Abstract
Introduction
Mesenchymal stromal/stem cells (MSCs) have been used in a wide variety of preclinical experiments and in an increasing number of human clinical trials. Although many of these studies have shown different levels of engraftment, the exact fate of MSCs after transplantation and the tissue response to their engraftment has not been investigated in detail. In the present work we studied the distribution of human MSCs in a rat hind limb ischemic injury model immediately after transplantation and also analyzed the recipient tissue response to transplanted cells.
Methods
We tracked the in vivo fate of the transplanted MSCs utilizing bioluminescence imaging, fluorescence microscopy, and gene/protein expression analysis in rat hind limb ischemia model. We also monitored the viability of transplanted cells by graft vs. recipient expression analysis and determined the angiogenic and proliferative effect of transplantation by histological staining.
Results
According to imaging analysis only a small portion of cells persisted for extended period of time at the site of injury. Interestingly, recipient versus graft expression studies showed increased synthesis of rat-origin angiogenic factors and no human-origin mRNA or protein synthesis in transplanted tissues. More importantly, despite the lack of robust engraftment or growth factor secretion the transplantation procedure exerted a significant pro-angiogenic and pro-proliferative effect, which we showed was mediated by angiogenic and mitogenic signaling pathways.
Discussion
Our results show an immediate temporal tissue effect in response to MSC transplantation that may represent a novel indirect paracrine mechanism for the beneficial effects of cell transplantation observed in injured tissues.
Keywords: Angiogenesis, Mesenchymal stem cell, Proliferation, Signal transduction
Introduction
Mesenchymal stromal/stem cells (MSCs), originally isolated from bone marrow (BM) (1–4), have recently generated excitement due to their potential in regeneration of different tissues through a variety of mechanisms, apparent lack of immunogenecity, favorable immunomodulatory functions, and an impressive safety record in clinical trials (5–7). To be recognized as MSCs cells must meet the currently accepted criteria: they should have plastic-adherence growth properties; should express specific pattern of cell surface antigens, e.g. CD73, and CD90, and CD105; should not express certain other (e.g. hematoendothelial) markers such as CD34 or CD31; and importantly they should be able to differentiate into osteogenic, chondrogenic, and adipogenic lineages (8). We have recently derived MSCs from human embryonic stem cells (hESCs), and further showed that they have phenotypic and immunological characteristics very similar to BM-derived MSCs (9, 10). Although myogenic cells differentiated from hESC-derived MSCs(11) and chondrogenically-committed hESC-derived MSCs (12) have been transplanted into rodent models so far there is no report on the potential of MSCs derived from hESCs, without any further differentiation, in an in vivo model.
Of direct relevance to our study, adult tissue derived MSCs have been shown to contribute to recovery from ischemic injury in rodent hind limb ischemia model (13–17). However, in these studies the overall engraftment levels vary largely, which may be due to the tissue of origin of MSCs, the culture conditions used to isolate MSCs, the type of injury model used, and the degree of injury (18–21). Furthermore, several in vitro and in vivo studies have shown that injection of MSC culture conditioned medium containing MSC-derived growth factors and cytokines can decrease apoptosis, improve cardiac function, and increase angiogenesis pointing to the myriad of beneficial properties of MSCs irrespective of their engraftment potential (13, 22, 23). Thus, it is now generally accepted that there is no single property but a variety of mechanisms, including paracrine effects of transplanted cells, which can explain many of the favorable results reported.
In the current work we utilized hESC-derived MSCs and adult human BM-derived MSCs to track the distribution and physiological effect of MSCs immediately after local transplantation into rat hind limb ischemia model. We used bioluminescence in vivo imaging (BLI) analysis and fluorescence microscopy for tracking of the cells, studied the angiogenic and cell proliferative effect of transplanted cells, and investigated their potential in growth factor synthesis in injured tissue.
Methods
Virus production and hESC-derived MSC transduction
GFP or luciferase lentiviruses were produced by calcium-phosphate transfection in HEK293T using pWPXLd, psPAX2 and pMD2G plasmids (Addgene, MA, USA). hESC-derived MSCs were transduced with MOI 3 for GFP lentivirus and MOI 20 for Luciferase lentivirus. Cells were cultured for several days and washed before transplantation procedure. The presence of senescent cells was checked with Senescence β-Galactosidase Staining Kit (Cell Signaling, MA, USA) by counting an average of 300 cells from 3 different cell culture dishes. The ratio of blue to normal cells represents the percentage of senescence.
Animals and the surgical procedure
Five to six weeks old male Fisher 344 rats, 86–115 g weight (Harlan, Netherlands) (n=4 in each group) were used in this study. The animals were anesthetized with fentanyl-fluanisone (370 µg/100 g) (Janssen Pharmaceutica, Beerse, Belgium) and midazolame (180 µg/100 g) (Roche, Basel, Switzerland). Hind limb ischemic injury was induced by ligation of the femoral artery, lateral circumflex femoral artery, popliteal artery, and the saphenous artery. All animals received daily injections of Cyclosporin A (1 mg/100 g) (Fluka Biochemica, Buchs, Switzerland). Animals were transplanted with intramuscular injection of either 1 × 106 Luc+ hESC-derived MSCs and followed with BLI for 24 hours, or 0.5 × 106 GFP+ hESC-derived MSCs for immunohistochemistry analysis at 72-hour time point. The transplantation was done 24 hours after ligation of the vessels with 5 separate injections into femoral biceps muscle, semitendinous muscle, semimembranous muscle and adductor muscle. As controls we used non-injured animals, injured animals that were not transplanted, injured animals that received sonicated cell homogenate, injured animals that received primary BM-derived MSCs, and injured animals that received fixed hESC-derived MSCs. A small volume of cells was saved and plated into culture dishes at the time of transplantation to check the viability of MSCs. The experimental animal committee of the University of Turku approved all experimental procedures.
Sonication and fixation
The hESC-derived MSCs were disrupted by sonication with Labsonic U (B. BRAUN, Melsungen, Germany) at 20 kHz frequency for three seconds. Disrupted cells were injected into injured tissues (0.5 × 106 cells/rat in 150 µl volume) immediately after sonication. A small aliquot of the homogenate was plated into culture dishes to check the viability of the cells. Approximately 0.01% of cells were able to attach the culture dishes and to continue growth. Cells were fixed with 4% paraformaldehyde/PBS 20 minutes at RT.
In vivo bioluminescent imaging
Due to limited sensitivity of bioluminescent imaging (BLI) (Xenogen, MA, USA) we used 1×106 cells to improve the tracking of the graft. The animals were imaged immediately after transplantation, 6 hours after transplantation, and 24 hours after transplantation. D-luciferin (Synchem, Felsberg, Germany) was administered intraperitoneally (15 mg/100 g of weight) 10 minutes prior to imaging.
Histology
The muscles were frozen in 2-methylbutane, embedded in optimal cutting temperature compound (OCT) (Tissue-Tek, CA, USA), and cut into 10 µm sections. The sections were stained with von Willebrand Factor (Abcam, Cambridge, UK), CD68 (Serotec, Oxford, UK), Ki67 (DakoCytomation, Glostrup, Denmark), and CD3 (Abcam). In Ki67 staining only muscle cell associated positive nuclei were counted. All hematopoietic cells were excluded from the analysis.
Genomic-PCR, RT-PCR, and quantitative RT-PCR
For genomic PCR and expression analysis we used 1 × 106 cells or frozen tissue samples. Human Alu primers, forward: CAT GGT GAA ACC CCG TCT CTA, reverse: GCC TCA GCC TCC CGA GTA G, were used for both RT-PCR and genomic PCR with annealing temperature of 60 °C. Species specific VEGF-primers were: human VEGF-A forward: TCC GGG TTT TAT CCC TCT TC, reverse: TCT GCT GGT TTC CAA AAT CC, rat VEGF-A forward: CAA TGA TGA AGC CCT GGA GT, reverse: TTT TTC GCG CTT TCG TTT TT, human VEGF-D forward: CGG CAT ACG TTG GAG AGA TT, reverse: ATC TTA GGG GTG GGG AGA GA, rat VEGF-D forward: ATT ATT TGT GCA GCG GGA AA, reverse: GGC ATT CTC CAG AAG CAA AG. Species specific PDGFR-β primers were: human forward: GTG AAC GCA GTG CAG ACT GT, reverse: AGG TGT AGG TCC CCG AGT CT rat forward: GGA GCT TCA CAG AGG ACT GG, reverse: GAT CTG GGT GCC ATG AGA GT. Primers recognizing both human and rat sequences were: VEGF-A forward: GGA CAT CTT CCA GGA GTA, reverse: TGC AAC GCG AGT CTG TGT, VEGF-D forward: GTT GCA ATG AAG AGA GCC TT, reverse: TCC CAT AGC ATG TCA ATA GG. Primers for β-actin were: human forward: TGC GTG ACA TTA AGG AGA AG, and reverse: GCT CGT AGC TCT TCT CCA, rat forward: TCG TGC GTG ACA TTA AGG AG, and reverse: GTC AGG CAG CTC GTA GCT CT. The annealing temperature for VEGF-A and rat VEGF-D was 55 °C, for human VEGF-D 58 °C, and PDGFR-β 55 °C.
Western blotting
Tissue samples or cells were homogenized in lysis buffer (50 mmol/L HEPES pH 7.5, 150 mmol/L NaCl2, 10% glycerol, 1% Triton X-100, 1 mmol/L EGTA, 1.5 mmol/L MgCl2, 10 mmol/L NaF, 10 mmol/L sodium pyrophosphate, 1 mmol/L Na3VO4, 10 µg approtinin/ml, 10 µg leupeptin/ml) (Sigma). Antibodies were human PDGFR-β (Santa Cruz, CA, USA) and α-tubulin (Sigma), 44/42 MAPK (Cell Signaling, MA, USA), p44/42 MAPK (Cell Signaling), Akt (Cell Signaling), pAkt (Cell Signaling).
Statistical analysis
Statistical analyses were done with t-test for means of three different experiments.
Results
hESC-derived MSCs reach senescence in vitro
The hESC-derived MSCs used in the study (9, 10) were readily transducible with GFP-or luciferase lentiviral vectors at passage 8, and sustained normal growth for several passages after lentiviral transduction showing no or a very low amount of senescent cells. Under culture conditions used in these experiments the cells assumed stagnant growth after 16 passages with 56% senescence level as compared to 3% senescence at the passage 8 (Fig. 1D–F).
Figure 1.
Labeling and imaging of transplanted hESC-derived MSCs. (A) Phase-contrast, (B) Fluorescent microscope, and (C) BLI images of lentivirally transduced passage 8 hESC-derived MSCs. Senescence staining of MSCs; (D) Control MSCs, (E) passage 8 MSCs, (E) passage 16 cells. At passage 16 the cells obtained senescent phenotype as shown by x-gal staining of endogenous β-galactosidase. (G–J) The decrease of the relative luminescent signal intensity (p/sec/cm2/sr) observed in animals (H) 0 hours, (I) 6 hours, and (J) 24 hours post-transplantation. (K) Fluorescent microscopy analysis for GFP+ cells at 72-hour time point (white arrows). (L) Genomic AluI PCR (upper panel) and RT-PCR (lower panel) from transplanted tissues. Samples: (1, 5) hESC-derived MSCs, (2, 6) Transplanted tissue, (3, 7) Non-transplanted tissue, (4, 8) Negative control. 10× magnification.
In vivo tracking of transplanted stem cells
We investigated the survival and distribution of hESC-derived MSCs after local delivery utilizing in vivo bioluminescent imaging (BLI), fluorescence microscopy, and PCR analysis. Prior to transplantation luciferase lentivirus transduced cells emitted 1.5439 × 1010 p/sec/cm2/sr photon flux as measured by BLI (Fig. 1C). Subsequent to their transplantation into hind limb ischemia the BLI imaging showed decreased signal intensity measurement from the initial 100% (3.6694 × 106 p/sec/cm2/sr) by 71% (1.0352 × 106 p/sec/cm2/sr) in 6 hours, and by 98.5% (0.055 × 106 p/sec/cm2/sr) in 24 hours (Fig. 1G–J). To confirm the data obtained by BLI hESC-derived MSCs were transduced with GFP lentivirus for fluorescence microscopy analysis. After 72-hour follow-up we were able to detect single GFP+ cells scattered randomly throughout the tissue (Fig. 1K), though mostly present near the damage site caused by the injecting needle. The GFP+ cells did not form colonies that could have indicated cell proliferation process in the transplanted hESC-derived MSCs. The presence of human cells was confirmed by genomic PCR for AluI, while AluI RT-PCR showed that the transplanted cells were alive and able to continue the mRNA synthesis (Fig. 1L). The 30-cycle RT-PCR amplification yielded only a weak signal from pooled transplanted tissues supporting the data from BLI imaging and fluorescence microscopy analysis.
Transplantation related inflammation
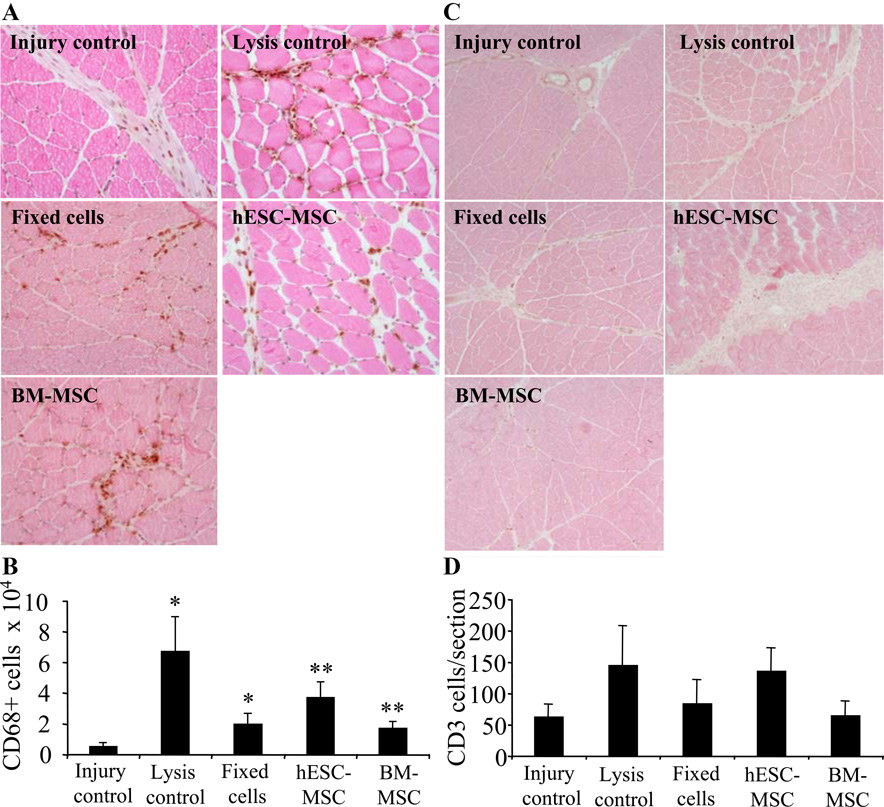
Since in xenograft models T-lymphocyte mediated reactions may affect the fate of transplanted cells we used Cyclosporine A, which is widely used to block recipient T-lymphocytes’ reactions against transplanted cells, but has, however, minor effect on granulocyte or macrophage migration. Since hind limb model is characterized by leukocyte, especially macrophage, migration (24, 25) we next determined the inflammatory cell migration into the injury region by counting CD68+ macrophages and CD3+ T-cells at 72-hour time point and normalized the data against the section size. The CD68+ staining showed significantly increased inflammation in tissues transplanted with hESC-derived MSCs and BM-derived MSCs (p<0.001) as compared to injury controls. Also injection of sonicated cells and fixed MSCs increased macrophage migration (p<0.01) showing increased inflammatory reaction regardless of transplanted material (Fig. 2A,B). Even though lymphocyte infiltration normally occurs later than macrophage and granulocyte migration, and is diminished by cyclosporine A, we analyzed the number of T cells to further study the effect of immune response on observed stem cell clearance. CD3+ staining of T-cells did not show significant differences between groups (Fig. 2C,D).
Figure 2.
Inflammatory cell accumulation into injury region at 72-hour time point. (A) CD68+ macrophage staining and (B) histogram. Both injection of sonicated cells and transplantation of intact stem cells significantly increased the CD68+ inflammatory cell migration into the damaged tissue. (C,D) CD3+ lymphocyte staining showed no differences between groups. 10× magnification.
Transplantation related angiogenesis and proliferation
Previous reports have suggested increased tissue recovery caused by stem cell transplantation (16, 26) could be due to growth factor and cytokine secretion from the graft or engraftment of the transplanted cells into the target tissue. To determine the tissue response on transplantation we studied the angiogenesis and cell proliferation at 3-day and 9-day time points by analyzing the whole tissue sections to cover areas the transplanted cells could have migrated from the original transplantation site (Fig. 3). We found that hESC-derived MSC transplantation increased new vessel formation by 32% (311±30 capillaries/mm2, p<0.01) as compared to injury controls (210±28 capillaries/mm2) and by 23% (p<0.05) as compared to sonicated lysed cell injections (237±43 capillaries/mm2). BM-derived MSC control transplantation had a similar effect as hESC-derived MSCs resulting significantly increased angiogenesis (309±22 capillaries/mm2, p<0.05) as compared to injury control. Interestingly, transplantation of paraformaldehyde fixed hESC-derived MSC controls showed improved vascularization at a level similar to intact cells (288±12 capillaries/mm2, p<0.05 as compared to injury control) (Fig. 3A,B). At 9-day time point the corresponding values were injury control (367±10), lysis control (417±11), fixed cells (.430±17), hESC-derived MSCs (395±14), and BM-derived MSCs (385±24) showing no significance differences between groups (Fig 3C).
Figure 3.
Proangiogenic effect induced by MSCs. Histological staining of 3-day tissues and histograms of 3-day and 9-day analysis (A–C). Analysis of capillary formation showed that transplantation of intact MSCs induced new vessel formation/mm2 significantly 72 hours after transplantation. The 9-day time point analysis showed no differences between groups. 10x magnification. Proproliferative effect induced by MSCs. Histological staining of 3-day tissues and histograms of 3-day and 9-day analysis (D–F). Staining for proliferating nuclei (arrows) showed significantly increased number of Ki67 positive nuclei associated with muscle cells at 72-hour time point. No significant differences were seen at 9-day time point. 20× magnification.
To confirm the recipient tissue response to transplantation we investigated cell proliferation, which is another important parameter in ischemic injury recovery. Ki67 staining analysis was in line with angiogenic analysis showing 8-fold increased proliferation at 3-day time point hESC-derived MSC transplanted muscles (38±2.6 p<0.001) and 5-fold increased proliferation in BM-derived MSC transplanted rat hind limbs (21±3.4 p<0.05) as compared to injury controls (4.7±0.6). The analysis of Ki67 positive nuclei in lysis control (15±4.1) was non-significant (p=0.09) but did reach statistically significant level in fixed cell transplantation (21±4.2 p<0.05). At 9-day time point Ki67 staining showed no differences in proliferation between groups being in line with the analysis of new vessel formation (Fig 3D–F).
Recipient versus graft expression analysis
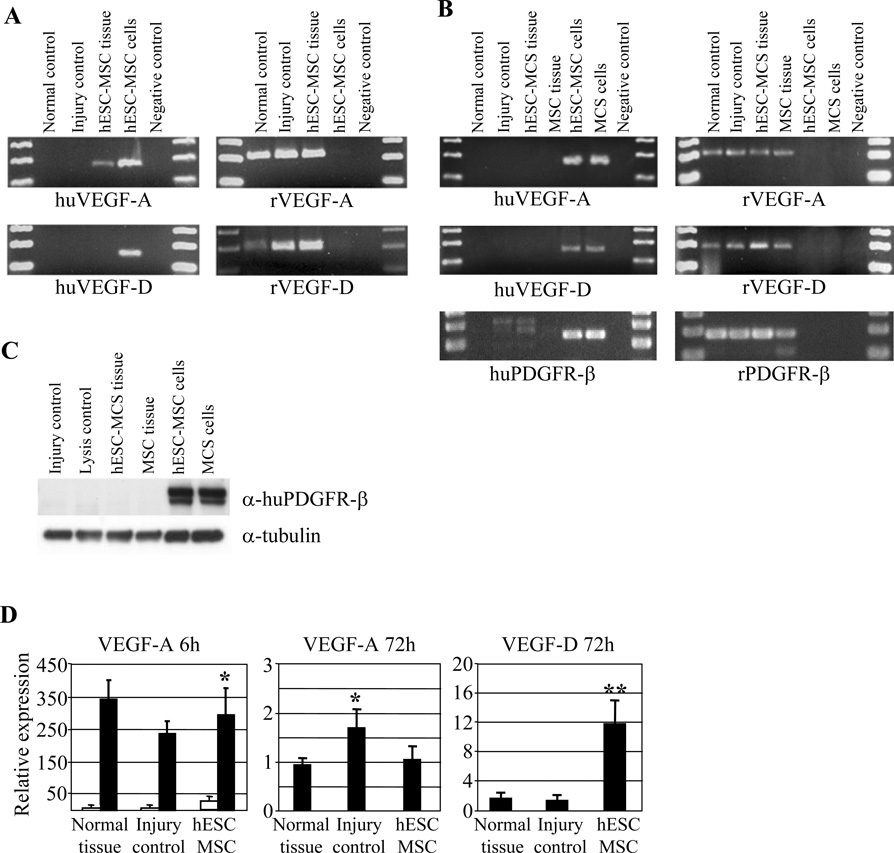
Since VEGF proteins play an essential role in tissue angiogenesis (27) and in cell proliferation (28) we next investigated the level of expression of VEGF-A and VEGF-D in transplanted tissues. RT-PCR analysis from 6-hour tissue samples showed rat VEGF-A amplification from normal, injured control, and hESC-derived MSC transplanted tissues, whereas human specific primers showed VEGF-A amplification only from the transplanted tissues in vivo and hESC-derived MSC positive control cells in vitro (Fig. 4A). At 72-hour time point the human VEGF-A and VEGF-D amplification was detectable only from hESC-derived MSC and BM-derived MSC positive control cells culture in vitro whereas rat specific primers showed clear mRNA synthesis from rat control and transplanted tissues. The data was further confirmed by PDGFR-β RT-PCR showing the same amplification pattern as VEGF-A and VEGF-D suggesting no human originated mRNA in transplanted tissues. However, amplification of VEGF-A, VEGF-D, and PDGFR-β from hESC-derived MSC and BM-MSC cells in vitro suggests a potential role for these molecules in promoting injury recovery (Fig. 4B). The RT-PCR data was further confirmed by western blotting using α-human PDGFR-β, which showed protein expression only from in vitro cultures hESC-derived MSCs and BM-derived MSCs (Fig. 4C).
Figure 4.
(A) RT-PCR showing species-specific VEGF-A and VEGF-D amplification at 6-hour time point. Human specific primers show hESC-derived MSC-originated VEGF-A amplification in transplanted tissues and in positive cell controls. (B) RT-PCR analysis at 72-hour time point. VEGF-A, VEGF-D, and PDGFR-β amplification were seen only with rat specific primers in transplanted tissues. (C) Western blot analysis for human PDGFR-β for 72-hour tissue homogenates. Only control MSC cells yielded a positive staining. α-Tubulin was used to normalize the samples. (D) Quantitative RT-PCR shows that majority of VEGF-A at 6-hour time point was synthesized by rat specific primers. White bars represent human specific amplification and black bars rat specific amplification. Quantification of the VEGF-A and VEGF-D production at 72-hour time point with primers recognizing both rat and human sequences show increased VEGF-D expression in hESC-derived MSC animals as compared to injured control group.
To determine more thoroughly the graft versus host angiogenic growth factor expression we analyzed human versus rat VEGF-A expression at 6-hour time point by quantitative RT-PCR, which suggested markedly higher expression of VEGF-A (p<0.05) from the recipient as compared to the graft (Fig 4D). Since conventional RT-PCR from 72-hour time point showed no human VEGF-A or VEGF-D mRNA expression and since especially VEGF-A is expressed in different splice variants (29) we used more general primers common to human and rat sequences to quantify the overall expression of VEGF-A and VEGF-D. The analysis resulted in significantly increased rat-originated VEGF-D expression in animals that had received hESC-derived MSC treatment (p<0.01). Differently, rat VEGF-A expression increased in injured tissues (p<0.05) but was not influenced by transplantation (Fig. 4D).
Transplantation activates mitogenic and angiogenic signal transduction
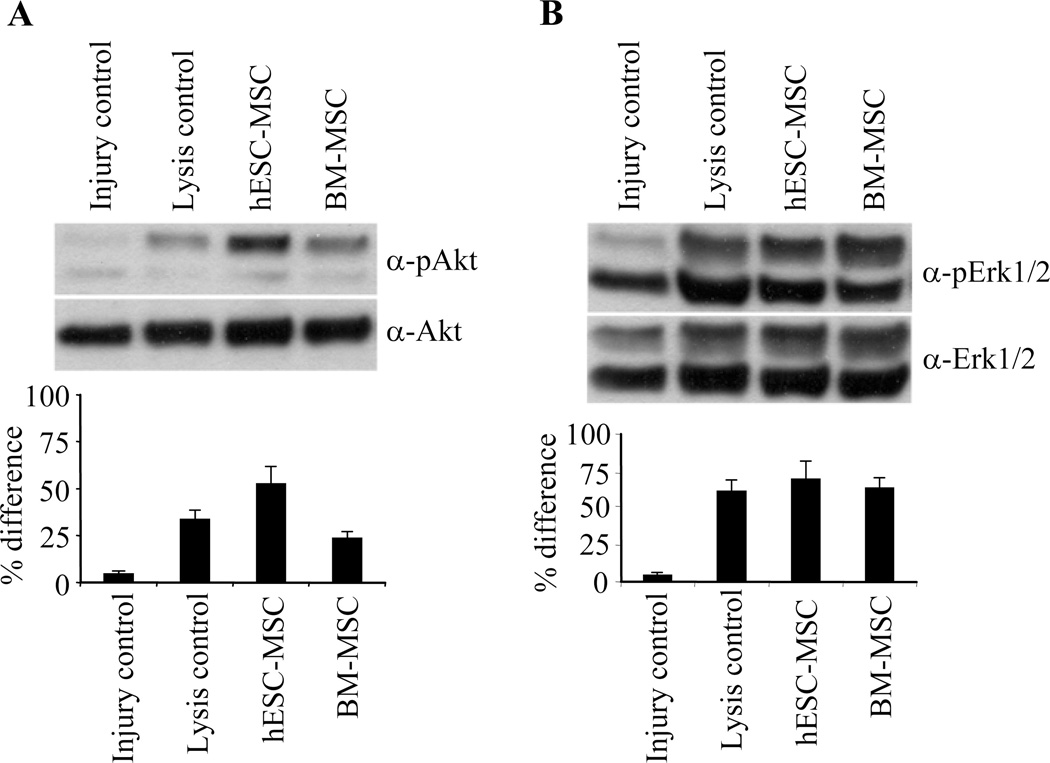
Finally, we analyzed the activation of signal transduction pathways related to angiogenesis and cell proliferation to further confirm the effect of stem cell transplantation in injury recovery. Both hESC-derived MSC and BM-derived MSC transplantation increased phosphorylation of Erk1/2 and Akt confirming the data that transplantation of MSCs potentially lead to angiogenic and mitogenic response in the recipient tissue (Fig 5).
Figure 5.
Western blot analysis for transplanted tissues from 72-hour time point showed markedly increased Erk 1/2 and Akt phosphorylation after injection of sonicated cells and MSCs. Histograms show the relative increase in intensities.
Discussion
MSCs have been extensively studied for cardiovascular repair and have been shown to attenuate the ischemia induced myocardial injury through a variety of mechanisms such as differentiation to cells having cardiomyocyte-like phenotype and increasing angiogenesis (30, 31). In a recent study it was shown that human MSC transplantation increased blood flow in murine hind limb ischemia model 24 hours after transplantation; however, the amount of cells that survived for 2 weeks was only 0.2% (32). hESCs could be differentiated into highly pure populations of MSCs with immunophenotypic and functional characteristics very similar to BM-derived MSCs(10). Due to lack of HLA-matching requirement for use of MSCs in patients(33) hESC could potentially provide an unlimited source of cells for clinical applications without the need for invasive procedures such as bone marrow aspiration, which is needed to obtain cells from adult donors. hESC-derived MSCs could be specially useful for enhancement of engraftment of other cells/tissues derived from the same hESC lines(34). In the present work we specifically focused on the immediate early distribution of hESC-derived MSCs and determined the impact of transplantation on rat hind limb ischemia.
Since the migration and biodistribution of stem cells shortly after transplantation is poorly characterized we focused on tracking the homing and engraftment of the transplanted stem cells into the injured tissue by BLI. Our study showed persistence of a small number of transplanted cells (1.5%) 72 hours after transplantation, which corresponds to approximately 1–2 × 104 cells out of the 1 × 106 initially transplanted MSCs. Even though the estimation of the number of the cells based on BLI signal is not accurate due to several reasons, e.g. size of the cells and nucleus-cytoplasm ratio, the decreased signal observed suggests rapid degradation or migration of the cells from the transplantation site. We estimated, based on our data considering the size of the cells, that approximately 1 × 103–1 × 104 cells at the same tissue location are needed to yield a reliably detectable signal. Monitoring the whole animal with BLI did not show signal elsewhere in the body suggesting clearance of the graft locally at the injury site with no large-scale migration to other tissues (Fig. 1G–J). The low engraftment at the site of injury was confirmed by GFP fluorescence microscopy 72 hours after transplantation, which showed only a few single cells scattered randomly in the proximity of injury region (Fig. 1K). The presence of single cells instead of groups of GFP+ cells suggested that the cells were not proliferating but rather were dormant or dying locally. The low abundance of the transplanted cells was further verified by genomic and RT-PCR for human specific AluI sequences that yielded comparably weak signals (Fig. 1L).
To study the biological effect of the transplantation procedure we analyzed the neovascularization and mitogenic response 72 hours after transplantation and detected significantly increased angiogenesis and cell proliferation in animals treated with hESC-derived MSCs and BM-derived MSCs as compared to control injury animals. Since the differentiation of transplanted stem cells into vascular or vascular supporting cells is expected to last longer than 72 hours the transplantation may have had an indirect supporting effect on new vessel formation. The indirect effect could also elucidate the presence of increased number of Ki67 positive proliferative muscle associated nuclei in injured regions. Even though MSCs have been shown to be able to escape immunodefence system (34–36) we cannot rule out the possibility that the local clearance of the transplanted cells could be due to increased inflammatory cell migration in response to stem cell transplantation into the injury region (Fig. 2). Granulocytes and macrophages are among the first inflammatory cells that migrate into damaged tissues as a natural defense mechanism in response to injury to protect the tissue from invading exogenous particles and their action might at least partly explain the rapid clearance of the graft. Macrophages are also essential mediators of tissue response to injury. Recently, it has been shown that inflammation is an important factor in the induction of angiogenesis and disruption of inflammatory cell-derived oxidative burst has been shown to impair angiogenesis (37). Even though inflammatory cells, especially macrophages, are known to secrete different growth factors increased inflammation alone, however, may not explain the observed angiogenesis or proliferation in our work, since both alive and sonicated stem cell injections increased inflammation whereas only the intact cells resulted in increased new vessel formation and cell proliferation. In addition, analysis of T-cell migration showed no differences between groups suggesting that the angiogenic and proliferative effect was not solely mediated by immune system.
Several studies have implicated VEGFs to have a role in vascular development after mesenchymal stromal cell transplantation (13, 22, 23). Furthermore, it has been recently shown that conditioned MSC culture media induced VEGF-A production apparently through VEGF receptor-PI3K-Akt pathway, and activated vascular tube formation in vitro in human aortic endothelial cells. VEGF receptor blocking antibody had a negative effect on tube formation but VEGF-A blocking antibody did not inhibit the angiogenic effect in cultured cells, further suggesting other factors than VEGF-A to mediate human aortic endothelial cell activation (38, 39).
Species-specific quantitative and qualitative detection of VEGF-A 6 hours after transplantation suggested that grafted cells were only a minor source for VEGF expression, which then became undetectable at 72-hour time point. Interestingly, the VEGF-D expression was solely originated from recipient rat cells both at 6-hour and 72-hour time points suggesting a major role for the recipient tissue in the synthesis of growth factors after transplantation of hESC-derived MSCs. VEGF-D has been shown to be a more potent angiogenic growth factor compared to VEGF-A, which alone cannot support new vessel formation (40, 41). The data was further confirmed by PDGFR-β RT-PCR analysis and western blotting supporting the rapid clearance of human originated cells. Increased host VEGF-D mRNA synthesis could at least partially explain the increased angiogenesis after transplantation in our model.
In several recent publications it has been shown that different MSC populations are secreting angiogenic growth factors in vitro providing indirect evidence for a paracrine effect of the graft in vivo (13, 22, 23). Our data shows that hESC-derived MSCs are capable of secreting VEGF-A growth factor only for a short period of time immediately after transplantation at levels markedly lower than the recipient tissues. Since the number of transplanted MSCs is dramatically decreased within hours after transplantation and VEGF growth factor secretion levels from the graft are low, it can be argued that other paracrine effects may play a role in the increased VEGF-D and angiogenic response. This is further supported by the increased angiogenesis and cell proliferation observed after transplantation of fixed MSCs, unable to metabolize or secrete growth factors, suggesting a potential role for direct physical MSC-tissue contact in promoting the transplantation response, which lead to increased PI3K-Akt and Erk phosphorylation. Both of these pathways are known to increase VEGF expression causing either increased angiogenesis or cell proliferation and migration (27, 28, 42). The immediate physical contact response in recipient tissue is supported by the temporal angiogenic and proliferative effect observed after transplantation and could potentially represent a novel mechanism for MSCs to mediate tissue response.
In conclusion, we have shown that after local transplantation of MSCs into hind limb ischemic injury model a small portion of the cells remain alive after 24 hours. Interestingly, despite the low level of engraftment the transplanted MSC cells were able to increase angiogenic and cell proliferative response by activating the synthesis of growth factors originated from host tissues. To our knowledge the current work is the first to show that increased new vessel formation and cell proliferation observed after MSC transplantation is not due to secretion of growth factors from the grafted MSCs but due to secretion of such factors from local recipient tissues. We believe this observation provides a novel paradigm in explaining the observed effects of transplanted MSCs in different experimental/pre-clinical/clinical models. Even though the exact mechanism mediating the observed effects requires further investigations we suggest that the parallel contribution of immune response together with the direct physical contact between the grafted cells and the recipient tissue could explain the activation of PI3K-Akt and Erk1/2 pathways leading to angiogenic and proliferative response.
Acknowledgements
The research was supported by grants from the Academy of Finland, Sigrid Juselius Foundation, Paolo Foundation and University of Turku Foundation.
Abbreviations
- MSC
mesenchymal stem cell
- BM
bone marrow
- hESC
human embryonic stem cell
- BLI
bioluminescence imaging
- VEGF
vascular endothelial growth factor
- PDGFR
platelet derived growth factor receptor
Footnotes
Declaration of interests: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32(5):414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow.Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–247. [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Porada CD, Zanjani ED, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1(3):365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- 5.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211(1):27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 6.Picinich SC, Mishra PJ, Mishra PJ, Glod J, Banerjee D. The therapeutic potential of mesenchymal stem cells. Cell- & tissue-based therapy. Expert Opin Biol Ther. 2007;7(7):965–973. doi: 10.1517/14712598.7.7.965. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 8.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 9.Trivedi P, Hematti P. Simultaneous generation of CD34+ primitive hematopoietic cells and CD73+ mesenchymal stem cells from human embryonic stem cells cocultured with murine OP9 stromal cells. Exp Hematol. 2007;35(1):146–154. doi: 10.1016/j.exphem.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36(3):350–359. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med. 2007;13(5):642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 12.Hwang NS, Varghese S, Lee HJ, Zhang Z, Ye Z, Bae J, et al. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A. 2008;105(52):20641–20646. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 14.Koponen JK, Kekarainen T, S EH, Laitinen A, Nystedt J, Laine J, et al. Umbilical Cord Blood-derived Progenitor Cells Enhance Muscle Regeneration in Mouse Hindlimb Ischemia Model. Mol Ther. 2007 doi: 10.1038/sj.mt.6300302. [DOI] [PubMed] [Google Scholar]
- 15.Kim SW, Han H, Chae GT, Lee SH, Bo S, Yoon JH, et al. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger's disease and ischemic limb disease animal model. Stem Cells. 2006;24(6):1620–1626. doi: 10.1634/stemcells.2005-0365. [DOI] [PubMed] [Google Scholar]
- 16.Ishikane S, Ohnishi S, Yamahara K, Sada M, Harada K, Mishima K, et al. Allogeneic injection of fetal membrane-derived mesenchymal stem cells induces therapeutic angiogenesis in a rat model of hind limb ischemia. Stem Cells. 2008;26(10):2625–2633. doi: 10.1634/stemcells.2008-0236. [DOI] [PubMed] [Google Scholar]
- 17.Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66(3):543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Muller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41(5):876–884. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Song P, Tang Y, Zhang XL, Zhao SH, Wei YJ, et al. Injection of bone marrow mesenchymal stem cells in the borderline area of infarcted myocardium: heart status and cell distribution. J Thorac Cardiovasc Surg. 2007;134(5):1234–1240. doi: 10.1016/j.jtcvs.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Iop L, Chiavegato A, Callegari A, Bollini S, Piccoli M, Pozzobon M, et al. Different cardiovascular potential of adult- and fetal-type mesenchymal stem cells in a rat model of heart cryoinjury. Cell Transplant. 2008;17(6):679–694. doi: 10.3727/096368908786092739. [DOI] [PubMed] [Google Scholar]
- 21.Feng SW, Lu XL, Liu ZS, Zhang YN, Liu TY, Li JL, et al. Dynamic distribution of bone marrow-derived mesenchymal stromal cells and change of pathology after infusing into mdx mice. Cytotherapy. 2008;10(3):254–264. doi: 10.1080/14653240802020381. [DOI] [PubMed] [Google Scholar]
- 22.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20(6):661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 23.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112(8):1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 24.Egami K, Murohara T, Aoki M, Matsuishi T. Ischemia-induced angiogenesis: role of inflammatory response mediated by P-selectin. J Leukoc Biol. 2006;79(5):971–976. doi: 10.1189/jlb.0805448. [DOI] [PubMed] [Google Scholar]
- 25.Shireman PK. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg. 2007;45(Suppl A):A48–A56. doi: 10.1016/j.jvs.2007.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du YY, Zhou SH, Zhou T, Su H, Pan HW, Du WH, et al. Immuno-inflammatory regulation effect of mesenchymal stem cell transplantation in a rat model of myocardial infarction. Cytotherapy. 2008;10(5):469–478. doi: 10.1080/14653240802129893. [DOI] [PubMed] [Google Scholar]
- 27.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84(5):1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, et al. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord. 1999;9(2):72–80. doi: 10.1016/s0960-8966(98)00111-4. [DOI] [PubMed] [Google Scholar]
- 29.Ballaun C, Weninger W, Uthman A, Weich H, Tschachler E. Human keratinocytes express the three major splice forms of vascular endothelial growth factor. J Invest Dermatol. 1995;104(1):7–10. doi: 10.1111/1523-1747.ep12613450. [DOI] [PubMed] [Google Scholar]
- 30.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz E, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy. 2008;10(8):771–774. doi: 10.1080/14653240802618085. [DOI] [PubMed] [Google Scholar]
- 32.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic Preconditioning Results in Increased Motility and Improved Therapeutic Potential of Human Mesenchymal Stem Cells. Stem Cells. 2008 doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 34.Hematti P. Role of mesenchymal stromal cells in solid organ transplantation. Transplant Rev (Orlando) 2008;22(4):262–273. doi: 10.1016/j.trre.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niemeyer P, Vohrer J, Schmal H, Kasten P, Fellenberg J, Suedkamp N, et al. Survival of human mesenchymal stromal cells from bone marrow and adipose tissue after xenogenic transplantation in immunocompetent mice. Cytotherapy. 2008:1–12. doi: 10.1080/14653240802419302. [DOI] [PubMed] [Google Scholar]
- 36.Chang C, Niu D, Zhou H, Zhang Y, Li F, Gong F. Mesenchymal stromal cells improve hyperglycemia and insufficient insulin upon diabetic pancreatic microenvironment in pigs. Cytotherapy. 2008:1–10. doi: 10.1080/14653240802461924. [DOI] [PubMed] [Google Scholar]
- 37.Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111(18):2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 38.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25(9):2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 39.Kasper G, Dankert N, Tuischer J, Hoeft M, Gaber T, Glaeser JD, et al. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25(4):903–910. doi: 10.1634/stemcells.2006-0432. [DOI] [PubMed] [Google Scholar]
- 40.Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, et al. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92(10):1098–1106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- 41.Milkiewicz M, Hudlicka O, Shiner R, Egginton S, Brown MD. Vascular endothelial growth factor mRNA and protein do not change in parallel during non-inflammatory skeletal muscle ischaemia in rat. J Physiol. 2006;577(Pt 2):671–678. doi: 10.1113/jphysiol.2006.113357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Angelo G, Struman I, Martial J, Weiner RI. Activation of mitogen-activated protein kinases by vascular endothelial growth factor and basic fibroblast growth factor in capillary endothelial cells is inhibited by the antiangiogenic factor 16-kDa N-terminal fragment of prolactin. Proc Natl Acad Sci U S A. 1995;92(14):6374–6378. doi: 10.1073/pnas.92.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]