Abstract
This article provides a detailed review of the extant gene-environment interaction (GxE) research in the etiology of posttraumatic stress disorder (PTSD). We begin by a discussion of why PTSD is uniquely fitting for the innovative framework of GxE methodology, followed by a review of the heritability and main effect molecular genetics studies of PTSD. Next, we discuss the six GxE investigations to date on PTSD. We end with a discussion of future directions and significance of this research, with an emphasis on the expansion of psychosocial factors that may be fitting ‘E’ variables for inclusion in this new research area. We posit that GxE research is vital to elucidating risk and resilience following exposure to a potentially traumatic event.
Although the majority of individuals have been exposed to at least one potentially-traumatic event (PTE) during their lifetime, trauma-exposed individuals evidence heterogeneous responses, including resilience, rapid recovery, or the development of psychopathology (e.g., posttraumatic stress disorder (PTSD), depressive and anxiety disorders, and a range of comorbidities) (Copeland, Keeler, Angold, & Costello, 2007; Kessler, Sonnega, Bromet, Hughes, & Nelson, 1995). Differential responding to environmental pathogens is perhaps one of the most important indicators of a gene-environment interaction (GxE), in which the effects of environmental exposure are moderated by genotype (Moffitt, Caspi, & Rutter, 2005). In the present paper, we discuss the compatibility of the GxE paradigm with research on PTSD, review the six published GxE investigations of PTSD, and briefly highlight strategies for future studies.
PTSD as an Ideal Candidate for GxE Methodology
In a seminal paper outlining strategies for examining interactions between candidate genes and measured environments, Moffitt and colleagues (Moffitt et al., 2005) provide an overview of the GxE paradigm, recommend steps for conducting GxE research, and highlight the benefits of GxE strategies for understanding the etiology of common mental disorders. PTSD, as one of the few psychiatric diagnoses requiring exposure to an environmental pathogen (e.g., exposure to a PTE), is particularly suited to GxE research.
PTEs as Candidate Environmental Pathogens
PTEs are excellent candidates for GxE research according to the criteria set out by Moffitt et al. (2005). First, there is variability in response even to the most severe PTEs; most exposed individuals do not develop PTSD. Second, PTE’s as reviewed in detail elsewhere (Delahanty, 2008), appear to exert measurable and causal effects on neurobiological pathways relevant to the development of PTSD. Third, evidence from epidemiologic and twin studies suggest severity of PTE’s, such as combat exposure, are causally related to the development of PTSD (Dohrenwend et al., 2006; Goldberg, True, Eisen, & Henderson, 1990).
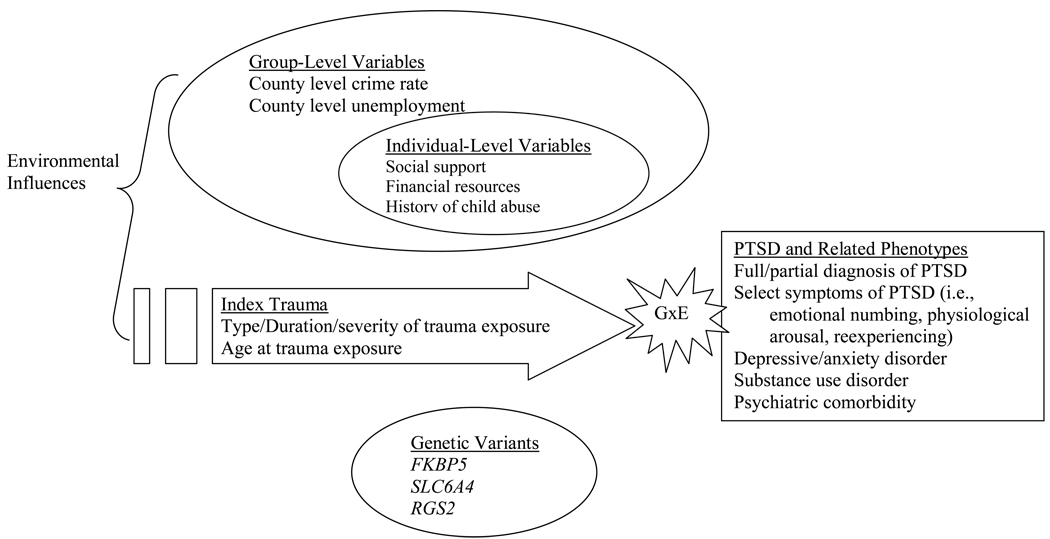
However, it is not merely the occurrence of PTEs that confers risk for PTSD but also important contextual and descriptive aspects of the trauma. As shown in Figure 1, specific aspects of the trauma (e.g., extent of exposure such as level of hurricane exposure) may be important for understanding the degree and nature of influence of the environmental pathogen. Identifying relevant aspects of the traumatic experience can be somewhat informed by the expected neurobiological mechanisms of effect on subsequent PTSD. For example, emergent research suggests that maternal hormones and concomitant pre- and peri-natal environment may influence glucocorticoid functioning in offspring (de Kloet, Sibug, Helmerhorst, & Schmidt, 2005; Yehuda et al., 2005). Given this evidence for possible developmentally-sensitive processes, GxE studies examining aspects of the glucocorticoid system may need to carefully quantify age-related factors such as age at index trauma, age at first trauma, or maternal peri-partum symptoms.
Figure 1.
An thematic overview of GxE studies of PTSD
Genetic Investigations of PTSD
Evidence for genetic influences on posttrauma development of PTSD comes from both family and twin studies (see (Koenen, 2007) for a review of genetic methodology pertaining to PTSD). Offspring whose parents have PTSD evidence higher rates of PTSD as adults (Yehuda, Halligan, & Bierer, 2001) and during childhood (Hall et al., 2005). Similarly, twin studies of PTSD suggest that genetic influences account for about one-third of the variance in risk of developing PTSD (Stein, Jang, Taylor, Vernon, & Livesley, 2002; True et al., 1993). However, familial risk of developing PTSD may be partly mediated by genetic influences on exposure to trauma (Koenen et al., 2002), referred to as the gene-environment correlation, and twin studies of both veteran and civilian samples have supported a role for genetic factors in exposure to PTEs (Lyons et al., 1993; Stein et al., 2002). Nonetheless, genetic influences on exposure to PTEs do not fully account for genetically-conferred risk for PTSD, as 30% of the variance in PTSD in the Vietnam Era Twin Registry was associated with genetic factors even after controlling for combat exposure (True et al., 1993).
Reviewed in detail elsewhere (Amstadter, Nugent, & Koenen, in press; Broekman, Olff, & Boer, 2007; Koenen, 2007; Nugent, Amstadter, & Koenen, 2008), 17 candidate gene studies of PTSD have been conducted, most of which have focused on the dopaminergic system (i.e., DRD2, DAT). Other neurobiologic systems have been studied, including the serotonergic system (i.e., SLC6A4, 5-HTR2A), markers of the hypothalamic pituitary adrenal axis (i.e., GCCR, FKBP5, CNR1), components of the locus coeruleus/noradrenergic system (i.e., NPY, DBH), and neurotrophins (i.e., BDNF). Reviews detailing the hypothesized underlying neurobiological mechanisms whereby these genes are believed to exert their effects are available elsewhere (Amstadter et al., in press; Broekman et al., 2007). Of brief note, however, apparent discrepancies in this literature may be partly resolved by attention to environmental factors (such as whether control participants were trauma-exposed) by careful and neurobiologically-informed characterization of the PTSD-related phenotype (such as comorbid concerns such as harmful drinking in PTSD cases). However, beyond the diagnostically-inherent inclusion of trauma exposure in most investigations, only six of these investigations (reviewed below) have formally tested GxE.
Nonetheless, published candidate gene studies can inform selection of genes for GxE investigations. Perhaps most notably, many of the published genetic variants are ideal candidates for GxE investigations according to Moffitt and colleagues (2005). More specifically, some of the aforementioned genes have putative functional effects on neurobiological systems affected by exposure to PTEs (Amstadter et al., in press), appear to be associated with PTSD or PTSD-related phenotypes, and are common polymorphisms. Studies may also benefit from consideration of the combined environmental effects of PTEs and additional environmental constructs (e.g., social support) on neurobiological systems believed to influence development of PTSD. For example, nonhuman animal models have shown interactions between the promoter region of the serotonin transporter gene and early rearing environment (maternal separation vs maternal rearing), suggesting a possible role for early or possibly peritrauma social relationships in models involving the promoter region of the serotonin transporter gene (Suomi, 2006).
Moreover, as neurobiological mechanisms are unlikely to show effects on “diagnostic status” as a construct, careful consideration of the PTSD-related phenotype is also warranted. For example, a series of studies first noted that a deletion variant of ADRA2B, the gene coding for the alpha2b-adrenergic receptor was associated with enhanced memory for emotional information in a sample of young adults (de Quervain et al., 2007). The subsequent study examined ADRA2B in trauma-exposed Rwandan refugees, with higher re-experiencing found in deletion carriers than in non-carriers, though no differences were found in hyperarousal, avoidance, or diagnostic status. In this case, given the mechanism whereby ADRA2B influences posttraumatic phenotypes, the role for ADRA2B would have been missed if PTSD, instead of the refined phenotype of reexperiencing symptoms, had been measured.
GxE PTSD Investigations
Although the phenotype of PTSD is uniquely well-suited for GxE research, only six GxE studies of PTSD have been published or are in press (see Table 1). Consistent with recommended GxE methodology, these investigations have generally examined common polymorphic variants that have shown prior support for gene-disorder associations. Further, these studies selected environmental risks that (1) have been previously shown to buffer/exacerbate the potentially deleterious effects of PTEs and (2) may be plausibly expected to interact with the candidate genes investigated.
Table 1.
Review of published GxE studies of PTSD
| First author | Year | Trauma Exposed Controls? | Trauma Type | Gene Name (Symbol) | Environmental Variables | Finding |
|---|---|---|---|---|---|---|
| Binder et al. | 2008 | Yes | Various | FKBinding Protein 5 (FKBP5) | Childhood trauma exposure and adult trauma exposure. The Trauma Events Inventory was used to measure trauma exposure. A 3 level variable was used for childhood events (no abuse; physical or sexual abuse; physical and sexual abuse). A 4 level non-child abuse variable was created (no adult trauma, one type, two types, three or more types). | 4 SNPs in FKBP5 significantly interacted with severity of child abuse in prediction of adult PTSD symptoms |
| Kilpatrick et al. | 2007 | Yes | Hurricane | Serotonin Transporter (SLC6A4) | Hurricane exposure and social support. Five hurricane exposure characteristics were assessed; endorsement of 2 or more was dichotomized as high hurricane exposure. Social support was measured via a modified Medical Outcomes Study Module (5 items on a 4 point scale); a score of 15 and below was dichotomized into low social support. | Significant association between s/s genotype and PTSD in adults with high hurricane exposure and low social support |
| Koenen et al. | 2009 | Yes | Hurricane; Various | Serotonin Transporter (SLC6A4) | County crime rate and county level-unemployment. Both variables were dichotomized into high vs. low, where high was defined as above the mean of studied counties | Significant interaction between genotype and both county-level environmental variables. ‘s’ allele was associated with decreased risk of PTSD in low-risk environments (low crime/unemployment), and increased risk of PTSD in high risk environments |
| Kolassa et al. | In Press | Yes | War | Serotonin Transporter (SLC6A4) | Number of traumatic events. Number of trauma experiences assessed using a structured interview administration of a checklist of 36 war- and non-war-related trauma events (e.g., rape, bombing or shelling). | The fitted probability of PTSD in ‘s’ homozygotes, regardless of number of traumas reported. However, participants with the s’/l’ and l’/l’ genotypes showed an S-shaped doseresponse, with increasing rates of PTSD reported as a function of number of traumatic experiences. Little difference for genotype was observed in participants reporting 15+ traumas. |
| Amstadter et al. | 2009 | Yes | Hurricane; Various | Regulator of G-Protein Signaling 2 (RGS2) | Hurricane exposure, prior trauma, and social support. Five hurricane exposure characteristics were assessed; endorsement of 2 or more was dichotomized as high hurricane exposure. Five Criterion A events were assessed, dichotomized into prior trauma positive or negative. Social support was measured via a modified Medical Outcomes Study Module (5 items on a 4 point scale); a score of 15 and below was dichotomized into low social support. | Significant association between CC genotype and post-hurricane PTSD in adults with high hurricane exposure and low social support; significant association between CC genotype and lifetime PTSD in adults with prior trauma exposure and low social support |
| Nelson et al. | 2009 | No | Various | Gamma-Aminobutyric Acid Receptor, Alpha-2 (GABRA2) | Childhood trauma exposure assessed via a modified version of the Christchurch Trauma Inventory. Five types of childhood trauma were assessed: sexual abuse; physical abuse by mother, father or other adult household member; emotional and physical partner maltreatment by a parent. A single second-order childhood trauma factor score was created for analyses. | 3 SNPs in GABRA2 significantly interacted with the childhood factor score in prediction of adult PTSD. Elevated risk was found only for homozygous individuals. |
PTSD = posttraumatic stress disorder; SNPs = single nucleotide polymorphisms; s allele = short version (vs. long) of the serotonin transporter promoter polymorphism.
The Florida Hurricanes Study
Three of the six published GxE investigations have come from our research team using data from the 2004 Florida Hurricanes Study (Amstadter et al., 2009; Kilpatrick et al., 2007; Koenen et al., in press), an NIMH funded project designed to assess hurricane related outcomes in a representative sample of older adults (n = 1130, age 60 and over) and a comparison sample of younger adults (n = 413, age 21–59) residing in Florida disaster sites. This household probability sample of adults was interviewed 6–9 months after the hurricanes about demographic variables, exposure to the hurricane(s), PTSD status, prior exposure to potentially traumatic events (PTEs), and social support (Acierno, Ruggiero, Kilpatrick, Resnick, & Galea, 2006). The study design included collecting saliva samples via mail from adults who had been interviewed about their exposure to the 2004 hurricanes, and over 600 participants returned saliva samples. Comparisons of participants who did and did not return saliva samples found that there were no significant differences on any major variables (Galea, Acierno, Ruggiero, Resnick, & Kilpatrick, 2006), underscoring the viability of biologic data collection via US mail for the purposes of genetic analyses. The saliva collection kits used in the Florida Hurricanes Study obtained approximately 10–30 µg of DNA from each sample, with a failure rate of about 3% (no higher than found in studies in which samples are not mailed to the laboratory prior to DNA extraction), which is an adequate yeild to support a plethora of genetic assays.
The first paper published from this project, and notably, the first published GxE PTSD study, tested the hypothesis that the polymorphism in the serotonin transporter gene (also known as 5-HTTLPR or SLC6A4) would modify risk of post-hurricane PTSD under the high environmental risk conditions (Kilpatrick et al., 2007). This commonly studied polymorphism is a variable number tandem repeat (VNTR) located in the promoter region of the gene. The 5-HTTLPR is a functional polymorphism, with the short, “s,” allele being less transcriptionally efficient than the long, “l,” allele (Lesch et al., 1996). This polymorphism has since been found to be triallelic, in that a third functional allele, ‘LG’, has been identified (Nakamura, Ueno, Sano, & Tanabe, 2000); LG is characterized by an A>G substitution at nucleotide 6 of the first of two extra 22-bp repeats in the ‘l’ allele, resulting in transcriptional capacity comparable to the ‘s’ allele. In this study, the LG and s alleles were coded as s’ alleles; LA were coded as l’ alleles. Environmental stress conditions were dichotomized into high versus low hurricane exposure, and high versus low social support. As illustrated by Figure 2, the low expression (s) variant of the 5-HTTLPR increased risk of post-hurricane PTSD, but only under the high stress conditions of high hurricane exposure and low social support. At Highest risk (n = 27) were those with the s’/s’ genotype and high stress exposure. Medium risk (n = 54) were those with the l/s genotype and high stress exposure. All others (n=498) all others were considered low risk. There was a strong association between risk group and prevalence of PTSD, χ2(2, n = 579) = 19.94, p<.001. High-risk individuals (high hurricane exposure, the low expression 5-HTTLPR variant, low social support) were at 4.5 times (95% CI = 1.2, 17.9) the risk of developing PTSD as compared to low-risk individuals.
Figure 2.
Level of stress exposure modifies the association between the 5-HTTLPR genotype and post-hurricane PTSD.
Note: Low stress exposure was defined as low hurricane exposure and high social support. High stress exposure was defined as high hurricane exposure and low social support. 5-HTTLPR genotypes were triallelic with lg categorization.
A second paper was published by our team that examined the 5-HTTLPR gene and PTSD (Koenen et al., 2009). Whereas previous GxE investigations of psychiatric phenotypes have only examined individual-level environmental variables (e.g., (Caspi et al., 2003; Kilpatrick et al., 2007), this study investigated the role of group-level social environment. Specifically, crime rate (taken from the Serious Crimes Known to Police U.S. Federal Bureau of Investigation, Uniform Crime Reporting Program unpublished data from 1999; high crime counties were defined as counties in which the crime rate was above the mean crime rate of the counties in our study) and percent unemployment (taken from the 2000 U.S. Census Summary File 3; high unemployment was defined as counties in which the unemployment rate was above the mean of the studied counties) were the examined environmental variables. Of particular note, county-level crime rate data provide an example of an environmental variable that may be objectively observed. Results indicated that county-level crime rate and percent unemployment modified the association between the 5-HTTLPR genotype and PTSD; low expression allele carries (‘s’) were at increased risk for PTSD in high environmental stress conditions (high unemployment; high crime), and were at decreased risk for PTSD in low-risk environments (low unemployment, low crime). This paper underscores the importance of investigation of not just individual level variables, but also group level factors of the overall social environment.
The third PTSD GxE paper using Florida Hurricane Study data examined a polymorphism that had not previously been studied in the context of PTSD (Amstadter et al., 2009). Polymorphisms in the RGS2 (regulator of G-protein signaling 2) gene were found to be associated with anxious behavior in mice (Leygraf et al., 2006) and anxiety in humans (Smoller et al., 2008). We examined whether rs4606, a single nucleotide polymorphism (SNP) of RGS2, and social support moderated risk for post-hurricane and lifetime PTSD. Rs4606 (‘C’ allele was the risk allele) was associated with increased symptoms of post-hurricane PTSD symptoms under conditions of high hurricane exposure and low social support (p < .05). Further, this polymorphism was associated with lifetime PTSD symptoms under conditions of lifetime exposure to a PTE (other than the current hurricane), and low social support (p<0.001). These GxE interactions remained significant after adjustment for sex, ancestry, and age, indicating that RGS2 rs4606 modifies risk of post-disaster and lifetime PTSD under conditions of high stressor exposure.
GxE Under Conditions of High Trauma Exposure
The 5-HTTLPR polymorphism of SLC6A4 was recently examined in 408 Rwandan refugees (Kolassa et al., in press). Participants endorsed extremely high levels of trauma exposure, reporting an average of 12.6 different traumas; not surprising, given the rates of traumatic experiences, 81% of participants met full criteria for lifetime PTSD and the probability of PTSD was nonlinearly associated with number of traumas, asymptotically approaching a probability of 1 for participants reporting 15 or more traumatic events. Findings supported a main effect for genotype, with the resiliency associated with the presence of an l allele diminishing across exposure to increasing numbers of traumatic events. More specifically, whereas the fitted probability of lifetime PTSD for participants homozygous for the low expression allele (s’/s’) was 100% regardless of the number of traumatic experiences, carriers of the l’ alleles evidenced lower levels of PTSD following exposure to few trauma experiences but showed significantly increased risk associated with increasing numbers of traumatic experiences. The extremely high levels of trauma exposure, and concomitant high rates of PTSD, found in this sample demonstrate the overwhelming effects of trauma under conditions of extreme exposure and highlight the importance of careful characterization of trauma exposure in GxE research.
Child Abuse as a GxE Moderator
A recent paper by Ressler’s research team at Emory University also tested GxE models for PTSD (Binder et al., 2008). The sample (n = 762) consisted predominately of low income African American patients who presented to general medical clinics at a large urban hospital. Current PTSD was assessed, as well as a retrospective assessment of exposure to child abuse. Fine mapping of the FKBP5 locus was undertaken, as FKBP5 is a glucocorticoid regulating gene, and has been previously found to be related to peritraumatic dissociation (a potent predictor of PTSD) (Koenen et al., 2005), it was a fitting candidate gene. No main effects of polymorphisms in FKBP5 were identified, but a significant GxE interaction between severity of child abuse (but not adult traumatic events) and FKBP5 polymorphisms was found for PTSD. Figure 3 illustrates the interaction findings for one of the FKBP5 polymorphisms that remained significant after correction for multiple testing. The results show that the ‘C’ allele was associated with higher levels of PTSD symptoms, but only among individuals exposed to two or more types of child abuse. There was no effect of genotype for individuals who experienced only one type of child abuse or no child abuse. Dexamethasone suppression test data also demonstrated that these polymorphisms have functional consequences for glucocorticoid system response sensitivity. They concluded that variation in FKBP5, in the presence of child abuse, may alter sensitivity of this stress-response pathway, “placing those individuals who have had significant child abuse at significant risk for PTSD in the face of other traumatic experiences” (p. 1304).
Figure 3.
Severity of child abuse modifies the association between FKBP5 polymorphisms and adult PTSD symptoms.
Created based on data presented in Binder et al., JAMA, 2008.
Finally, Nelson et al (2009) examined the interaction of gamma-aminobutyric acid receptor, alpha-2 (GABRA2) polymorphisms and childhood trauma in risk of lifetime PTSD diagnosis in adults (n=259). The authors found significant (p<.05) interactions between three SNP and childhood abuse; only homozygous individuals were at significantly increased risk of PTSD. Findings remained similar after controlling for nicotine and alcohol dependence. No evidence for significant GxE interactions were found for major depression.
Novel Environmental Variables
An important direction for PTSD GxE research to advance in is not only the expansion of studied ‘G’ variables, but also the broadening of ‘E’ variables that are investigated. Environmental variables previously found to moderate the relationship between genetic variants and PTSD include individual-level variables (e.g., social support, level of trauma exposure), and group-level environmental variables (i.e., county level crime rate, county level unemployment rate). Although the studied ‘E’ variables have been promising, they likely only represent a small fraction of the possible ‘E’ characteristics that may play a role in the etiology and maintenance of PTSD, leaving a wide-open field of research in need of being conducted. Expansion of both individual-level and group-level environmental variables is warranted.
Extant psychosocial studies of PTSD may help to inform the selection of ‘E’ variables for inclusion in GxE studies. A number of psychosocial risk factors for PTSD have been identified (Brewin, Andrews, & Valentine, 2000) that may be fitting ‘E’ variables for use in future research (e.g., education, general childhood adversity, current life stressors, female gender). Further, aspects of an individuals’ trauma history (e.g., PTE severity, duration, interpersonal victimization, age of onset, multiple victimization history) have been related to increased risk for the disorder (Brewin et al., 2000), and could also be studied in the context of a GxE study of PTSD. Additionally, findings from the non-human primate literature may help inform novel ‘E’ variable selection, particularly for pediatric PTSD, as early rearing environment has also been shown to moderate the relationship between serotonin transporter variation and central nervous system function (Bennett et al., 2002) as well as hypothalamic adrenal axis response to stress (Barr et al., 2004). PTSD GxE studies may also benefit from findings in other areas of psychiatric genetic study. For example, a recent GxE study of depression found that parenting style, specifically perceived maternal rejection, moderated the relationship between genetic variants and depression in juvenile detainees (Haeffel et al., 2008), suggesting that family environment variables (e.g., family cohesion, communication, conflict) may be fitting ‘E’ variables in the study of child and adolescent PTSD.
Limitations/Challenges in GxE Research in PTSD
Our review of the literature identified only six GxE investigations of PTSD, three of which report findings from the same larger study. Thus, few conclusions may be drawn about the complicated interplay of genes and environment in PTSD. Because psychiatric genetic research requires large samples and has a history of multiple failed attempts at replication, quantitative reviews of the literature have been critical for thorough appraisal of candidate genes in more extensively studied disorders (i.e., schizophrenia, depression, etc). The scarcity of published GxE investigations of PTSD prevents quantitative examination at this time. Moreover, the large number of plausible candidate genes and environmental variables present a further challenge to the field both through the risk of Type I error and through the reduced likelihood that comparable genetic and, in particular, environmental variables will be examined across investigations. For example, Kilpatrick and colleagues’ (2007) examined only two environmental risk factors, including level of hurricane exposure and of social support, with genotype risk varying as a function of both environmental influences. Further, to the degree that environmental risk factors rely on self-report, particularly retrospective self-report, the presence of PTSD may color reported environmental risks and inflate associations between environment and PTSD. The effect of PTSD symptoms on retrospective recall of trauma exposure has been well-documented in veteran samples (Koenen, Stellman, Dohrenwend, Sommer, & Stellman, 2007; Roemer, Litz, Orsillo, Ehlich, & Frieman, 1998; Southwick, Morgan, Nicolaou, & Charney, 1997). Hence, researchers interested in GxE research with PTSD are encouraged to consider objective measures of risk (such as county crime/unemployment rates) or to conduct longitudinal investigations that attenuate recall biases. Finally, it is important to note that PTSD is only one possible outcome of trauma exposure. As we have discussed elsewhere, research supports considerable genetic overlap between PTSD and other psychiatric disorders (Koenen, Nugent, & Amstadter, 2008) and our own work with trauma-exposed populations has identified posttrauma GxE effects on depression similar to those for PTSD (Kilpatrick et al., 2007).
Significance and Future Directions
PTSD is a prevalent disorder that represents a significant public health burden to adults, adolescents, and children. GxE research in PTSD has the potential to substantially improve our understanding of the specific genetic variants that are associated with PTSD, as well as the environmental conditions that influence these effects. Of note, only one of the six GxE studies of PTSD identified a significant main effect for genotype (Kolassa et al., in press). Thus, the potential role of these specific genes in PTSD etiology would not have detected without testing for their interaction with specific stressor variables. Understanding both genetic and environmental influences, as well as their interactions, has the potential to inform neurobiological models of PTSD and to inform treatment efforts. For example, given the consistently identified interactions between social environment and the serotonin promoter gene, as well as evidence that posttraumatic adjustment may be partly predicted by the serotonin promoter gene, genetically-informed interventions may benefit from the incorporation of family members or other sources of social support in individuals with low-expressing alleles. This is particularly promising as family environment and social supports have been shown to be modifiable in the context of ecologically-based approached to early intervention (Dadds, Spence, Holland, Barrett, & Laurens, 1997) and psychosocial treatment (Henggeler, Clingempeel, Brondino, & Pickrel, 2002). GxE research in PTSD is in its infancy and many important lines of research warrant study (e.g., developmental trajectories, various neurobiologic systems as ‘G’ variables, expansion of ‘E’ variables, possible sex differences, examination of endophenotypes of PTSD). These lines of research have clear public health significance and will be able to inform primary prevention of disorder among those at risk, secondary prevention of PTSD development among trauma exposed individuals, and the allocation of limited treatment resources to those who are most likely to be affected.
Acknowledgments
Dr. Amstadter is supported by US-NIMH 083469. Dr. Nugent is supported by US-NIMH T32 MH078788. Dr. Koenen is supported by US-NIMH K08 MH070627 and MH078928.
References
- Acierno R, Ruggiero KJ, Kilpatrick D, Resnick H, Galea S. Risk and protective factors for psychopathology among older versus younger adults after the 2004 Florida hurricanes. American Journal of Geriatric Psychiatry. 2006;14:1051–1059. doi: 10.1097/01.JGP.0000221327.97904.b0. [DOI] [PubMed] [Google Scholar]
- Amstadter AB, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Kilpatrick DG, et al. Variant in RGS2 moderates posttraumatic stress symptoms following potentially traumatic event exposure. Journal of Anxiety Disorders. 2009;23:369–373. doi: 10.1016/j.janxdis.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Koenen KC. Genetics of PTSD: Fear conditioning as a model for future research. Psychiatric Annals. doi: 10.3928/00485713-20090526-01. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biological Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Molecular Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Journal of the American Medical Association. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology. 2000;68:748–766. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Broekman BFP, Olff M, Boer F. The genetic background to PTSD. Neuroscience & Biobehavioral Reviews. 2007;31:348–362. doi: 10.1016/j.neubiorev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig I, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Keeler G, Angold A, Costello EJ. Traumatic events and posttraumatic stress in childhood. Archives of General Psychiatry. 2007;64:577–584. doi: 10.1001/archpsyc.64.5.577. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Spence SH, Holland DE, Barrett PM, Laurens KR. Prevention and early intervention for anxiety disorders: A controlled trial. Journal of Consulting & Clinical Psychology. 1997;65:627–635. doi: 10.1037//0022-006x.65.4.627. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MH. Stress, genes and the mechanism of programming the brain for later life. Neuroscience and Biobehavioral Reviews. 2005;29:271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- de Quervain DJF, Kolassa I-T, Ertl V, Onyut PL, Neuner F, Elbert T, et al. A deletion variant of the [alpha]2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nature Neuroscience. 2007;10:1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, editor. The Psychobiology of Trauma. Jason Aronson; 2008. [Google Scholar]
- Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313:979–982. doi: 10.1126/science.1128944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, Acierno R, Ruggiero KJ, Resnick HS, Kilpatrick DG. Social context and the psychobiology of trauma. Annals of the New York Academy of Sciences. 2006;1071:231–241. doi: 10.1196/annals.1364.018. [DOI] [PubMed] [Google Scholar]
- Goldberg J, True WR, Eisen S, Henderson W. A twin study of the effects of the Vietnam War on posttraumatic stress disorder. Journal of the American Medical Association. 1990;263:1227–1232. [PubMed] [Google Scholar]
- Haeffel GJ, Getchell M, Koposov RA, Yrigollen CM, DeYoung CG, af Klinteberg B, et al. Association between polymorphisms in the dopamine transporter gene and depression. Evidence for a gene-environment interaction in a sample of juvenile detainees. Psychological Science. 2008;19:62–69. doi: 10.1111/j.1467-9280.2008.02047.x. [DOI] [PubMed] [Google Scholar]
- Hall E, Saxe G, Stoddard F, Kaplow J, Koenen K, Chawla N, et al. Posttraumatic stress symptoms in parents of children with acute burns. Journal of Pediatric Psychology. 2005;31:403–412. doi: 10.1093/jpepsy/jsj016. [DOI] [PubMed] [Google Scholar]
- Henggeler SW, Clingempeel WG, Brondino MJ, Pickrel SG. Four-year follow-up of multisystemic therapy with substance abusing and dependent juvenile offenders. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:868–874. doi: 10.1097/00004583-200207000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, et al. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. American Journal of Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Koenen KC. Genetics of posttraumatic stress disorder: review and recommendations for future studies. Journal of Traumatic Stress. 2007;20:737–750. doi: 10.1002/jts.20205. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Aiello AE, Bakshis E, Amstadter AB, Ruggiero KJ, Acierno R, et al. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. American Journal of Epidemiology. 169:704–711. doi: 10.1093/aje/kwn397. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Harney R, Lyons MJ, Wolfe J, Simpson JC, Goldberg J, et al. A twin registry study of familial and individual risk factors for trauma exposure and posttraumatic stress disorder. Journal of Nervous and Mental Disease. 2002;190:209–218. doi: 10.1097/00005053-200204000-00001. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Nugent NR, Amstadter A. Posttraumatic Stress Disorder: A Review and Agenda for Gene-Environment Interaction Research in Trauma. European Archives of Psychiatry and Clinical Neuroscience: Special Issue on Gene-Environment Interaction and the Anxiety Disorders. 2008;258:82–96. doi: 10.1007/s00406-007-0787-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, et al. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Molecular Psychiatry. 2005;10:1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Stellman SD, Dohrenwend BP, Sommer JF, Jr, Stellman JM. The consistency of combat exposure reporting and course of PTSD in Vietnam War veterans. Journal of Traumatic Stress. 2007;20:3–13. doi: 10.1002/jts.20191. [DOI] [PubMed] [Google Scholar]
- Kolassa IT, Ertl V, Eckart MS, Glockner F, Kolassa S, Papassotiropoulos A, et al. Association study of trauma load and SCL6A4 promoter polymorphism in PTSD: evidence from survivors of the Rwandan genocide. Journal of Clinical Psychiatry. doi: 10.4088/JCP.08m04787blu. (in press) [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Leygraf A, Hohoff C, Freitag C, Willis-Owen SAG, Krakowitzky P, Fritze J, et al. Rgs 2 gene polymorphisms as modulators of anxiety in humans? Journal of Neural Transmission. Journal of Neural Transmission. 2006;113:1921–1925. doi: 10.1007/s00702-006-0484-8. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Goldberg J, Eisen SA, True W, Tsuang MT, Meyer JM. Do genes influence exposure to trauma? A twin study of combat. American Journal of Medical Genetics. 1993;48:22–27. doi: 10.1002/ajmg.1320480107. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Molecular Psychiatry. 2000;5:32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Lynskey MT, Todorov AA, Wang JC, Todd RD, Martin NG, Heath AC, Goate AM, Montgomery GQ, Madden PAF. Association with childhood trauma exposure and GABRA2 polymorphisms with risk of posttraumatic stress disorder in adults. Molecular Psychiatry. 2009;14:234–235. doi: 10.1038/mp.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent NR, Amstadter AB, Koenen KC. Genetics of post-traumatic stress disorder: informing clinical conceptualizations and promoting future research. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2008;148:127–132. doi: 10.1002/ajmg.c.30169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roemer L, Litz BT, Orsillo SM, Ehlich PJ, Frieman MJ. Increases in retrospective accounts of war-zone exposure over time: The role of PTSD symptom severity. Journal of Traumatic Stress. 1998;11:597–605. doi: 10.1023/A:1024469116047. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Paulus MP, Fagerness JA, Purcell S, HYamaki LH, Hirshfeld-Becker D, et al. RGS2 influences anxiety-related temperament, personality and brain function. Archives of General Psychiatry. 2008;65:298–308. doi: 10.1001/archgenpsychiatry.2007.48. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Morgan CAI, Nicolaou AL, Charney DS. Consistency of memory for combat-related traumatic events in veterans of Operation Desert Storm. American Journal of Psychiatry. 1997;154:173–177. doi: 10.1176/ajp.154.2.173. [DOI] [PubMed] [Google Scholar]
- Stein MB, Jang KJ, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder: A twin study. American Journal of Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Risk, resilience, and gene x environment interactions in rhesus monkeys. Annals of the New York Academy of Science. 2006;1094:52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- True WJ, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Archives of General Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Bierer LM. Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. Journal of Psychiatric Research. 2001;35:261–270. doi: 10.1016/s0022-3956(01)00032-2. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Mulherin Engel S, Brand SR, Seckl J, Marcus SM, Berkowitz GS. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. The Journal of Clinical Endocrinology & Metabolism. 2005;90:4115–4118. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]