Abstract
Currently, no single marker is sensitive and specific enough to be considered a reliable biomarker for prenatal alcohol exposure. To identify a proteomic signature profile for maternal alcohol consumption, we performed high throughput proteomics on maternal endothelial caveolae exposed to moderate binge-like alcohol conditions. In these specialized lipid ordered microdomains which contain a rich assembly of proteins, we demonstrate that moderate binge-like alcohol resulted in a distinctive maternal caveolar proteomic signature with important proteins being dramatically decreased/knocked out in the alcoholic profile. These proteins span from histones and basic structural proteins like tubulin α to proteins involved in trafficking, deubiquitination, cell signaling, and cell-cell adhesion. The profile also suggests an important role for the mother and the utero-placental compartment in the pathogenesis of Fetal Alcohol Spectrum Disorders (FASD). These data demonstrate that the caveolar proteomic signature created by alcohol shows a promising direction for early detection of FASD.
INTRODUCTION
Early in utero detection of FASD is highly desired for commencing therapeutic intervention and for stopping alcohol use for the remainder of pregnancy (Bearer, 2001). Biomarkers developed for maternal alcohol consumption include increases in: 1) blood gamma-glutamyltransferase, 2) blood carbohydrate-deficient transferrin, 3) mean corpuscular volume, 4) blood hemaglobin-acetaldehyde adduct, 5) specific fatty acid ethyl esters (FAEE) and ethyl glucuronide in meconium and hair, and 6) neonatal urine dolichols (Hannuksela et al., 2007; Bearer et al., 2004). Although these are useful indicators of heavy alcohol consumption, no single marker is sensitive and specific enough to be considered a reliable biomarker for prenatal alcohol exposure (Bearer, 2001; Stoler et al., 1998; reviewed by Bearer et al., 2004).
The advancing field of proteomics offers promise for developing state of the art biomarkers that can detect extremely subtle physiologic changes associated specifically with alcohol use (Bearer et al., 2004). It is critically important to identify pregnant women who drink, to start them on suitable nutritional/ pharmacologic/ behavioral therapies and also test the putative efficacy of these treatments by repeated measurements of these markers over the course of pregnancy. So far, few investigators have focused attention on proteomic analyses designed to establish potential biomarkers for prenatal alcohol exposure (Bearer et al., 2004). Robinson and colleagues (1995) reported eight serum proteins whose concentrations differed significantly between the FASD and control children. However, this study neither utilized high throughput proteomics, nor did it conclusively identify a reliable marker that can predict prenatal alcohol exposure.
In this study, we specifically exploited the caveolae which are specialized lipid ordered microdomains containing assemblies of proteins (receptors, channels, signaling complexes). The caveolae are found in many cell types including the red blood cells (RBCs) and the endothelial cell (Ozuyaman et al., 2008; Parton and Simons, 2007). We hypothesize that high-throughput proteomic analysis will identify a distinctive proteomic signature profile for maternal alcohol consumption in these membrane structures. The first theory that alcohol might disrupt the caveolae comes from the observation that alcohol affects major signalosomes that are located in the caveolae. Ronis et al., 2007 speculated that this action is due to alcohol-induced caveolar cholesterol/lipid depletion. Recently, Mao et al., 2009 demonstrated that alcohol disrupts the interaction of proteins with the caveolar scaffolding protein caveolin-1 (cav-1), resulting in dissociation of these complexes from the lipid rafts. In this study, we specifically utilized fully validated ovine maternal uterine artery endothelial cells as the caveolae are best characterized in this cell type. Maternal cells were isolated during a period when blood flow to the uteroplacental unit is ~25 fold greater than the non-pregnant state (Magness, 1998). This is also a period when alcohol decreases uterine perfusion (Falconer, 1990), reduces fetal growth (Ramadoss et al., 2006) and produces fetal neuronal loss and behavioral deficits (Goodlett and Eilers, 1997; Ramadoss et al., 2008; Thomas et al., 1996). In addition to signature profile development, the strategical utilization of endothelial cells from the uterus served a twin purpose of providing some novel mechanistic insights on the role of intra-uterine environment in disorders associated with prenatal alcohol exposure. Finally, the ovine system is ideal for this purpose as the third-trimester equivalent of human gestation occurs in utero in this species (Cudd, 2005).
METHODS
Alcohol Binging
The Animal Care Committee approved procedures for obtaining uterine arteries from pregnant ewes (Day 120–130; term = 147) for endothelial cells isolation using collagenase digestion procedures (Bird et al., 2000). Cells were further purified using fluorescence activated cell sorting (FACS), devoid of vascular smooth muscle cell contamination and maintained in culture to passage 4. To mimic maternal binge drinking patterns, uterine artery endothelial cells were cultured to 70% confluence in the absence (0 mg/dl; control) or presence of alcohol (150 mg/dl) in sealed, humidified chambers equilibrated with aqueous alcohol for 3 h on 3 consecutive days (Eysseric et al., 1997; Ramadoss et al., 2007a; Ramadoss et al., 2007b). Five replicates in each group were utilized. Cell viability was validated by trypan blue exclusion microscopy, Calcein AM imaging, and immunoblotting. Trypan blue stained cell count demonstrated that the number of viable cells in the control and alcohol groups were not different (control, 891,250 ± 11,433; alcohol, 834,000 ± 49,784; p = 0.73). We also found that uncleaved caspase 3 was unaltered and cleaved caspase 3 was not detectable. Further, 150 mg/dl is easily achieved in mothers of children with FAS (Church and Gerkin, 1988) and in women who abuse alcohol (Urso et al., 1981). This level of alcohol produces a decrease in ovine maternal uterine blood flow by nearly 20% (Falconer, 1990), and produces fetal neuronal deficits (Parnell et al., 2007).
Caveolar Isolation
Caveolar isolation was performed as described previously (Liao et al., 2009; Song et al., 1996). Cells were collected in a 0.5 ml sodium carbonate buffer (pH 11) containing phosphatase inhibitors and protease inhibitors. A 5–35% discontinuous sucrose gradient was formed above (3.5 ml of 35% sucrose/0.5 ml of 5% sucrose; in MBS containing 250 mM sodium carbonate). The samples were centrifuged at 116,000 × g for 16–20 hours in a SW55Ti rotor (Beckman Instruments, Palo Alto, CA). Fractions (0.5 ml) were collected from the top of the tube. A light scattering band confined to the 5–35% sucrose interface was observed when enriched with caveolar membranes.
Proteomic Analysis
Caveolar proteins were extracted by precipitation with equal volume of 15% ice-cold TCA and incubated for 1hr on ice, centrifuged for 10 min at 16,000g and pellets washed (3×) with ice-cold acetone. Pelleted proteins were denatured in 10µl of 6M Urea/ 100mM NH4HCO3 for 10min then diluted to 50µl for tryptic digestion with: 1µl of 25mM DTT, 7µl Acetonitrile, 22µl MilliQ water and 10µl trypsin solution (20ng/µl Trypsin Gold from PROMEGA Corp. in 25mM NH4HCO3). Reaction was terminated by acidification with 2.5% Trifluoroacetic Acid to 0.5% final. Peptides generated from digestion were directly loaded for nanoLC-MS/MS analysis. Peptides were analyzed by nanoLC-MS/MS using the Agilent 1100 nanoflow system (Agilent, Palo Alto, CA) connected to a hybrid linear ion trap-orbitrap mass spectrometer (LTQ-Orbitrap, Thermo Fisher Scientific, San Jose, CA) equipped with a nanoelectrospray ion source. Capillary HPLC was performed using an in-house fabricated column with integrated electrospray emitter essentially as described previously (Martin et al., 2000) except for the use of 360µm × 75µm fused silica tubing. Sample loading and desalting were done at 10uL/min with the loading solvent delivered from an isocratic pump. The column was packed with 5µm C18 particles (Column Engineering, Ontario, CA) to approximately 12cm. Sample loading (8µl) and desalting were achieved using a trapping column in line with the autosampler (Zorbax 300SB-C18, 5µM, 5×0.3mm, Agilent). HPLC solvents were as follows: Loading: 1% (v/v) acetonitrile (ACN), 0.1M acetic acid; A: 0.1M acetic acid in water, and B: 95% (v/v) ACN, 0.1M acetic acid in water. Gradient elution was performed at 200nL/min and increasing %B in A of 0 to 40 in 200 min, 40 to 60 in 20 min, and 60 to 100 in 5 min. The LTQ-Orbitrap was set to acquire MS/MS spectra in data-dependent mode. Raw MS/MS data was searched against NCBI non-redundant Bos Taurus amino acid sequence database using in-house Sequest search engine with methionine oxidation, asparagine and glutamine deamidation and cysteine carbamidomethylation as variable modifications. Protein abundance score was determined by normalizing the spectrum counts within untreated control and alcohol groups using Scaffold (Proteomics Software Inc.). Specificity was set a priori at an abundance score value ≥ 20 for control state and the protein identification probability at p < 0.05.
Validation
Proteomic profiles were confirmed by immunoblotting for cav-1, and endothelial nitric oxide synthase (eNOS). Cav-1 was selected because it is the major caveolar scaffolding protein and is a mandatory marker for the caveolae. Signaling of the endothelial marker eNOS is compartmentalized within the caveolae under control conditions (Chen et al., 2001). In brief, all fractions (1–13) from sucrose gradient centrifugation were loaded (16 ul/lane) on 4–20% polyacrylamide gels and probed for cav-1 (Cell Signaling Technologies) and eNOS (BD Transduction Laboratories) following transfer.
RESULTS
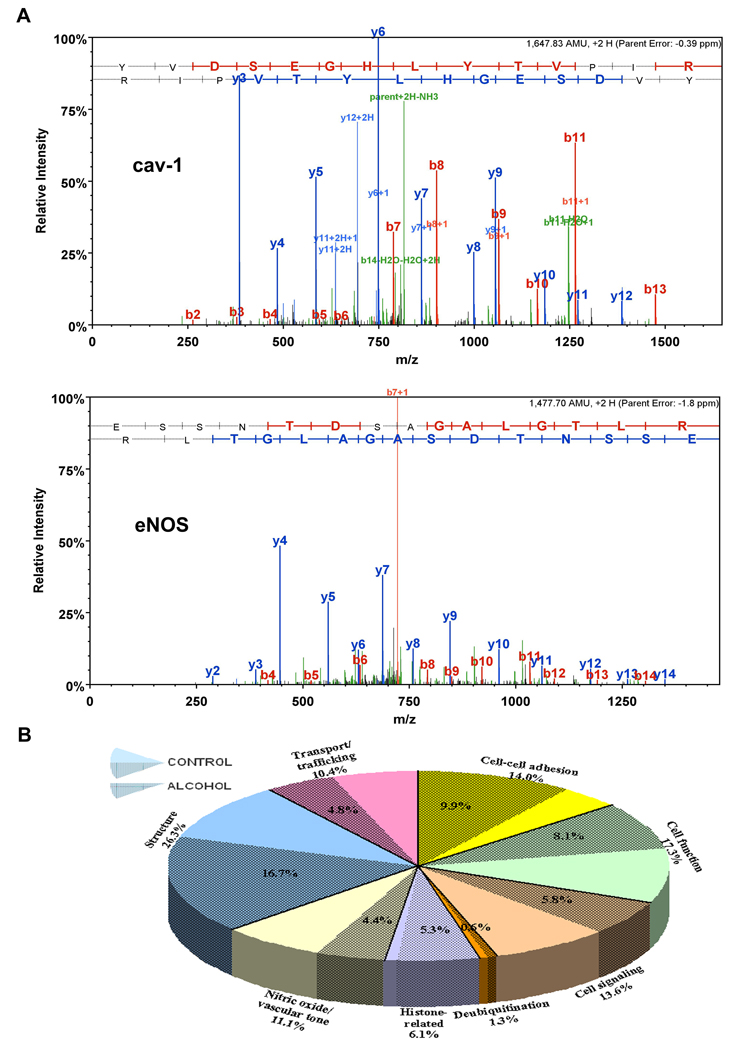
Representative MS/MS spectra of cav-1 and eNOS illustrate a nearly complete Y ion series assignment (figure 1A). B ion series confirm peptide assignment. All major signals in the spectra are explained by the assigned sequences. Sequest cross correlation scores for cav-1 and eNOS are 4.58 and 4.08 respectively. Cav-1 and eNOS parent mass error are −0.39 PPM and −1.8 PPM respectively. Chronic-binge alcohol exposure resulted in decreases in the abundance of caveolar proteins related to cell-cell adhesion (by 29%), cell function (by 53.3%), cell signaling (by 57.2%), deubiquitination (by 51.5%), histones (by 13.2%), nitric oxide regulation (by 60.2%), structure (by 36.4%), and transport/trafficking (by 53.3%) compared with those in the control state (figure 1B). The caveolar proteomic signature profile in response to binge-like alcohol is depicted in figure 2. Caveolar profile of proteins related to cell-cell adhesion/interaction consisted of platelet endothelial cell adhesion molecule (Pecam1), integrin α5, fibronectin receptor (Fibronectin rp), integrin β1, vascular endothelial (VE) cadherin, integrin β3, synaptosomal-associated protein 23 (SAP23), serpin 1, catenin α1 and fibronectin. Alcohol altered this signature pattern with the most dramatic decrease seen with Pecam1 abundance (by ~64%). The only protein that exhibited a marked increase in response to alcohol was fibronectin (~164%). Several proteins normally associated with cell function and/or cellular organelles (e.g. golgi, endoplasmic reticulum, and mitochondria) exhibited a distinct profile in response to alcohol including hexokinase, CD109, ribophorin 2 (RPN2), dolichyl-diphosphooligosaccharide-protein glycotransferase (DDOPG), translocon-associated protein δ (TAP δ), inner mitochondrial membrane protein (mito membr), cytochrome b5 reductase 3 (CYB5R3), transmembrane emp24 transporter (emp24), prohibitin 2, ribophorin 1 (RPN 1), prohibitin, and nicotinamide nucleotide transhydrogenase (NNT). Alcohol altered this profile dramatically with hexokinase, a glycolytic enzyme decreasing by ~84% and CD 109, a protein associated with cell differentiation / transformation decreasing by ~73%. We note that it has already been demonstrated that presence of cellular organelle-associated proteins in caveolae is not a result of contamination as these proteins are integral parts of caveolae (McMahon et al., 2006). Numerous cell signaling associated proteins were found in the caveolae including Ca++ transporting ATP synthase (Ca++ transport), ATP synthase β unit, Ca++ ATPase, ATP synthase, Na+K+ATPase, RAS family protein RAB1A, G αi, calnexin (canX), and stator of ATP synthase (St ATP synthase). Alcohol dramatically altered this profile with Ca++ transporter decreasing by 84% and other ATP synthases or ATPases decreasing by nearly 40–70%. We also found the major deubiquitination enzyme ubiquitin specific peptidase 2 (USP2) in the caveolae which was decreased by ~52% by alcohol. It is noteworthy that three histone proteins were found in great abundance in the caveolae and alcohol reduced H2A by ~53%. The caveolar proteins related to nitric oxide signaling including heat shock protein (HSP)70 V, cav-1, and eNOS were dramatically decreased by binge-like alcohol exposure. However, prostacyclin synthase (PGI2 syn) located in the caveolae was marginally increased by ~7% in response to alcohol. A number of structural proteins including tubulin α3c, lamin B1 (LMNB1), cytoskeletal-associated protein 4 (CAP4), myoferlin, leucine rich repeat (LRR), vimentin, actin γ1, and tubulin β were identified in the caveolae and alcohol produced a dramatic alteration in this profile with α tubulin being knocked out of the caveolar profile. In contrast, tubulin β increased ~18% above control. Finally, important solute carriers, trafficking proteins and transporters were detected in the caveolae, including protein translocator SEC61, SNARE 22 (SEC22), lectin-mannose binding protein (LMAN2), voltage dependent anion channel 2 (VDAC2), adenosine translocator (Adenine tr), voltage dependent anion channel 1 (VDAC1), and phosphate solute carrier 25A3 (SLC25A3). Of these, SEC 61 was dramatically decreased (~90%) in the alcoholic profile.
Figure 1.
(A) Representative MS/MS spectra of cav-1 and eNOS illustrate a nearly complete Y ion series assignment. The B ion series confirm peptide assignment. All major signals in the spectra are explained by the assigned sequences. Sequest cross correlation scores for cav-1 and eNOS are 4.58 and 4.08 respectively. Cav-1 and eNOS parent mass error are −0.39 PPM and −1.8 PPM respectively. (B) Chronic binge-like alcohol exposure resulted in dramatic decreases in the abundance of endothelial caveolar proteins related to cell-cell adhesion, cell function, cell signaling, deubiquitination, histones, nitric oxide/vascular tone, structure, and transport/trafficking. Pie chart slices bound by solid lines represent the caveolar protein abundance in control state. The shaded subset in each slice represents the caveolar protein abundance in response to chronic binge alcohol.
Figure 2.
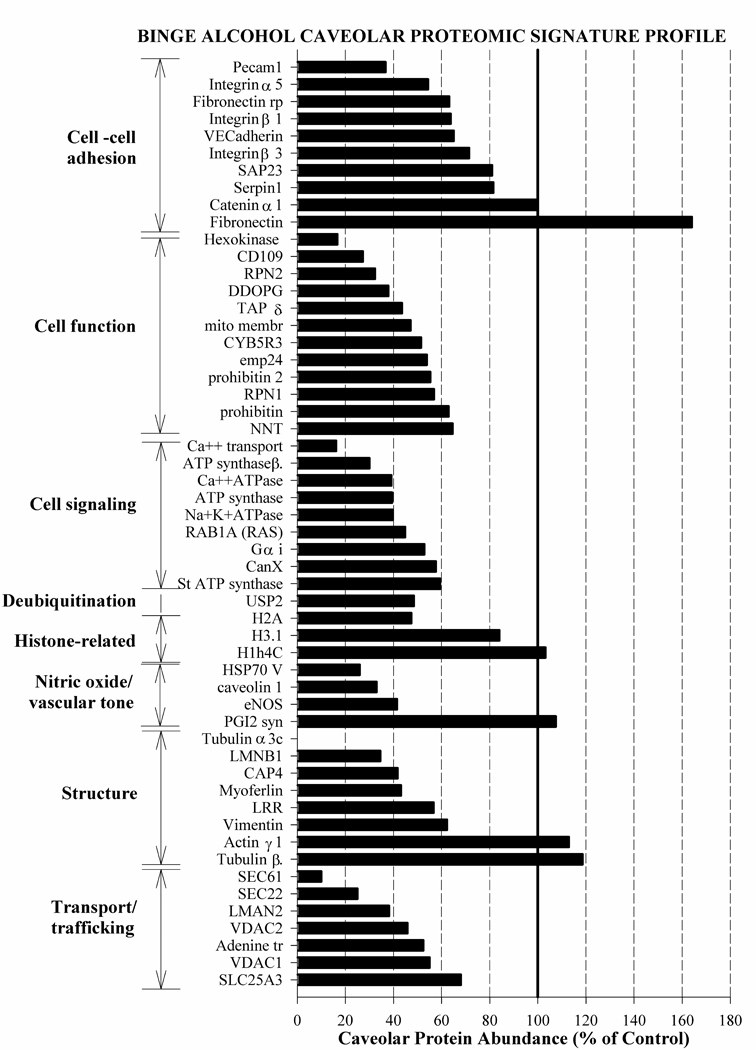
Chronic binge-like alcohol exposure resulted in a distinctive endothelial caveolar proteomic signature profile that included proteins related to cell-cell adhesion, cell function, cell signaling, deubiquitination, histones, nitric oxide/vascular tone, structure, and transport/trafficking. Profile details and abbreviations are described in the Results section. The caveolar protein profile is depicted as % of control.
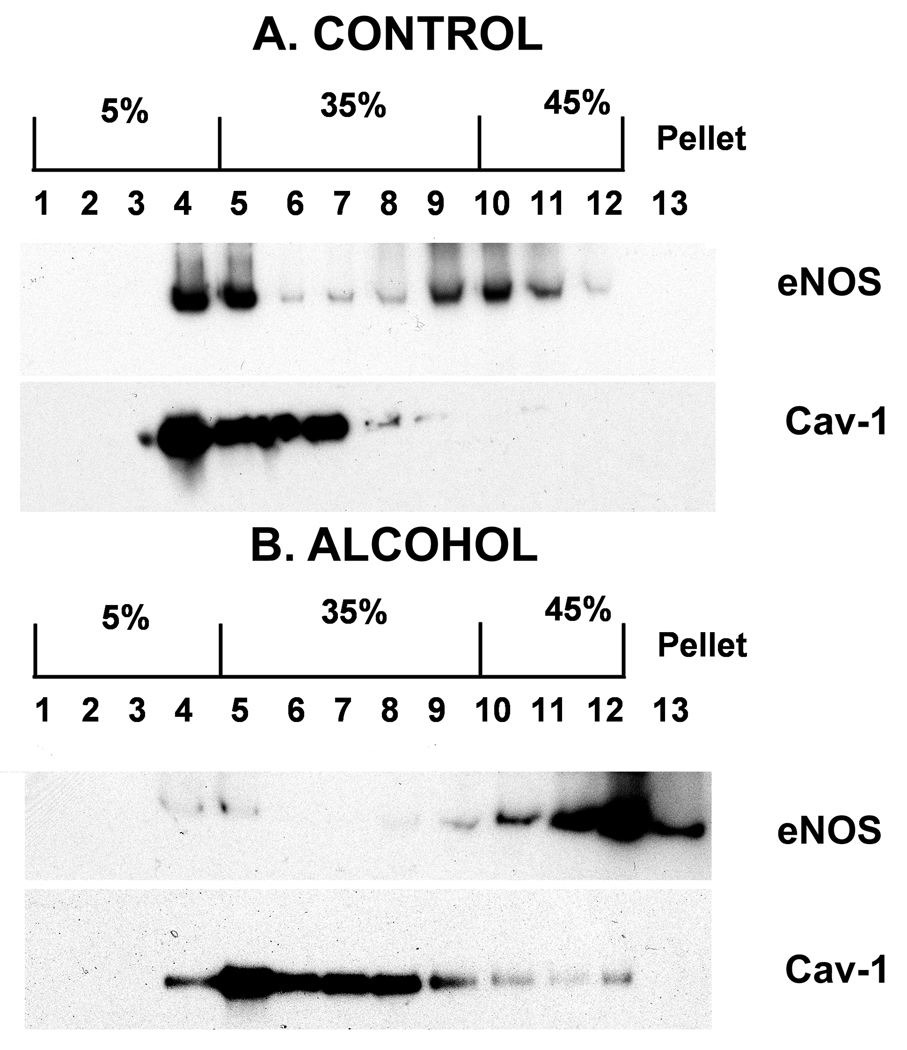
Immunoblot validation of cav-1, the major caveolar scaffolding protein, and eNOS, an enzyme that is compartmentalized to the caveolae confirmed high throughput proteomic findings (figure 3). Further, whole cell eNOS level was comparable between high throughput proteomics and Western blotting (eNOS decrease: with proteomics, by 58.54%; with immunoblotting, by 57%) (data not shown). These alcohol responses for eNOS and Cav-1 are both novel observations.
Figure 3.
Western immunoblot analysis of gradient density centrifugation of subcellular fractions fractions prepared from (A) control (0 mg/dl) and (B) binge alcohol (150 mg/dl) treated uterine arterial endothelial cells from pregnant ewes. Under control conditions, cav-1 and eNOS were both predominantly located in the caveolar pool (lanes 4–5; figure 2A) whereas endothelial nitric oxide synthase was entirely depleted from this caveolar pool in response to binge-like alcohol (figure 2B).
DISCUSSION
Though a wealth of information can be gleaned from the proteomic profile, we will focus on seven major and salient observations. First, binge-like alcohol results in a distinctive caveolar proteomic signature profile with several abundant caveolar proteins being dramatically decreased or knocked out by alcohol. Though the mechanisms underlying the effects of alcohol on the caveolar proteome are unknown, these results are in agreement with earlier studies on the overall negative effects of alcohol on the caveolar rafts (Mao et al., 2009; Ronis et al., 2007; Wang and Abdel-Rahman, 2005; Wood et al., 2001). Second, a moderate concentration of alcohol (150 mg/dl) has a substantial impact on the caveolar proteome. The concentration of alcohol utilized in this study is also clinically relevant compared to many FAS studies utilizing other model systems which assess alcohol-induced neuro-structural/ behavioral deficits at doses > 300 mg/dl (Livy et al., 2003). Further, the magnitude of caveolar lipid raft disruption may be proportional to the level of alcohol insult and since a number of proteins are dramatically decreased or knocked out by a moderate alcohol concentration, it suggests that the proteome will reflect alcohol exposure much beyond the time of insult. However, these temporal studies have not yet been performed. Third, the proteins that decreased dramatically or knocked out in the alcoholic profile span from basic structural proteins like actin γ to proteins involved in trafficking, cell signaling, and cell-cell adhesion. Fourth, we observed a nearly 164% increase of fibronectin, a multifunctional extracellular matrix protein that forms a kind of scaffolding in which the cells are embedded (Tuma and Casey, 1998). In adult non-pregnant humans, chronic alcohol has been demonstrated to increase levels of fibronectin in the lung (Burnham et al., 2007) and the perivenular zone of the liver (Savolainen et al., 1995). In rats, short term alcohol leads to accumulation of fibronectin in the heart (Vendemiale et al., 2001). In pregnant women, increased plasma fibronectin levels is utilized as a marker for gestational hypertensive disorders (Paarlberg et al., 1998) and fetal fibronectin is associated with the onset of labor (Cunze et al., 1996). Finally, these data may also have physiologic implications on uterine endothelial cell shape and function (Grinnell et al., 1982). Fifth, the fact that alcohol dramatically decreases the caveolar abundance of histone H2A family proteins provides novel cues about epigenetic effects of alcohol. Sixth, dramatic alteration in all caveolar proteins associated with nitric oxide regulation, and numerous ATP-related proteins are noteworthy with reference to regulation of uterine blood flow during pregnancy. The immunoblot not only validates the proteomic data but also clearly demonstrates movement of eNOS away from its caveolar “home” with every bout of alcohol, a finding that suggests significant utero-placental vascular effects of alcohol during the third trimester-equivalent of human gestation. Further, dramatic decrease of many ATP-associated proteins may exacerbate alcohol-induced impaired vasodilation as ATP is a powerful physiologic agonist for nitric oxide release in the uterine vascular bed during pregnancy (Bird et al., 2003). The fact that nitric oxide also regulates the intricate coordinated growth and remodeling of the entire uterine circulation, as well as the creation of a placenta suggests a very important role for the maternal utero-placental vascular compartment in the pathogenesis of FASD. Finally, the direct impact of the nitric oxide system on the fetal compartment is substantiated by reports that deficiency in nitric oxide production renders the developing fetal neuronal cells more vulnerable to the toxic effects of alcohol and that the nitric oxide-cGMP-PKG pathway has protective effect against alcohol-induced injury (Bonthius et al., 2003; 2008; 2009). Seventh, alcohol-induced dramatic reduction of USP2, the major deubiquitinating enzyme may provide mechanistic perspectives underlying caveolar protein depletion and/or degradation as USP2 depletion has been documented to significantly enhance protein degradation (Tirat et al., 2005).
These data demonstrate for the first time that the caveolar proteomic signature for alcohol consumption shows a promising direction for early detection of FASD. As proof of principle, this study exploited the unique advantages of the well characterized endothelial caveolae for biomarker(s) development. A logical extension of this study would be to demonstrate this caveolar signature pattern for maternal alcohol consumption in the RBCs, a blood-borne cell type that is readily accessible. Follow up studies will also include characterization of caveolae from uterine and umbilical endothelial cells from alcohol exposed ewes as well as from human umbilical cord endothelial cells which are easily obtained from mothers following parturition. Further, in vivo studies employing patterns of ethanol consumption that more closely resemble human drinking may produce very different protein signatures than that observed in vitro studies using relatively acute ethanol exposure paradigms.
Acknowledgements
We wish to thank Mr. Kreg Grindle and Ms. Gladys Lopez for their assistance in this study.
Supported by NIH grants HL49210, HD38843 HL89144, HL70562.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bearer CF. Markers to detect drinking during pregnancy. Alcohol Res. Health. 2001;25:210–218. [PMC free article] [PubMed] [Google Scholar]
- Bearer CF, Stoler JM, Cook JD, Carpenter SJ. Biomarkers of alcohol use in pregnancy. Alcohol Res. Health. 2004;28:38–43. [PMC free article] [PubMed] [Google Scholar]
- Bird IM, Sullivan JA, Di T, Cale JM, Zhang L, Zheng J, Magness RR. Pregnancy-dependent changes in cell signaling underlie changes in differential control of vasodilator production in uterine artery endothelial cells. Endocrinology. 2000;141:1107–1117. doi: 10.1210/endo.141.3.7367. [DOI] [PubMed] [Google Scholar]
- Bird IM, Zhang L, Magness RR. Possible mechanisms underlying pregnancy-induced changes in uterine artery endothelial function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R245–R258. doi: 10.1152/ajpregu.00108.2002. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Karacay B, Dai D, Pantazis NJ. FGF-2, NGF and IGF-1, but not BDNF, utilize a nitric oxide pathway to signal neurotrophic and neuroprotective effects against alcohol toxicity in cerebellar granule cell cultures. Brain Res. Dev. Brain Res. 2003;140:15–28. doi: 10.1016/s0165-3806(02)00549-7. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Bonthius NE, Li S, Karacay B. The protective effect of neuronal nitric oxide synthase (nNOS) against alcohol toxicity depends upon the NO-cGMP-PKG pathway and NF-kappaB. Neurotoxicology. 2008;29:1080–1091. doi: 10.1016/j.neuro.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Luong T, Bonthius NE, Hostager BS, Karacay B. Nitric oxide utilizes NF-kappaB to signal its neuroprotective effect against alcohol toxicity. Neuropharmacology. 2009;56:716–731. doi: 10.1016/j.neuropharm.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Burnham EL, Moss M, Ritzenthaler JD, Roman J. Increased fibronectin expression in lung in the setting of chronic alcohol abuse. Alcohol. Clin. Exp. Res. 2007;31:675–683. doi: 10.1111/j.1530-0277.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- Chen D, Zangl AL, Zhao Q, Markley JL, Zheng J, Bird IM, Magness RR. Ovine caveolin-1: cDNA cloning, E. coli expression, and association with endothelial nitric oxide synthase. Mol. Cell. Endocrinol. 2001;175:41–56. doi: 10.1016/s0303-7207(01)00403-8. [DOI] [PubMed] [Google Scholar]
- Church MW, Gerkin KP. Hearing disorders in children with fetal alcohol syndrome: findings from case reports. Pediatrics. 1988;82:147–154. [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Exp. Biol. Med. (Maywood) 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Cunze T, Osmers R, Lefhalm B, Wieding J, Fadaian-Motlagh S, Kuhn W. Fibronectin in the plasma during pregnancy and parturition. Gynecol. Obstet. Invest. 1996;41:183–188. doi: 10.1159/000292265. [DOI] [PubMed] [Google Scholar]
- Eysseric H, Gonthier B, Soubeyran A, Bessard G, Saxod R, Barret L. There is not simple method to maintain a constant ethanol concentration in longterm cell culture: keys to a solution applied to the survey of astrocytic ethanol absorption. Alcohol. 1997;14:111–115. doi: 10.1016/s0741-8329(96)00112-7. [DOI] [PubMed] [Google Scholar]
- Falconer J. The effect of maternal ethanol infusion on placental blood flow and fetal glucose metabolism in sheep. Alcohol Alcohol. 1990;25:413–416. [PubMed] [Google Scholar]
- Goodlett CR, Eilers AT. Alcohol-induced Purkinje cell loss with a single binge exposure in neonatal rats: a stereological study of temporal windows of vulnerability. Alcohol. Clin. Exp. Res. 1997;21:738–744. [PubMed] [Google Scholar]
- Grinnell F, Head JR, Hoffpauir J. Fibronectin and cell shape in vivo: studies on the endometrium during pregnancy. J. Cell Biol. 1982;94:597–606. doi: 10.1083/jcb.94.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannuksela ML, Liisanantti MK, Nissinen AE, Savolainen MJ. Biochemical markers of alcoholism. Clin. Chem. Lab. Med. 2007;45:953–961. doi: 10.1515/CCLM.2007.190. [DOI] [PubMed] [Google Scholar]
- Liao WX, Feng L, Zhang H, Zheng J, Moore TR, Chen DB. Compartmentalizing VEGF-induced ERK2/1 signaling in Placental Artery Endothelial Cell Caveolae: a Paradoxical Role of Caveolin-1 in Placental Angiogenesis in vitro. Mol. Endocrinol. 2009;23:1428–1444. doi: 10.1210/me.2008-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol. Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Magness RR. The Endocrinology of Pregnancy Bazer. Humana Press; 1998. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy; pp. 507–539. [Google Scholar]
- Mao H, Diehl AM, Li YX. Sonic hedgehog ligand partners with caveolin-1 for intracellular transport. Lab. Invest. 2009;89:290–300. doi: 10.1038/labinvest.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SE, Shabanowitz J, Hunt DF, Marto JA. Subfemtomole MS and MS/MS peptide sequence analysis using nano-HPLC micro-ESI fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 2000;72:4266–4274. doi: 10.1021/ac000497v. [DOI] [PubMed] [Google Scholar]
- McMahon KA, Zhu M, Kwon SW, Liu P, Zhao Y, Anderson RG. Detergent-free caveolae proteome suggests an interaction with ER and mitochondria. Proteomics. 2006;6:143–152. doi: 10.1002/pmic.200500208. [DOI] [PubMed] [Google Scholar]
- Ozuyaman B, Grau M, Kelm M, Merx MW, Kleinbongard P. RBC NOS: regulatory mechanisms and therapeutic aspects. Trends Mol. Med. 2008;14:314–322. doi: 10.1016/j.molmed.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Paarlberg KM, de Jong CL, van Geijn HP, van Kamp GJ, Heinen AG, Dekker GA. Total plasma fibronectin as a marker of pregnancy-induced hypertensive disorders: a longitudinal study. Obstet. Gynecol. 1998;91:383–388. doi: 10.1016/s0029-7844(97)00683-2. [DOI] [PubMed] [Google Scholar]
- Parnell SE, Ramadoss J, Delp MD, Ramsey MW, Chen WJ, West JR, Cudd TA. Chronic ethanol increases fetal cerebral blood flow specific to the ethanol-sensitive cerebellum under normoxaemic, hypercapnic and acidaemic conditions: ovine model. Exp. Physiol. 2007;92:933–943. doi: 10.1113/expphysiol.2007.038091. [DOI] [PubMed] [Google Scholar]
- Parton RG, Simons K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Hogan HA, Given JC, West JR, Cudd TA. Binge alcohol exposure during all three trimesters alters bone strength and growth in fetal sheep. Alcohol. 2006;38:185–192. doi: 10.1016/j.alcohol.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Chen WJ, West JR, Cudd TA. Temporal vulnerability of fetal cerebellar Purkinje cells to chronic binge alcohol exposure: ovine model. Alcohol. Clin. Exp. Res. 2007a;31:1738–1745. doi: 10.1111/j.1530-0277.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Ouyang N, Chen WJ, Cudd TA. Acid-sensitive channel inhibition prevents fetal alcohol spectrum disorders cerebellar Purkinje cell loss. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R596–R603. doi: 10.1152/ajpregu.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Pina KB, Chen WJ, Cudd TA. All three trimester binge alcohol exposure causes fetal cerebellar purkinje cell loss in the presence of maternal hypercapnea, acidemia, and normoxemia: ovine model. Alcohol. Clin. Exp. Res. 2007b;31:1252–1258. doi: 10.1111/j.1530-0277.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- Robinson MK, Myrick JE, Henderson LO, Coles CD, Powell MK, Orr GA, Lemkin PF. Two-dimensional protein electrophoresis and multiple hypothesis testing to detect potential serum protein biomarkers in children with fetal alcohol syndrome. Electrophoresis. 1995;16:1176–1183. doi: 10.1002/elps.11501601195. [DOI] [PubMed] [Google Scholar]
- Ronis MJ, Wands JR, Badger TM, de la Monte SM, Lang CH, Calissendorff J. Alcohol-induced disruption of endocrine signaling. Alcohol. Clin. Exp. Res. 2007;31:1269–1285. doi: 10.1111/j.1530-0277.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Savolainen V, Perola M, Lalu K, Penttila A, Virtanen I, Karhunen PJ. Early perivenular fibrogenesis--precirrhotic lesions among moderate alcohol consumers and chronic alcoholics. J. Hepatol. 1995;23:524–531. doi: 10.1016/0168-8278(95)80057-3. [DOI] [PubMed] [Google Scholar]
- Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J. Biol. Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Stoler JM, Huntington KS, Peterson CM, Peterson KP, Daniel P, Aboagye KK, Lieberman E, Ryan L, Holmes LB. The prenatal detection of significant alcohol exposure with maternal blood markers. J. Pediatr. 1998;133:346–352. doi: 10.1016/s0022-3476(98)70267-7. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Wasserman EA, West JR, Goodlett CR. Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: importance of developmental timing and number of episodes. Dev. Psychobiol. 1996;29:433–452. doi: 10.1002/(SICI)1098-2302(199607)29:5<433::AID-DEV3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Tirat A, Schilb A, Riou V, Leder L, Gerhartz B, Zimmermann J, Worpenberg S, Eidhoff U, Freuler F, Stettler T, et al. Synthesis and characterization of fluorescent ubiquitin derivatives as highly sensitive substrates for the deubiquitinating enzymes UCH-L3 and USP-2. Anal. Biochem. 2005;343:244–255. doi: 10.1016/j.ab.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Tuma DJ, Casey CA. Dangerous byproducts of alcohol breakdown--focus on adducts. Alcohol. Res. Health. 2003;27:285–290. [PMC free article] [PubMed] [Google Scholar]
- Urso T, Gavaler JS, Van Thiel DH. Blood ethanol levels in sober alcohol users seen in an emergency room. Life. Sci. 1981;28:1053–1056. doi: 10.1016/0024-3205(81)90752-9. [DOI] [PubMed] [Google Scholar]
- Vendemiale G, Grattagliano I, Altomare E, Serviddio G, Portincasa P, Prigigallo F, Palasciano G. Mitochondrial oxidative damage and myocardial fibrosis in rats chronically intoxicated with moderate doses of ethanol. Toxicol. Lett. 2001;123:209–216. doi: 10.1016/s0378-4274(01)00401-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Abdel-Rahman AA. Effect of chronic ethanol administration on hepatic eNOS activity and its association with caveolin-1 and calmodulin in female rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G579–G585. doi: 10.1152/ajpgi.00282.2004. [DOI] [PubMed] [Google Scholar]
- Wood WG, Avdulov NA, Chochina SV, Igbavboa U. Lipid carrier proteins and ethanol. J. Biomed. Sci. 2001;8:114–118. doi: 10.1007/BF02255979. [DOI] [PubMed] [Google Scholar]