Abstract
Background
Surgical resection of the ileum, cecum and proximal right colon (ICR) is common in the management of Crohn’s disease, yet little is known about the effect of active inflammation on the adaptive response following intestinal loss. We recently developed a surgical model of ICR in germ-free (GF) IL-10 null mice that develop small intestinal inflammation only when mice undergo conventionalization with normal fecal microflora (CONV) before surgical intervention. In this study, we examined the effects of post-surgical small bowel inflammation on adaptive growth after ICR.
Methods
8-10 week old GF 129SvEv IL-10 null mice were allocated to GF or CONV groups. Non-operated GF and CONV mice provided baseline controls. Two weeks later GF and CONV mice were further allocated to ICR or sham operation. Small intestine and colon were harvested 7d after surgery for histological analysis.
Results
All mice within the gnotobiotic facility maintained GF status and did not develop small intestinal or colonic inflammation. CONV resulted in colitis in all groups, whereas small intestinal inflammation was only observed following ICR. Resection-induced small intestinal inflammation in CONV mice was associated with increases in proliferation, crypt depth and villus height when compared to GF mice after ICR. Resection-induced increases in crypt fission only occurred in CONV mice.
Conclusion
ICR-dependent small intestinal inflammation in CONV IL-10 null mice dramatically enhances early adaptive growth of the small intestine. Additional studies utilizing our model may provide clinical insight leading to optimal therapies in managing IBD patients after surgical resection.
Keywords: IL-10 null, Inflammatory Bowel Disease, IBD, ileocecal resection, ICR, adaptation, crypt fission, proliferation, microbial effect, conventionalization
Introduction
Inflammatory bowel disease (IBD) is a common indication for intestinal resection. It has been estimated that upwards of 75% of patients suffering from Crohn’s disease will require surgical intervention at some point for complications of their disease [1;2]. As many of these patients will ultimately undergo multiple bowel resections for recurrent disease during their lifetime, they are at risk of developing intestinal failure due to short bowel syndrome. Clinically, the most frequent site of disease onset involves the ileum and proximal right colon, making an ileo-cecal resection (ICR) the most common intestinal surgical procedure in patients with Crohn’s disease [3]. Multiple studies in animal resection models have demonstrated that the remaining intestine undergoes a process of adaptive growth to compensate for the loss of bowel [4-8]. We have recently developed and characterized a murine model of ICR in wild type (WT) mice to study adaptive growth response of the small intestine and colon after this operative procedure [9]. Data from our initial characterization of the ICR model in WT mice demonstrated marked early increases in the presence of crypt fission after resection that was temporally associated with expansion of intestinal stem cells (ISC) leading to sustained long-term adaptive growth. Several other published studies have also associated the incidence of crypt fission and the expansion of ISC, notably during development [10], following chemo-radiation [11] and in pre-malignant conditions [12]. Despite the high incidence of intestinal surgery required to treat the complications of Crohn’s disease and the extensive research that has focused on the adaptive growth of the small intestine, surprisingly little is known about the effect of active inflammation on the adaptive response of the remaining small bowel after resection.
The ideal animal model to study the surgical effects of Crohn’s disease currently does not exist as the etiology has not been well established. We therefore sought to identify a murine model that develops reproducible, spontaneous, transmural small intestinal inflammation. Unfortunately, to date the vast majority of IBD models predominantly develop spontaneous colitis without small intestinal inflammation. Although a few models of ileitis have been described [13-15], there is not one that reproducibly involves the small intestine in a healthy animal that can undergo surgical resection. In this study we utilized the IL-10 null mouse as it provides a well characterized, reproducible model of colitis that develops only when exposed to microbiota and not when housed under germ free (GF) conditions [13;16-22]. The small bowel in un-operated or sham-operated IL-10 null mice does not become inflamed following conventionalization (CONV), thus bacteria alone are insufficient to induce small bowel inflammation in IL-10 null mice. We have recently demonstrated that ICR results in chronic small intestinal inflammation at the anastomosis and importantly within the distal jejunum remote from the anastomosis that persists following resection [23], thus providing a valuable model of post-surgical small intestinal inflammation in a widely used animal model of IBD. Importantly, IL-10 null mice maintained in a GF environment remain disease free after resection, providing genetically matched controls to assess the effects of small bowel inflammation on the adaptive response to ICR.
The effect of microbiota on intestinal adaptation following resection has been previously examined. Juno et al demonstrated that a small but significant increases in both villus height and proliferation occurred within the ileum in GF rats after massive proximal small bowel resection when compared to CONV rats [4]. As regional differences in luminal bacteria and adaptation following resection exist when comparing the distal versus proximal small bowel [4], we have recently developed and characterized the effect of distal small bowel resection (ICR) in mice [9]. In addition, we have created a surgical isolator in our gnotobiotic facility that allows us to perform ICR and maintain GF status in mice post-operatively [24]. An important observation from studies in GF and CONV WT C57BL6 mice was that baseline differences in intestinal morphology and homeostasis exist when comparing un-operated GF and CONV animals. These differences have been described in a few animal models including the pig where GF status has been shown to result in significantly larger villi and smaller crypts when compared to CONV pigs [25], but have not been well characterized in the small intestine from WT or IL-10 null mice. In our previous study in WT C57BL6 mice, when we corrected for these baseline differences, no obvious differences in the magnitude of small intestinal adaptive responses occurred in GF versus CONV mice after ICR. However, bacteria-dependent up-regulation of intestinal bile acid binding protein (IBABP), and colonic adaptation occurred in CONV mice when compared to GF following ICR. In the current study, we sought to use our ICR model in GF and CONV IL-10 null mice to determine the early effects of post-surgical small intestinal inflammation on adaptive growth in the small intestine and colon. We hypothesized that bacterially-mediated intestinal inflammation will enhance the adaptive response after ICR in this genetic model of IBD.
Methods
Animals
The University of North Carolina Institutional Animal Care and Use Committee approved the protocol for this study (IACUC #07-229.0). Male 129SvEv IL-10 null mice that were born and raised in the UNC gnotobiotic rodent facility were utilized for all experiments. At eight weeks of age mice were randomly allocated to either GF or CONV groups (n = 4-12 mice/group). All mice maintained in the gnotobiotic facility were confirmed to be GF at the conclusion of the experiment by stool viral, bacterial and fungal cultures. Additionally, sentinel mice from the litter were kept in the same incubator after the conclusion of the experiment as controls and were also confirmed to be GF. Mice allocated to be in the CONV groups were transferred to our specific pathogen free (SPF) housing facility where conventionalization was achieved by standard fecal slurry [18;19]. Mice were considered to be CONV after 2 weeks.
Operative Procedure
Two days prior to the start of the experiment, all animals were switched from regular chow to a sterilized liquid diet (Jevity 1cal, Isotonic Nutrition with fiber, Ross Laboratories, Columbus, Ohio), which was changed daily throughout the study. Mice were then further allocated to either operative or non-operative groups. The non-operative (nonop) groups were handled identically to the operative groups with regards to feeding regimen and bedding. The operative groups underwent either intestinal transection approximately 13cm proximal to the ileocecal junction with primary anastomosis (sham) or an ileo-cecal resection (ICR) as previously described [9;24]. Of note, GF surgical procedures were performed in a customized surgical isolator in the exact same manner as utilized for CONV animals (Figure 1). Liquid diet was provided ad libitum immediately post-operatively through post-operative day 7, at which time the mice were sacrificed.
Figure 1. Surgical Isolator in UNC Gnotobiotic Facility.
The surgical isolator allows GF mice to undergo the exact same operative procedure as CONV mice while remaining free of all microbiota post-operatively.
Tissue Harvest
All mice harvested for histology received an IP injection of bromodeoxyuridine (BrdU 120mg/kg) 90 minutes prior to sacrifice. Great care was taken to ensure we were comparing identical segments of distal jejunum between operative and non-operative groups, with all samples measuring 1cm. The small intestine and colon were harvested at the same location in all mice as previously described [9]. The small intestine 1cm proximal to the anastomosis in operative groups or 13cm proximal to the ileocecal junction in the non-operative groups and colon 1cm distal to the anastomosis in the ICR group, and 1cm distal to the cecum in the sham and non-operative groups were harvested. The intestine was irrigated with cold PBS and placed into labeled cassettes for perpendicular and longitudinal sectioning and fixed overnight in 10% unbuffered zinc formalin (Fisher Scientific, Kalamazoo, MI). Twenty-four hours later, the cassettes were washed with double distilled water (ddH20) and transferred to 70% alcohol for tissue processing.
Histology and Analysis of Intestinal Morphometrics
Hematoxylin and Eosin (H&E) stained histological sections were analyzed for intestinal morphology in a blinded manner using digital images acquired with an Axio Imager microscope (Zeiss). To determine crypt depth (CD) and villus height (VH), at least 10 well-oriented, full-length crypt-villus units were measured and averaged for each sample. The identification of the crypt-villus junction required that a well visualized single epithelial layer with a central lumen was visualized in all scored crypts and villi. The end of the crypt lumen marked the junction of the two structures as previously described [9]. BrdU immunohistochemistry was utilized to determine proliferative index by calculating the ratio of BrdU positive cells to total cells within at least 10 intact crypts. We have previously defined the criteria for crypt fission (CF) as a bifurcating crypt with a bisecting fissure creating at least two flask-shaped bases with a shared single crypt-villus junction [9]. Using this method we determined the percent CF in ~50 well-oriented crypts from both perpendicular and longitudinal sections per mouse.
Statistical Methods
All quantitative results are presented as mean values ± standard error of the mean (SEM). A one-way ANOVA was initially used to compare all data in non-operated GF and CONV IL-10 null mice to assess if baseline values differed. Since significant differences were observed, data in sham and ICR GF or CONV IL-10 null mice were expressed as a percentage change compared with their respective non-operated controls unless stated otherwise. Additionally, a two-way ANOVA was performed comparing GF and CONV operative groups to test for main effects of bacteria (GF vs. CONV) and surgery (sham vs. ICR). Post hoc pair-wise comparisons were then used to compare data in two specific groups. For all tests, nominal p-values were generated and less than 0.05 was considered statistically significant. Statistical analyses were performed using SAS statistical software, Version 9.2, SAS Institute, Inc., Cary, NC.
Results
Animals
All mice utilized in this study were healthy at the time of harvest. Although we were unable to obtain daily weights within the gnotobiotic facility, we noted similar weight changes in both sham operated and ICR GF and CONV mice at the time of harvest when compared to non-operative controls. Similar excellent survival (>90 percent) occurred in both GF and CONV mice. Groups of mice housed within the gnotobiotic facility maintained GF status throughout the study and did not develop small intestinal or colonic inflammation.
Non-Operative Controls
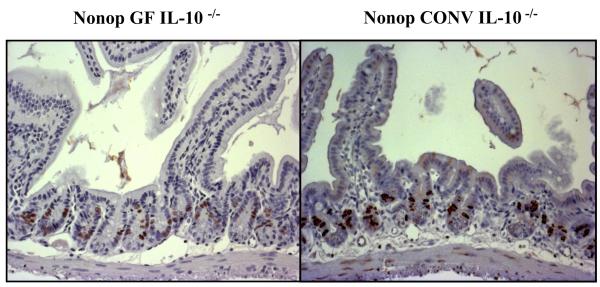
After conventionalization all 129SvEv IL-10 null mice consistently developed gross and histological evidence of colitis, as recently reported [23]. Representative sections of BrdU immunostaining in the small intestine of non-operated GF and CONV IL-10 null mice are presented in Figure 2. Differences in numbers of BrdU labeled cells, crypt depth and villus height between GF and CONV mice were clearly evident in all histological sections. To quantitate these differences, histological sections were scored for proliferative index, crypt depth, villus height and crypt fission in non-operated GF and CONV IL-10 null mice (Table 1). These data confirmed that conventionalization results in proliferation, whereas GF mice have significantly taller villi when compared to CONV mice. However, similar low levels of crypt fission were observed in both GF and CONV non-operated mice. Of note, significant increases in colonic crypt depth also occurred following CONV (77.3±5.1 (GF) vs. 102.3±6.6 (CONV), p<0.02) in our non-operative controls. Given the differences in morphology between GF and CONV IL-10 null non-operative controls, subsequent sham-operated and ICR results are expressed primarily as a percentage change relative to the corresponding non-operative data.
Figure 2. Non-operative BrdU Immunohistochemistry.
Representative BrdU immunostained sections from the jejunum of non-operative GF and CONV IL-10 null mice (20x). Note the increase in BrdU labeled cells in the CONV mouse compared to GF.
Table 1. Non-operative Baseline Morphometrics.
Morphometric and proliferative data from GF and CONV IL-10 null non-operative controls (n ≥ 5).
| Parameter | GF | CONV | P value |
|---|---|---|---|
| Proliferative Index (%) | 9.8 ± 0.6 | 16.2 ± 1.1 | <0.001 * |
| Crypt Depth (μm) | 70.1 ± 4.6 | 81.5 ± 2.2 | <0.01 * |
| Villus Height (μm) | 387.2 ± 35.1 | 232.1 ± 11 | <0.001 * |
| Crypt Fission (%) | 1.2 ± 0.01 | 0.5 ± 0.003 | NS (0.12) |
P value is calculated comparing GF to CONV.
statistically significant.
Operative Group
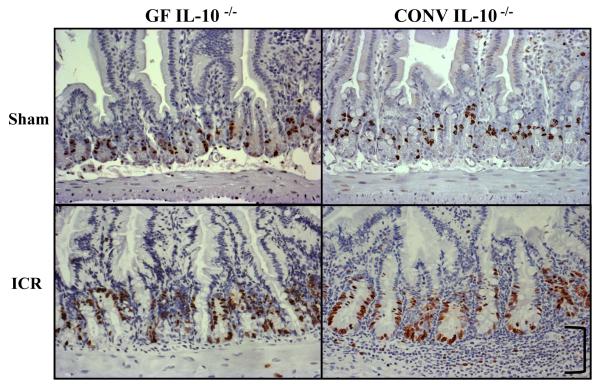
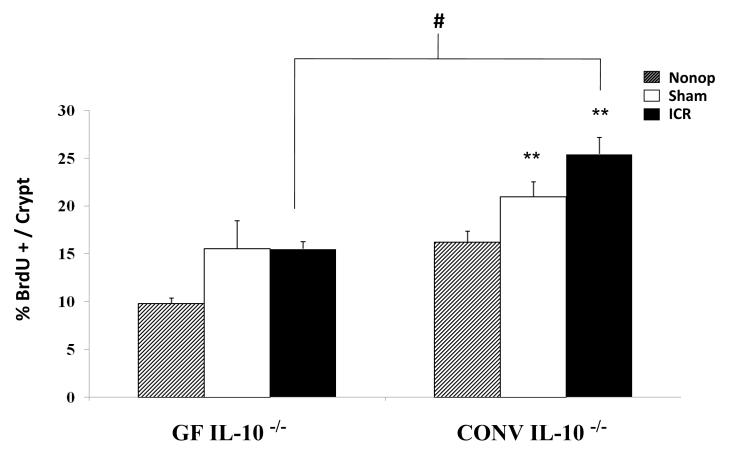
Initial evaluation of small intestinal histological sections confirmed significant inflammation at 7d after ICR in CONV IL-10 null groups [23], while non-operated and sham-operated GF and CONV IL-10 null mice showed little or no small intestinal inflammation. Representative BrdU immunostained histological sections in Figure 3 demonstrate the marked inflammation that occurs following ICR in CONV IL-10 null mice as well as the adaptive morphological and proliferative differences that occur following ICR in CONV compared with GF mice. Most obvious is the dramatic increase in crypt proliferation as demonstrated by the increase in BrdU labeled cells in CONV compared to GF IL-10 null mice after ICR. Quantitative data in Figure 4 demonstrate that operation (both sham and ICR) results in significant increases in proliferation when compared to non-operative baseline levels in CONV mice (p<0.05), but similar increases in GF mice did not reach statistical significance. In addition, resection did not further increase proliferative index in either group when compared to sham-operated groups, but significant adaptive increases in proliferation were observed in CONV mice when compared to GF following ICR (p<0.01).
Figure 3. Operative BrdU Immunohistochemistry.
Representative BrdU immunostained sections from the jejunum of sham-operated and ICR mice 7d following operation (20x). Note the increases in BrdU labeled cells and crypt depth that occur in both GF and CONV mice after ICR. In addition, marked submucosal inflammation (bracket) is only present in the CONV mouse after ICR when compared to GF and sham-operated groups.
Figure 4. Proliferative Index.
Proliferative index is determined by the percent BrdU labeled cells per crypt in non-operative, sham-operative and ICR CONV and GF IL-10 null mice. ** represents statistically significant differences comparing ICR and sham-operated to non-operated mice (p < 0.01), # represents significant difference between CONV and GF IL-10 null mice after ICR (p < 0.01). Error bars represent ± SEM.
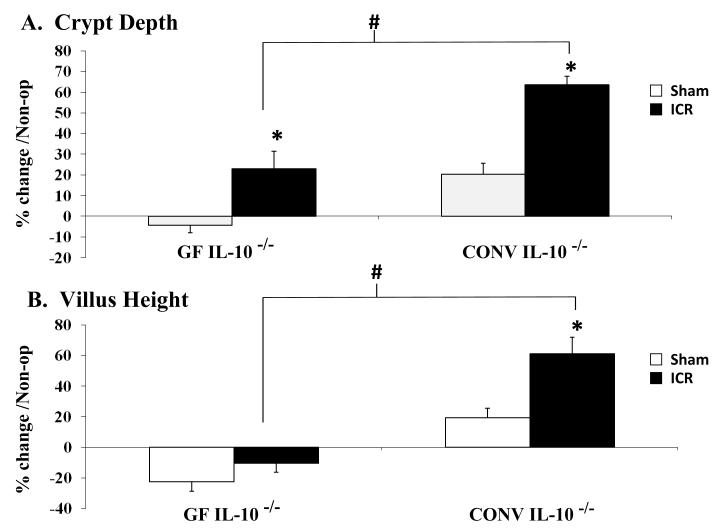
Also evident in Figure 3 is the dramatic increase in crypt depth that occurs after ICR in CONV IL-10 null mice, which was more evident than resection-induced increases in crypt depth in GF IL-10 null mice. These differences are notably greater when compared to sham-operated mice as well (p<0.02). Figure 5A shows quantitative data to illustrate that both GF and CONV IL-10 null mice demonstrate increases in crypt depth after ICR, but the magnitude of change was significantly greater in CONV when compared to GF IL-10 null mice (p<0.01). Of interest, ICR resulted in similar significant increases in colonic crypt depth in both GF and CONV mice when compared to their respective non-operative controls. Based on these observations, we focused our study on small intestinal adaptive growth following ICR. Measurement of villus height (Figure 5B) in GF IL-10 null mice demonstrated an overall significant reduction occurred in both sham-operated and ICR groups compared to non-operative controls, without significant increases following ICR when compared to sham. In contrast, CONV IL-10 null mice showed marked and significant increases in villus height following ICR compared to sham (p<0.02). The increase in villus height in CONV IL-10 null mice after ICR was significantly greater than in GF IL-10 null mice after ICR (p<0.01).
Figure 5.
A. Crypt Depth and B. Villus Height represented as the percent change compared to non-operative controls (Table 1). * significant difference Sham: ICR (p<0.02), # significant difference GF ICR: CONV ICR (p<0.01). Error bars represent ± SEM.
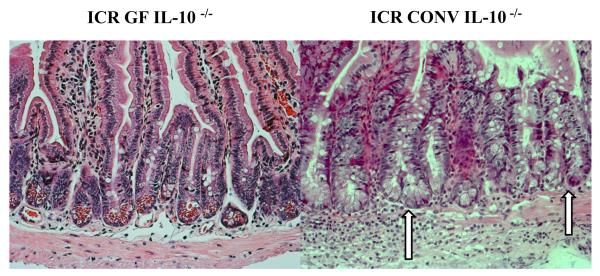
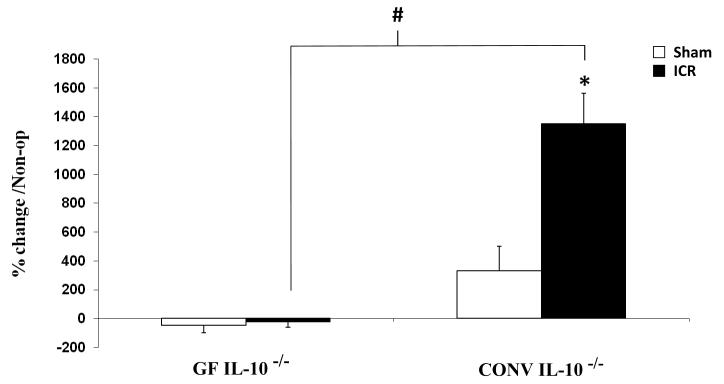
Following ICR, marked increases in crypt fission associated with intestinal inflammation were observed in all histological sections from CONV IL-10 null mice. This is in contrast to the infrequent crypt fission typically seen in both un-operated GF IL-10 null mice and GF IL-10 null mice after resection (Figure 6). Figure 7 compares the percent change in crypt fission in sham-operated and ICR GF and CONV IL-10 null mice. No changes in crypt fission were observed in GF IL-10 null mice after sham operation or ICR, whereas dramatic increases in crypt fission were observed in CONV IL-10 null mice following ICR compared to sham-operated CONV IL-10 null mice (p<0.02). Crypt fission in CONV IL-10 null mice after ICR was also significantly greater than in GF IL-10 null ICR groups (p<0.01).
Figure 6. Crypt Fission.
Representative histological H&E sections of jejunum (20x) demonstrating normal crypt morphology in GF IL-10 null mice compared to the observation of increased crypt fission and inflammation in CONV IL-10 null mice after ICR. Arrows denote bifurcating crypts.
Figure 7. Percent Change in Crypt Fission.
Percent increase in crypt fission observed following sham-operation or ICR, normalized to non-operative GF and CONV levels. * represents significant difference between CONV Sham and ICR (p<0.02), # represents significant difference between GF and CONV IL-10 null mice 7d after ICR (p<0.01). Error bars represent ± SEM.
Discussion
In this study we sought to determine the effect of small intestinal inflammation in IL-10 null mice on the adaptive response following the loss of the ileum, cecum and proximal colon, similar to the clinical situation that occurs in many patients requiring surgical resection for Crohn’s disease. We specifically utilized IL-10 null mice in this study, as the presence of both microbiota and ICR in this model are required to initiate the small intestinal inflammatory response [23]. This enabled the resected GF IL-10 null mice to serve as genetically matched, non-inflamed controls. Notably, this is the first description of postoperative small bowel adaptation in the face of ongoing small intestinal and colonic inflammation.
When comparing the normalized adaptive response in CONV to GF mice 7d after ICR, we demonstrated that the presence of microbiota-induced inflammation is associated with significant increases in crypt depth, villus height, proliferation and crypt fission only in the small intestine and not the colon after resection. These data support our hypothesis that luminal bacteria and intestinal inflammation augment the small intestinal adaptive response. It is noteworthy that prior studies suggest that GF status either does not influence or slightly enhances adaptive growth responses after resection [4;9]. Thus the dramatic increase in adaptive growth in CONV IL-10 null mice likely reflects the presence of inflammation in CONV IL-10 null mice, although it could also reflect synergistic effects of inflammation and microbiota. We recognize that the synergistic effect of microbiota and the lack of IL-10 may have contributed to these findings, as a modest adaptive response occurred in GF mice. This point is supported by previous studies, including our own, which demonstrate a similar adaptive response in GF and CONV WT mice and rats [4;24]. The observation that inflammation after ICR dramatically enhances adaptive growth of the small intestine is clinically relevant. This suggests that the adaptive growth effects of anti-inflammatory drugs given to post-surgical IBD patients should be explored. Further research will be necessary to delineate the effects of individual microbiota capable of inducing inflammation [16], which can be accomplished utilizing our model.
Similar to other GF animal models [4;25], significant baseline differences exist between GF and CONV IL-10 null mice. These differences highlight the importance of normalizing our operative data to non-operative controls. Consistent with previously published reports, we have demonstrated that villus height is notably greater in GF mice when compared to CONV litter mates, whereas the introduction of microbiota stimulates an increase in proliferation and crypt depth. Our findings that crypt fission is rarely observed in GF mice have not been previously published. We were further surprised to find that ICR did not result in any increases in crypt fission in GF IL-10 null mice. As crypt fission is closely associated with expansion of ISC, these data indicate an important role of microbiota or inflammation in driving this process. Our previous published data characterizing the ICR model in WT mice demonstrates that the early expansion of ISC sustains the long-term adaptive response [9]. It will be of great interest to assess what effect the lack of increase in crypt fission in GF IL-10 null mice will have on the long-term adaptive response.
The potential role of IL-10 in regulating adaptive growth of the small intestine is not defined. In the current studies we included IL-10 null mice since we were testing whether ongoing post-surgical inflammation affects adaptive growth. Future studies evaluating the role of IL-10 and adaptive ISC expansion are of great interest. Ongoing studies to directly compare WT and IL-10 null mice are a future direction of our laboratory. In addition, the specific role of microbiota driving this process will have significant clinical relevance. To address this, mono-association studies with colitogenic and non-colitogenic bacterial strains given to IL-10 null mice maintained within our gnotobiotic facility will help determine the specific role of these bacteria and inflammation contributing to the adaptive effects seen in our model.
Clinically, the robust adaptive response that occurs following intestinal resection in the presence of active inflammation may be beneficial or detrimental. It remains a possibility that increased inflammation and adaptation may be associated with fibrostenotic disease [23;26]. Furthermore, the potential effect of intestinal resection on the risk and prevalence of small intestinal adenocarcinoma in IBD patients has not been clearly defined [27;28]. As patients with Crohn’s disease frequently require multiple intestinal resections, further studies characterizing the regulation of ISC expansion following resection will provide important insight into a potential role of adaptive increase in ISC and adenocarcinoma risk. These data may impact clinical management, as the prolonged use of antibiotics, immunosuppression and probiotics can alter luminal microbiota which, based on findings in this study, may augment the adaptive response. In addition, delineation of the signaling pathways associated with ISC expansion in a setting of post-surgical IBD may prove useful in both the management of patients with intestinal failure as well as prevention of long-term surgical complications in patients with IBD.
Acknowledgements
The authors would like to express our gratitude to Kirk McNaughton and his staff, as well as Carolyn Suitt and Nikki McCoy in the Histology department for their expertise. We also thank Brooks Scull for assistance during surgeries, conventionalization and tissue harvest. Additionally, we would like to thank Maureen Bower with the UNC Gnotobiotic facility for the use of germ free mice and the operative suite. Great appreciation is given to Dominic Moore and Wonil Chung in the UNC Department of Biostatistics and Data Management for their assistance with statistical analysis.
Grant support: NIH R21-DK80283-01 (MAH & PKL), NIH T32-GM0845015 (KES), National Gnotobiotic Rodent Resource Center P40 RR018603 and P30 DK34987.
Abbreviations
- ICR
ileocecal resection
- nonop
nonoperative
- IBD
inflammatory bowel disease
- IBABP
intestinal bile acid binding protein
- WT
wild type
- SPF
specific pathogen free
- SP
side population
- NS
not significant
- IP
intraperitoneal
- ddH20
double distilled water
- H&E
Hematoxylin and Eosin
Footnotes
Financial Disclosures and conflicts of interest: No conflicts of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Penner RM, Madsen KL, Fedorak RN. Postoperative Crohn’s disease. Inflamm. Bowel. Dis. 2005;11:765–777. doi: 10.1097/01.mib.0000171273.09757.f2. [DOI] [PubMed] [Google Scholar]
- 2.Regueiro M. Management and prevention of postoperative Crohn’s disease. Inflamm. Bowel. Dis. 2009 doi: 10.1002/ibd.20909. [DOI] [PubMed] [Google Scholar]
- 3.Tilney HS, Constantinides VA, Heriot AG, Nicolaou M, Athanasiou T, Ziprin P, Darzi AW, Tekkis PP. Comparison of laparoscopic and open ileocecal resection for Crohn’s disease: a metaanalysis. Surg. Endosc. 2006;20:1036–1044. doi: 10.1007/s00464-005-0500-3. [DOI] [PubMed] [Google Scholar]
- 4.Juno RJ, Knott AW, Jarboe MD, Profitt SA, Erwin CR, Warner BW. Characterization of small bowel resection and intestinal adaptation in germ-free rats. Surgery. 2003;134:582–589. doi: 10.1016/s0039-6060(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien DP, Nelson LA, Huang FS, Warner BW. Intestinal adaptation: structure, function, and regulation. Semin. Pediatr. Surg. 2001;10:56–64. doi: 10.1053/spsu.2001.22383. [DOI] [PubMed] [Google Scholar]
- 6.Wolvekamp MC, Heineman E, Taylor RG, Fuller PJ. Towards understanding the process of intestinal adaptation. Dig. Dis. 1996;14:59–72. doi: 10.1159/000171539. [DOI] [PubMed] [Google Scholar]
- 7.Levine GM, Deren JJ, Yezdimir E. Small-bowel resection. Oral intake is the stimulus for hyperplasia. Am. J. Dig. Dis. 1976;21:542–546. doi: 10.1007/BF01464760. [DOI] [PubMed] [Google Scholar]
- 8.Haxhija EQ, Yang H, Spencer AU, Sun X, Teitelbaum DH. Intestinal epithelial cell proliferation is dependent on the site of massive small bowel resection. Pediatr. Surg. Int. 2007 doi: 10.1007/s00383-006-1855-9. [DOI] [PubMed] [Google Scholar]
- 9.Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am. J. Physiol Gastrointest. Liver Physiol. 2007;293:G1013–G1022. doi: 10.1152/ajpgi.00218.2007. [DOI] [PubMed] [Google Scholar]
- 10.Cummins AG, Catto-Smith AG, Cameron DJ, Couper RT, Davidson GP, Day AS, Hammond PD, Moore DJ, Thompson FM. Crypt fission peaks early during infancy and crypt hyperplasia broadly peaks during infancy and childhood in the small intestine of humans. J. Pediatr. Gastroenterol. Nutr. 2008;47:153–157. doi: 10.1097/MPG.0b013e3181604d27. [DOI] [PubMed] [Google Scholar]
- 11.Booth C, Booth D, Williamson S, Demchyshyn LL, Potten CS. Teduglutide ([Gly2]GLP-2) protects small intestinal stem cells from radiation damage. Cell Prolif. 2004;37:385–400. doi: 10.1111/j.1365-2184.2004.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat. Rev. Cancer. 2008;8:415–424. doi: 10.1038/nrc2392. [DOI] [PubMed] [Google Scholar]
- 13.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol. Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 14.Wirtz S, Neurath MF. Mouse models of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2007;59:1073–1083. doi: 10.1016/j.addr.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Faure M, Moennoz D, Mettraux C, Montigon F, Schiffrin EJ, Obled C, Breuille D, Boza J. The chronic colitis developed by HLA-B27 transgenic rats is associated with altered in vivo mucin synthesis. Dig. Dis. Sci. 2004;49:339–346. doi: 10.1023/b:ddas.0000017462.75257.70. [DOI] [PubMed] [Google Scholar]
- 16.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 17.Sartor RB. Role of commensal enteric bacteria in the pathogenesis of immune-mediated intestinal inflammation: lessons from animal models and implications for translational research. J. Pediatr. Gastroenterol. Nutr. 2005;40(Suppl 1):S30–S31. doi: 10.1097/00005176-200504001-00018. [DOI] [PubMed] [Google Scholar]
- 18.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoentjen F, Harmsen HJ, Braat H, Torrice CD, Mann BA, Sartor RB, Dieleman LA. Antibiotics with a selective aerobic or anaerobic spectrum have different therapeutic activities in various regions of the colon in interleukin 10 gene deficient mice. Gut. 2003;52:1721–1727. doi: 10.1136/gut.52.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson NJ, Leach MW, Fort MM, Thompson-Snipes L, Kuhn R, Muller W, Berg DJ, Rennick DM. T helper cell 1-type CD4+ T cells, but not B cells, mediate colitis in interleukin 10-deficient mice. J. Exp. Med. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rennick DM, Fort MM. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10(-/-) mice and intestinal inflammation. Am. J. Physiol Gastrointest. Liver Physiol. 2000;278:G829–G833. doi: 10.1152/ajpgi.2000.278.6.G829. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 23.Rigby RJ, Hunt MR, Scull BP, Simmons JG, Speck KE, Helmrath MA, Lund PK. A new animal model of post-surgical bowel inflammation and fibrosis: the effect of commensal microflora. Gut. 2009 doi: 10.1136/gut.2008.157636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dekaney CM, von Allmen DC, Garrison AP, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Bacterial-dependent up-regulation of intestinal bile acid binding protein and transport is FXR-mediated following ileo-cecal resection. Surgery. 2008;144:174–181. doi: 10.1016/j.surg.2008.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willing BP, Van Kessel AG. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J. Anim Sci. 2007;85:3256–3266. doi: 10.2527/jas.2007-0320. [DOI] [PubMed] [Google Scholar]
- 26.Burke JP, Mulsow JJ, O’Keane C, Docherty NG, Watson RW, O’Connell PR. Fibrogenesis in Crohn’s disease. Am. J. Gastroenterol. 2007;102:439–448. doi: 10.1111/j.1572-0241.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- 27.Feldstein RC, Sood S, Katz S. Small bowel adenocarcinoma in Crohn’s disease. Inflamm. Bowel. Dis. 2008;14:1154–1157. doi: 10.1002/ibd.20393. [DOI] [PubMed] [Google Scholar]
- 28.Palascak-Juif V, Bouvier AM, Cosnes J, Flourie B, Bouche O, Cadiot G, Lemann M, Bonaz B, Denet C, Marteau P, Gambiez L, Beaugerie L, Faivre J, Carbonnel F. Small bowel adenocarcinoma in patients with Crohn’s disease compared with small bowel adenocarcinoma de novo. Inflamm. Bowel. Dis. 2005;11:828–832. doi: 10.1097/01.mib.0000179211.03650.b6. [DOI] [PubMed] [Google Scholar]