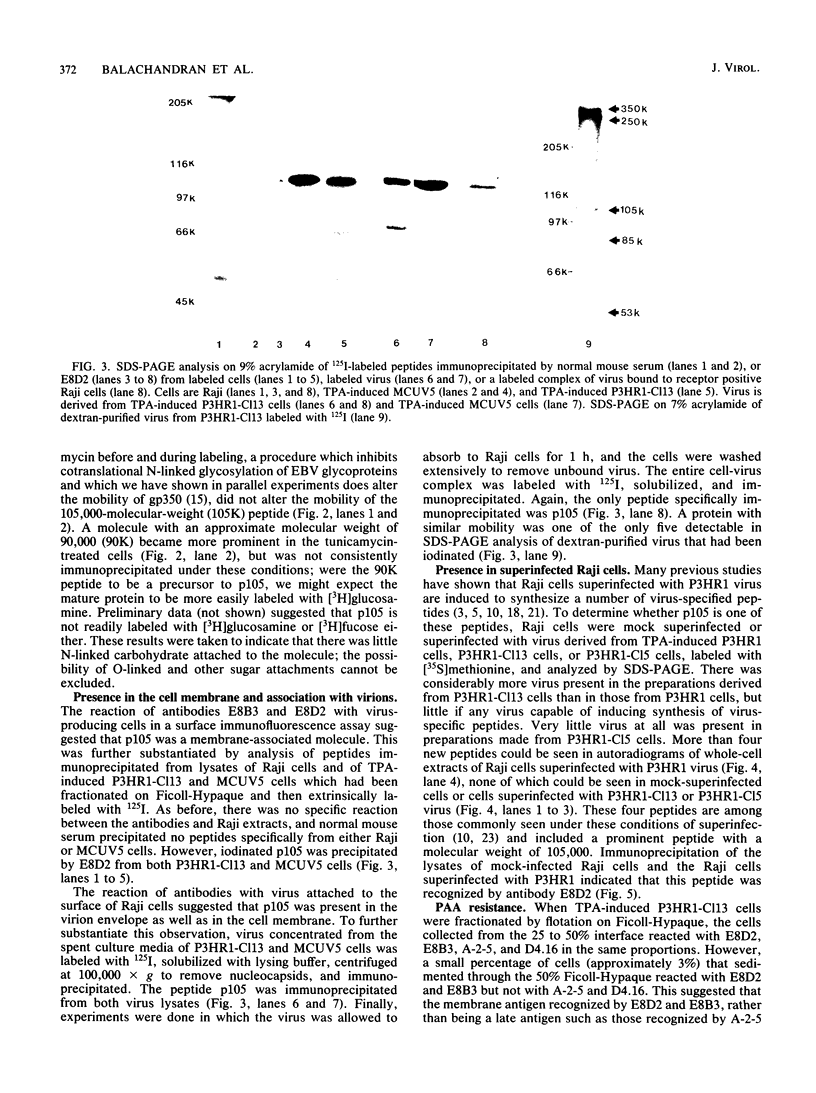
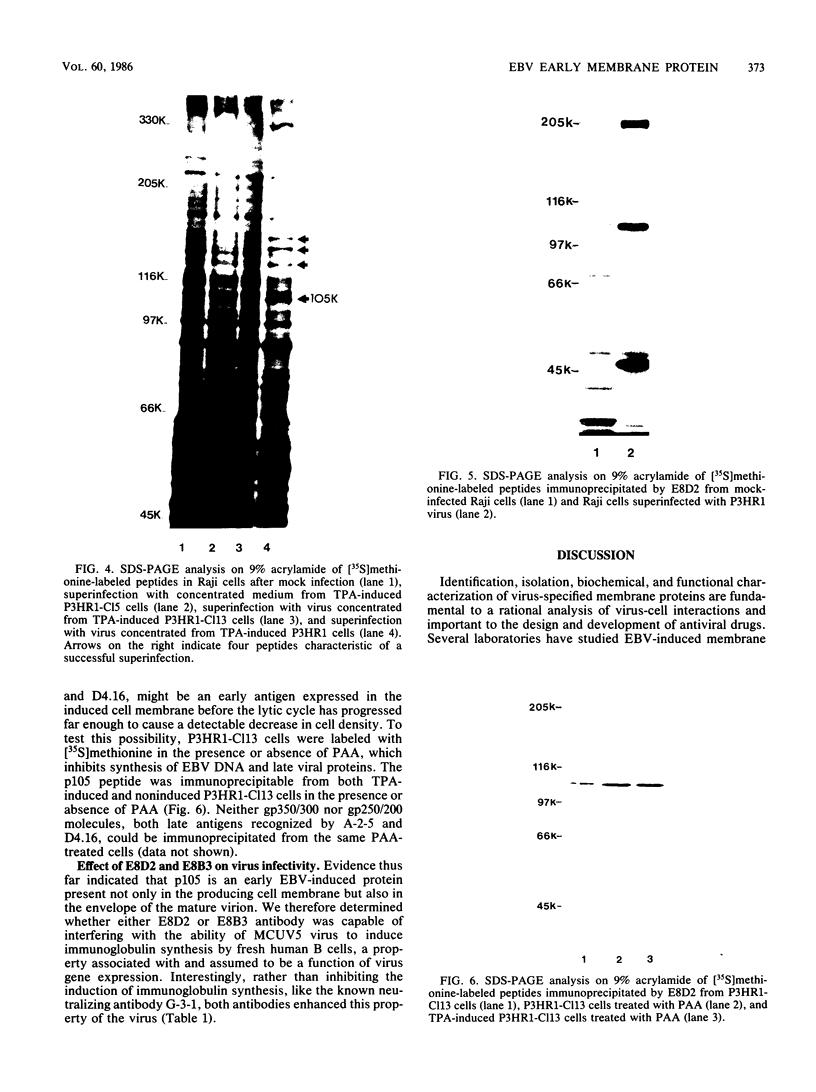
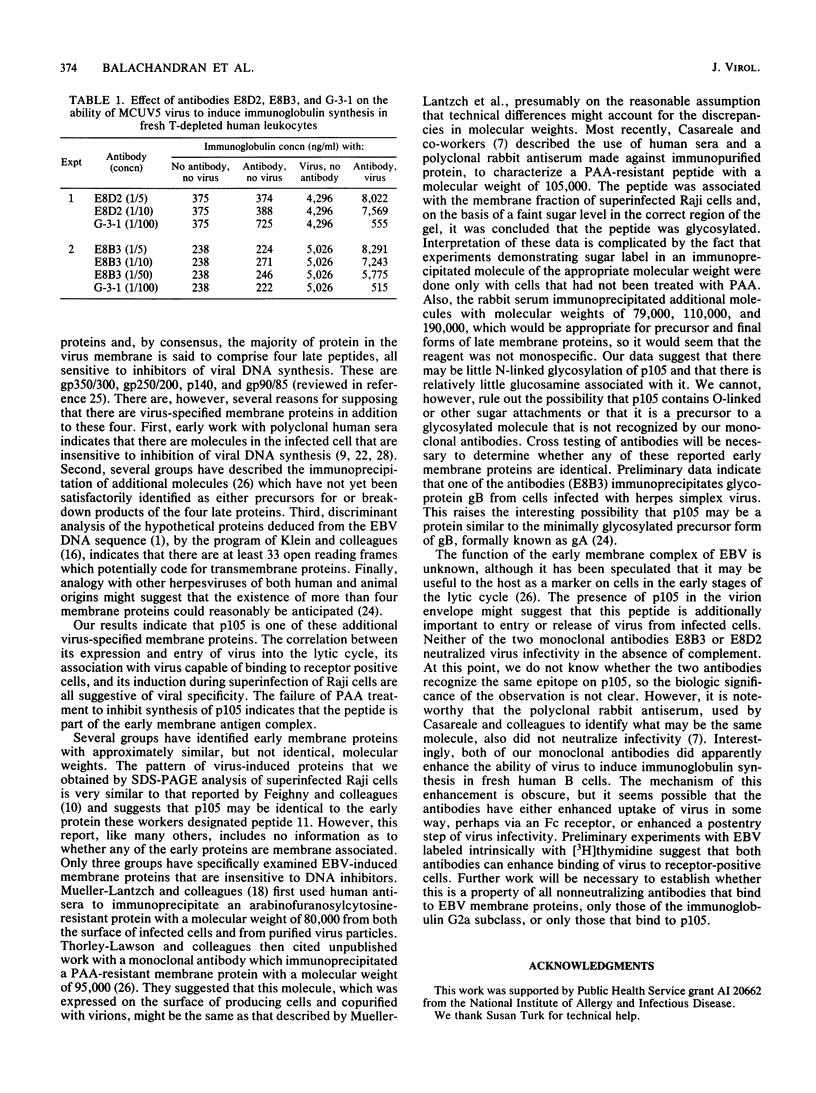
Two monoclonal antibodies, E8B3 and E8D2, were raised against Epstein-Barr virus (EBV)-producing cells and were shown to immunoprecipitate a protein with an approximate molecular weight of 105,000 (p105). The protein was detectable only in EBV-containing cells which were supporting the virus lytic cycle, and its synthesis increased after cells were induced with phorbol esters. The molecule was radiolabeled and immunoprecipitated from virus-producing cells that had been extrinsically labeled with 125I, and the antibodies E8B3 and E8D2 reacted in immunofluorescence assays with infected cells; the molecule was also associated with virion particles. Synthesis of p105 was not blocked by phosphonoacetic acid and could be induced in Raji cells by superinfection with virus derived from P3HR1 cells. These data support the conclusion that p105 is an EBV-specific early membrane protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Balachandran N., Harnish D., Killington R. A., Bacchetti S., Rawls W. E. Monoclonal antibodies to two glycoproteins of herpes simplex virus type 2. J Virol. 1981 Aug;39(2):438–446. doi: 10.1128/jvi.39.2.438-446.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss G. J., Nonoyama M. Mechanisms of infection with Epstein-Barr virus. III. The synthesis of proteins in superinfected Raji cells. Virology. 1978 Jun 1;87(1):204–207. doi: 10.1016/0042-6822(78)90173-3. [DOI] [PubMed] [Google Scholar]
- Bird A. G., Britton S. A new approach to the study of human B lymphocyte function using an indirect plaque assay and a direct B cell activator. Immunol Rev. 1979;45:41–67. doi: 10.1111/j.1600-065x.1979.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Bodemer W. W., Summers W. C., Niederman J. C. Detection of virus-specific antigens in EB-(P3HR1) virus-superinfected Raji cells by immunoprecipitation. Virology. 1980 Jun;103(2):340–349. doi: 10.1016/0042-6822(80)90192-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Casareale D., Jones W., Sairenji T., Humphreys R. E. p105, an Epstein-Barr virus-induced, phosphonoacetic acid-insensitive glycoprotein target of the anti-Epstein-Barr virus immune response. Infect Immun. 1983 Jan;39(1):85–90. doi: 10.1128/iai.39.1.85-90.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernberg I., Klein G., Kourilsky F. M., Silvestre D. Differentiation between early and late membrane antigen on human lymphoblastoid cell lines infected with Epstein-Barr virus. I. Immunofluorescence. J Natl Cancer Inst. 1974 Jul;53(1):61–65. doi: 10.1093/jnci/53.1.61. [DOI] [PubMed] [Google Scholar]
- Feighny R. J., Henry B. E., 2nd, Pagano J. S. Epstein-Barr virus polypeptides: effect of inhibition of viral DNA replication on their synthesis. J Virol. 1981 Jan;37(1):61–71. doi: 10.1128/jvi.37.1.61-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Heston L., Rabson M., Brown N., Miller G. New Epstein-Barr virus variants from cellular subclones of P3J-HR-1 Burkitt lymphoma. Nature. 1982 Jan 14;295(5845):160–163. doi: 10.1038/295160a0. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Balachandran N., Elkins M. H. B cell activation by cytomegalovirus. J Exp Med. 1983 Dec 1;158(6):2171–2176. doi: 10.1084/jem.158.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutt-Fletcher L. M., Balachandran N., LeBlanc P. A. Modification of Epstein-Barr virus replication by tunicamycin. J Virol. 1986 Jan;57(1):117–123. doi: 10.1128/jvi.57.1.117-123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Meuller-Lantzsch N., Georg B., Yamamoto N., zur Hausen H. Epstein-Barr virus-induced proteins. II. Analysis of surface polypeptides from EBV-producing and -superinfected cells by immunoprecipitation. Virology. 1980 Apr 30;102(2):401–411. doi: 10.1016/0042-6822(80)90107-5. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Nemerow G. R., Cooper N. R. Isolation of Epstein Barr-virus and studies of its neutralization by human IgG and complement. J Immunol. 1981 Jul;127(1):272–278. [PubMed] [Google Scholar]
- PULVERTAFT J. V. CYTOLOGY OF BURKITT'S TUMOUR (AFRICAN LYMPHOMA). Lancet. 1964 Feb 1;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- Qualtiere L. F., Pearson G. R. Epstein-Barr virus-induced membrane antigens: immunochemical characterization of Triton X-100 solubilized viral membrane antigens from EBV-superinfected Raji cells. Int J Cancer. 1979 Jun 15;23(6):808–817. doi: 10.1002/ijc.2910230612. [DOI] [PubMed] [Google Scholar]
- Sairenji T., Hinuma Y., Sekizawa T., Yoshida M. Appearance of early and late components of Epstein-Barr virus-associated membrane antigen in Daudi cells superinfected with P3HR-1 virus. J Gen Virol. 1978 Jan;38(1):111–120. doi: 10.1099/0022-1317-38-1-111. [DOI] [PubMed] [Google Scholar]
- Simmons J. G., Hutt-Fletcher L. M., Fowler E., Feighny R. J. Studies of the Epstein-Barr virus receptor found on Raji cells. I. Extraction of receptor and preparation of anti-receptor antibody. J Immunol. 1983 Mar;130(3):1303–1308. [PubMed] [Google Scholar]
- Strnad B. C., Schuster T., Klein R., Hopkins R. F., 3rd, Witmer T., Neubauer R. H., Rabin H. Production and characterization of monoclonal antibodies against the Epstein-Barr virus membrane antigen. J Virol. 1982 Jan;41(1):258–264. doi: 10.1128/jvi.41.1.258-264.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Edson C. M., Geilinger K. Epstein-Barr virus antigens-a challenge to modern biochemistry. Adv Cancer Res. 1982;36:295–348. doi: 10.1016/s0065-230x(08)60428-5. [DOI] [PubMed] [Google Scholar]
- Yata J., Klein G., Hewetson J., Gergely L. Effect of metabolic inhibitors on membrane immunofluorescence reactivity of established Burkitt lymphoma cell lines. Int J Cancer. 1970 May 15;5(3):394–403. doi: 10.1002/ijc.2910050314. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]