Abstract
Microglia have neuroprotective capacities, yet chronic activation can promote neurotoxic inflammation. Neuronal fractalkine (FKN), acting on CX3CR1, has been shown to suppress excessive microglia activation. We found that disruption in FKN/ CX3CR1 signaling in young adult rodents decreased survival and proliferation of neural progenitor cells through IL-1β. Aged rats were found to have decreased levels of hippocampal FKN protein; moreover, interruption of CX3CR1 function in these animals did not affect neurogenesis. The age-related loss of FKN could be restored by exogenous FKN reversing the age-related decrease in hippocampal neurogenesis. There were no measureable changes in young animals by the addition of exogenous FKN. The results suggest that FKN/ CX3CR1 signaling has a regulatory role in modulating hippocampal neurogenesis via mechanisms that involve indirect modification of the niche environment. As elevated neuroinflammation is associated with many age-related neurodegenerative diseases, enhancing FKN/ CX3CR1 interactions could provide an alternative therapeutic approach to slow age-related neurodegeneration.
Keywords: CX3CR1, fractalkine, neurogenesis, aging, microglia, neuroinflammation
1. Introduction
Adult neurogenesis is a lifelong process, continuing even in elderly humans (Eriksson et al., 1998). However, studies in rodents have demonstrated a continual age-related decline in neurogenesis (Ben Abdallah et al., 2008; Rao et al., 2006). An extensive list of neurogenic regulators has been identified, many of which change as a result of aging (Drapeau and Abrous, 2008) making a unified theory to account for the age-related decrease in neurogenesis unlikely. Yet, the potential importance of neurogenesis in some affective (Sahay and Hen, 2007) and cognitive behaviors (Drapeau and Abrous, 2008), as well as endogenous tissue repair mechanisms, makes further investigation of neurogenic regulators warranted.
Microglia, the immune cells of the brain, are neuroprotective, but following excessive or prolonged activation, microglia can become neurotoxic (Aloisi, 2001). Microglia have also been shown to impair neurogenesis (Ekdahl et al., 2003; Monje et al., 2003), in part through the secretion of proinflammatory cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, which act directly on neural stem/progenitor cells (NPC) (Iosif et al., 2006; Koo and Duman, 2008; Monje et al., 2003). Microglia can also support neurogenesis through the production of growth factors (Ziv and Schwartz, 2008). Therefore the involvement of microglia, likely depends on the activating signals and state of the microglia in the neurogenic niche, which determines if microglia will support or impair neurogenesis. Until recently, neurons were believed to be submissive to the effects of microglia; however, a number of neuronal signals were found that can regulate microglia activation (Biber et al., 2007), suggesting a neuron-microglia dialogue.
One neuronally derived signal that has been shown to be important in regulating the neurotoxic affects of microglia is the chemokine fractalkine (FKN; CX3CL1; neurotactin). In contrast to many other chemokines, FKN binds and activates a single receptor, CX3CR1. Although there is some debate concerning the cell types expressing these two molecules, in vivo FKN is principally expressed on neurons while CX3CR1 is found on microglia (Cardona et al., 2006; Harrison et al., 1998; Lauro et al., 2008). Previous reports establish that interactions between FKN and CX3CR1 contribute to maintaining microglia in a resting phase, partially controlling their neurotoxicity. FKN acts in vitro as an anti-inflammatory molecule by down-regulating IL-1β, TNFα, and IL-6 production (Zujovic et al., 2000; Zujovic et al., 2001). FKN can also elicit neuroprotective effects on pure neuronal cultures (Meucci et al., 1998; Meucci et al., 2000; Tong et al., 2000). Moreover, mRNA and protein expression of CX3CR1 were found in isolated NPCs (Ji et al., 2004; Krathwohl and Kaiser, 2004).
With age there is an increase in the number of activated microglia, which can suppress neurogenesis (Bachstetter et al., 2008; Gemma et al., 2007). We hypothesized that, as a consequence of aging, FKN signaling becomes disregulated, which leads to increased microglial activation and decreased neurogenesis. Our findings demonstrate for the first time that FKN/CX3CR1 signaling is critical for the regulation of hippocampal neurogenesis.
2. Materials and Methods
2.1 Animals
All experiments were conducted in accordance with the National Institute of Health Guide and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use committee of the University of South Florida, College of Medicine or the University of Florida as appropriate. CX3CR1-deficient (CX3CR1GFP/GFP) mice, backcrossed to the C57BL/6 background for greater than 10 generations were obtained from JAX Laboratories (Bar Harbor, Maine). Colonies of the CX3CR1+/GFP and CX3CR1GFP/GFP mice were maintained at the University of Florida. Four-month-old male CX3CR1+/GFP and CX3CR1GFP/GFP littermates were used in the experiments. Male Fisher 344 (F344) rats (NIA contract colony, Harlan Sprague Dawley, Indianapolis, IN), were pair-housed in environmentally controlled conditions (12:12 h light: dark cycle at 21 ± 1°C) and provided food and water ad libitum. Three age groups of rats used in this study included: young (3 months old), middle aged (12 months old) and aged (22 months old). Animals were excluded from the study if they became jaundiced, had pituitary tumors, or developed post-surgery infections.
2.2 Surgical procedure
For all surgical procedures, rats were anaesthetized with isofluorane. For intracerebroventricular infusion, a guide cannula was stereotaxically implanted in the left ventricle (AP, −1.0; ML, 1.6; DV, −3.5 mm) and connected to an osmotic minipump, which was inserted subcutaneously. For the first 7 days all rats received sterile saline, to allow time for the rats to heal before drug treatment was started. After the first 7 days, a mid-scapular incision was made and the saline pump was switched for the treatment pump for either an additional 7 days (Alzet Model, 2001: pumping rate, 1.0 µL/h; total volume, 200 µL), 14 days (Alzet Model, 2002: pumping rate, 0.5 µL/h; total volume, 200 µL), or 28 days (Alzet Model, 2004: pumping rate, 0.25 µL/h; total volume, 200 µL). The treatments used in this study included: (1) rabbit-anti rat CX3CR1 blocking antibody (α-CX3CR1) (10µg per day; Torrey Pines Biolabs, San Diego, CA; Cat no. TP 501)(Milligan et al., 2004); (2) rabbit non-immune IgG (10µg per day; Sigma-Adrich; Cat no. I-5006); (3) recombinant rat FKN (aa 22–100) chemokine domain (30ng per day R & D systems, Inc.; Cat no. 568-FR/CF) (Milligan et al., 2004); (4) r-metHu IL-1Ra (10µg per day; kind gift from Amgen, Thousand Oaks CA). For controls, the proteins were heat-inactivated for 45 minutes in a water bath at 90°C.
2.3 Thymidine analog labeling
Following the time line in Figure 2A animals received two intraperitoneal (i.p.) injections of one or more thymidine analogs within a 12-hour interval. Bromodeoxyuridine (BrdU) (5-bromo-2-deoxyuridine; Sigma, St. Louis, MO) was injected at dose of 50 mg/kg. Equimolar solutions, to be equivalent to the 50 mg/kg of BrdU, were prepared from chlorodeoxyuridine (CldU) (42.5 mg/kg; Sigma, St. Louis, MO) and iododeoxyuridine (IdU) (57.5 mg/kg; MP Biomedicals) as previously described (Vega and Peterson, 2005).
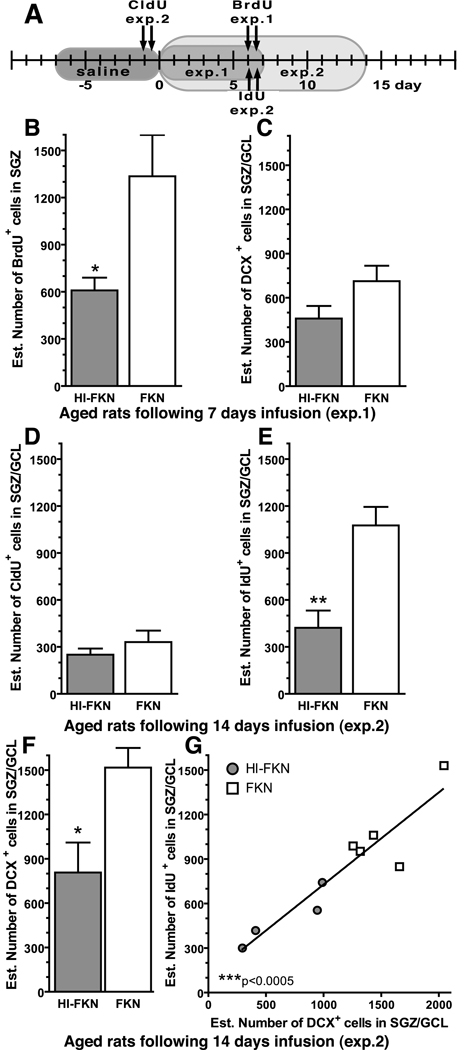
Figure 2. FKN reverses the age-related decrease in neurogenesis.
(A) Timeline: In experiment 1 treatment lasted for 7 days, with injections of BrdU occurring at day 6. In experiment 2, treatment lasted for 14 days, with injections of CldU occurring at day -1 and injections of IdU occurring at day 6. BrdU was used to study the effects of the treatments on proliferation of hippocampal NPC. CldU was used to study the effects of treatment on the cells born prior to the treatment. IdU was used to study the effects of survival of the cells born after treatment and to make direct comparisons with BrdU data. (B) A significant increase in proliferation as measured by the number of BrdU+ cells was found in the aged rats treated with FKN for 6 days. *(t(10)=2.639; p=0.0248; FKN(n=6) vs. HI-FKN(n=6)). (C) After 7 days of FKN treatment in age rats there was no significant difference in the number of DCX+ cells (FKN n=4; HI-FKN n=6). (D) In the second experiment, in the cells born before treatment began (labeled with CldU) with 15 days of time for the labeled cells to survive, no difference was found between groups (FKN n=5; HI-FKN n=4). (E) When cells were labeled with IdU on day 7 (same time point as BrdU (B)) a significant increase (p=0.0065; E) in number of IDU+ cells was found in the FKN treated group. *(t(7)=3.831; p=0.0065; FKN(n=5) vs. HI-FKN(n=4)). (F) After 14 days of treatment in aged rats, FKN significantly increased the number of DCX+ cells. *(t(8)=2.945; p=0.0116; FKN(n=5) vs. HI-FKN(n=5)). (G) The number of IdU+ cells was found to strongly correlate with the number of DCX+. ***(Pearson r=0.933; p=0.0002).
2.4 Tissue collection and processing
To account for diurnal differences, one animal from each group was euthanized before a second animal from the same group was euthanized. Studies were conducted during the light phase of the 12:12 h light: dark cycle.
For immunohistochemistry studies animals were anaesthetized with pentobarbital (50 mg/kg, i.p.). The rats were transcardially perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PBS. The brains were postfixed in 4% paraformaldehyde for 12 h, after which they were transferred into 30% sucrose in PBS for at least 16 h at 4°C. Exhaustive sagittal sections of the left hemisphere were made at 40µm using a Microm cryostat (Richard-Allan Scientific, Kalamazoo Michigan) and stored in cryoprotectant at 4°C. For biochemical experiments, animals were deeply anaesthetized with isofluorane before decapitation. The brain was quickly removed and the brain regions were dissected. Hippocampal tissues were dissected from both hemispheres and collected separately. In animals that received treatment, only the hemisphere that received the treatment was used. In naïve animals, both hemispheres were included.
2.5 IL-1β ELISA
Homogenization of tissues was performed using an ultrasonic cell disrupter, in a 1:10 weight/volume of ice-cold cell lysis buffer (Cell Signaling Technology, Inc.; Danvers, MA; Cat no. 9803) and phenylmethylsulphonyl fluoride, 1 mm (Sigma, St. Louis, MO). Samples were centrifuged at 21,000 g at 4 °C for 15 min and supernatant was collected. Determination of total protein, using a Bradford protein assay (BIO-RAD Laboratories, Hercules, CA, USA) and an enzyme-linked immunosorbent assay (ELISA) were performed on the same day to avoid repetitive thawing of samples. The rat IL-1β ELISA (eBioscience, Inc.; San Diego, CA; cat no. 88-6010-22) was performed using a commercially available kit following the manufacturer's protocol.
2.6 FKN ELISA
Frozen hippocampal samples were thawed on ice and homogenized in TBS (pH 7.4) buffer containing protease inhibitor cocktail plus EDTA (Pierce Biotechnology, Rockford, IL). Tissue homogenization was achieved using a 26-gauge needle and a 1.0mL syringe and triturating 20 times. Soluble proteins were collected with supernatant following centrifugation at 1000 g for 10 minutes at 4°C. Subsequently, pellets were homogenized in TBS (pH 7.4) buffer containing 0.1% Triton X-100, protease inhibitor cocktail plus EDTA (Pierce). A second fraction was collected from supernatant following centrifugation at 17,950g for 90 minutes at 4°C. Lastly, pellets were homogenized in RIPA cell lysis buffer (Millipore, Billerica, MA) containing protease inhibitor cocktail plus EDTA (Pierce). After homogenization, samples were agitated for 15 minutes at 2000 rpm (MixMate; Eppendorf, Westbury, NY) at 4°C. Membrane-bound proteins were removed with supernatant following centrifugation at 17,950g for 90 minutes 4°C. BCA protein assay (Pierce) was used to determine total protein concentration for each sample. Fractionation efficiency was analyzed by Western blot. For each sample 50 µg of total protein was separated by electrophoresis on denaturing 4–15% SDS-polyacrylamide gel (BioRad, Hercules, CA) and transferred to PVDF membranes (Millipore). The membranes were probed with an antibody to the chemokine domain of FKN (Torrey Pines Biolabs, San Diego, CA; Cat no. TP203). Following incubation, a peroxidase conjugated secondary antibody to rabbit (Pierce Biotechnology, Rockford, IL; Cat no. 34260) was used. Visualization of the peroxidase reaction was achieved using West Dura substrate (Pierce) and ChemiDoc XRS Molecular Imager with Quantity One software (BioRad). Soluble FKN (~65 kDa) existed predominantly in the first protein fraction, while membrane-bound FKN (~87 kDa) was found predominantly in the third fraction. Soluble fraction (20 µg of total protein) were used for a FKN ELISA (RayBiotech, Inc, Norcross, GA; Cat no. ELR-Fractalkine-001C) incubated overnight at 4°C and processed according to manufacturer’s protocol.
2.7 Real-Time RT-PCR
Dissected tissues stored at −80°C were used for RNA isolation using RNeasy mini-columns (Qiagen; Cat no. 74104) with on-column DNase treatment (Qiagen; Cat no. 79254) according to the manufacturer’s protocol. RNA quantity was determined and normalized using Quant-iT™ RiboGreen® RNA Assay Kit (Invitrogen; Cat no. R11490). Integrity of the RNA was confirmed on an agarose gel assessing the 18s and 28s rRNA bands. Reverse transcription (RT) was done following the manufacturer’s protocol using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems; Cat no. 4368814). A no template and a no RT control were conducted to control for contamination. qPCR reaction was performed using SYBR® Green PCR Master Mix (Applied Biosystems; Cat no. 4309155) following the manufacturer’s protocol, with the exception of variable annealing temperatures (55°C–65°C) as determined most optimal during primer validation. A melt curve beginning at 55°C and increasing by 0.5°C to 95°C every 10 sec with fluorescence measured at each interval. A single peak in the melt curve was used to check for a single product. A standard curve that covers 3 logs was made of pooled cDNA from all the rats and used in each plate to check the efficiency of the reaction as determined by the slope of the standard curve and to assess plate to plate variations. All samples were run in triplicate. The primers that were used included; Rpl19 (NM_031103; sense: AATCGCCAATGCCAACTC; antisense: CCCTTCCTCTTCCCTATGC) as the reference gene, to normalize the expression of FKN (NM_134455; sense: CGAGTTCTGCTGTCTACCAATCTG; antisense: GAAGTGGTGGACGCTTGAGTAG). Relative gene expression was calculated by the 2−ΔΔCT method.
2.8 Immunohistochemistry and Immunofluorescence
Except where specifically indicated, standard staining procedures were conducted on free-floating sections using every sixth section for the entire hippocampus beginning with a random start and including sections before and after the hippocampus to ensure that the entire structure was sampled. The standard staining procedures used began with 0.3% H2O2 solution in 30% methanol to block endogenous peroxidase activity (this step was omitted for immunofluorescence). Sections were blocked in 10% normal serum from the species that secondary antibody was raised in, with the addition of 0.1% Triton X-100. Sections were incubated, with primary antibody diluted in 3% normal serum with 0.1% Triton X-100, overnight at 4°C. For immunohistochemistry biotinylated secondary antibodies were diluted in 3% normal serum with 0.1% Triton X-100 and were incubated for 2 hours at room temperature. For immunofluorescence appropriate secondary antibody conjugated to an Alexafluor probe (Molecular Probes, Eugene, OR) was applied for 2 hours. For immunohistochemistry, enzyme detection was done using avidin-biotin substrate (ABC kit, Vector Laboratories, Burlingame, CA) followed by color development in diaminobenzidine solution (Sigma, St. Louis, MO). For BrdU, sections were pretreated with 50% formamide/2× SSC (0.3 M NaCl, 0.03 M sodium citrate) at 65°C for 2 hours, rinsed in 2× SSC, incubated in 2N HCL for 30 minutes at 37°C, and washed with borate buffer (pH 8.5). For IdU and CldU, sections were pretreated 2N HCL for 20 minutes at 37°C followed by a wash in borate buffer (pH 8.5). BrdU was detected using a mouse anti-BrdU (1:100; Roche; Indianapolis IN; Cat no.11 170 376 001, clone BMC 9318). CldU was detected with rat anti-BrdU (Accurate Chemicals, Westbury, NY Cat no OBT003 clone: BU1/75 (ICR1)). For IdU, mouse anti-BrdU (Becton Dickinson Bioscience, San Jose, CA; Cat no.347580, clone B44), was used at a dilution of 1:500. Doublecortin (DCX) is a marker of migrating neurons that is expressed for approximately three weeks after the cell is born and has been shown to be a reliable indicator of neurogenesis(Couillard-Despres et al., 2005; Rao and Shetty, 2004). For DCX immuno-detection, incubation in primary antibody was done for 36 hours at 4°C using a polyclonal goat antibody C-terminus of human DCX (1:200; SC-8066, Santa Cruz biotechnology, Santa Cruz, CA, USA). Ki67 is expressed in cells G1 through M phase of the cell cycle (Scholzen and Gerdes, 2000). For detection of Ki67, a rabbit anti-human Ki67 antibody (NCL-Ki67p; Novocastra Laboratories/Vision BioSystems, Newcastle upon Tyne, UK) was used at a dilution of 1:500. Mature neurons were stained with the marker NeuN (1:100; Chemicon, Temecula, CA). For OX-6 immuno detection a monoclonal antibody directed against the rat major histocompatibility II (MHCII) (RT1B, Becton, Dickinson Pharmingen, San Diego, CA, USA) was used at a concentration of 1:750. For microglia analysis in mice, two antibodies were used; mouse major histocompatibility class II (I-A/I-E, Becton, Dickinson Pharmingen, San Diego, CA, USA; Cat no. 556999 Clone: M5/114.15.2); and rat anti-mouse CD45 (AbD Serotec; Raleigh, NC; 1:10,000; Clone: YW62.3; Cat no. MCA1031G). Detection of FKN was accomplished using a goat anti-rat polyclonal antibody that recognizes the chemokine domain of FKN at a concentration of 1:100 (R&D Systems; Minneapolis, MN; Cat no. AF537).
2.9 Quantification and imaging
To determine cell numbers, the optical fractionator method of unbiased stereological cell counting techniques (West et al., 1991) was used with a Nikon Eclipse 600 microscope and quantified using Stereo Investigator software (MicroBrightField, Colchester, VT). Due to the low number of BrdU+, CldU+, Idu+, and DCX+ cells in the aged animals, the virtual grid and counting frame were both 125µm×125µm in order to count all the cells that were present in the section. For all other counts, sampling was optimized to count at least 200 cells per animal with error coefficients less than 0.07. Outlines of the anatomical structures were done using a 10x/0.45 objective and cell quantification was conducted using a 60x/1.40 objective. An Olympus FluoView FV1000 confocal microscope was used for all Immunofluorescence photomicrographs, only linear adjustments (brightness and contrast) were made to the figures. When quantification of percentage of positive cells was determined, Z stacks were created at 1µm intervals throughout the 40µm of the sections with a guard region of 2µm excluded from top and bottom of the Z stack. The Z stacks were rotated in all planes to verify double labeling.
2.10 Statistical analyses
Data are presented as mean±SEM. Statistical analysis was performed using an unpaired, two-side t-test, or a one-way ANOVA followed by unpaired two-side t-test. Correlations were tested using a Pearson product-moment correlation coefficient. A value of p<0.05 was considered to be significant.
3. Results
3.1 CX3CR1-deficient mice have decreased hippocampal NPC proliferation and neurogenesis
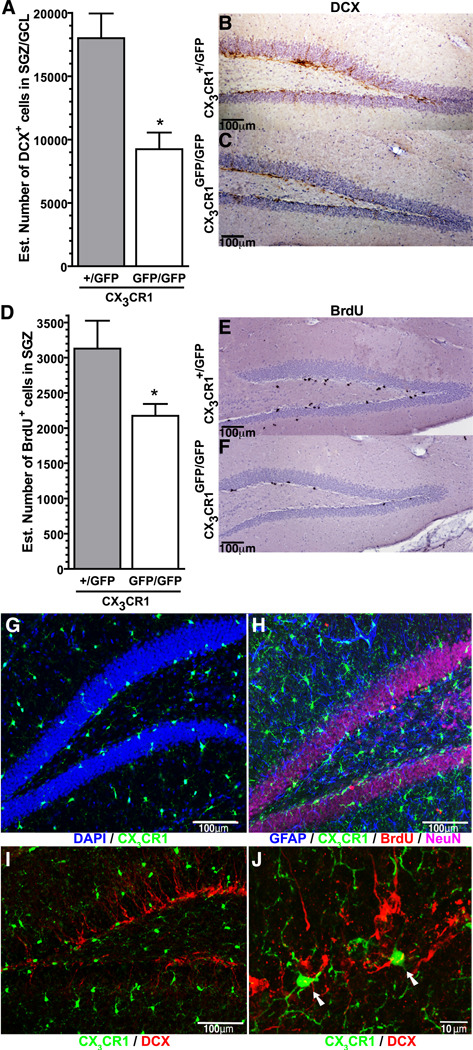
Previous studies suggest that CX3CR1 suppresses the neurotoxic effects of activated microglia (Cardona et al., 2006). In the absence of a neurotoxic insult, CX3CR1GFP/GFP mice lack any obvious phenotype, and appear to have normal brain development (Cook et al., 2001; Haskell et al., 2001; Jung et al., 2000); however no studies have investigated whether loss of CX3CR1 signaling results in changes in adult neurogenesis. Using the Optical fractionator method of design-based stereology a significant decrease in the number of DCX+ cells in the subgranular zone (SGZ) and granular cell layer (GCL) of the dentate gyrus was found in the CX3CR1GFP/GFP mice compared to the CX3CR1+/GFP mice (t(9)=3.857; p=0.0062; Fig. 1A). Figure 1B and 1C shows representative photomicrographs of the DCX immunohistochemistry. A significant decrease in proliferation was also found in the CX3CR1GFP/GFP mice compared to the CX3CR1+/GFP mice as determined by the number of BrdU+ cells labeled by two injections of BrdU (50mg/kg; 8 hour intervals) with the mice euthanized 24 hours post-injection (t(13)=2.513; p=0.026; Fig. 1D). BrdU immunohistochemistry is shown for CX3CR1+/GFP mice (Fig.1E) and CX3CR1GFP/GFP mice (Fig.1F) in the representative photomicrographs.
Figure 1. CX3CR1GFP/GFP mice have diminished hippocampal neurogenesis.
(A) Unbiased stereology revealed a significant decrease in the number of DCX+ cells in the hippocampus of adult male CX3CR1GFP/GFP mice compared to heterozygote control *(t(9)=3.857; p=0.0062; CX3CR1GFP/GFP(n=5) vs. CX3CR1+/GFP (n=4)). Representative photomicrographs of the DCX+ cells in the brown immunohistochemical staining can be seen in the CX3CR1+/GFP mice (B) with fewer DCX+ cells seen in the CX3CR1GFP/GFP mice (C). (D) Quantification of the number of cells that were proliferating during the proceeding 24 hours, as determined by the incorporation of BrdU, was significantly fewer in the CX3CR1GFP/GFP mice compared to control. * (t(13)=2.513; p=0.026; CX3CR1GFP/GFP(n=9) vs. CX3CR1+/GFP (n=6)). The BrdU immunohistochemistry (black staining) is shown in the representative photomicrographs from the CX3CR1+/GFP mice (E) and CX3CR1GFP/GFP mice (F). (G) Confocal photomicrogaph of CX3CR1 (GFP) and DAPI (blue), demonstrate the localization of the CX3CR1 cells in the dentate gyrus. (H) Localization of CX3CR1 (GFP) cells was not found in NeuN+ cells (magenta) or in GFAP+ cells (blue), and only rarely in BrdU+ cells (red). (I) Low power confocal photomicrograph of DCX+ cells (red) and CX3CR1 (GFP) cells are also (J) shown in higher power in maximum projection of confocal z-stack. Arrows indicate CX3CR1 (GFP) cells that are in close proximity to the DCX cells. Video of the z stack used for the maximum projection can be found in the supplementary material.
Figure 1G shows that CX3CR1+ cells (green) were widely distributed in the dentate gyrus, with a typical appearance of ramified microglia. The CX3CR1+ cells in the SGZ had a morphological appearance of microglia and were located in close proximity to the NPC. To determine whether neurons or astrocytes also expressed CX3CR1, sections from CX3CR1+/GFP mice and CX3CR1GFP/GFP mice were stained for GFAP (blue), BrdU (red), and NeuN (Magenta) (Fig. 1H) and for DCX (red) (Fig.1I and 1J). Colocalization of GFP with GFAP, NeuN, or DCX was not observed. Colocalization of BrdU and GFP was rarely seen, and most likely represented proliferating microglia and not expression of CX3CR1 on a NPC. Figure 1J and supplementary video shows CX3CR1+ cells (GFP) adjacent to the DCX+ cells (red) suggesting an important cell-to-cell regulation of the NPCs and the maturation and survival of the adult born neurons.
3.2 Proliferation of NPCs is decreased by α-CX3CR1 treatment in young but not middle aged or old rats
To develop a pharmacological model of decreased FKN signaling, a blocking antibody to CX3CR1 was employed. This model allowed for transient loss of signaling as compared to the mouse model that, as a result of the permanent developmental loss of FKN signaling, might evoke compensatory mechanisms. Microglia are the only known cell in the CNS to express CX3CR1 (Cardona et al., 2006). In fixed rat tissue sections stained with the blocking antibody to CX3CR1, cells with the morphological appearance of microglia were the only cells observed confirming the specificity of the blocking antibody (Supplementary.Fig.1).
To determine if blocking of CX3CR1 would cause a decreased NPC proliferation and neurogenesis as seen in the mouse model, we infused blocking antibody to CX3CR1 for 7 days via an osmotic minipump to the left lateral ventricle. Young adult rats (3 months old), middle aged rats (12 months old) and old aged rats (22 months old) were injected with BrdU on the 6th day of treatment. The animals were euthanized on the following day and sections of the hippocampus were evaluated for proliferation and short-term survival of the NPCs (see Fig.2A for timeline). Quantification of the number of BrdU+ cells in the SGZ showed a significant decrease (t(10)=4.688; p=0.0009) in the α-CX3CR1-treated rats compared to the non-immune IgG-treated animals (Supplementary.Fig.2A). Confirming the BrdU results, a significant decrease (t(5)=3.596; p=0.0156) was found in the number of Ki-67+ cells (Supplementary.Fig.2B) and DCX+ cells (t(5)=2.629; p=0.0466; Supplementary.Fig.1C) in the α-CX3CR1-treated rats compared to the non-immune IgG-treated animals. However, no significant differences in the number of BrdU+ cells (Supplementary.Fig.2A), or in the number of DCX+ cells (Supplementary.Fig.1C) were found in the middle aged rats or old aged rats following treatment with the blocking antibody
3.3 FKN reversed the age-related decrease in neurogenesis, but had no effect in young or middle aged rats
To further investigate whether FKN/ CX3CR1 signaling could modulate hippocampal neurogenesis, we treated the three different age groups of rats with 30ng/d of recombinant rat FKN following the same 7 day protocol as described earlier (Fig.2A). A significant increase in the number of BrdU+ cells in the aged rats treated with FKN was found as compared to the aged rats treated with heat-inactivated (HI)-FKN (t(10)=2.639; p=0.0248; Fig.2B). No significant difference was found in the number of DCX+ cells in the aged rats (Fig 2C). In the young adult and middle-aged rats, FKN treatment did not produce any measurable changes in the number BrdU+ cells (Supplementary.Fig.3A), or in the number of DCX+ cells (Supplementary.Fig.3B).
3.4 FKN treatment in aged rats mainly affects proliferation
To determine whether the disruption in FKN/ CX3CR1 signaling induced changes in proliferation or survival of the newly born cells, we used a multiple thymidine analog approach. Both CldU and IdU were used to date newborn cells at 2 different time points. (see timeline Fig.2A). One day prior to the beginning of the treatment aged rats were injected with CldU (b.i.d.; 42.5 mg/kg; equimolar to BrdU). IdU was injected on day 6 (b.i.d.; 57.5 mg/kg; equimolar to BrdU), allowing an additional 7-days of treatment to measure the effect of treatment on the survival of IdU+ cells. No effect of FKN treatment was found in the number of CldU+ cells (Fig 2D). In contrast, there was significantly more IdU+ cells in the FKN-treated rats compared to the HI-FKN-treated rats (t(7)=3.831; p=0.0065; Fig.2E). Comparison of BrdU+ cells (first experiment) to IdU+ cells (second experiment) it is possible to determine if there were combined effects on survival and proliferation following treatment, or if these effects were limited to proliferation. Through this comparison it was found that FKN treatment increased survival of proliferating cells by about 18%, but these changes were within 95% confidence interval (FKN 82.7%±16.45%; HI-FKN 64.7%±21.0%; mean±SD). After 14 days of treatment there was a significant increase (t(8)=2.945; p=0.0116; Fig.2F) in the number of DCX+ cells in the aged FKN group compared to the aged HI-FKN group. The increase in the number of IdU+ cells was found to significantly correlate with the number of DCX+ cells (Pearson r=0.933; p=0.0002; Fig 2G).
3.5 Expression of FKN in the rat hippocampus
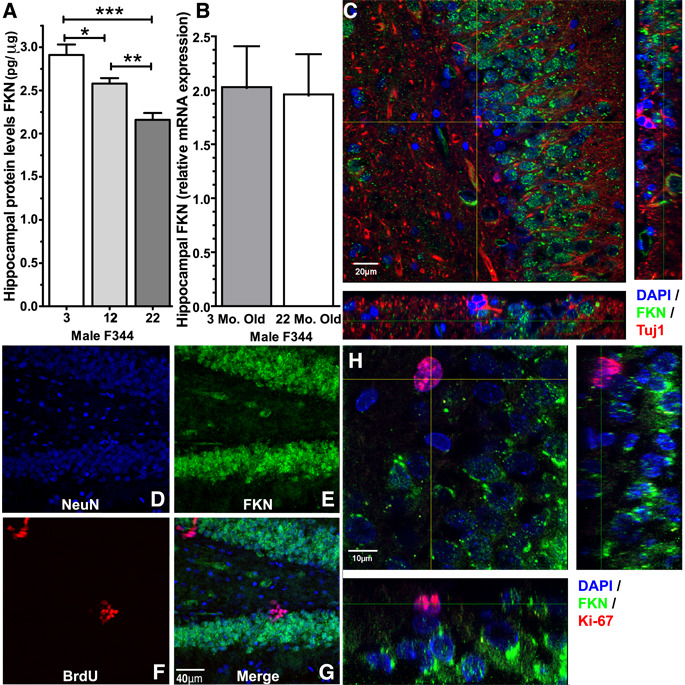
As we were able to reverse the age-related decline in neurogenesis through the addition of recombinant FKN in the aged rats, we hypothesized that FKN might be altered with age. A significant effect in the levels of shed FKN protein in hippocampal tissue homogenates from young, middle aged, and aged rats was found by ELISA (F(2,17)=17.08; p=0.0001; Fig. 3A). Aged rats were found to have significantly less shed FKN protein compared to the young rats (t(10)=5.190; p=0.0004). Middle aged rats had significantly less FKN then young rats (t(10)=2.436; p=0.0351), yet significantly more then aged rats (t(10)=4.130; p=0.002). However, mRNA levels of FKN were unaltered in the aged rats compared to the young rats (Fig.3B). We further investigated the protein localization of FKN in the dentate gyrus. Figure 3C shows a photomicrograph of staining with DAPI (blue), FKN (green), and Tuj1 (red). While FKN was abundantly expressed on the cell bodies in the GCL, FKN staining was not found on Tuj1+ cells. Mature neurons labeled with NeuN (blue) (Fig.3D) were found to extensively express FKN (green) (Fig.3E). BrdU+ cells (red), one-day post injection with BrdU, did not express FKN (Fig.3F). The colocalization of the three markers is shown in the merged figure 3G. Moreover, photomicrographs of Ki-67 (red) and FKN (green) (Fig.3H) also demonstrated a lack of FKN expression on the proliferating cells. These data indicated that FKN is not expressed on immature neuronal cells; however, it is not clear exactly when FKN begins to be expressed on neurons.
Figure 3. Expression of FKN in the hippocampus.
Quantification of protein (A) and mRNA (B) levels of FKN in the hippocampus of young adult and aged rat, demonstrated a significant decrease in protein levels of FKN, but not in mRNA levels (n=6 per group). *(t(10)=2.436; p=0.0351; 3 Mo. old(n=6) vs. 12 Mo. old(n=6)).**(t(10)=4.130; p=0.002; 12 Mo. old(n=6) vs. 22 Mo. old(n=6)).***(t(10)=5.190; p=0.0004; 3 Mo. old(n=6) vs. 22 Mo. old(n=6)). (C) FKN expression (green) was not found on Tuj1+ (red) cells, but was expressed on the majority of the cells (DAPI: blue) in the GCL. (D–G) FKN expression (green) was found on the mature neurons labeled with NeuN (blue) but not on the BrdU+ cells (red) in rats one day post injection with BrdU, as seen in the merged figure (G). (H) Shows a high magnification photomicrogaph of Ki-67 staining (red) and FKN staining (green), with DAPI (blue). Similar to the Tuj1+ cells and BrdU+ cells, the Ki-67+ cells also lack FKN expression.
3.6 CX3CR1 blocking antibody increased IL-1β
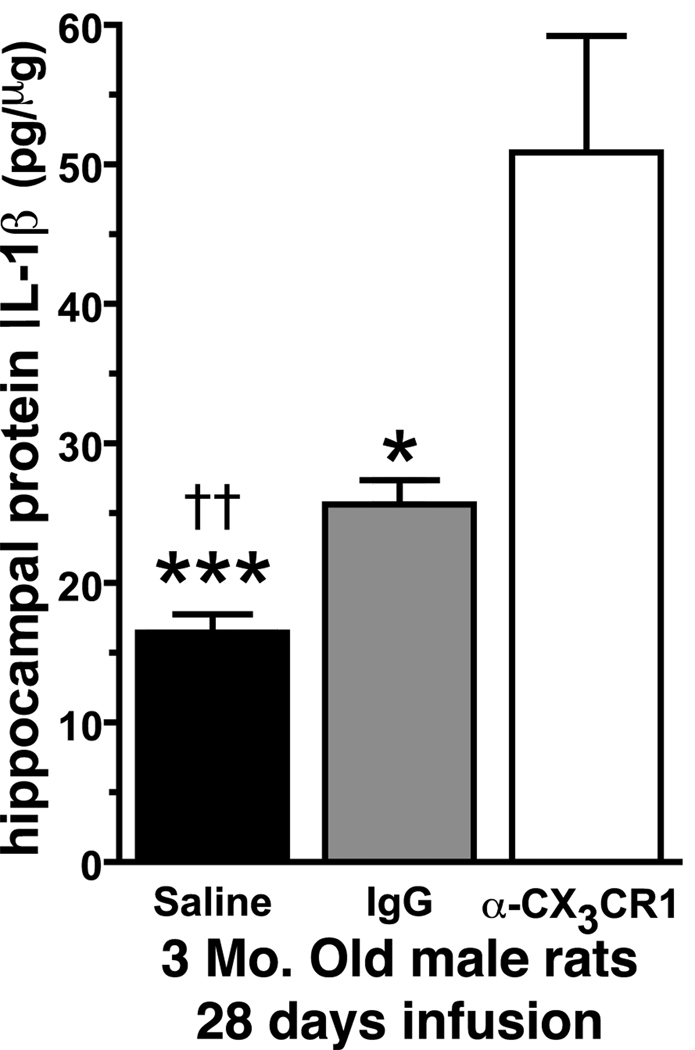
To determine whether inhibition of CX3CR1 activity would lead to an increase in IL-1β protein levels, the CX3CR1 blocking antibody was infused in young rats for 28 days via an osmotic minipump. The 28 days time point was chosen to ensure any difference would be large enough to be easily detected by a standard ELISA. In Figure 4, we found that there was a significant effect in the amount of IL-1β following treatment for 28 days with the CX3CR1 blocking antibody (F(2,16)=16.89; p=0.0002). Compared to either the saline treated rats (t(11)=5.179; p=0.0003) or the non-immune IgG treated rats (t(7)=2.630; p=0.0339) the α-CX3CR1 treated rats had a significant elevation in IL-1β. The saline treated rats and the non-immune IgG treated rats were also significantly different from each other (t(10)=4.064; p=0.0023). Nevertheless, the data indicates that the pharmacological antagonism of CX3CR1 leads to increased production of IL-1β.
Figure 4. CX3CR1 blocking antibody increases hippocampal IL-1β levels.
In 3 month old male rats treated for 28 days with the blocking antibody we found a significant increase in IL-1β protein levels compared to non-immune IgG or saline control animals. (††p=0.0023 saline vs. IgG) (***p=0.0003 saline vs. α-CX3CR1) (*p=0.039 IgG vs. α-CX3CR1) (Saline n=8; IgG n=4; α-CX3CR1 n=5).
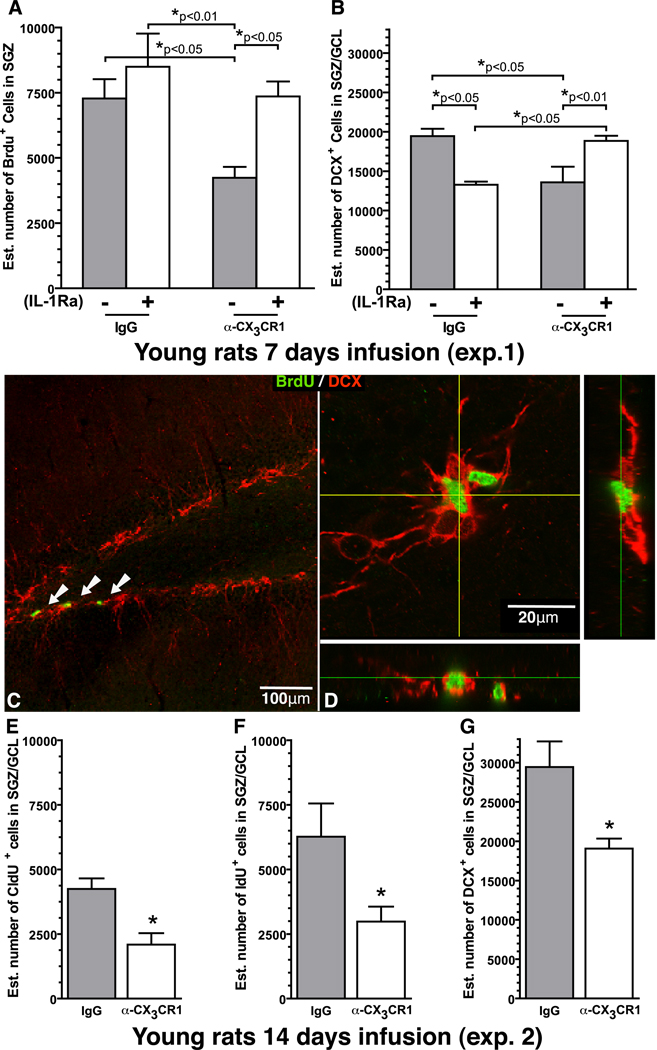
3.7 IL-1β mediates the effects of α-CX3CR1 treatment
To determine if the anti-proliferative effects of the CX3CR1 blocking antibody were dependent on IL-1β, young rats were infused with IL-1 receptor antagonist (IL-1Ra) along with either the α-CX3CR1 or non-immune IgG, following the same 7-day protocol as described earlier. A One-Way ANOVA revealed a significant effect in the number of BrdU+ cells (F(3,17)=5.476; p=0.0081; Fig.5A). In the α-CX3CR1 treated rats the addition of IL-1Ra was able to prevent the decrease in proliferation that occurred following treatment with the α-CX3CR1; such that, the rats which received the blocking antibody with the inactive IL-1Ra had significantly fewer BrdU+ cells than the rats that received the blocking antibody and an active IL-1Ra (t(9)=4.220; p=0.0022). No difference was found as a result of IL-1Ra treatment between the non-immune IgG groups that received an active IL-1Ra or a heat-inactivated (HI)-IL1Ra. A significant decrease (t(7)=3.499; p=0.010) was found in the number of BrdU+ cells in the rats that received the α-CX3CR1 compared to the IgG group, replicating the findings in Sup.Fig.1B., and demonstrating that without active IL-1Ra, treatment with α-CX3CR1 decreases proliferation of NPCs. A significant decrease was also found between the α-CX3CR1+HI-IL1Ra treated group and the non-immune IgG treated group that received the active IL-1Ra (t(9)=3.373; p=0.0082).
Figure 5. IL-1Ra reverses the effects of α-CX3CR1.
To determine if the decrease neurogenesis caused by blocking antibody to α-CX3CR1 was mediated by IL-1β we infused IL-1Ra along with the blocking antibody for 7 days of treatment. On day 6, young adults rat were injected with BrdU. Gray bars are the groups that received heat-inactivated IL-1Ra. White bars are groups that received active IL-1Ra. (A) In the α-CX3CR1/HI-IL-1ra group, there was significantly fewer BrdU+ cells then the three other groups which were not different from each other. (B) IL-1ra blocked the decrease in DCX+ cells caused by α-CX3CR1. In the non-immune IgG group IL-1Ra (white) caused a significant decrease in the number of DCX+ cells compared to the IL-1Ra inactive control (grey). (C) A representative photomicrograph of the BrdU (green) and DCX (red) double labeling. The white arrows point to the BrdU+ cells in the SGZ. (D) Orthogonal view of the confocal z-stack of BrdU+ DCX+ cells. Following the 14 day infusion paradigm (see Fig.2A), (E) we found a significant decrease in the number of CldU+ cells which were born the day before we started infusion in the α-CX3CR1. * (t(7)=3.566; p=0.0091; IgG (n=5) vs. α-CX3CR1 (n=4)). (F) In the 14 day experiment, a significant decrease was also found in the number of IdU+ cells. * (t(7)=2.506; p=0.0406; IgG (n=5) vs. α-CX3CR1 (n=4)). (G) Quantification of the number of DCX+ cells also demonstrated a significant decrease following treatment with α-CX3CR1. * (t(7)=2.690; p=0.0311; IgG (n=5) vs. α-CX3CR1 (n=4)).
To determine if IL-1β also mediated the decrease in neurogenesis found after treatment with α-CX3CR1, the number of DCX+ cells was also quantified revealing a significant effect (F(3,15)=7.615; p=0.0025; Fig.5B). The addition of active IL-1Ra along with α-CX3CR1 was able to prevent the decrease DCX+ cells caused by treatment with α-CX3CR1 and inactive IL-1Ra (t(8)=2.441; p=0.0358). Confirming the earlier experiments, α-CX3CR1 resulted in a significant decrease in the number of DCX+ compare to non-immune IgG without the addition of active IL-1Ra (t(7)=2.441; p=0.0447). Rats that received the IL-1Ra with α-CX3CR1 were not significantly different than the IgG+HI-IL-1Ra group with respect to the number of DCX+ cells. The results demonstrate that the decrease in DCX+ cells following blocking antibody treatment was mediated through IL-1β.
Previous studies have shown that when IL-1β is elevated above a physiological level, elevated levels of IL-1β can impair neurogenesis (Gemma et al., 2007; Koo and Duman, 2008). An unexpected finding was that when we decreased IL-1β signaling below physiological levels in the young rats the loss of IL-1β also impaired neurogenesis. When IL-1Ra was given to the non-immune IgG group (IL-1Ra+IgG n=5) a significant reduction in the number of DCX+ cells was found compared to the non-immune IgG group that received the heat-inactivated IL-1Ra (t(7)=6.573; p=0.0003). As there was not a significant decrease in proliferation in the IL-1Ra+IgG group the DCX data suggests that a physiological level of IL-1β is important for the survival of the DCX+ cells, a similar finding was previously reported in a mouse that overexpressed human IL-1Ra (Spulber et al., 2008). The numbers of BrdU+ cells (green) that were also DCX+ (red) were quantified (Fig.5C), and no differences in the number of BrdU+/DCX+ cells was found between any of the groups in the percentage of double-labeled cells (81.75%±6.3%).
3.8 CX3CR1 blocking antibody decreased survival of cells born prior to treatment
After seven days of α-CX3CR1 treatment, the number of DCX+ cells was significantly decreased in the young adult rats (Fig.5B). The decrease in DCX+ cells could be due to decreased proliferation, as measured at day six (Fig.5A). Alternatively, treatment with α-CX3CR1 could also decrease the survival of the immature neurons leading to a decrease in DCX+ cells. To determine if α-CX3CR1 treatment affected survival of cells born prior to treatment, CldU was injected one day before the beginning of the 14 days of infusion of non-immune IgG or α-CX3CR1. Figure 2A shows the different time points that were compared. Quantification of the number of CldU+ cells demonstrated a significant decrease in the number of CldU+ cells (t(7)=3.566; p=0.0091; Fig. 5E). After 14 days of treatment, in the rats that received α-CX3CR1 fewer of the cells that were born the day before treatment began survived compared to the non-immune IgG group. Supplementary Figure 4 shows CldU+/NeuN+ cells in the non-immune IgG group (Supplementary.Fig.4A) and α-CX3CR1 group (Supplementary.Fig.4B).
The preceding experiments demonstrated that α-CX3CR1 treatment decreased proliferation of NPCs. To determine if α-CX3CR1 treatment could alter the survival of cells born during the treatment, IdU was injected on day six of a 14 day of treatment. Day six was chosen so that a comparison could be made between the number of IdU+ cells and BrdU+ cells quantificated from the earlier experiment (Fig.5A). In the α-CX3CR1 treatment group significantly fewer IdU+ cells were found compared to the non-immune IgG treatment group(t(7)=2.506; p=0.0406; Fig. 5F).
Comparisons of BrdU+ to IdU+ revealed no difference between the treatment groups (α-CX3CR1 49.6%±10.11%; IgG 50.7%±7.9%; mean ± SD), demonstrating that the decrease in the number of IdU+ cells was due to a decrease in proliferation and not survival. A decrease in survival was seen in the cells born prior to treatment. These results suggest, that following treatment with α-CX3CR1, the niche environment becomes unfavorable for the survival of the newborn cells, and the cells born before the change in environment die. After the change in environment, there is a decrease in proliferation but not survival as the number of cells born is limited to what the environment can support. The changes in the niche environment translated to a decrease in neurogenesis in the α-CX3CR1 group as there was a significant decrease in the number of DCX+ cells in the α-CX3CR1 group compared to the non-immune IgG treated group (t(7)=2.690; p=0.0311; Fig. 5G).
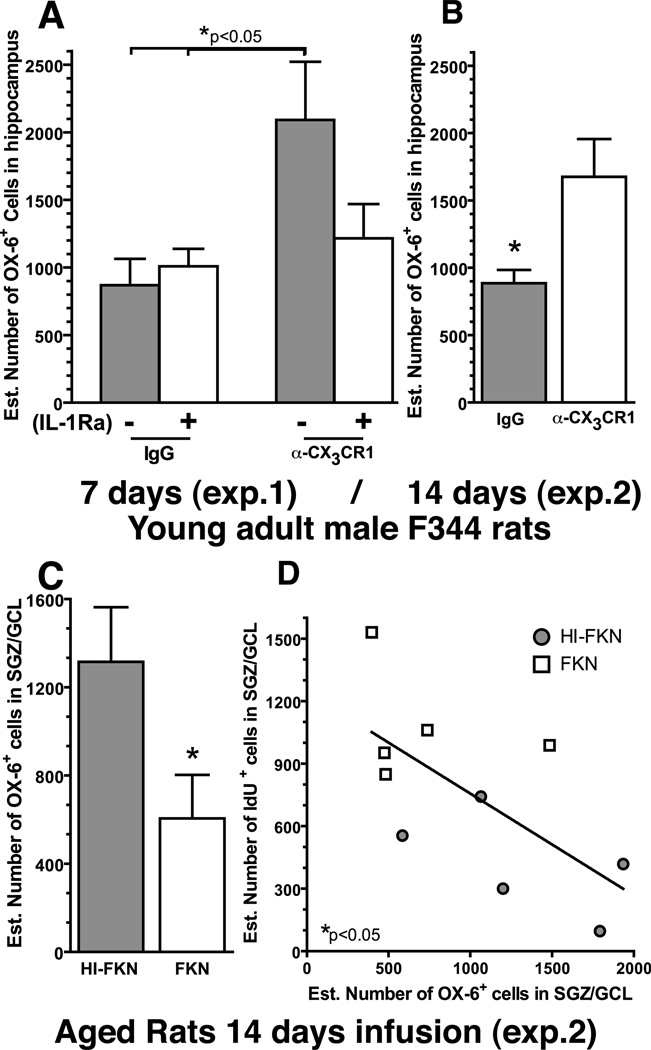
3.9 FKN is necessary to maintain microglia in an inactivated state
FKN/CX3CR1 has been proposed to maintain microglia in a quiescent resting state. The expression of MHC Class II on microglia is induced when the cell becomes activated. To determine if microglia became activated following the CX3CR1 blocking antibody treatment, the number of cells expressing MHC Class II was quantified using the marker OX-6. In the young adult rats the entire hippocampus was used as the region of interest in order to sample a large enough population of cells, due to the few OX-6+ cells in young adult control rats. Following 7 days (F(3,18)=3.644; p=0.0326; Fig.6A) or 14 days (t(8)=2.653; p=0.0291; Fig.6B) of treatment there was a significant effect in the number of OX-6+ cells. At 7 days, the group of rats that were treated with the α-CX3CR1 and the heat-inactivated IL-1Ra (HI-IL-1Ra+ α-CX3CR1; n=5) had significantly more OX-6+ cells compared to the non-immune IgG treated rats with either the inactive IL-1Ra (HI-IL-1Ra+IgG; n=5) (t(8)=2.591; p=0.0321) or active IL-1Ra (IL-1Ra+IgG; n=5) (t(8)=2.412; p=0.0423). The group that received the α-CX3CR1 blocking antibody along with the active IL-1Ra was not significantly different from any of the other groups (IL-1Ra+ α-CX3CR1; n=6). At 14 days a similar significant increase in the number OX-6+ cells was found in the α-CX3CR1 treated group compared to the non-immune IgG treated group.
Figure 6. FKN signaling regulates microglia activation.
Quantification of the number of OX-6+ cell, which is a marker for MHC class II, (A) we found in the rats that received the blocking antibody with an inactive IL-1Ra (gray) a significant increase in the number of OX-6+ cells compared to the two non-immune IgG groups. (B) After 14 days of blocking antibody treatment in the young rats a significant increase in the number of OX-6+ cells was found in the young α-CX3CR1 treated rats (n=5) compared to the non-immune IgG treated rats (n=5). * (t(8)=2.653; p=0.0291; IgG (n=5) vs. α-CX3CR1 (n=5)). (C) In the aged rats after 14 days of treatment with FKN significantly decreased the number of OX-6+ cells. * (t(8)=3.030; p=0.0163; HI-FKN (n=6) vs. FKN (n=4)). (D) The number of OX-6+ cells was also found to significantly correlate with the number of IDU+ cells * (Pearson r(9)=−0.7425; p=0.0219).
Aging is associated with increased activation of microglia. We hypothesized that a portion of the microglia activation might be a result of a decreased inhibitory signaling by FKN. In the aged rats, we quantified the number of OX-6+ cells in SGZ/GCL only as an adequate number of OX-6+ cells were available in both treatment groups. After 14 days (Exp.2) of FKN treatment, a significant decrease in the number of activated OX-6+ cells was found (t(8)=3.030; p=0.0163; Fig.6C) in the FKN group compared to the HI-FKN group. Previous studies have shown a negative correlation between the number of activated microglia and the amount of new cells that are born (Bachstetter et al., 2008; Ekdahl et al., 2003); a correlation analysis was conducted to determine if similar effect was observed following FKN treatment. In the aged rats, after 14 days of treatment with FKN, the number of OX-6+ cells was found to significantly negatively correlate (r(9)=-0.7425; p=0.0219; Fig.6D) with the number of IdU+ cells (Exp.2; Fig.3E). We did not find a significant correlation between the number of CldU+ cells or DCX+ cells and the number of OX-6+ cells (data not shown). Furthermore, in aged rats treated for 7 days with FKN we did not find any differences in the number of OX-6+ cells (data not shown).
4. Discussion
Two questions were addressed by the present study: Is CX3CR1/FKN signaling important for maintaining adult hippocampal neurogenesis? Could a disruption in CX3CR1/FKN signaling contribute to the age-related decline in neurogenesis? We demonstrated three main findings: first, loss of function of CX3CR1 in young adult rodents, mice and rats, resulted in a significant decrease in hippocampal neurogenesis; second, administration of exogenous FKN reversed the decline in neurogenesis associated with aging; third, IL-1Ra protected against the decrease in hippocampal neurogenesis induced by blocking CX3CR1 function.
Our results suggest that neurons, which are the major producers of FKN, and microglia, which express CX3CR1, are actively involved in a cross-talk to regulate the production of new neurons. During development the expression of FKN in the brain has been shown to increase nearly 10 fold in four week old mice compared to one day old mice (Labrada et al., 2002). In our study, we found that FKN expression was absent on immature neurons, suggesting that FKN might be important in protecting mature neurons from the consequences of overactive microglia. It is also possible that mature neurons, through an indirect mechanism, could communicate with microglia to regulate the addition of new neurons into the mature circuit. This could occur via IL-1β decreasing the proliferation of the NPCs. Furthermore, FKN might also be involved in the removal of apoptotic cells, as FKN has been shown to enhance phagocytosis of apoptotic cells (Fuller and Van Eldik, 2008). Removal of apoptotic cells is an important mechanism to make room for new cells to be added, and to prevent secondary necrosis of the apoptotic cell, which occurs if dead cells are not quickly removed.
FKN is anchored to the cell membrane, but can be cleaved off the membrane by metalloproteinase 10 (ADAM10) (Hundhausen et al., 2003) or by TNF-a converting enzyme (TACE / ADAM17) (Garton et al., 2001). FKN signaling, when decreased beyond a physiological level, as in the case of young rats treated with α-CX3CR1, was found to decrease neurogenesis. On the other hand, when FKN signaling was already decreased, as we observed in the aged control rats, a further loss did not affect neurogenesis. Similarly in the young control rats, in which FKN levels are normal, addition of exogenous FKN did not alter neurogenesis. However, our current study doesn’t address if the membrane or shed form of FKN might have unique roles in regulating neurogenesis. Therefore, future studies are warranted to discern if there are different mechanisms of actions produced by the different forms of FKN.
CX3CR1/FKN signaling is proposed to keep microglia in a non-proinflammatory state (Cardona et al., 2006), as inhibition of FKN/CX3CR1 function has been shown to increase microglial activation and increase production of TNFα and IL-1β (Cardona et al., 2006; Mizuno et al., 2003; Zujovic et al., 2000). We found that inhibition of FKN/ CX3CR1 function increased microglia activation. This finding is in agreement with our hypothesis that disruption of CX3CR1 function leads to an increase in microglia activation, which could be responsible for the negative regulation of neurogenesis. However, we did not find increased microglia activation as measured by MHC-II expression, in the CX3CR1GFP/GFP mice compared to the heterozygote littermates (data not shown). A possible explanation could be that permanent loss of CX3CR1 since birth results in compensatory changes in the expression of cell surface markers of microglia activation. An additional discrepancy was found in the aged rats treated with FKN for 7 days, where we saw an increase in proliferation but no changes in the number of MHC-II+ cells, or DCX+ cells. Thus, it is possible that FKN does not exert its effect directly through microglia. Alternatively, alterations in MHC-II expression may not be the best indicator of the activation state of microglia induced by alteration in FKN/ CX3CR1 axis. This may be because MHC-II expression can occur in activated microglia which can be classically activated to produce pro-inflammatory cytokines, as well as in microglia that are alternatively activated to produce growth factors and anti-inflammatory cytokines. However after 14 days of FKN treatment there was a significant reduction in the number of MHC class II+ cells. Furthermore, we found a significant negative correlation between the number of MHC-II+ cells with the number of IdU+ cells. While we cannot rule out the possibility that other pathways are involved in the effects observed in our study, our data in aged rats strongly indicate that FKN/ CX3CR1 suppression of microglia activation, at least in part, modulates hippocampal neurogenesis in aged rats.
Aging is associated with chronically elevated levels of IL-1β in the hippocampus (Gemma and Bickford, 2007). IL-1β has been shown to act directly at the NPC via the IL-1R1 to block cell cycle progression and thereby decrease proliferation (Koo and Duman, 2008). Moreover, we have recently shown that reducing the levels of IL-1β in aged rats is able to reverse some of the age-related decreases in neurogenesis (Gemma et al., 2007). CX3CR1 regulates the PI3K pathway in microglia resulting in inhibiting the production of IL-1β (Re and Przedborski, 2006). We found disruption of FKN signaling by a blocking antibody to the FKN receptor CX3CR1 caused an increased production of IL-1β. In the young adult rats we found that IL-1Ra completely reversed the decrease in proliferation and neurogenesis that resulted after blocking CX3CR1, suggesting that the effects of FKN on NPC proliferation are mediated through inhibition of IL-1β.
The results of the current study suggest that FKN acts via the microglia expressed CX3CR1 to regulate IL-1β, which then acts on the NPCs and neurons. Several studies have shown that FKN can have direct effects on neurons in vitro (Meucci et al., 1998; Meucci et al., 2000; Tong et al., 2000). Using the GFP expression in the CX3CR1+/GFP mice we found that the receptor for CX3CR1 was not found on neurons in the GCL, which confirmed earlier findings in vivo that found CX3CR1 expression only in microglia (Cardona et al., 2006; Harrison et al., 1998; Jung et al., 2000). Moreover, it has been recently shown that the survival effect of FKN on primary neuronal culture was dependent on microglia (Lauro et al., 2008). Earlier studies have shown that NPCs have the message and express CX3CR1 when isolated and cultured in vitro (Ji et al., 2004; Krathwohl and Kaiser, 2004); however, in vivo we were not able to detect the expression of CX3CR1 on NPCs. We did not measure mRNA in NPCs in vivo, or protein or mRNA in isolated NPCs in vitro; therefore, it is possible that NPC could express very low levels of CX3CR1 that we were not able to detect.
In rats to suppress FKN/CX3CR1 signaling we used a blocking antibody to the FKN receptor. The use of the CX3CR1 blocking antibody raises questions concerning effects that can be attributed to the antibody that are independent of the effects of blocking FKN/CX3CR1 signaling. This is an important caveat of our findings. However, a decrease in neurogenesis was found not only in the rats with the blocking antibody treatment but also in mice that lack the FKN receptor. Suggesting that loss of FKN/CX3CR1 is the critical event that is occurring in the mice treated with the CX3CR1 blocking antibody.
The second major finding of this study is an age-related disruption of FKN/CX3CR1 signaling. Cardona et al. (2006) demonstrated in a number of models of neurodegeneration that loss of neuron-microglia interactions by disruption of FKN/CX3CR1 signaling results in increased microglia neurotoxicity and an associated worsening in disease pathology. It is unclear if the disregulation of FKN signaling observed in our study is a cause or consequence of the increased activation of microglia and neuroinflammation that occurs as a result of normal aging. Levels of FKN in aged control rats were decreased in the hippocampus compared to young adult rats. The lack of alteration in FKN mRNA suggests that post-translational changes are responsible for the age-related decrease in FKN. Therefore, as a result of normal aging, the alterations in FKN/CX3CR1 signaling are mostly likely a result of changes in ligand levels or post-translational processing and not alterations to the receptor as administration of exogenous FKN restored the age-related loss in neurogenesis.
The age related decrease in FKN was found to occur in the middle-aged rats, but the decrease was not as great as that seen in the aged rats. Interestingly, the middle-aged rats were found unresponsive to alterations in FKN/ CX3CR1 axis. Neither the addition of exogenous FKN or blocking the receptor had an effect on modulating neurogenesis. This suggests that there is a physiological threshold where FKN/CX3CR1 axis is active in regulating neurogenesis. Only when FKN/CX3CR1 axis is outside that threshold is neurogenesis affected.
Age-related changes in the FKN/CX3CR1 axis have been characterized in other scenarios. There are at least two common single nucleotide polymorphisms in the coding region of CX3CR1, which cause reduced CX3CR1 function, including decreased adhesion, signaling, and chemotaxis of CX3CR1+ cells. These polymorphisms have been associated with reduced risk for atherosclerosis (McDermott et al., 2003) and increased risk of age-related macular degeneration (Combadiere et al., 2007; Tuo et al., 2004). Additionally, APP transgenic mice showed a decrease in neuronal levels of FKN at 9 months of age (Duan et al., 2008). Moreover, soluble plasma levels of FKN are elevated in patients with mild cognitive impairment and mild/moderate Alzheimer’s disease, with lower levels of soluble plasma FKN correlated with lower mini-mental status examination score (Kim et al., 2008). The significance of the alterations in FKN/CX3CR1 signaling in disease are not yet clear and need to be considered in the context of other age-related alterations in immune homeostasis and oxidative stress.
5. Conclusion
Microglia have been demonstrated to be both pro and anti-neurogenic depending upon their activation state. This study demonstrates that neurons may actively regulate microglia in the neurogenic niche, and are not necessarily passive actors to the effects of microglia. However with age, the dialog between neuron and microglia via FKN appears to be disrupted, but it can be re-established through the addition of recombinant FKN. Inflammation is believed to be a contributing factor to the pathogenesis of a number of neurodegenerative diseases, many of which are age-related, including: Alzheimer’s disease, Parkinson’s disease, and age-related macular degeneration. Understanding the mechanism by which age-related alterations in the inflammatory response contribute to the progression of the aforementioned neurodegenerative diseases is key to developing therapeutic interventions for the age-related neurodegenerative condition.
Supplementary Material
A naïve tissue section from a young adult male F344 rat was stained with the α-CX3CR1 blocking antibody. Staining with the blocking could be see throughout the dentate gyrus on cells with the morphological appearance of microglia.
Three ages of male F344 rats were treated with α-CX3CR1 or the IgG control for 7 days via an osmotic minipump to the left lateral ventricle. On day 6 of treatment BrdU was injected twice 8 hours apart. The animals were sacrificed on day 7. (A) Quantification of the number of BrdU+ cells in the subgranular zone, in the young adult rats revealed a significant decrease (***p=0.0009) in the number of adult young rats following α-CX3CR1 (n=6) compare to IgG control (n=6). In the middle aged rats and the aged rats there was no significant differences in the number of BrdU+ cells in the IgG control group compare to the α-CX3CR1 treated group. (B) The decrease in BrdU+ cells in the young rats treated with α-CX3CR1 (n=4) compare to IgG control (n=3) was confirmed by Ki-67, as a significant decrease was also found in the number of Ki-67+ cells (*p=0.0156). Confirming the BrdU data, in the aged rats no difference in the number of Ki-67+ cells was found. (C) The number of DCX+ cells was also found to be significantly decreased (*p=0.0466) in the young adult rats following α-CX3CR1 treatment (α-CX3CR1 n=3; IgG n=4), but no effect was found in the middle aged rats or old aged rats.
Rats were injected with BrdU on day 6 of a 7 day treated of FKN (30ng/d) or a heat-inactivated (HI)-FKN control. (A). No differences were found in the number of BrdU+ cells in the young or middle aged animals between treatment groups. As shown in figure 2B, in the aged rats a significant increase in the number of BrdU+ cells was found (*p=0.0248) in the FKN treated rats compare to the control rats. (B) Quantification of DCX+ cells demonstrated an absence of a treatment effect in all three ages after 7 days of treatment. BrdU and DCX data from the 22 mo old rats was also presented in Fig 2B and 2C respectively, and was re-presented in the supplementary data for comparison purposes.
CldU+ cells (red) were found to co-localize with NeuN+ cells (green) in the IgG treated group (A) and α-CX3CR1 treated group (B).
CX3CR1-GFP+ cells can be seen adjacent to the DCX+ as demonstrated by the 3 dimensional localization of the Green (GFP) and Red (DCX) staining in the video.
Acknowledgements
Research supported by: This work was supported by the National Institutes of Health (AG024165A CG: AG004418 PCB; AI058256 JKH), the VA Medical Research Service, and USF Signature Interdisciplinary Program in Neuroscience Research. Rashid Laboratory for Developmental Neurobiology, Silver Child Development Center, Department of Psychiatry and Behavioral Medicine, for use of confocal microscope. Amgen (Thousand Oaks CA) provide the IL-1Ra as a kind gift
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: ADB, JKH, PCB, CG designed research. ADB, JMM, JJ, AS, SHM, CEH, MJC, CG performed research. ADB, CG wrote paper
Author Conflict of Interest: none
Disclosure Statement
All authors have not any actual or potential conflicts of interest including any financial, personal or other relationships with other people or organizations within 3 years of beginning the work submitted that could inappropriately influence their work. The research was conducted in accordance with the Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use committee of the University of South Florida, College of Medicine or the University of Florida as appropriate.
References
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Pabon MM, Cole MJ, Hudson CE, Sanberg PR, Willing AE, Bickford PC, Gemma C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008;9:22. doi: 10.1186/1471-2202-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Abdallah NM, Slomianka L, Vyssotski AL, Lipp HP. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DN, Chen SC, Sullivan LM, Manfra DJ, Wiekowski MT, Prosser DM, Vassileva G, Lira SA. Generation and analysis of mice lacking the chemokine fractalkine. Mol Cell Biol. 2001;21:3159–3165. doi: 10.1128/MCB.21.9.3159-3165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Drapeau E, Nora Abrous D. Stem cell review series: role of neurogenesis in age-related memory disorders. Aging Cell. 2008;7:569–589. doi: 10.1111/j.1474-9726.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan RS, Yang X, Chen ZG, Lu MO, Morris C, Winblad B, Zhu J. Decreased fractalkine and increased IP-10 expression in aged brain of APP(swe) transgenic mice. Neurochem Res. 2008;33:1085–1089. doi: 10.1007/s11064-007-9554-z. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fuller AD, Van Eldik LJ. MFG-E8 regulates microglial phagocytosis of apoptotic neurons. J Neuroimmune Pharmacol. 2008;3:246–256. doi: 10.1007/s11481-008-9118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) J Biol Chem. 2001;276:37993–38001. doi: 10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- Gemma C, Bachstetter AD, Cole MJ, Fister M, Hudson C, Bickford PC. Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur J Neurosci. 2007;26:2795–2803. doi: 10.1111/j.1460-9568.2007.05875.x. [DOI] [PubMed] [Google Scholar]
- Gemma C, Bickford PC. Interleukin-1beta and caspase-1: players in the regulation of age-related cognitive dysfunction. Rev Neurosci. 2007;18:137–148. doi: 10.1515/revneuro.2007.18.2.137. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell CA, Hancock WW, Salant DJ, Gao W, Csizmadia V, Peters W, Faia K, Fituri O, Rottman JB, Charo IF. Targeted deletion of CX(3)CR1 reveals a role for fractalkine in cardiac allograft rejection. J Clin Invest. 2001;108:679–688. doi: 10.1172/JCI12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, Kallen KJ, Rose-John S, Ludwig A. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Expression of chemokine receptors CXCR4, CCR2, CCR5 and CX3CR1 in neural progenitor cells isolated from the subventricular zone of the adult rat brain. Neurosci Lett. 2004;355:236–240. doi: 10.1016/j.neulet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Lim HK, Lee JY, Kim DJ, Park S, Lee C, Lee CU. Changes in the levels of plasma soluble fractalkine in patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2008;436:196–200. doi: 10.1016/j.neulet.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells. 2004;22:109–118. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- Labrada L, Liang XH, Zheng W, Johnston C, Levine B. Age-dependent resistance to lethal alphavirus encephalitis in mice: analysis of gene expression in the central nervous system and identification of a novel interferon-inducible protective gene, mouse ISG12. J Virol. 2002;76:11688–11703. doi: 10.1128/JVI.76.22.11688-11703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauro C, Di Angelantonio S, Cipriani R, Sobrero F, Antonilli L, Brusadin V, Ragozzino D, Limatola C. Activity of adenosine receptors type 1 Is required for CX3CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. J Immunol. 2008;180:7590–7596. doi: 10.4049/jimmunol.180.11.7590. [DOI] [PubMed] [Google Scholar]
- McDermott DH, Fong AM, Yang Q, Sechler JM, Cupples LA, Merrell MN, Wilson PW, D'Agostino RB, O'Donnell CJ, Patel DD, Murphy PM. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest. 2003;111:1241–1250. doi: 10.1172/JCI16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci U S A. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O'Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Kawanokuchi J, Numata K, Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979:65–70. doi: 10.1016/s0006-8993(03)02867-1. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell. 2006;5:545–558. doi: 10.1111/j.1474-9726.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Re DB, Przedborski S. Fractalkine: moving from chemotaxis to neuroprotection. Nat Neurosci. 2006;9:859–861. doi: 10.1038/nn0706-859. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Spulber S, Oprica M, Bartfai T, Winblad B, Schultzberg M. Blunted neurogenesis and gliosis due to transgenic overexpression of human soluble IL-1ra in the mouse. Eur J Neurosci. 2008;27:549–558. doi: 10.1111/j.1460-9568.2008.06050.x. [DOI] [PubMed] [Google Scholar]
- Tong N, Perry SW, Zhang Q, James HJ, Guo H, Brooks A, Bal H, Kinnear SA, Fine S, Epstein LG, Dairaghi D, Schall TJ, Gendelman HE, Dewhurst S, Sharer LR, Gelbard HA. Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J Immunol. 2000;164:1333–1339. doi: 10.4049/jimmunol.164.3.1333. [DOI] [PubMed] [Google Scholar]
- Tuo J, Smith BC, Bojanowski CM, Meleth AD, Gery I, Csaky KG, Chew EY, Chan CC. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega CJ, Peterson DA. Stem cell proliferative history in tissue revealed by temporal halogenated thymidine analog discrimination. Nat Methods. 2005;2:167–169. doi: 10.1038/nmeth741. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Schwartz M. Immune-based regulation of adult neurogenesis: implications for learning and memory. Brain Behav Immun. 2008;22:167–176. doi: 10.1016/j.bbi.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Zujovic V, Benavides J, Vige X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. [PubMed] [Google Scholar]
- Zujovic V, Schussler N, Jourdain D, Duverger D, Taupin V. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNFalpha and 8-isoprostane production induced by intracerebroventricular injection of LPS. J Neuroimmunol. 2001;115:135–143. doi: 10.1016/s0165-5728(01)00259-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A naïve tissue section from a young adult male F344 rat was stained with the α-CX3CR1 blocking antibody. Staining with the blocking could be see throughout the dentate gyrus on cells with the morphological appearance of microglia.
Three ages of male F344 rats were treated with α-CX3CR1 or the IgG control for 7 days via an osmotic minipump to the left lateral ventricle. On day 6 of treatment BrdU was injected twice 8 hours apart. The animals were sacrificed on day 7. (A) Quantification of the number of BrdU+ cells in the subgranular zone, in the young adult rats revealed a significant decrease (***p=0.0009) in the number of adult young rats following α-CX3CR1 (n=6) compare to IgG control (n=6). In the middle aged rats and the aged rats there was no significant differences in the number of BrdU+ cells in the IgG control group compare to the α-CX3CR1 treated group. (B) The decrease in BrdU+ cells in the young rats treated with α-CX3CR1 (n=4) compare to IgG control (n=3) was confirmed by Ki-67, as a significant decrease was also found in the number of Ki-67+ cells (*p=0.0156). Confirming the BrdU data, in the aged rats no difference in the number of Ki-67+ cells was found. (C) The number of DCX+ cells was also found to be significantly decreased (*p=0.0466) in the young adult rats following α-CX3CR1 treatment (α-CX3CR1 n=3; IgG n=4), but no effect was found in the middle aged rats or old aged rats.
Rats were injected with BrdU on day 6 of a 7 day treated of FKN (30ng/d) or a heat-inactivated (HI)-FKN control. (A). No differences were found in the number of BrdU+ cells in the young or middle aged animals between treatment groups. As shown in figure 2B, in the aged rats a significant increase in the number of BrdU+ cells was found (*p=0.0248) in the FKN treated rats compare to the control rats. (B) Quantification of DCX+ cells demonstrated an absence of a treatment effect in all three ages after 7 days of treatment. BrdU and DCX data from the 22 mo old rats was also presented in Fig 2B and 2C respectively, and was re-presented in the supplementary data for comparison purposes.
CldU+ cells (red) were found to co-localize with NeuN+ cells (green) in the IgG treated group (A) and α-CX3CR1 treated group (B).
CX3CR1-GFP+ cells can be seen adjacent to the DCX+ as demonstrated by the 3 dimensional localization of the Green (GFP) and Red (DCX) staining in the video.