Abstract
Despite abundant evidence that aberrant Rho-family GTPase activation contributes to most steps of cancer initiation and progression, there is a dearth of inhibitors of their effectors (e.g., p21-activated kinases). Through high-throughput screening and structure-based design, we identify PF-3758309, a potent (Kd = 2.7 nM), ATP-competitive, pyrrolopyrazole inhibitor of PAK4. In cells, PF-3758309 inhibits phosphorylation of the PAK4 substrate GEF-H1 (IC50 = 1.3 nM) and anchorage-independent growth of a panel of tumor cell lines (IC50 = 4.7 ± 3 nM). The molecular underpinnings of PF-3758309 biological effects were characterized using an integration of traditional and emerging technologies. Crystallographic characterization of the PF-3758309/PAK4 complex defined determinants of potency and kinase selectivity. Global high-content cellular analysis confirms that PF-3758309 modulates known PAK4-dependent signaling nodes and identifies unexpected links to additional pathways (e.g., p53). In tumor models, PF-3758309 inhibits PAK4-dependent pathways in proteomic studies and regulates functional activities related to cell proliferation and survival. PF-3758309 blocks the growth of multiple human tumor xenografts, with a plasma EC50 value of 0.4 nM in the most sensitive model. This study defines PAK4-related pathways, provides additional support for PAK4 as a therapeutic target with a unique combination of functions (apoptotic, cytoskeletal, cell-cycle), and identifies a potent, orally available small-molecule PAK inhibitor with significant promise for the treatment of human cancers.
Keywords: chemical biology, high-content screening, p53, signaling signature
Although many components of oncogenic signaling have been identified, the current clinically effective signal transduction therapeutics are primarily confined to a subset of targets such as receptor tyrosine kinases (1). In the search for new therapeutics, whole regions of signaling space have gone underexplored despite the mounting evidence of their essential roles. One such area is Rho GTPase signaling, which has been hard to directly target (2) despite the extensive experimental evidence of its role in most aspects of cancer initiation and progression including uncontrolled proliferation, evasion of apoptosis, actin cytoskeletal remodeling, invasion, and metastasis (3–8). Members of the Rho-family GTPases (e.g., cdc42) in the activated, GTP-bound state bind directly to p21-activated kinases (PAKs) through an amino-terminal p21 binding domain to affect kinase activation and signaling (9–12). The PAKs are members of the STE20 serine/threonine kinase family (13) with two subgroups: PAK1, PAK2, and PAK3 (group A) and PAK4, PAK5, and PAK6 (group B). These kinases have been shown to have major yet varied roles in oncogenic processes (9–12, 14). Active PAK4 (15–18) is required for Ras-driven NIH 3T3 cell transformation to facilitate anchorage-independent growth (19, 20) and affects profound cell morphological and cytoskeletal effects (20, 21). Expression of activated PAK4 in NIH 3T3 cells is tumorigenic in nude mice, with tumors having less apoptosis (activated caspase 3 staining) and increased proliferation relative to control tumors (22). Deletion of PAK4 has the opposite functional and biochemical effects. PAK4−/− mouse embryos have an increase in apoptosis (active caspase 3, sub-G0 population) and decreased proliferation (Ki67 staining) (23). PAK4−/− mouse embryonic fibroblast cells significantly attenuate oncogenic Ras-driven tumorigenesis and tumor growth (22), whereas PAK4 knockout cells completely abrogate tumor formation by an oncogenic variant of the direct upstream GTPase (cdc42V12) (22). In HeLa tumor cells, expression of active PAK4 blocks TNFα-induced apoptosis (PARP cleavage, caspase 3) (24), whereas siRNA PAK4 knockdown sensitizes tumor cells to TNFα-induced apoptosis and blocks anchorage-independent growth (25). A limitation of PAK4 siRNA knockdown is that PAK4 has been reported to have functions dependent on and independent of kinase activity (22, 26). A complementary approach, expression of kinase-inactive PAK4, demonstrated that anchorage-independent growth of HCT116 tumor cells requires PAK4 kinase activity (19). The role of PAK4 in cancer is further supported by studies that show elevated PAK4 activity in a broad range of human tumor lines (19), activated in archived primary tumor tissues (19), with a PAK4 locus present on an amplicon associated with colorectal and pancreatic cancers (27–30). PAK4 substrates identified to date are consistent with its observed cellular functions: Rho GTPase activator guanine nucleotide exchange factor-H1 (GEF-H1) (21), apoptotic regulatory protein BAD (24), and cytoskeletal effector protein LIMK (31).
Taken together, previous data identified some of the determinants of PAK4 biology, and also indicated that a drug discovery effort focused on this unique drug target could yield a potentially unique and useful therapeutic. However, most details regarding PAK4 biology, including the nature of pathways controlling tumor cell growth and survival, are largely unknown, and no potent inhibitors of PAK-family kinases have been reported. We therefore set out to develop and validate potent and selective PAK4 inhibitors, with the dual goals of better understanding PAK4 biology and providing a novel therapeutic option for tumor types with a dysregulated PAK4-signaling axis.
Results
Discovery and Characterization of PF-3758309 as a PAK Inhibitor.
The small-molecule pyrrolopyrazole inhibitor PF-3758309 was rationally designed through a structure-based design approach from multiple distinct chemical series identified in separate screening campaigns, including the assessment of over 1.3 million unique compounds in one screening campaign. PF-3758309 was kinetically determined to be a potent, ATP-competitive inhibitor in a PAK4-kinase domain biochemical assay (Ki = 18.7 ± 6.6 nM; Fig. S1). Because the peptide substrate used in the enzymatic assay may alter the PAK4 active site conformation, two direct binding technologies were used to characterize binding of PF-3758309 to PAK4. Isothermal calorimetric analysis determined the PF-3758309 equilibrium dissociation constant (Kd) of 2.7 ± 0.3 nM, a binding stoichiometry of 0.91 ± 0.08, and an enthalpy change (ΔH) of −5.0 ± 0.1 kcal/mol (Fig. S1). Thus, the binding free energy (ΔG = −11.7 kcal/mol) results from equal contributions from enthalpy and entropy (TΔS = 6.7 kcal/mol) of binding. A surface plasmon resonance study determined the dissociation constant of PF-3758309 with PAK4 to be 4.5 ± 0.07 nM, with the off-rate koff = 0.010 s−1 (t1/2 = 68 s) (Fig. S1). As such, PF-3758309 binds directly to PAK4 with an in vitro potency of 2.7–4.5 nM. Using peptide substrates, PF-3758309 had similar enzymatic potency against the kinase domains of the other group B PAKs (PAK5, Ki = 18.1 ± 5.1 nM; PAK6, Ki = 17.1 ± 5.3 nM) and group A PAK1 (Ki = 13.7 ± 1.8 nM), but was less active against the other two group A PAKs (PAK2, IC50 = 190 nM; PAK3, IC50 = 99 nM). The similar biochemical activity of PF-3758309 across the group B PAKs may be attributed to catalytic domain amino acid identity and structural similarity (32).
PF-3758309 Is a Potent PAK4 Inhibitor in Living Cells and Blocks Tumor Cell Growth in Vitro.
Due to the lack of known unique substrates for PAK4, a PAK4-specific assay was constructed with the inducible expression of PAK4 catalytic domain in cells that harbor a deletion mutant of GEF-H1 (amino acids 210–921). In this system, PAK4 phosphorylates GEF-H1 on a previously characterized serine residue 810 (21) and is inhibited by PF-3758309 (IC50 = 1.3 ± 0.5 nM). PF-3758309 also inhibits endogenous pGEF-H1 accumulation in HCT116 cells (Fig. S2). PF-3758309 was evaluated for its ability to inhibit anchorage-independent growth, a hallmark characteristic of cell transformation, in HCT116 colon carcinoma cells (IC50 = 0.24 ± 0.09 nM) previously shown to be PAK4-dependent (19). Across a panel of 20 tumor cell lines, 15 had an anchorage-independent growth inhibition IC50 < 10 nM with an average of 4.7 ± 3.0 nM (Table S1). To further establish causality (33), we found that the potency of 59 pyrrolopyrazole inhibitors in the biochemical cellular assay (phospho-GEF-H1 assay) was highly correlated (R2 = 0.90) with anchorage-independent growth (Fig. S3). PF-3758309 potently inhibits cellular proliferation (IC50 = 20 nM) and anchorage-independent growth (IC50 = 27 nM) of A549 cells, which we show to be PAK4-dependent (SI Appendix). RNAi-mediated PAK4 protein knockdown induces A549 tumor cell apoptosis as measured by caspase 3 activation. Transfection of A549 tumor cells with 100 nM PAK4 siRNA resulted in 45% of A549 cells positive for caspase 3 activation at 72 h, whereas A549 cells transfected with control siRNAs showed less than 0.29% of cells with activated caspase 3 staining (SI Appendix). Furthermore, PAK4 knockdown in A549 cells also inhibits anchorage-independent growth and affects pronounced morphological changes in cell morphology and the actin cytoskeleton (SI Appendix). To evaluate the breadth of antiproliferative activity, PF-3758309 was profiled for its growth-inhibitory activity in a panel of 92 tumor cell lines derived from colorectal, non-small-cell lung cancer, pancreatic, and breast tumors, with 46% exhibiting IC50 values less than 10 nM (Table S1). Of note, PF-3758309 is not antiproliferative in primary hepatocyte cells (IC50 > 1,000 nM). PF-3758309 is potent toward a broad array of tumor cell lines from different tumor types.
Breadth of PF-3758309 Biochemical Activity.
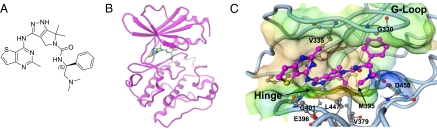
Molecules targeting large gene families must be well-characterized to allow for meaningful interpretation of biological effects and clinical findings. Achieving the appropriate level of kinase selectivity is essential to identify the appropriate clinical patients, rationally select therapeutic combinations, and identify efficacy biomarkers. The molecular underpinnings of inhibitor interactions with PAK4 were characterized by x-ray crystallography to design out off-target activities. PF-3758309 binds to the PAK4 catalytic domain in the ATP binding site and makes multiple contacts with the hinge region through hydrogen-bond interactions with the pyrrolopyrazole core and the amine linker to the thienopyrimidine ring (Fig. 1). The urea carbonyl oxygen makes a critical hydrogen bond with a conserved water molecule, which also solvates neighboring charged residues (Lys350, Asp458) through hydrogen-bond interactions. The dimethylamine group forms a strong charge–charge interaction with Asp458, which is essential for potency. Hydrophobic interactions between the thienopyrimidine of PF-3758309 and the PAK4 protein are also critical for potent activity including (i) pyrimidine C2 methyl and Gly328/Val335 and (ii) thiophene and Gly401. The gem-dimethyl group of the pyrrolopyrazole core makes effective hydrophobic interactions with Met395 [the “gate keeper of the deep pocket” (34)], Val379, Leu447, and Ala348. In addition, the gem-dimethyl moiety of the pyrrolopyrazole core augments selectivity through steric clashes in other kinases (e.g., AUR2 L194/PAK4 V447 and LCK T316/PAK4 M305). The phenyl ring makes a hydrophobic interaction with the glycine loop, a critical interaction for selectivity over other kinases such as Cdk2. The structure-based design of PF-3758309 facilitated the optimization of potency and the enhancement of kinase selectivity.
Fig. 1.
Structural characterization of PF-3758309 binding to the PAK4 catalytic domain. (A) Chemical structure of PF-3758309. (B) PF-3758309 in 2.1 Å PAK4 catalytic domain x-ray cocrystal structure (Protein Data Bank ID code 2x4z). (C) Binding of PF-3758309 in the active site of PAK4 (surface color-coded by hydrophobicity).
To begin to understand the potential range of biological activities, PF-3758309 was broadly profiled in enzymatic assays of recombinant kinase domains. PF-3758309 was screened at a permissive ATP concentration (Km) against 146 of the 518 known human kinases to estimate off-target activities with a potential for cellular activity (IC50 < 5,000 nM) assuming ATP-competitive inhibition, published Km,ATP values, and a cellular ATP concentration of 2 mM (SI Materials and Methods). The biochemical screening hits estimated to have a potential for cellular activity were identified: Src family (Src, Yes, Fyn), AMPK, RSK (1/2/3), CHK2, FLT3, PKC (β, γ, μ, θ), PDK2, TRKα, AKT3, PRK1, and FGR (Table S2). Many factors affect translatability of biochemical potency to the appropriate physiological context which could produce false positives. To better define the pathways regulated by PF-3758309 in a cellular context, we used specific cellular readouts, broad pathway analyses, and tumor proteomic studies.
System-Wide High-Content Analysis of PF-3758309 Selectivity and Mechanism of Action.
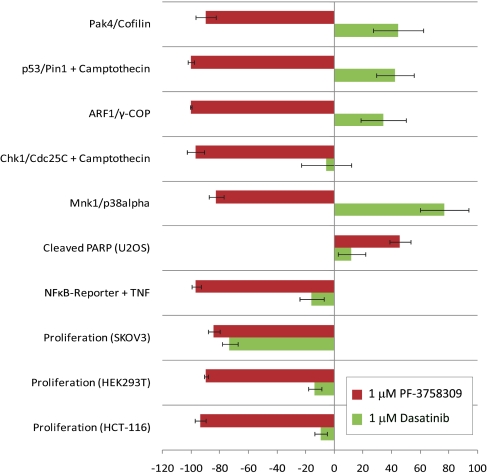
Specific pathway activities of PF-3758309 in cells, including the level and subcellular localization of specific signaling complexes, were explored with a broad and diverse panel of 113 protein-fragment complementation assays (PCA) (35) and other high-content assays to measure pathway activities. Use of this pathway analysis strategy for PF-3758309 characterization enabled confirmation of on-target activities, exploration of off-target biochemical screening hits, and the identification of unexpected pathway connections to PAK4. A subset of these high-content cellular assays was consistently modulated by PAK4 inhibitors. The use of 65 chemically diverse PAK inhibitors enabled the exclusion of cellular activities unique to a particular chemical structure and likely to be off-target effects. We were therefore able to define a PAK4 inhibitor-specific biological “fingerprint” or “signature” (Fig. 2) which was compared with the signatures of well-characterized inhibitors of kinases identified as PF-3758309 biochemical screening hits. Related potent pyrrolopyrazole inhibitors designed to inhibit PKC (36) (e.g., PF-04577806) did not inhibit PAK4 (PAK4, Ki = 52 μM, pGEF-H1 cellular IC50 = 10 μM), nor did they modulate any of the assays that constitute the PAK4 signature at 1 μM. As PF-3758309 had biochemical activity toward Src-family kinases in vitro, we compared PF-3758309 cellular signaling activity with the well-characterized Src-family inhibitor Dasatinib (Fig. 2). The starkly different profile of PF-3758309 is not consistent with a common mechanism of action. As such, the Src- and PKC-family activities detected in biochemical screens do not appear to translate to cells.
Fig. 2.
PF-3758309 has a unique profile of cellular activity. Cellular analysis was performed using a panel of over 113 cell-based assays, composed of both high-content and functional readouts, most performed at multiple time points. The group of assays shown was consistently regulated by diverse PAK4 inhibitors. Displayed in red is the core assay profile for PF-3758309 (1 μM); the Src-family kinase inhibitor Dasatinib (1 μM; green bars) is included for comparison. Each bar represents percent of inhibition stimulation relative to assay-specific controls from at least three independent experiments, ± SD (SI Materials and Methods).
To further enhance confidence in the signaling signature, we evaluated the constituents for PAK4 connectivity. A number of the assays in which PF-3758309 was active measure well-established “on-target” compound activities (e.g., PAK4/cofilin; Fig. 2) (LIMK2/cofilin). The IC50 values for the on-target PCAs were similar to values determined by biophysical and cellular methods (e.g., PAK4/cofilin, IC50 = 10.4 ± 4.0 nM), indicating that PAK-specific activity of PF-3758309 is recapitulated in PCA methodology. In addition, the PAK4 PCA activity of PF-3758309 and a related group of pyrrolopyrazole inhibitors correlates remarkably well (R2 = 0.92) with functional cellular efficacy as measured by proliferation in tumorigenic human cell lines, indicating that functional activity is likely to be related to PAK-inhibitory activity. Other PF-3758309 cellular findings (Fig. 2) may be linked to either poorly characterized or new PAK4 functions. The observed PF-3758309 activities on NF-κB signaling (Fig. 2; IC50 = 24.2 ± 14.8 nM) and PARP cleavage (Fig. 2) are consistent with PAK4 knockdown findings (25). Inhibition of the ARF1/γ-COP protein complex (Fig. 2; IC50 = 7.9 ± 4.7 nM) can be correlated to PAK4 biology. PAK4 colocalizes with the β-COP subunit of the COPI Golgi complex which regulates actin cytoskeleton dynamics, a known PAK4 function (19, 21). In addition, the PAK4 activator, cdc42, is recruited to COPI vesicles (37), and is required for Arf1 signaling (38). The observed inhibition of Mnk1/p38α complexes (Fig. 2) may also be linked to PAK biology: Mnk was previously shown to be phosphorylated by caspase-activated PAK2 and an unidentified cdc42-dependent kinase (39). PF-3758309 was particularly active in p53-containing assays (p53/Pin1, IC50 = 5.7 ± 1.7 nM; p53/Mdm2, IC50 = 5.6 ± 3.6 nM), yet its connection to PAK biology unknown. This is an important finding, because the tumor suppressor p53 cooperates with Ras to transform cells, acts as a DNA damage checkpoint in the cell cycle, and is mutated in over 50% of human tumors (40). The p53 PCA findings were substantiated by profiling seven structurally distinct pyrrolopyrazole PAK4 inhibitors in dose–response analyses. The correlation between IC50 values in the PAK4/cofilin and the Pin1/p53 assays was striking (R2 = 0.98), implicating these PCA findings as PAK-family-dependent. These global cellular screening methods were valuable in generating hypotheses for unprecedented biology.
PAK Is Upstream of p53.
The involvement of p53 and related pathways in endogenous PAK4 signaling was unexpected and has important implications for development of the chemotherapeutic agent. We therefore confirmed and extended these results with endogenous proteins in HCT116 cells. PF-3758309 did in fact cause cell-cycle effects. Follow-up studies with fluorescence-activated cell sorter analysis of 100 nM PF-3758309-treated HCT116 cells (48 h) exhibited cell-cycle arrest and apoptosis (DMSO/PF-3758309): sub-G1 (1.8/12.4), G1 (50.7/49.4), S (26.2/14.4), and G2 (19.8/23.1) (Fig. S4). Next, the effects of PF-3758309 and PAK4 on p53 signaling were explored. After 15 min of pretreatment with 1 μM PF-3758309 or the p53 degradation inhibitor Nutlin-3, cells were stimulated with the DNA damaging agent camptothecin. Phosphorylated and total protein levels were measured with Western blots for PAK4 substrates (PAK4 autophosphorylation and GEF-H1) as well as p53 and a p53-regulated protein (p21waf1/cip1) (Fig. S2). PF-3758309 and a structurally related PAK4 inhibitor (PF-4644904) significantly reduced camptothecin-induced phospho-S474-PAK4, phospho-S810-GEF-H1, p53, and p21waf1/cip1 levels (Fig. S2). As expected (41), Nutlin-3 treatment resulted in the accumulation of p53. However, Nutlin-3 treatment had no effect on pPAK4 and pGEF-H1 levels. Previous reports also noted p53 and p21cip1 induction following exogenous expression of activated PAK4 (42). In addition, PAK-family kinases and p53 expression have been reported to be coregulated (43, 44). Recently, a role for p53 in cytoskeletal regulation has been suggested (40), but the hierarchy of events in the signaling processes linking p53 and PAK4 is unclear. Nevertheless, our results are consistent with PAK4, and by extension certain Rho GTPases, as upstream regulators of p53, and also indicate that pharmacologic modulation of p53 does not directly affect PAK4 expression or activity in these settings. Furthermore, based the tumor cell line panel screens with PF-3758309, tumor sensitivity is not dependent on p53 status (Table S1).
PF-3758309 Is a Potent Antitumor Agent in Human Xenograft Tumor Models.
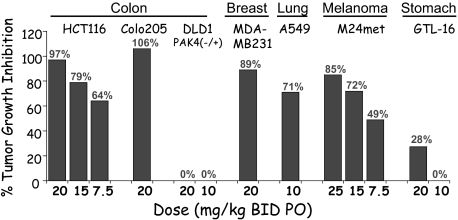
To further investigate the biological effects of PF-3758309 in more physiologically relevant systems, human xenograft tumor models were used. The antitumor efficacy of PF-3758309 was evaluated in a panel of human xenograft tumor models (Fig. 3). Twice daily oral dosing of PF-3758309 (7.5–30 mg/kg BID) for 9–18 days resulted in statistically significant tumor growth inhibition (TGI) in five models including HCT116 and A549 models (Fig. 3; >70% TGI, P < 0.01). HCT116 cells are known to be PAK4-dependent (19), and A549 tumor cells are now shown to be PAK4-dependent (SI Appendix). PF-3758309 was not active in DLD1 cells, which have a loss-of-function mutation in one of the PAK4 alleles, nor in the GTL16 model that has been shown to be cMet-driven (45). Time-course studies of HCT116 tumor growth and PF-3758309-driven TGI were conducted to develop a PK/PD model using Alzet osmotic minipumps to deliver finely controlled drug exposures (SI Materials and Methods). In the model, the plasma concentration time course of PF-3758309 drives the effective inhibition of the tumor growth rate, where the estimated IC50 was determined to be 0.40 nM (unbound).
Fig. 3.
Tumor growth inhibition of human xenograft tumor models: HCT116 (CRC), M24met (melanoma), Colo205 (CRC), A549 lung carcinoma, GTL-16 (GIST), DLD1 (CRC), and MDA-MB231 (BC). All TGI measurements were statistically significant except 20 mg/kg dosing of the c-Met-driven GTL-16 model (45).
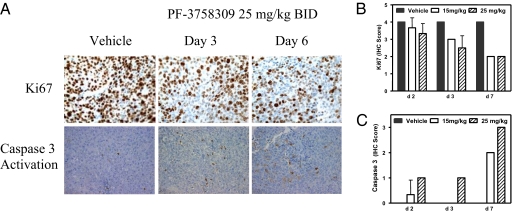
We next explored mechanisms underlying the observed antitumor efficacy. Phosphorylation levels of a bona fide PAK4 substrate, GEF-H1 S810, were measured in HCT116 tumors following 6 days of PF-3758309 s.c. dosing. At efficacious doses (1.1–3.3 mg·kg−1·day−1, 0.14–1.3 nM unbound steady-state exposures), a 54–59% reduction of phosphorylated GEF-H1 was measured by an ELISA method. Fluorodeoxythymidine (FLT) uptake has been used as a surrogate marker for tumor cell proliferation (46, 47). PF-3758309 (15 mg/kg BID) was found to inhibit [3H]FLT uptake 32.5% in the HCT116 tumor xenografts by day 6. With 25 mg/kg (QD) dosing, statistically significant FLT uptake inhibition was observed on day 1 (33.8%), day 2 (35.3%), and day 6 (43.1%). The proliferation marker Ki67, previously shown to be inhibited in PAK4 knockout studies (22), was demonstrated to be inhibited by PF-3758309 in a dose- and time-dependent manner (Fig. 4). PF-3758309 treatment showed a significant increase in the apoptotic marker activated caspase 3 in HCT116 tumors (Fig. 4), which was also observed in cellular studies (Fig. 2). These results are consistent with the functional responses reported for PAK4 knockdown and knockout studies (19, 22–25) and indicates that both cytostatic and cytoreductive mechanisms contribute to antitumor activity.
Fig. 4.
PF-3758309 is antiproliferative and induces apoptosis in a HCT116 tumor model. PF-3758309 functional activity was measured by IHC in an HCT116 model with Ki67 expression and caspase 3 activation endpoints. (A) Ki67 and caspase 3 activation IHC analysis at 25 mg/kg PF-3758309. (B and C) Quantitation of the IHC images. Tumor-bearing mice were p.o. administered with vehicle (solid bars) and 15 mg/kg (open bars) and 25 mg/kg (striped bars) PF-3758309 twice daily and tumors were harvested at the indicated times.
Phosphoproteomic Analysis Maps the Molecular Underpinnings of PF-3758309 Activity in Tumors.
Reverse-phase protein array technology was used to monitor the amount and activation status of signaling proteins extracted from treated and untreated tumors. Tumor lysates from PF-3758309-treated HCT116 tumor-bearing mice were screened against 98 antibodies (Table S3). A dose–response analysis of PF-3758309 activity was performed at high exposure levels via an Alzet minipump (15, 50, 75, 150 mg·kg−1·day−1) to observe all possible effects after 18 h of exposure (Table S3). Of the 98 antibodies evaluated, 11 antibodies yielded a statistically significant dose–response: BAD (pS136), BAD (pS112), AKT1 (pS473), Src (pY527), Src (pY416), GSK3β (pS21), MEK1 (pS221), Myc (total), Elk (pS383), PKCα (pS569), and STAT6 (pY641). Tumor proteomic studies have revealed that PF-3758309 modulates pathways and signaling nodes expected for a PAK4 inhibitor in a dose-dependent manner: Raf (MEK, Elk1, cMyc) and Akt (Akt, BAD, PKA) (10, 12, 14). For example, BAD was shown to be phosphorylated on S112 and S136 by PAK4 (24, 26). These modulated pathways define a subset of possible signaling events that underlie the observed apoptotic and antiproliferative activities of PF-3758309.
Discussion
Based on genetically derived validation studies (current and published studies), PAK4 represents an intriguing therapeutic target with functional roles in apoptosis, cytoskeletal remodeling, and the cell cycle. We undertook a large-scale, systematic drug discovery and preclinical development campaign to identify and optimize potent and selective small-molecule inhibitors of PAK4.
We report the identification and characterization of PF-3758309, a potent, reversible ATP-competitive inhibitor of PAK4 with the expected cellular functions of a PAK4 inhibitor: inhibition of anchorage-independent growth, induction of apoptosis, cytoskeletal remodeling, and inhibition of proliferation. A unique and unusually broad panel of in vitro, cell-based, and in vivo profiling assays was used to define selectivity, mechanisms, and efficacy of this molecule and, by extension, the pathways regulated by PAK4. PF-3758309 was found to regulate expected targets and functions in vitro and in vivo, including key signaling nodes at the nexus of cytoskeletal, cell-cycle, and apoptotic signaling processes. These activities are likely responsible for the potent activity of PF-3758309 in blocking the growth of tumors. Activities beyond those previously reported are equally intriguing. In particular, the further definition and mapping of the link between PAK4 and p53 signaling may have important implications for the use of PAK inhibitors in the treatment of human disease.
The described studies demonstrate the utility of integrating traditional approaches with emerging technologies—including crystallography, high-content global signaling analyses, tumor proteomics, and systems/chemical biology—in the characterization of a novel experimental agent before clinical trials. This integrated approach captures a wider array of target and compound biology which enables more informed decision making. The strategy described here may represent a generally applicable strategy for optimizing preclinical development to drive better-informed clinical strategies. We expect that PF-3758309 will contribute to future mechanistic studies, and may have promise as a therapeutic agent for human cancers.
Materials and Methods
Reagents and procedures used in this report are described in detail in SI Materials and Methods.
Phospho-GEF-H1 Cellular Assay.
TR-293-KDG cells were constructed from HEK293 cells stably transfected with tetracycline-inducible PAK4-kinase domain (amino acids 291–591) and constitutively expressed HA-tagged GEFH1ΔDH (amino acids 210–921). TR-293-KDG cells were incubated for 3 h with PF-3758309, captured on an anti-HA antibody-coated plate (Invitrogen), detected with an anti-phospho-S810-GEF-H1 antibody, and quantified with a horseradish peroxidase-goat anti-rabbit antibody conjugate (Invitrogen).
Colony Formation Cellular Assay.
Ninety-six-well plates contained a lower layer (50 μL 1% wt/wt agarose) and an upper layer (50 μL 0.7% wt/wt agarose and 4,000 cells). PF-3758309 (100 μL in DMEM) was added and incubated for 6 days. Alamar blue (10 μL) fluorescence (540 nm) was quantified.
High-Content and Functional Analysis of Cellular Signaling Pathways.
PCA methods have been previously described (ref. 35 and references therein). For NF-κB pathway analysis, HEK293T cells were transfected with the pNF-κB-TA-Luc luciferase plasmid (Clontech/Takara Bio), grown for 48 h, stimulated with 20 ng/mL TNFα (7 h), and quantified using the Steady-Glo Luciferase Assay System (Promega). Cleaved PARP (U2OS cells) was detected with an antibody raised against Asp214 of cleaved PARP (Cell Signaling Technology).
In Vivo Function: Tumor Growth Inhibition, Histopathology, and [3H]FLT Uptake.
Xenograft tumors in nude mice were quantitated with calipers or harvested and fixed. Anti-caspase 3 (Cell Signaling) and anti-Ki67 (Lab Vision) -stained slides were analyzed using the Chromovision automated cell-imaging system: Ki67-positive cells: 0 < 40%, 1 = 40–50%, 2 = 50–60%, 3 = 60–70%, 4 > 70%, and caspase 3-positive cells: 0 = <2%, 1 = 2–3%, 2 = 4–5%, 3 = 6–7%, 4 > 7%. [3H]FLT uptake was performed similarly except that [3H]FLT was administrated 2 h before tumor harvest.
Supplementary Material
Acknowledgments
We thank Gabriele Troche, Joseph Lee, and David Looper for their technical expertise in performing the in vivo studies.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 2x4z).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0911863107/-/DCSupplemental.
References
- 1.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59:111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 2.Onesto C, Shutes A, Picard V, Schweighoffer F, Der CJ. Characterization of EHT 1864, a novel small molecule inhibitor of Rac family small GTPases. Methods Enzymol. 2008;439:111–129. doi: 10.1016/S0076-6879(07)00409-0. [DOI] [PubMed] [Google Scholar]
- 3.Qiu RG, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zohn IM, Campbell SL, Khosravi-Far R, Rossman KL, Der CJ. Rho family proteins and Ras transformation: The RHOad less traveled gets congested. Oncogene. 1998;17(11 Reviews):1415–1438. doi: 10.1038/sj.onc.1202181. [DOI] [PubMed] [Google Scholar]
- 5.Sahai E, Olson MF, Marshall CJ. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001;20:755–766. doi: 10.1093/emboj/20.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 7.Ellenbroek SI, Collard JG. Rho GTPases: Functions and association with cancer. Clin Exp Metastasis. 2007;24:657–672. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 8.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 9.Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100:97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 10.Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51–63. doi: 10.1007/s10555-008-9168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eswaran J, Soundararajan M, Kumar R, Knapp S. UnPAKing the class differences among p21-activated kinases. Trends Biochem Sci. 2008;33:394–403. doi: 10.1016/j.tibs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. PAK signaling in oncogenesis. Oncogene. 2009;28:2545–2555. doi: 10.1038/onc.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strange K, Denton J, Nehrke K. Ste20-type kinases: Evolutionarily conserved regulators of ion transport and cell volume. Physiology (Bethesda) 2006;21:61–68. doi: 10.1152/physiol.00139.2005. [DOI] [PubMed] [Google Scholar]
- 14.Eswaran J, Soundararajan M, Knapp S. Targeting group II PAKs in cancer and metastasis. Cancer Metastasis Rev. 2009;28:209–217. doi: 10.1007/s10555-008-9181-4. [DOI] [PubMed] [Google Scholar]
- 15.Abo A, et al. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 1998;17:6527–6540. doi: 10.1093/emboj/17.22.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minden A. US Patent 6,103,500. 1998 [Google Scholar]
- 17.Plowman G, Martinez R. US Provisional Patent Appl 60/081,784. 1998 [Google Scholar]
- 18.Plowman G, Martinez R, Whyte D. US Appl 09/291,417. 1999 [Google Scholar]
- 19.Callow MG, et al. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem. 2002;277:550–558. doi: 10.1074/jbc.M105732200. [DOI] [PubMed] [Google Scholar]
- 20.Qu J, et al. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol Cell Biol. 2001;21:3523–3533. doi: 10.1128/MCB.21.10.3523-3533.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci. 2005;118:1861–1872. doi: 10.1242/jcs.02313. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, et al. The Pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Mol Cancer Res. 2008;6:1215–1224. doi: 10.1158/1541-7786.MCR-08-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian Y, Lei L, Cammarano M, Nekrasova T, Minden A. Essential role for the Pak4 protein kinase in extraembryonic tissue development and vessel formation. Mech Dev. 2009;126:710–720. doi: 10.1016/j.mod.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Gnesutta N, Qu J, Minden A. The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. J Biol Chem. 2001;276:14414–14419. doi: 10.1074/jbc.M011046200. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Minden A. PAK4 functions in tumor necrosis factor (TNF) α-induced survival pathways by facilitating TRADD binding to the TNF receptor. J Biol Chem. 2005;280:41192–41200. doi: 10.1074/jbc.M506884200. [DOI] [PubMed] [Google Scholar]
- 26.Gnesutta N, Minden A. Death receptor-induced activation of initiator caspase 8 is antagonized by serine/threonine kinase PAK4. Mol Cell Biol. 2003;23:7838–7848. doi: 10.1128/MCB.23.21.7838-7848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahlamäki EH, et al. High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia. 2004;6:432–439. doi: 10.1593/neo.04130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons DW, et al. Colorectal cancer: Mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, et al. Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol Ther. 2008;7:1793–1802. doi: 10.4161/cbt.7.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimmelman AC, et al. Genomic alterations link Rho family of GTPases to the highly invasive phenotype of pancreas cancer. Proc Natl Acad Sci USA. 2008;105:19372–19377. doi: 10.1073/pnas.0809966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dan C, Kelly A, Bernard O, Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J Biol Chem. 2001;276:32115–32121. doi: 10.1074/jbc.M100871200. [DOI] [PubMed] [Google Scholar]
- 32.Eswaran J, et al. Crystal structures of the p21-activated kinases PAK4, PAK5, and PAK6 reveal catalytic domain plasticity of active group II PAKs. Structure. 2007;15:201–213. doi: 10.1016/j.str.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss WA, Taylor SS, Shokat KM. Recognizing and exploiting differences between RNAi and small-molecule inhibitors. Nat Chem Biol. 2007;3:739–744. doi: 10.1038/nchembio1207-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alton GR, Lunney EA. Targeting the unactivated conformations of protein kinases for small molecule drug discovery. Expert Opin Drug Discov. 2008;3:595–605. doi: 10.1517/17460441.3.6.595. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald ML, et al. Identifying off-target effects and hidden phenotypes of drugs in human cells. Nat Chem Biol. 2006;2:329–337. doi: 10.1038/nchembio790. [DOI] [PubMed] [Google Scholar]
- 36.Grant S, et al. Discovery of a novel class of targeted kinase inhibitors that blocks protein kinase C signaling and ameliorates retinal vascular leakage in a diabetic rat model. Eur J Pharmacol. 2010;627:16–25. doi: 10.1016/j.ejphar.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 37.Chen JL, et al. Coatomer-bound Cdc42 regulates dynein recruitment to COPI vesicles. J Cell Biol. 2005;169:383–389. doi: 10.1083/jcb.200501157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heuvingh J, Franco M, Chavrier P, Sykes C. ARF1-mediated actin polymerization produces movement of artificial vesicles. Proc Natl Acad Sci USA. 2007;104:16928–16933. doi: 10.1073/pnas.0704749104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orton KC, et al. Phosphorylation of Mnk1 by caspase-activated Pak2/γ-PAK inhibits phosphorylation and interaction of eIF4G with Mnk. J Biol Chem. 2004;279:38649–38657. doi: 10.1074/jbc.M407337200. [DOI] [PubMed] [Google Scholar]
- 40.Roger L, Gadea G, Roux P. Control of cell migration: A tumour suppressor function for p53? Biol Cell. 2006;98:141–152. doi: 10.1042/BC20050058. [DOI] [PubMed] [Google Scholar]
- 41.Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: A novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–241. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cammarano MS, Nekrasova T, Noel B, Minden A. Pak4 induces premature senescence via a pathway requiring p16INK4/p19ARF and mitogen-activated protein kinase signaling. Mol Cell Biol. 2005;25:9532–9542. doi: 10.1128/MCB.25.21.9532-9542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 α and CDC42. Nat Struct Mol Biol. 2009;16:23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 44.Swami S, Raghavachari N, Muller UR, Bao YP, Feldman D. Vitamin D growth inhibition of breast cancer cells: Gene expression patterns assessed by cDNA microarray. Breast Cancer Res Treat. 2003;80:49–62. doi: 10.1023/A:1024487118457. [DOI] [PubMed] [Google Scholar]
- 45.Francone TD, et al. Novel xenograft model expressing human hepatocyte growth factor shows ligand-dependent growth of c-Met-expressing tumors. Mol Cancer Ther. 2007;6:1460–1466. doi: 10.1158/1535-7163.MCT-06-0466. [DOI] [PubMed] [Google Scholar]
- 46.Bading JR, Shields AF. Imaging of cell proliferation: Status and prospects. J Nucl Med. 2008;49(Suppl 2):64S–80S. doi: 10.2967/jnumed.107.046391. [DOI] [PubMed] [Google Scholar]
- 47.Apisarnthanarax S, et al. Early detection of chemoradioresponse in esophageal carcinoma by 3′-deoxy-3′-3H-fluorothymidine using preclinical tumor models. Clin Cancer Res. 2006;12:4590–4597. doi: 10.1158/1078-0432.CCR-05-2720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.