Abstract
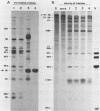
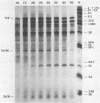
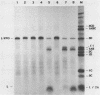
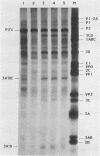
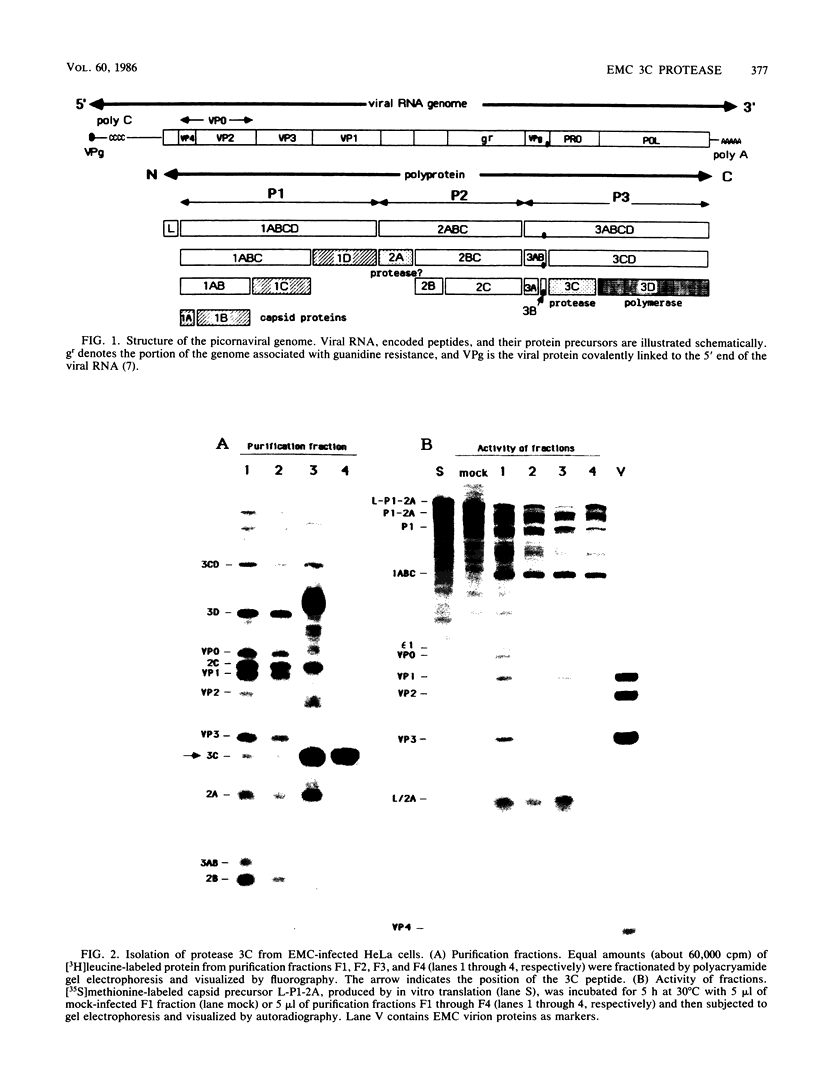
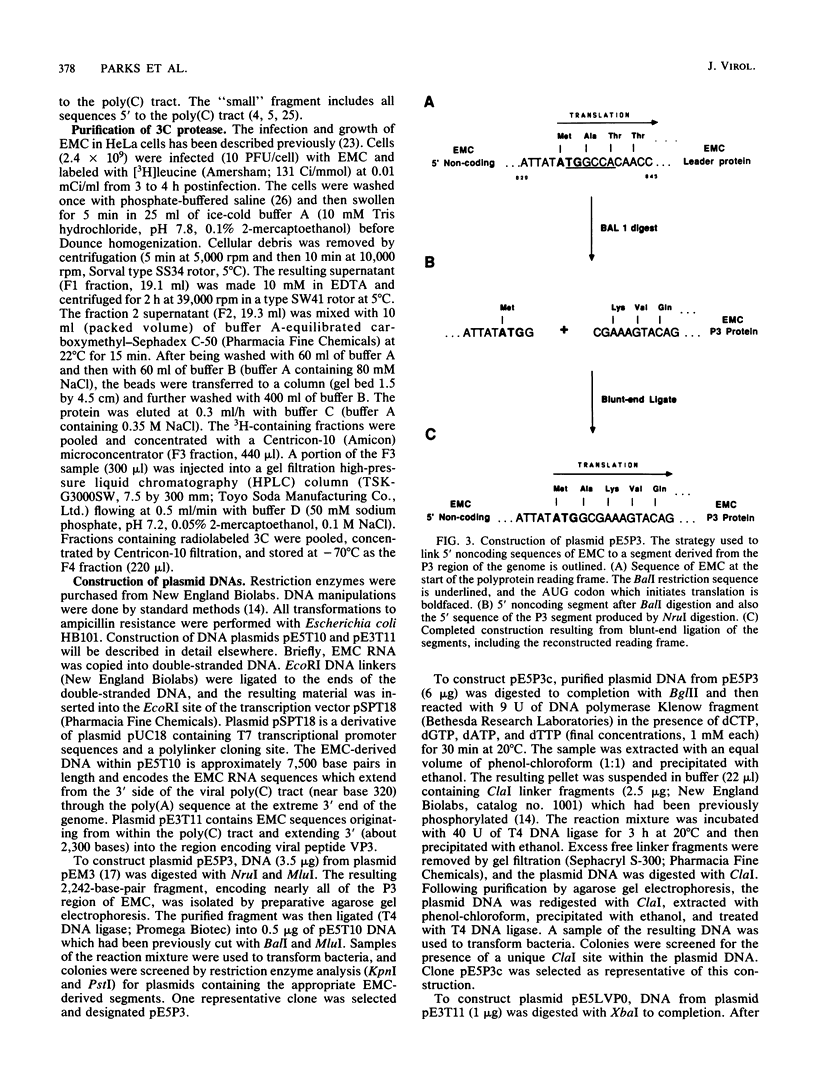
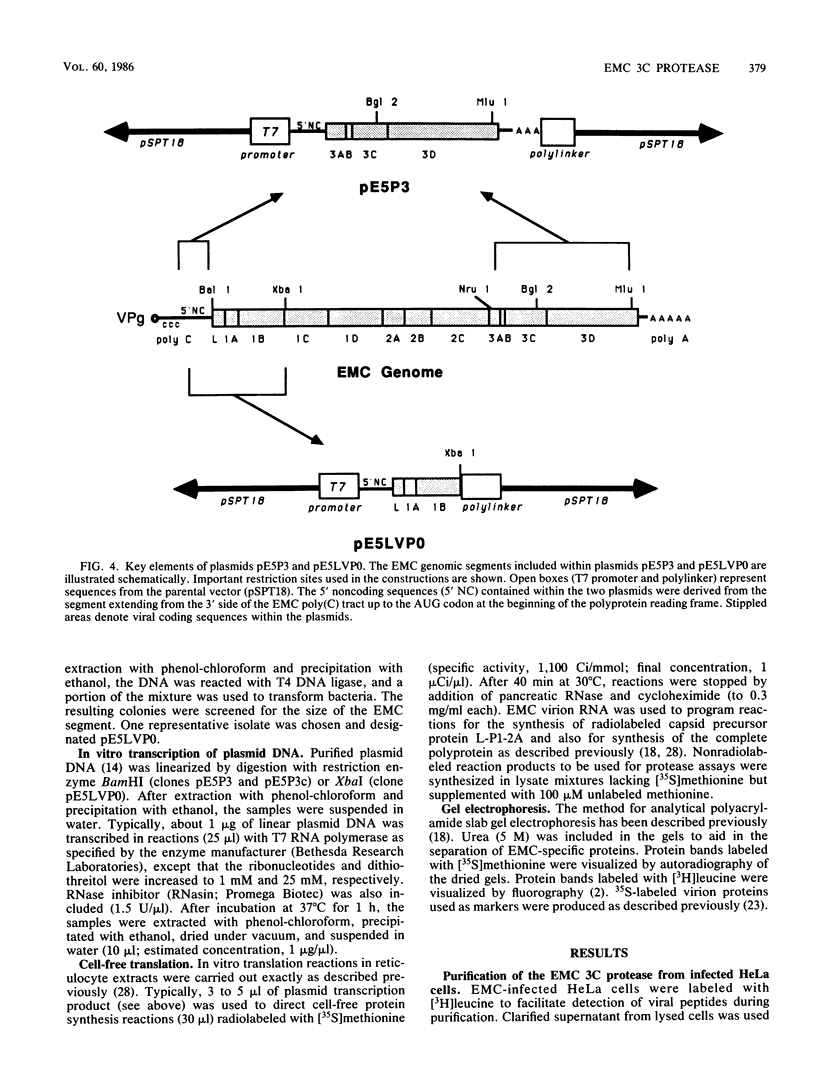
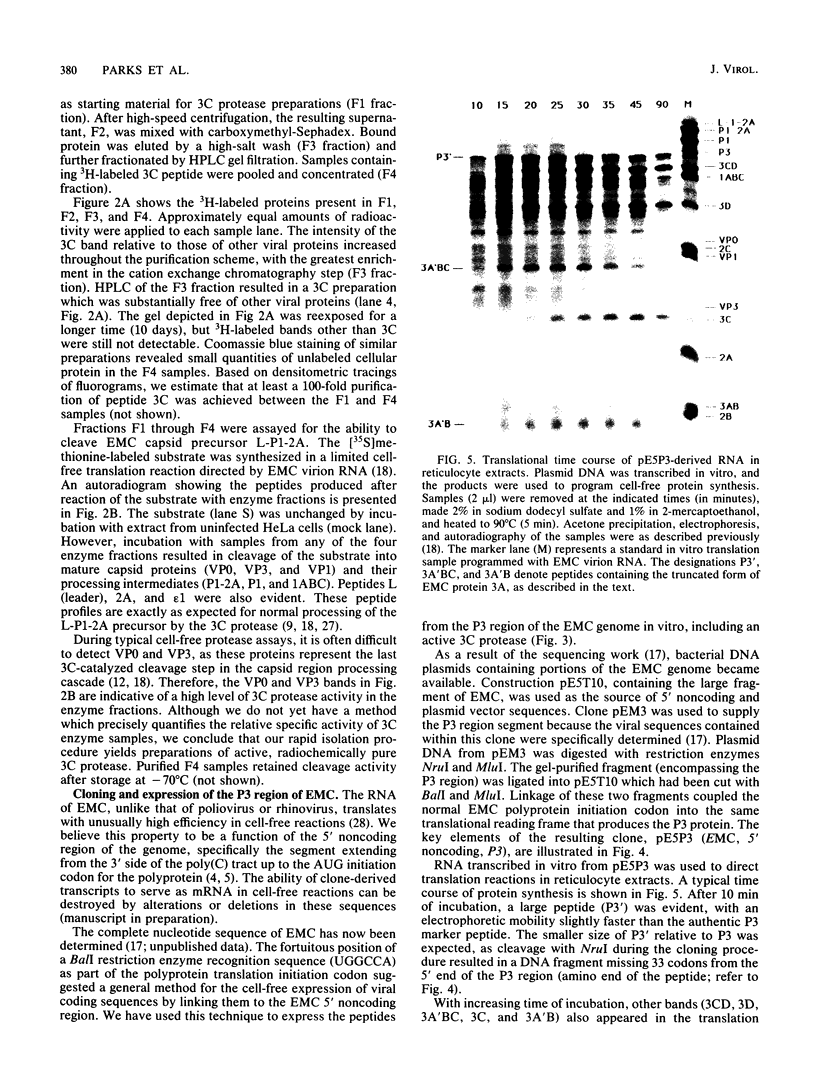
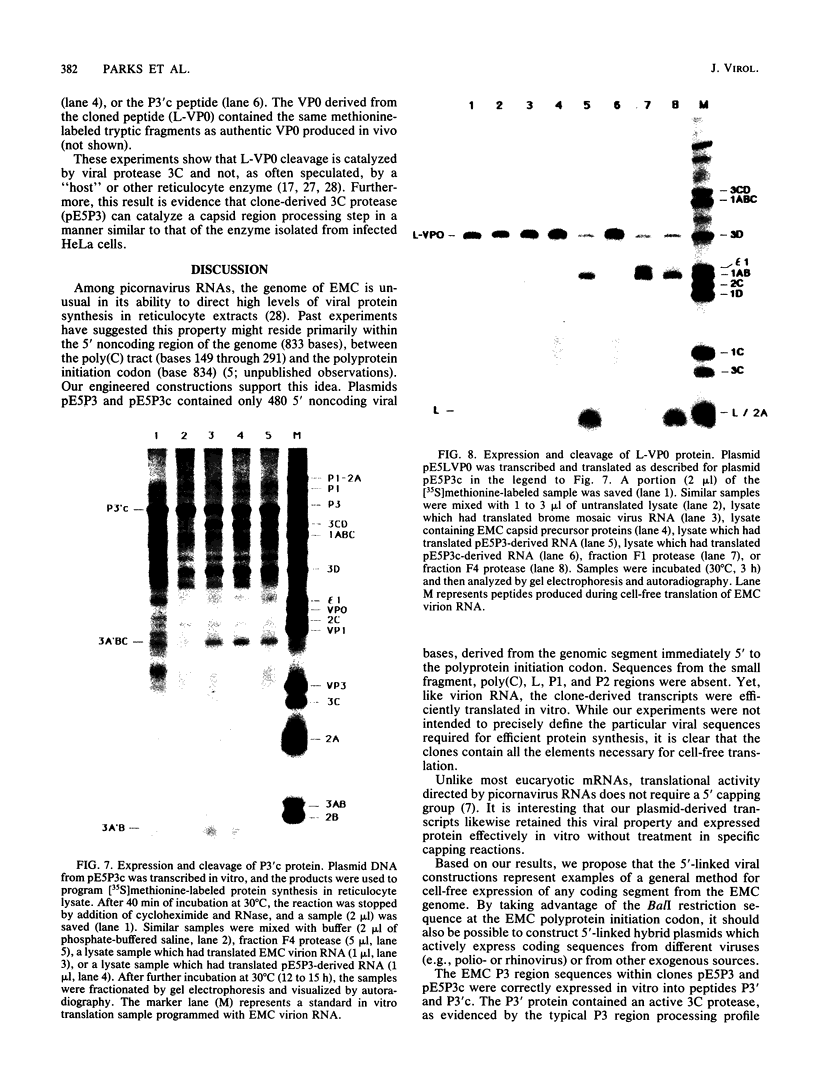
All picornaviral peptides are derived by progressive posttranslational cleavage of a giant precursor polyprotein. Translation of encephalomyocarditis virus (EMC) RNA in rabbit reticulocyte extracts produces active viral peptides, including protease 3C, which is responsible for many cleavage reactions within the processing cascade. DNA plasmids containing 5' noncoding sequences of EMC linked to other portions of the viral genome were constructed and transcribed into RNA. Like virion RNA, the clone-derived transcripts directed efficient protein translation in vitro. The 5'-linked constructions may represent examples of a general method for cell-free expression of any cloned gene segment. One construction produced a self-cleaving P3 region precursor, which contained active 3C protease. A genetically engineered insertion within the 3C sequences eliminated endogenous self-cleavage activity without altering the ability of the P3 peptide to serve as substrate in bimolecular reactions with added 3C. Another plasmid encoding the L-VP0 portion of the capsid region was used to demonstrate that scission between the leader peptide (L) and capsid protein VP0 can be catalyzed by 3C. The enzyme responsible for this step was previously unidentified. A rapid purification scheme for isolation of 3C from EMC-infected HeLa cells is also presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Kamer G., Nicklin M. J., Wimmer E. Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res. 1984 Sep 25;12(18):7251–7267. doi: 10.1093/nar/12.18.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Campbell E. A., Jackson R. J. Processing of the encephalomyocarditis virus capsid precursor protein studied in rabbit reticulocyte lysates incubated with N-formyl-[35S]methionine-tRNAfMet. J Virol. 1983 Jan;45(1):439–441. doi: 10.1128/jvi.45.1.439-441.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov K. M., Agol V. I. Poly(C) sequence is located near the 5'-end of encephalomyocarditis virus RNA. Biochem Biophys Res Commun. 1976 Jul 26;71(2):551–557. doi: 10.1016/0006-291x(76)90822-6. [DOI] [PubMed] [Google Scholar]
- Churchill M. A., Radloff R. J. Two-dimensional electrophoretic analysis of encephalomyocarditis viral proteins. J Virol. 1981 Mar;37(3):1103–1106. doi: 10.1128/jvi.37.3.1103-1106.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golini F., Semler B. L., Dorner A. J., Wimmer E. Protein-linked RNA of poliovirus is competent to form an initiation complex of translation in vitro. Nature. 1980 Oct 16;287(5783):600–603. doi: 10.1038/287600a0. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Svitkin YuV, Agol V. I. Proteolytic activity of the nonstructural polypeptide p22 of encephalomyocarditis virus. Biochem Biophys Res Commun. 1981 Feb 27;98(4):952–960. doi: 10.1016/0006-291x(81)91203-1. [DOI] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Ariga H., Anderson C. W., Wimmer E. Expression of a cloned gene segment of poliovirus in E. coli: evidence for autocatalytic production of the viral proteinase. Cell. 1984 Jul;37(3):1063–1073. doi: 10.1016/0092-8674(84)90441-0. [DOI] [PubMed] [Google Scholar]
- Jackson R. J. A detailed kinetic analysis of the in vitro synthesis and processing of encephalomyocarditis virus products. Virology. 1986 Feb;149(1):114–127. doi: 10.1016/0042-6822(86)90092-9. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C. In vitro synthesis and assembly of picornaviral capsid intermediate structures. J Virol. 1982 Dec;44(3):900–906. doi: 10.1128/jvi.44.3.900-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Kirby E. M., Janda M. R., Drake N. L., Duke G. M., Potratz K. F., Collett M. S. The nucleotide and deduced amino acid sequences of the encephalomyocarditis viral polyprotein coding region. Nucleic Acids Res. 1984 Mar 26;12(6):2969–2985. doi: 10.1093/nar/12.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Pallansch M. A., Rueckert R. R. Protease required for processing picornaviral coat protein resides in the viral replicase gene. J Virol. 1979 Dec;32(3):770–778. doi: 10.1128/jvi.32.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Rueckert R. R. Evidence for intramolecular self-cleavage of picornaviral replicase precursors. J Virol. 1982 Jan;41(1):244–249. doi: 10.1128/jvi.41.1.244-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Translation of encephalomyocarditis virus RNA in vitro yields an active proteolytic processing enzyme. Eur J Biochem. 1978 Apr 17;85(2):457–462. doi: 10.1111/j.1432-1033.1978.tb12260.x. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Rueckert R. R., Pallansch M. A. Preparation and characterization of encephalomyocarditis (EMC) virus. Methods Enzymol. 1981;78(Pt A):315–325. [PubMed] [Google Scholar]
- Rueckert R. R., Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984 Jun;50(3):957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangar D. V., Black D. N., Rowlands D. J., Harris T. J., Brown F. Location of the initiation site for protein synthesis on foot-and-mouth disease virus RNA by in vitro translation of defined fragments of the RNA. J Virol. 1980 Jan;33(1):59–68. doi: 10.1128/jvi.33.1.59-68.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985 Jan;53(1):137–143. doi: 10.1128/jvi.53.1.137-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. Y., Shih D. S. Cleavage of the capsid protein precursors of encephalomyocarditis virus in rabbit reticulocyte lysates. J Virol. 1981 Dec;40(3):942–945. doi: 10.1128/jvi.40.3.942-945.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]