Abstract
β-methylamino-L-alanine (BMAA), a neurotoxic nonprotein amino acid produced by most cyanobacteria, has been proposed to be the causative agent of devastating neurodegenerative diseases on the island of Guam in the Pacific Ocean. Because cyanobacteria are widespread globally, we hypothesized that BMAA might occur and bioaccumulate in other ecosystems. Here we demonstrate, based on a recently developed extraction and HPLC-MS/MS method and long-term monitoring of BMAA in cyanobacterial populations of a temperate aquatic ecosystem (Baltic Sea, 2007–2008), that BMAA is biosynthesized by cyanobacterial genera dominating the massive surface blooms of this water body. BMAA also was found at higher concentrations in organisms of higher trophic levels that directly or indirectly feed on cyanobacteria, such as zooplankton and various vertebrates (fish) and invertebrates (mussels, oysters). Pelagic and benthic fish species used for human consumption were included. The highest BMAA levels were detected in the muscle and brain of bottom-dwelling fishes. The discovery of regular biosynthesis of the neurotoxin BMAA in a large temperate aquatic ecosystem combined with its possible transfer and bioaccumulation within major food webs, some ending in human consumption, is alarming and requires attention.
Keywords: β-methylamino-L-alanine, Baltic Sea, cyanobacteria, liquid chromatography/mass spectrometry, bioaccumulation
Cyanobacteria are ancient and cosmopolitan microorganisms with a unique capacity to produce a rich arsenal of complex organic compounds, including numerous bioactive metabolites. Some are produced by polyketide/nonribosomal peptide synthetase, whereas others are derived from aromatic amino acids or ribosomally encoded cyclic peptides (1, 2). These include the well-known cyanotoxins, classified according to their effects on vertebrates as hepatotoxins or neurotoxins (3). Toxic effects often are associated with natural, or antropogenically stimulated, massive surface blooms of cyanobacteria of various limnic or marine water bodies. Acute toxins are restricted to certain cyanobacterial genera, such as nodularin by Nodularia, a bloom-forming genus in the temperate Baltic Sea (3). In contrast, the more recently discovered cyanobacterial toxin β-methylamino-L-alanine (BMAA) has been proposed to have a long latency period (4) and is synthesized by representatives from all five cyanobacterial sections (5). Thus, BMAA might be spread globally in terrestrial and aquatic ecosystems.
A potential link between this neurotoxic nonprotein amino acid and neurodegenerative diseases was originally discovered on the island of Guam in the Western Pacific Ocean. Here an exceptionally high incidence of amyotrophic lateral sclerosis (ALS), of somewhat late onset with added extrapyramidal features and dementia (ALS/PDC), was recorded in the late 1940s to early 1950s (6, 7). ALS/PDC is a progressive neurological disorder with clinical and neuropathological features resembling those of ALS, Parkinson's disease, and Alzheimer's disease (8). High incidences of ALS/PDC have been documented in three populations in the western Pacific Ocean: residents of the Japanese Kii Peninsula, the Auyu people of Irian Jaya, and the Chamorro population of Guam (9). When administered to primates at experimentally high dosages, BMAA was shown to induce specific motor system damages resembling that of ALS/PDC in humans (10). BMAA was later shown to cause selective motor neuron loss in dissociated mixed spinal cord cultures at concentrations ≈30 μM (11), and also to potentiate neuronal injury induced by other insults at concentrations as low as 10 μM (12). Hippocampal neurons are among the most BMAA-sensitive neuronal populations in vitro (13). The hypothesis that BMAA acts as a possible pathogenic factor in human neurodegenerative disease is further supported by the observation of high BMAA levels in relevant central nervous system (CNS) areas in Alzheimer's disease and ALS patients in North America (14). In addition, BMAA has been recorded in brain tissue of diseased individuals in the Chamorro population suffering from neurodegeneration (15).
BMAA was originally proposed to be produced by a cycad (Cycas micronesica), a gymnospermous tree common to the subtropical/tropical region of Guam, the seed flour of which is used for human consumption (e.g., in tortillas). Later, whether BMAA was the causative agent of the ALS/PDC in Guam was disputed, given that the concentration of BMAA in the Chamorro diet was far lower than that used in the primate experiments (16, 17). Subsequently, however, the BMAA hypothesis was reconsidered, based on several observations: (i) BMAA levels are 50–100 times higher in the protein-bound form in flour made from cycad seeds compared with the free-BMAA pool analyzed previously (4); (ii) BMAA may originate from a cyanobacterium (genus Nostoc), which inhabits the roots of cycads and provides the tree with nitrogen via nitrogen fixation (18); and (iii) a more likely route for BMAA exposure in the Chamorro population was via consumption of flying foxes, a bat of the Pteropus genus. These animals feed on cycad seeds, which causes a 10,000-fold increase in BMAA concentration in their tissues compared with that produced by cyanobacteria in the cycad roots (19). Thus, prerequisites are available not only for BMAA causing neurodegeneration among Chamorros, but also for BMAA being present in the environment outside Guam, because BMAA-producing cyanobacteria may exist worldwide. This was further substantiated by the finding that >90% of randomly selected cyanobacteria synthesize BMAA (5). Since then, attempts have been made to confirm the presence of BMAA in cultured and environmental cyanobacterial samples using various analytical techniques; however, inconclusive results (20–24), including detection of BMAA in other biological matrices (25, 26), and analytical difficulties have led to conflicting data. In addition, BMAA is a small, highly hydrophilic molecule with no natural chromophore or flourophore, which might be present in low quantities and be “embedded” in organismal matrices of varying complexity (27, 28). Another analytical issue involves the structural isomer of BMAA, α-γ-diaminobutyric acid (DAB), which might coelute with BMAA during separation by HPLC and potentially cause a false-positive result (24). Previous publications using different columns and gradients have reported separation of BMAA and DAB, although these data were based solely on nonselective fluorescent detection (29). The first indisputable mass spectrometric data on daughter ions distinguishing between BMAA and DAB were recently published by Rosen et al. (24).
In an attempt to overcome these analytical pitfalls, we recently introduced a sample workup scheme that includes solid-phase extraction of BMAA, followed by precolumn derivatization with AccQ-Tag and analysis by HPLC-MS/MS (30). Through this approach, BMAA can be reproducibly extracted, identified from a range of biological matrices with a limit of detection (LOD) as low as 70 fmol, and efficiently separated from DAB in time and space.
Introducing this method and selecting a cyanobacterial-dominated ecosystem “outside” the tropical island of Guam as a model, we set out to test the hypothesis that BMAA might be produced by natural populations of cyanobacteria in a temperate climate, and that BMAA might bioaccumulate in organisms at higher trophic levels, including those used for human consumption. The Baltic Sea is a highly productive brackish water ecosystem (31) that has provided services over millennia to the huge surrounding population (nine countries and ∼90 million people in the catchment area; Fig. S1). The regularly recurring cyanobacterial surface blooms of the Baltic Sea are dominated by the nitrogen-fixing cyanobacterial genera Nodularia and Aphanizomenon (32, 33). Through their photosynthetic and nitrogen-fixing capacity, these genera serve as extremely important primary producers and constitute a basis in the Baltic Sea food webs.
Previous analyses of cultured isolates of these two cyanobacteria found that they produce BMAA (5). Here we present data that extend that finding significantly by including several natural cyanobacterial populations over seasons, as well as organisms at higher trophic levels dependent on these primary producers, including representatives used for human consumption. The data demonstrate that BMAA is produced by the widespread cyanobacteria in an aquatic temperate ecosystem, and that BMAA occurs and is bioaccumulated at higher trophic levels, including fish and mollusks used for human consumption. The occurrence and bioaccumulation of BMAA in a temperate ecosystem outside Guam suggest that BMAA may be a globally widespread toxin that represents a potential threat to human health.
Results
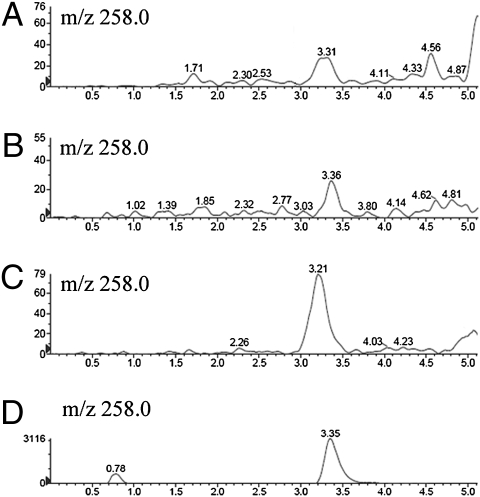
Figure 1 displays representative MS/MS spectra from the various species examined, including a natural population of cyanobacteria, a mixture of zooplankton and Osmerus eperlanus. These spectra show the singly-charged product ion (m/z 258.0) originating from the precursor ion, double-derivatized, singly-charged BMAA (m/z 459.1). Thus, the ion 258.0 m/z is a diagnostic fragment for BMAA. The BMAA peak elutes within the time range 3.20–3.36 min, and this slight variation in retention times is due to matrix effects (30). MS/MS spectra of all species analyzed are presented in Fig. S2.
Fig. 1.
BMAA spectra of representative organisms analyzed. (A) Cyanobacteria collected on July 15, 2008, containing a mixture of the bloom-forming cyanobacteria N. spumigena and Aphanizomenon spp. (B) Zooplankton collected on July 11, 2008, containing a mixture of A. bifilosa and C. pengoi. (C) O. eperlanus collected on October 10, 2008 (Fig. S1, location 2). (D) BMAA standard. Note the chromatographic peaks belonging to the product ion (m/z 258.0) originating from the precursor ion (459.1 m/z). The diagnostic fragment (258.0 m/z) is highly specific for BMAA. The scales on the y-axes have been adjusted in such a manner that the BMAA peak goes from the bottom to the top of each panel.
BMAA in Cyanobacteria and Zooplankton.
Plankton tows were performed in June through September 2007 and 2008 in surface waters at coastal station B1 and open-water station BY31 in the Baltic Sea (Fig. S1). The typical presence of mixed genera of cyanobacteria and zooplankton was verified by light microscopy. The cyanobacteria were separated from the zooplankton using a “light trap” (34). The composition of the cyanobacterial samples differed among the various sampling dates. Aphanizomenon spp. dominated early in the growth season (June–July) at station B1, whereas Nodularia spp. increased progressively, and in August approximately equal mixtures of Aphanizomenon spp. and Nodularia spp. were apparent. In contrast, Nodularia spp. always dominated at station BY31. The fluctuation in cyanobacterial diversity was consistent throughout the two seasons.
First, BMAA levels in the cyanobacterial and zooplankton fractions were measured by HPLC-MS/MS (Tables 1 and 2, Fig. 1, and Fig. S2). The total BMAA levels (free and protein-associated) were determined in all samples. BMAA levels were semiquantified and expressed as μg BMAA/g dry weight. The results show that BMAA was found in all cyanobacterial samples at both stations and throughout the two seasons (Table 1), although variations in BMAA levels were apparent between the stations and the sampling dates, ranging from 0.001 to 0.015 μg BMAA/g dry weight. The zooplankton-enriched fraction contained higher levels of BMAA than the cyanobacterial samples. The BMAA levels in zooplankton were 6-fold higher on average (Table 2).
Table 1.
Seasonal variations in BMAA levels in cyanobacteria sampled in the Baltic Sea, 2007–2008
| Date | BMAA, μg/g dry weight | |
| Coastal station B1 | ||
| 2007 | June 13 | 0.013 ± 0.005 |
| June 27 | 0.003 ± 0.0005 | |
| July 11 | 0.008 ± 0.0013 | |
| July 26 | 0.0054 ± 0.001 | |
| August 1 | 0.0023 ± 0.0003 | |
| August 16 | 0.005 ± 0.0002 | |
| August 24 | 0.008 ± 0.0005 | |
| September 12 | 0.003 ± 0.0009 | |
| 2008 | June 18 | 0.005 ± 0.0001 |
| June 26 | 0.004 ± 0.0006 | |
| July 1 | 0.008 ± 0.0004 | |
| July 8 | 0.010 ± 0.001 | |
| July 15 | 0.007 ± 0.0007 | |
| July 23 | 0.012 ± 0.002 | |
| July 30 | 0.005 ± 0.0004 | |
| August 6 | 0.001 ± 0.0006 | |
| August 21 | 0.008 ± 0.0014 | |
| August 30 | 0.015 ± 0.001 | |
| September 3 | 0.005 ± 0.0003 | |
| Open-water station BY31 | ||
| 2007 | June 20 | 0.0080 ± 0.0008 |
| 2008 | August 1 | 0.0023 ± 0.0001 |
BMAA levels in natural populations of cyanobacteria collected at two stations in the Baltic Sea with different characteristics, coastal station B1 and open-water station BY31 (Fig. S1). BMAA levels are expressed as μg BMAA/g dry weight ± SE (n = 3).
Table 2.
Seasonal variations in BMAA levels in zooplankton sampled in the Baltic Sea, 2007–2008
| Date | BMAA, μg /g dry weight | |
| Coastal station B1 | ||
| 2007 | June 13 | 0.024 ± 0.006 |
| July 11 | 0.045 ± 0.008 | |
| July 26 | 0.029 ± 0.003 | |
| August 1 | 0.030 ± 0.002 | |
| August 16 | 0.030 ± 0.005 | |
| August 29 | 0.042 ± 0.002 | |
| 2008 | June 18 | 0.087 ± 0.005 |
| July 1 | 0.076 ± 0.0097 | |
| July 11 | 0.0037 ± 0.0003 | |
| July 15 | 0.046 ± 0.001 | |
| July 30 | 0.048 ± 0.002 | |
| August 6 | 0.024 ± 0.002 | |
| August 30 | 0.026 ± 0.0012 | |
The zooplankton occurred naturally intermixed with the cyanobacteria in plankton tows and was collected at coastal station B1 (Fig. S1). BMAA levels are expressed as μg BMAA/g dry weight ± SE (n = 3).
BMAA in Vertebrates and Invertebrates.
Next, BMAA was extracted and determined using HPLC-MS/MS from selected Baltic Sea vertebrates, including the fish genera Osmerus eperlanus (smelt), Scophthalmus maximus (turbot), Triglopsis quadricornis (fourhorn sculpin), Clupea harengus (herring), Coregonus lavaretus (common whitefish), and Sander lucioperca (pike-perch), inhabiting various parts of the water body. The bottom-dwelling invertebrates Mytilus edulis (mussel) and Ostrea edulis (oyster) cultivated for human consumption on the west coast of Sweden were included as well. This water body lacks the massive cyanobacterial blooms seen in the Baltic Sea. Levels of BMAA (free and protein-associated) were expressed as μg/g dry weight (Tables 3 and 4). Muscle and brain tissues from the fish species were analyzed separately in triplicate. As shown in Table 3, BMAA was detected in several fish samples. Although the BMAA levels varied even within species, the technical triplicates within the same organism/sample showed highly consistent BMAA levels. The standard errors were calculated from the technical triplicates. In general, the fishes living closer to the bottom of the Baltic Sea contained higher levels of BMAA than the pelagic fishes, some of which even lacked BMAA. Moreover, the brain tissue from O. eperlanus, S. maximus, and T. quadricornis contained up to 82-fold higher levels of BMAA than the corresponding muscle tissue. Particularly high BMAA levels were found in the brain tissue of S. maximus, ranging from 0.99 to 1.29 μg BMAA/g dry weight. The situation was reversed in C. lavarentus, which contained high levels of BMAA in the muscle and lower levels in the brain (Table 3). C. harengus had low but similar BMAA levels in both tissue samples. BMAA was not detected in any tissues in the two pelagic species evaluated, S. lucioperca (Fig. S1, location 2) from the Baltic Sea, and Salmo salar (salmon) from the North Atlantic Ocean (Fig. S1, location 4). Surprisingly high levels of BMAA were detected in the two invertebrates, M. edulis and O. edulis, collected on the western coast of Sweden (Fig. S1, location 3).
Table 3.
BMAA levels in selected Baltic Sea vertebrates (fishes)
| BMAA, μg/g dry weight |
||||||
| Muscle tissue (n = 3) |
Brain tissue (n = 3) |
|||||
| Fish | n1 | n2 | n3 | n1 | n2 | n3 |
| Osmerus eperlanus (smelt) | 0.016 ± 0.0009 | 0.016 ± 0.0008 | 0.023 ± 0.000/9 | 0.161 ± 0.010 | 0.24 ± 0.003 | ND |
| Scophthalmus maximus (turbot) | 0.011 ± 0.0009 | 0.008 ± 0.0003 | ND | 0.99 ± 0.0009 | 0.047 ± 0.0009 | 1.29 ± 0.03 |
| Triglopsis quadricornis (fourhorn sculpin) | ND | ND | ND | 0.073 ± 0.002 | 0.012 ± 0.0023 | 0.124 ± 0.005 |
| Clupea harengus (herring) | ND | 0.010 ± 0.001 | ND | ND | 0.008 ± 0.001 | 0.007 ± 0.0008 |
| Coregonus lavaretus (common whitefish) | 0.059 ± 0.004 | 0.033 ± 0.0006 | ND | 0.0019 ± 7E−5 | ND | 0.002 ± 8E−5 |
| Sander lucioperca (pike-perch) | ND | ND | ND | ND | ND | ND |
| Salmo salar (Atlantic salmon) | ND | ND | ND | ND | ND | ND |
Levels of BMAA in muscle and brain tissues are presented in selected fishes collected at location 2 in the Baltic Sea and location 4 in the Atlantic Ocean (Salmo salar; salmon) (Fig. S1). BMAA levels are expressed as μg BMAA/g dry weight ± SE. n1–n3 denote biological replicate samples. ND, not detectable.
Table 4.
BMAA levels found in invertebrates (mollusks) on Sweden's west coast
| BMAA, μg/g dry weight |
|||
| n1 | n2 | n3 | |
| Mytilus edulis (common mussel) | 0.201 ± 0.07 | 0.185 ± 0.04 | 0.151 ± 0.009 |
| Ostrea edulis (common oyster) | 0.140 ± 0.006 | 0.022 ± 0.003 | 0.006 ± 0.0002 |
BMAA levels in invertebrates collected from the Kattegat Sea (west coast of Sweden; Fig. S1, location 3). BMAA levels are expressed as μg BMAA/g dry weight ± SE. BMAA levels in mussels and oysters are calculated from technical replicates (n = 3). ND, not detectable.
Discussion
Using our recently developed extraction and HPLC-MS/MS analysis method for robust BMAA identification (30), we were able to demonstrate the presence and spread of the cyanobacterial toxin BMAA outside the tropical terrestrial ecosystem of Guam. This conclusion is based on the finding of reoccurring biosynthesis of BMAA in a temperate aquatic ecosystem dominated by large BMAA-producing bloom-forming cyanobacteria collected at different time points during two consecutive growth seasons (2007 and 2008). Because BMAA was found in the cyanobacterial samples examined, with no pronounced variations linked to species composition and season, BMAA biosynthesis is proposed to be a constitutive feature of these primary producers of the Baltic Sea ecosystem. In addition, analyses of organisms at higher trophic levels sharing the same ecosystem clearly showed the spread of BMAA outside the cyanobacterial radiation. This might suggest that BMAA is transferred from cyanobacteria via zooplankton to organisms at higher trophic levels (fishes) inhabiting both pelagic and benthic water masses, thereby representing different niches in a cyanobacterial based food web.
Based on dry weight, and in agreement with earlier studies (35, 36), BMAA levels in the cyanobacteria in the Baltic Sea were low compared with those found in the terrestrial ecosystem of Guam. Compared with the BMAA levels in the symbiotic cyanobacterium (Nostoc) in the roots of cycads in the Guam ecosystem (19), those of the Baltic Sea cyanobacteria are two orders of magnitude lower (0.001–0.3 μg BMAA/g dry weight). These highly divergent BMAA levels might be explained by both biological and methodological differences. First, Guam is a tropical terrestrial ecosystem, and the cyanobacteria examined are symbiotic and well protected and fed with all of the nutrients required by the host plant (C. micronesica). In contrast, the planktonic cyanobacteria in the temperate aquatic ecosystem of the Baltic Sea belong to different genera, and they function as the primary producers in this extremely nutrient-poor and more “open” environment. Moreover, our analyses are based on and quantified using HPLC-MS/MS, whereas the BMAA data from Guam were based primarily on fluorescence detection (HPLC-FD) analyses, with nonselective detection and a potentially increased risk of coeluting substances (30).
BMAA levels were higher in zooplankton, which feed mainly on cyanobacteria (37), compared with the cyanobacteria. Even higher BMAA levels were found in some of the fish species examined (up to 200-fold higher levels in some fish tissues), suggesting a bioaccumulation of BMAA within this ecosystem. A remarkable finding was the high BMAA levels in the bottom-dwelling fish species S. maximus, T. quadricornis, and O. eperlanus and in the water filter-feeding mollusks M. edulis and O. edulis. The high BMAA levels in the bottom-feeding fish species might originate from descending and BMAA-decomposing cyanobacterial populations on which zooplankton and smaller fish feed (Fig. S3) (38). This might be the case for S. maximus, a common sediment flatfish feeding on bottom-living fishes such as O. eperlanus and T. quadricornis and, to a lesser extent, on crustaceans (39). O. eperlanus lives in coastal zones and moves to shallower shore areas for reproduction. The larvae feed mainly on copepods, other zooplankton, and phytoplankton (e.g., cyanobacteria, diatoms), whereas older O. eperlanus feed on shrimps and small crustaceans (40). BMAA was also found at high levels in brain tissue of T. quadricornis, a bottom-living fish that feeds mainly on mollusks, insects, and larvae (41). An unexpected finding was the lack of BMAA in fish species within the pelagic (bloom-forming) zone, such as S. lucioperca. This also was the case in the pelagic S. salar, collected in the North Atlantic. This clearly demonstrates that BMAA is not found in all eukaryotic organisms at a given time and suggests the action of regulatory mechanisms potentially related to the ecological niche, age, and lifestyle of the individual eukaryotic organisms.
The role of BMAA in cyanobacteria, as is the role of any cyanobacterial toxin, is unknown, as is indeed the effects of BMAA on any of the other organisms examined, for instance the implications for fishes with high levels of this neurotoxin in their brains (e.g., S. maximus). Earlier analyses have suggested that BMAA occurs primarily in a protein-bound/protein associated fraction in cyanobacteria, and that BMAA is released only after hydrolysis (4). The protein-associated BMAA pool also has been suggested to function as an endogenous neurotoxic “reservoir,” which is slowly released during protein metabolism (4). Both free and protein-associated BMAA also have been found in several tissue types, including brain, muscle, skin, intestine, kidney, and fur, in flying foxes (Pteropus mariannus and P. tonganus) (42). CNS uptake and long-term and short-term neurotoxic effects of BMAA have been demonstrated in rodents after perinatal administration (13, 43). The mechanisms of entry to the CNS of BMAA remain unclear, however. Even though it is water-soluble, BMAA might cross the blood-brain barrier, at least under certain circumstances (44, 45); other routes, such as the retrograde axonal transport system, should be considered as well (46). Thus, studies on the biosynthesis, distribution, turnover, and consequences of BMAA in cyanobacteria, as well as in vertebrates sharing their environment, are merited.
Another important question is to what extent the BMAA in Baltic Sea organisms is a threat to humans living in the catchment area. All of the fish species analyzed except T. quadricornis are frequently caught for human consumption, and low but detectable levels were found in the pelagic species C. harengus, considered as a delicacy in Sweden (Table 3). Because the highest BMAA levels were discovered in fish brains (Table 3), which are not normally consumed, transfer of BMAA to the human population might be restricted. Indeed, out of the six fish genera examined, C. lavaretus (a common whitefish) was the only that contained an appreciable BMAA level in its muscle tissue (7-fold higher than that in cyanobacteria).
Unexpectedly, comparatively high BMAA levels were found in mussels and oysters harvested on Sweden's west coast (Table 4), a fully marine ecosystem with potentially only a benthic “nonblooming” cyanobacterial population. However, the filter-feeding lifestyle of the mollusks exposes these organisms to large quantities of phytoplankton, possibly including unicellular cyanobacteria as well. On average, a 5- to 6-cm mussel filters up to 8 L of water per hour and digests all phytoplankton (cyanobacteria and diatoms) <0.5 mm in size. The average size of cyanobacteria is <25 μm (47). Given the rapidly increasing cultivation and human consumption of filter-feeding aquatic organisms on a global scale, consumption might act as a route of entry of BMAA into the human population.
The co-occurrence of BMAA and other cyanotoxins might further increase the risk of motor neuron toxicity (12, 36). Cyanotoxins, such as the well-studied hepatotoxins nodularin and microcystin, also have been found at high concentrations in mussels (M. edulis and M. balthica) and flounder (Platichthys flesus) (48, 49). This suggests that cyanobacterial toxins might follow the same routes of bioaccumulation through aquatic food webs and stresses the need for additional research on the accumulative effects of cyanobacterial toxins (50).
Taken together, the data presented here demonstrate the widespread natural occurrence of the potential neurotoxin BMAA in prokaryotic and eukaryotic organisms living in a temperate aquatic ecosystem, that is, an ecosystem located outside the tropical terrestrial ecosystem of Guam, where BMAA was first discovered. Because this finding raises questions of great potential relevance to public health, it is now urgent to extend the analyses for BMAA to additional ecologically and economically important eukaryotic organisms, such as fish and filter-feeders, and to examine the extent to which BMAA is transferred and bioaccumulated through natural food webs. This need is further emphasized by the increase in cyanobacterial blooms (38, 51) caused by anthropogenic activities [e.g., eutrophication (52)] and recent observations of increased incidence rates of ALS in Sweden (53). The occurrence and transfer of BMAA within a temperate ecosystem might also have far-reaching implications for other aquatic ecosystems worldwide (limnic and marine), and possibly explain the discovery of BMAA in the brain tissue of Alzheimer's patients in Canada (19) and ALS and Alzheimer's patients in the United States (14).
Material and Methods
Sample Collection.
Populations of cyanobacteria and zooplankton were collected in June through September 2007 and 2008 outside the island of Askö, located in the southern archipelago of Stockholm, Sweden, at sample station B1 (Fig. S1). Colonies of cyanobacteria and zooplankton were collected from surface drift tows using a HydroBios net (0.5 diameter, 90-μm mesh). Collections were performed at noon on each sampling day. In addition, samples of cyanobacteria from the open Baltic Sea were collected at station BY31 using plankton nets (Fig. S1). The various fish species examined were bought either directly from fishermen or at fish markets in Stockholm and were caught at different sample points (Fig. S1, locations 2, 3, and 4). The fish species from the Baltic Sea were selected to represent the pelagic or benthic aquatic zone and important species in the food chain or used directly for human consumption. The mussels and oysters analyzed were farmed for human consumption on the west coast of Sweden.
Sample Preparation.
The cyanobacteria and zooplankton populations collected were separated using a light trap (34). Each population of cyanobacteria and zooplankton was filtered and placed in liquid nitrogen for further analysis. The cyanobacteria were identified by light microscopy with an Olympus BX60.
Muscle and brain tissue samples from the fish were dissected using scalpels and frozen in liquid nitrogen until use. The soft bodies of the mussels and oysters were removed from their shells and stored at −80 °C until use.
BMAA Extraction.
BMAA extraction was performed according to the method described by Eriksson et al. (27) with minor modifications. Cyanobacterial samples were initially dissolved in 600 μL of 80% methanol (80/20 methanol/water). Cell lysis was ensured through three freeze/thaw cycles of the cell suspension in liquid nitrogen, followed by sonication (Bandelin Sonoplus HP 2070) in three cycles of 40 s at 70% intensity. To minimize protein degradation, all samples were kept in an ice-water bath during sonication cycles. Protein concentrations of the samples were determined using a BioRad RC/DC Kit. Samples were subsequently hydrolyzed in 6 M HCl for 20 h at 110 °C. Particulate matter was removed by ultrafiltration (Ultra-Free-MC; Millipore). The 6 M HCl was then allowed to evaporate, and samples were reconstituted in 20 mM HCl.
Before MS analysis, the samples were purified by solid-phase extraction (ISOLUTE HCX-3, 100 mg; Biotage) using the cleanup procedure described by Spacil et al. (30). First, 2.5 mg protein from each sample was passed through the cartridge. The adsorbed material was stepwise eluted with 0.1% formic acid/25% MeOH, followed by 2% NH4OH/MeOH. The eluted fractions were allowed to evaporate, and the remaining pellet was subsequently reconstituted in 20 μL of 0.1% formic acid. All samples were derivatized with AccQ-Tag (Waters) before analysis by HPLC-MS/MS.
HPLC/MS/MS Analysis.
HPLC-MS/MS analyses of BMAA were performed using an Applied Biosystems API 2000TM LC/MS/MS analyzer consisting of a binary pump (LC-10Advp), an autosampler (SIL-10Advp), and a benchtop triple quadrupole mass analyzer (API 2000). Settings were as reported by Spacil et al. (30).
Supplementary Material
Acknowledgments
This research was supported by the Foundation for Strategic Environmental Research; the Swedish Research Council for Environmental, Agricultural and Spatial Planning; the K. and A. Wallenberg Foundation; and the Czech Ministry of Education.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914417107/-/DCSupplemental.
References
- 1.Jones AC, Gu L, Sorrels CM, Sherman DH, Gerwick WH. New tricks from ancient algae: Natural products biosynthesis in marine cyanobacteria. Curr Opin Chem Biol. 2009;13:216–223. doi: 10.1016/j.cbpa.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welker M, von Döhren H. Cyanobacterial peptides: Nature's own combinatorial biosynthesis. FEMS Microbiol Rev. 2006;30:530–563. doi: 10.1111/j.1574-6976.2006.00022.x. [DOI] [PubMed] [Google Scholar]
- 3.Sivonen K, Börner T. In: The Cyanobacteria: Molecular Biology, Genomics and Evolution. Herrero A, Flores E, editors. Norfolk, UK: Caister Academic Press; 2008. pp. 159–197. [Google Scholar]
- 4.Murch SJ, Cox PA, Banack SA. A mechanism for slow release of biomagnified cyanobacterial neurotoxins and neurodegenerative disease in Guam. Proc Natl Acad Sci USA. 2004;101:12228–12231. doi: 10.1073/pnas.0404926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox PA, et al. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc Natl Acad Sci USA. 2005;102:5074–5078. doi: 10.1073/pnas.0501526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold A, Edgren DC, Palladino VS. Amyotrophic lateral sclerosis: Fifty cases observed on Guam. J Nerv Ment Dis. 1953;117:135–139. [PubMed] [Google Scholar]
- 7.Spencer PS, et al. Guam amyotrophic lateral sclerosis–parkinsonism–dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- 8.Hirano A, Malamud N, Elizan TS, Kurland LT. Amyotrophic lateral sclerosis and Parkinsonism-dementia complex on Guam: Further pathologic studies. Arch Neurol. 1966;15:35–51. doi: 10.1001/archneur.1966.00470130039004. [DOI] [PubMed] [Google Scholar]
- 9.Garruto RM, Yase Y. Neurodegenerative disorders of the western Pacific: The search for mechanisms of pathogenesis. Trends Neurosci. 1986;9:368–374. [Google Scholar]
- 10.Spencer PS, Nunn PB, Hugon J, Ludolph A, Roy DN. Motorneurone disease on Guam: Possible role of a food neurotoxin. Lancet. 1986;1:965. doi: 10.1016/s0140-6736(86)91059-7. [DOI] [PubMed] [Google Scholar]
- 11.Rao SD, Banack SA, Cox PA, Weiss JH. BMAA selectively injures motor neurons via AMPA/kainate receptor activation. Exp Neurol. 2006;201:244–252. doi: 10.1016/j.expneurol.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Lobner D, Piana PMT, Salous AK, Peoples RW. beta-N-methylamino-L-alanine enhances neurotoxicity through multiple mechanisms. Neurobiol Dis. 2007;25:360–366. doi: 10.1016/j.nbd.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlsson O, Lindquist NG, Brittebo EB, Roman E. Selective brain uptake and behavioral effects of the cyanobacterial toxin BMAA (beta-N-methylamino-L-alanine) following neonatal administration to rodents. Toxicol Sci. 2009;109:286–295. doi: 10.1093/toxsci/kfp062. [DOI] [PubMed] [Google Scholar]
- 14.Pablo J, et al. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer's disease. Acta Neurol Scand. 2009;120:216–225. doi: 10.1111/j.1600-0404.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- 15.Murch SJ, Cox PA, Banack SA, Steele JC, Sacks OW. Occurrence of beta-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol Scand. 2004;110:267–269. doi: 10.1111/j.1600-0404.2004.00320.x. [DOI] [PubMed] [Google Scholar]
- 16.Duncan MW, Steele JC, Kopin IJ, Markey SP. 2-Amino-3-(methylamino)-propanoic acid (BMAA) in cycad flour: An unlikely cause of amyotrophic lateral sclerosis and parkinsonism-dementia of Guam. Neurology. 1990;40:767–772. doi: 10.1212/wnl.40.5.767. [DOI] [PubMed] [Google Scholar]
- 17.Cox PA, Sacks OW. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology. 2002;58:956–959. doi: 10.1212/wnl.58.6.956. [DOI] [PubMed] [Google Scholar]
- 18.Vessey JK, Pawlowski K, Bergman B. Root-based N2-fixing symbioses: Legumes, actinorhizal plants, Parasponia sp, and cycads. Plant Soil. 2005;274:51–78. [Google Scholar]
- 19.Cox PA, Banack SA, Murch SJ. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc Natl Acad Sci USA. 2003;100:13380–13383. doi: 10.1073/pnas.2235808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moura S, Ultramari Mde A, de Paula DM, Yonamine M, Pinto E. (1)H NMR determination of beta-N-methylamino-l-alanine (l-BMAA) in environmental and biological samples. Toxicon. 2009;53:578–583. doi: 10.1016/j.toxicon.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Li A, et al. Detection of the neurotoxin BMAA within cyanobacteria isolated from freshwater in China. Toxicon. 2009;55:947–953. doi: 10.1016/j.toxicon.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Krüger T, Mönch B, Oppenhäuser S, Luckas B. LC-MS/MS determination of the isomeric neurotoxins BMAA (beta-N-methylamino-l-alanine) and DAB (2,4-diaminobutyric acid) in cyanobacteria and seeds of Cycas revoluta and Lathyrus latifolius. Toxicon. 2009;55:547–557. doi: 10.1016/j.toxicon.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Kubo T, Kato N, Hosoya K, Kaya K. Effective determination method for a cyanobacterial neurotoxin, beta-N-methylamino-l-alanine. Toxicon. 2008;51:1264–1268. doi: 10.1016/j.toxicon.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Rosen J, Hellenäs KE. Determination of the neurotoxin BMAA (beta-N-methylamino-L-alanine) in cycad seed and cyanobacteria by LC-MS/MS (liquid chromatography tandem mass spectrometry) Analyst (Lond) 2008;133:1785–1789. doi: 10.1039/b809231a. [DOI] [PubMed] [Google Scholar]
- 25.Montine TJ, Li K, Perl DP, Galasko D. Lack of beta-methylamino-l-alanine in brain from controls, AD, or Chamorros with PDC. Neurology. 2005;65:768–769. doi: 10.1212/01.wnl.0000174523.62022.52. [DOI] [PubMed] [Google Scholar]
- 26.Snyder LR, et al. Lack of cerebral BMAA in human cerebral cortex. Neurology. 2009;72:1360–1361. doi: 10.1212/WNL.0b013e3181a0fed1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksson J, Jonasson S, Papaefthimiou D, Rasmussen U, Bergman B. Improving derivatization efficiency of BMAA utilizing AccQ-Tag((R)) in a complex cyanobacterial matrix. Amino Acids. 2009;36:43–48. doi: 10.1007/s00726-007-0023-4. [DOI] [PubMed] [Google Scholar]
- 28.Banack SA, Johnson HE, Cheng R, Cox PA. Production of the neurotoxin BMAA by a marine cyanobacterium. Mar Drugs. 2007;5:180–196. doi: 10.3390/md504180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banack SA, Cox PA. Distribution of the neurotoxic nonprotein amino acid BMAA in Cycas micronesica. Bot J Linn Soc. 2003;143:165–168. [Google Scholar]
- 30.Spacil Z, et al. Analytical protocol for identification of BMAA and DAB in biological samples. Analyst (Lond) 2010;135:127–132. doi: 10.1039/b921048b. [DOI] [PubMed] [Google Scholar]
- 31.Boesch D, Hecky R, Chair CO, Schindler D, Seitzinger S. Report 5509. Swedish Environmental Protection Agency; 2006. pp. 1–68. [Google Scholar]
- 32.Gallon JR, et al. Maximum rates of N2 fixation and primary production are out of phase in a developing cyanobacterial bloom in the Baltic Sea. Limnol Oceanogr. 2002;47:1514–1521. [Google Scholar]
- 33.Degerholm J, Gundersen K, Bergman B, Söderbäck E. Phosphorus-limited growth dynamics in two Baltic Sea cyanobacteria, Nodularia sp. and Aphanizomenon sp. FEMS Microbiol Ecol. 2006;58:323–332. doi: 10.1111/j.1574-6941.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 34.Larsson U, Blomqqvist S, Abrahamsson B. A new sediment trap system. Mar Ecol Prog Ser. 1986;31:205–207. [Google Scholar]
- 35.Esterhuizen M, Downing TG. Beta-N-methylamino-L-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicol Environ Saf. 2008;71:309–313. doi: 10.1016/j.ecoenv.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Metcalf JS, et al. Co-occurrence of beta-N-methylamino-L-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environ Microbiol. 2008;10:702–708. doi: 10.1111/j.1462-2920.2007.01492.x. [DOI] [PubMed] [Google Scholar]
- 37.Turner JT, Tester PA. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol Oceanogr. 1997;42:1203–1214. [Google Scholar]
- 38.Karjalainen M, et al. Ecosystem consequences of cyanobacteria in the northern Baltic Sea. Ambio. 2007;36:195–202. doi: 10.1579/0044-7447(2007)36[195:ecocit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Murua H, Saborido-Rey F. Female reproductive strategies of marine fish species of the North Atlantic. J Northwest Atl Fish Sci. 2003;33:23–31. [Google Scholar]
- 40.Nyberg P, Bergstrand E, Degerman E, Enderlein O. Recruitment of pelagic fish in an unstable climate: Studies in Sweden's four largest lakes. Ambio. 2001;30:559–564. doi: 10.1579/0044-7447-30.8.559. [DOI] [PubMed] [Google Scholar]
- 41.Quéro J-C. Fishes of the Northeastern Atlantic and the Mediterranean. Paris: UNESCO; 1986. [Google Scholar]
- 42.Banack SA, Murch SJ, Cox PA. Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands. J Ethnopharmacol. 2006;106:97–104. doi: 10.1016/j.jep.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 43.Karlsson O, Roman E, Brittebo EB. Long-term cognitive impairments in adult rats treated neonatally with beta-N-methylamino-L-alanine. Toxicol Sci. 2009;112:185–195. doi: 10.1093/toxsci/kfp196. [DOI] [PubMed] [Google Scholar]
- 44.Smith QR, Nagura H, Takada Y, Duncan MW. Facilitated transport of the neurotoxin, beta-N-methylamino-L-alanine, across the blood-brain barrier. J Neurochem. 1992;58:1330–1337. doi: 10.1111/j.1471-4159.1992.tb11346.x. [DOI] [PubMed] [Google Scholar]
- 45.Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Histol Histopathol. 2004;19:535–564. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- 46.Loberg EM, Torvik A. Uptake of plasma proteins into damaged neurons: An experimental study on cryogenic lesions in rats. Acta Neuropathol. 1991;81:479–485. doi: 10.1007/BF00310126. [DOI] [PubMed] [Google Scholar]
- 47.Lindahl O, et al. Improving marine water quality by mussel farming: A profitable solution for Swedish society. Ambio. 2005;34:131–138. [PubMed] [Google Scholar]
- 48.Sipiä VO, et al. Bioaccumulation and detoxication of nodularin in tissues of flounder (Platichthys flesus), mussels (Mytilus edulis, Dreissena polymorpha), and clams (Macoma balthica) from the northern Baltic Sea. Ecotoxicol Environ Saf. 2002;53:305–311. doi: 10.1006/eesa.2002.2222. [DOI] [PubMed] [Google Scholar]
- 49.Gérard C, et al. Influence of toxic cyanobacteria on community structure and microcystin accumulation of freshwater molluscs. Environ Pollut. 2009;157:609–617. doi: 10.1016/j.envpol.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Sipiä VO, et al. Analysis of nodularin-R in eider (Somateria mollissima), roach (Rutilus rutilus L.), and flounder (Platichthys flesus L.) liver and muscle samples from the western Gulf of Finland, northern Baltic Sea. Environ Toxicol Chem. 2006;25:2834–2839. doi: 10.1897/06-185r.1. [DOI] [PubMed] [Google Scholar]
- 51.Jozwiak T, Mazur-Marzec H, Plinski M. Cyanobacterial blooms in the Gulf of Gdansk (southern Baltic): the main effect of eutrophication. Oceanol Hydrobiol Stud. 2008;37:115–121. [Google Scholar]
- 52.Larsson U, Hajdu S, Walve J, Elmgren R. Baltic Sea nitrogen fixation estimated from the summer increase in upper mixed layer total nitrogen. Limnol Oceanogr. 2001;46:811–820. [Google Scholar]
- 53.Fang F, et al. Amyotrophic lateral sclerosis in Sweden, 1991–2005. Arch Neurol. 2009;66:515–519. doi: 10.1001/archneurol.2009.13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.