Abstract
AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate) recep-tors desensitize rapidly and completely in the continued presence of their endogenous ligand glutamate; however, it is not clear what role AMPA receptor desensitization plays in the brain. We generated a knock-in mouse in which a single amino acid residue, which controls desensitization, was mutated in the GluA2 (GluR2) receptor subunit (GluA2L483Y). This mutation was homozygous lethal. However, mice carrying a single mutated allele, GluA2L483Y/wt, survived past birth, but displayed severe and progressive neurological deficits including seizures and, ultimately, increased mortality. The expression of the AMPA receptor subunits GluA1 and GluA2 was decreased, whereas NMDA receptor protein expression was increased in GluA2L483Y/wt mice. Despite this, basal synaptic transmission and plasticity in the hippocampus were largely unaffected, suggesting that neurons preferentially target receptors to synapses to normalize synaptic weight. We found no gross neuroanatomical alterations in GluA2L483Y/wt mice. Moreover, there was no accumulation of AMPA receptor subunits in intracellular compartments, suggesting that folding and assembly of AMPA receptors are not affected by this mutation. Interestingly, EPSC paired pulse ratios in the CA1 were enhanced without a change in synaptic release probability, demonstrating that postsynaptic receptor properties can contribute to facilitation. The dramatic phenotype observed in this study by the introduction of a single amino acid change demonstrates an essential role in vivo for AMPA receptor desensitization.
Keywords: GluA2 (GluR2), hippocampus, seizures, knock-in mouse
AMPA receptors are tetramers assembled from the four receptor subunits GluA1–GluA4 (GluR1–4) (1). These receptors are activated by their endogenous ligand glutamate, and rapidly undergo desensitization within milliseconds of glutamate binding. Desensitization involves a conformational change of the receptor complex that allows closure of the channel gate while glutamate remains bound to the receptor (2). Synaptic currents are predominantly mediated by AMPA receptors at most excitatory synapses; therefore there has been interest in the development of pharmacological agents that enhance AMPA receptor function by limiting receptor deactivation and desensitization (3). There are many clear examples of synapses at which postsynaptic receptor desensitization plays a major role in synaptic depression (4–6). Many of these synapses are specialized structures in which glutamate remains in the synaptic cleft for prolonged periods of time during normal operation of the synapse (7). In contrast, at synapses where cleft glutamate is cleared rapidly or where AMPA receptor stoichiometry has become specialized to support high frequency transmission, there is little evidence that synaptic receptor desensitization has much influence on shaping the kinetics of transmission, and it is likely that receptor deactivation is the primary determinant of EPSC time-course (8–10).
To determine the importance of AMPA receptor desensitization in vivo, we introduced the nondesensitizing L483Y mutation into the mouse gene encoding GluA2 (Gria2) (11). This mutation turned out to be homozygous lethal; however, heterozygous mice (GluA2L483Y/wt) were viable despite a severe and progressive neurological and developmental phenotype that included significant runting, abnormal gait, development of progressively severe seizures, and early mortality in the third postnatal weeks. Overall the very severe phenotype observed by a single amino acid alteration in the GluA2 receptor subunit indicates that AMPA receptor desensitization is critical for the viability of the animal and function of the CNS.
Results
Generation and Phenotype of GluA2L483Y/wt Mice.
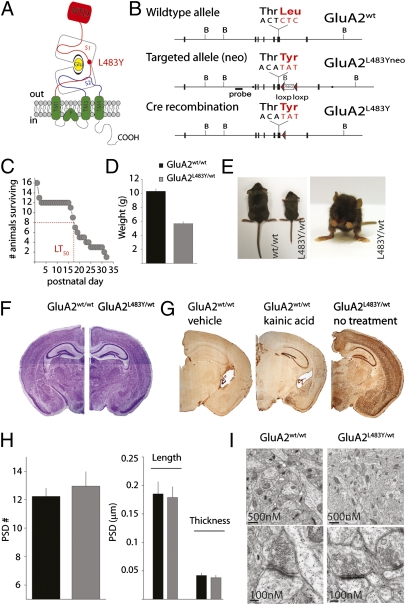
A single amino acid exchange in the S1 domain of the AMPA receptor subunits eliminates desensitization of recombinant receptors expressed in heterologous systems (11) (Fig. 1A). To introduce this mutation into the mouse genome, we generated a targeting construct containing exon 11 and the surrounding region of Gria2 with several mutated nucleotides to code for a tyrosine residue at position 483. In addition, a loxP flanked neomycin (neo) selection cassette was incorporated into the intronic region downstream of this exon (Fig. 1B). The targeting construct was integrated into mouse embryonic stem cells by standard techniques of homologous recombination to generate mice carrying the mutated allele GluA2L483Yneo. Heterozygous GluA2L483Yneo/wt mice, which were viable and fertile, were intercrossed with the intention of producing homozygote mutants; however, no offspring were generated that carried two mutant alleles. To remove the neo cassette from the intronic region of Gria2, GluA2L483Yneo/wt mice were crossed with a “Cre-deleter” line that expressed Cre recombinase in all tissues including the germ-line (CMV-Cre) (12). This cross produced offspring carrying a single mutated allele without the neo cassette (GluA2L483Y/wt). Surprisingly, removing the neo cassette uncovered a dramatic phenotype in heterozygote animals, suggesting that the presence of the neo cassette had caused unequal expression of the mutant allele; this was supported by Western analysis, which demonstrated that GluA2 expression is decreased in GluA2L483Yneo/wt mice (Fig. S1). Similar reductions in allele expression by intronic insertion of a neomycin cassette have been reported previously (13, 14).
Fig. 1.
Generation and characterization of GluA2L483Y mice. (A) Cartoon representation of the AMPA receptor subunit GluA2 depicting the position of the L483Y. (B) Schematic representation of the Gria2 genomic locus, targeting construct and targeted allele with position of codon for leucine 483 in exon 11. (C) Mortality curve for GluA2L483Y/wt mice. (D) Body weights comparisons in P15–P17 mice. (E) Comparison of size of GluA2wt/wt and GluA2L483Y/wt littermate mice at P15 (Left). Altered gait of GluA2L483Y/wt mice (Right). (F) Nissl stained coronal sections. (G) C-fos staining in section from vehicle treated GluA2wt/wt, kainic acid–treated GluA2wt/wt, and nontreated (P17) GluA2L483Y/wt mice. (H) Analysis of PSD density (number of PSDs per 32.5 μm2) and size (length and thickness) in GluA2L483Y/wt mice. (I) Example electron micrographs from hippocampal CA1 area sections.
GluA2L483Y/wt offspring were runted in comparison with their wild-type (WT) littermates with an approximate 45% decrease in body weight at postnatal days (P) 15–17 (Fig. 1 D and E). Moreover, GluA2L483Y/wt animals were prone to progressively severe spontaneous seizures. At P14, we observed no seizures when animals were observed for a 1-h period. At P16 and beyond, GluA2L483Y/wt mice exhibited multiple spontaneous generalized clonic/tonic convulsions when observed over a similar time period. Examination of c-fos expression in P16-18 mice demonstrated activation of neurons throughout the brain. C-fos reactivity was more widespread in the brains of GluA2L483Y/wt mice, which had been observed to have multiple seizures, than in WT animals that had undergone acute seizures induced by kainic acid injection (Fig. 1G). GluA2L483Y/wt mice were monitored from birth and it was found that the LT50 (the time to 50% lethality) was 17.5 days. Most mice died in the third postnatal week, with very few surviving past P30 (Fig. 1C). In Nissl-stained sections we observed no obvious alterations in cell layers or density of GluA2L483Y/wt mice (Fig. 1F), and analysis of synaptic structure at the electron microscopic level did not reveal any alterations in the density or size of asymmetric excitatory synapses in area CA1 of the hippocampus (Fig. 1 H and I).
Glutamate Receptor Expression Is Altered in GluA2L483Y/wt Animals.
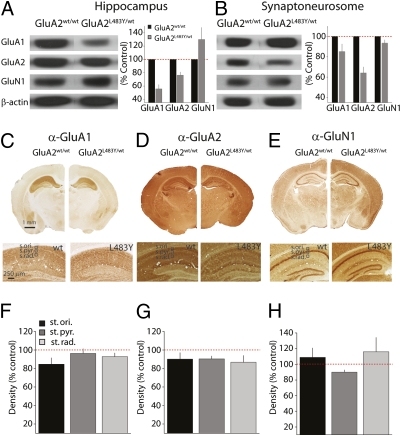
Western blot analysis of whole hippocampal homogenate demonstrated a clear reduction in the amount of GluA1 (44 ± 6.4%, n = 6, P < 0.001), and to a lesser degree GluA2 receptor subunit protein (24 ± 4.6%, n = 6, P < 0.01) in GluA2L483Y/wt (Fig. 2A). Membrane receptors were also reduced in the isolated synaptoneurosome fraction. In this case, we observed a clear reduction in GluA2 receptor protein (36 ± 5.8%, n = 6, P < 0.05) and a smaller decrease in GluA1 protein (15 ± 7.3%, n = 6, P < 0.05) (Fig. 2B). Because AMPA and NMDA receptors are colocalized at synapses and their relative contributions to synaptic signaling and expression are tightly linked, we also quantified the relative amount of GluN1 protein. Surprisingly, we observed an up-regulation of GluN1 expression in whole hippocampus (130 ± 18%, n = 6, P < 0.05), but again only a small alteration in the synaptoneurosome fraction (93 ± 3.2%, n = 6, P < 0.05) (Fig. 2B). These data suggest that multiple compensatory alterations in glutamate receptor expression occur in GluA2L483Y/wt mice. To validate these changes in receptor expression observed with Western blot analysis, we performed immunohistochemical analysis on sections from GluA2L483Y/wt and GluA2wt/wt. Using quantitative measurements of labeling density in sections from WT and mutant animals we compared expression of GluA1, GluA2, and GluN1 in the hippocampal regions stratum oriens (s.ori.), stratum pyramidale (s.pyr.), and stratum radiatum (s. rad.) (Fig. 2 C–H). Although we did not see as clear alterations in antibody density in sections as we had in protein blots, there was a reduction in hippocampal GluA1 and GluA2 and a small increase in GluN1 consistent with our preliminary finding. Overall these results demonstrate that introduction of the mutant allele causes a drastic alteration in glutamate receptor expression in GluA2L483Y/wt mice.
Fig. 2.
Analysis of glutamate receptor expression in GluA2L483Y/wt mice. (A) Western blots for GluA1, GluA2, and GluN1 from hippocampal homogenates. (B) Western blots for glutamate receptor expression in the synaptoneurosome fraction. (C) Immunohistochemical analysis of GluA1 expression in coronal sections from GluA2wt/wt and GluA2L483Y/wt mice. (Lower) Hippocampal sections. (D) Expression of GluA2 in coronal section. (E) Expression of GluN1 (F–H) Densitometric analysis of antibody staining in hippocampal sections. Density analysis was carried out by defining three regions of interest in hippocampal section in the stratum oriens (st. oriens), stratum pyramidale (st. pyr.), and stratum radiatum (st. rad.) in the CA1 region.
Glutamate Receptors Are Not Sequestered in the ER in GluA2L483Y/wt Mice.
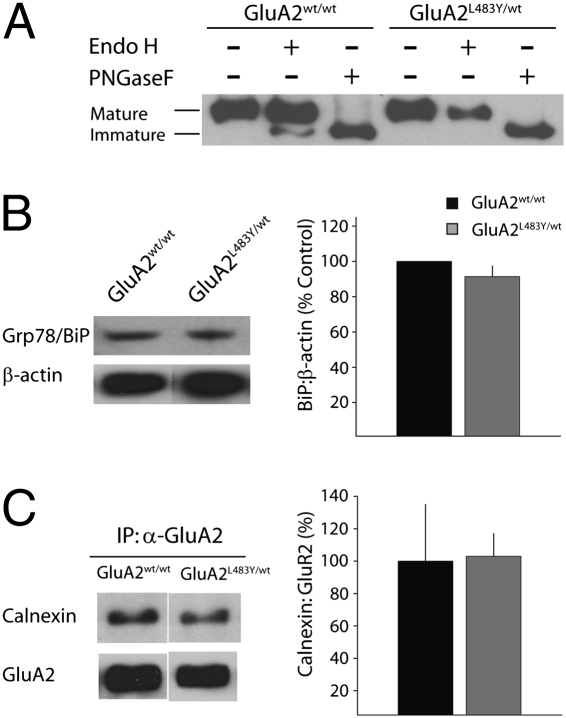
The expression of glutamate receptors is controlled by mechanisms that regulate biogenesis and assembly of membrane proteins in the endoplasmic reticulum (ER). Misfolded or improperly assembled receptors are not further trafficked into the secretory pathway, becoming “trapped” in the ER. A previous study demonstrated that when GluA2(L483Y) was exogenously expressed in cultured neurons, receptor subunits assembled normally in tetrameric complexes but ER exit of this mutant receptor was reduced (15). Using an EndoH assay to determine the glycosylation state of GluA2 receptor subunits, we found that AMPA receptors did not appear to be accumulating in intracellular compartments in GluA2L483Y/wt mice. There was no increase in the immature ER resident GluA2 protein, and in fact we observed less immature protein, which is likely due to a decrease in the overall abundance of GluA2 (Fig. 3A). As an alternative approach to examine whether the intracellular trafficking of glutamate receptor subunits was disrupted in GluA2L483Y/wt mice, we examined ER stress proteins. The accumulation of misfolded proteins in the ER lumen induces prolonged ER stress, resulting in the activation of an adaptive response known as the unfolded protein response (UPR). This is commonly detected by an up-regulation of the ER chaperone protein Grp78/BiP (78-kDa glucose regulated protein). In quantitative Western blots for Grp78/BiP, we found no evidence of Grp78/BiP up-regulation in GluA2L483Y/wt mice (91 ± 5%, n = 6, P > 0.05) (Fig. 3B). In addition, we found no alteration in the amount of calnexin (a chaperone molecule that assists in folding and quality control of proteins as they pass along the secretory pathway), that coimmunoprecipitated with GluA2 in GluA2L483Y/wt mice (103 ± 14%, n = 3, P > 0.05) (Fig. 3C). Therefore, there is little evidence for the accumulation of glutamate receptor subunits in intracellular compartments in GluA2L483Y/wt mice.
Fig. 3.
Intracellular trafficking of GluA2 receptor subunits is not disrupted in GluA2L483Y/wt mice. (A) Representative example gel from Endo H assay. (B) Representative Western blot analysis of the expression of the ER chaperone protein Grp78/BiP (Left) and quantification of grouped data (Right). (C) Representative gel of interaction of GluA2 with chaperone molecule calnexin (Left). Quantified grouped data from three separate experiments (Right).
Glutamate Receptors Redistribute to Synaptic Sites in GluA2L483Y/wt Mice.
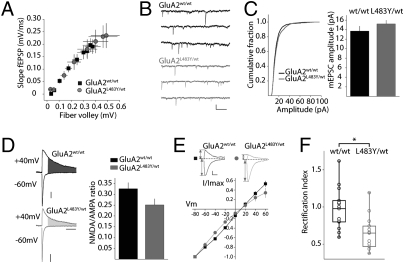
The overall expression of glutamate receptor subunits is altered in GluA2L483Y/wt mice, with a less dramatic change in expression observed in the synaptoneurosome fraction. To determine whether there were any alterations in basal levels of synaptic transmission in GluA2L483Y/wt mice, we performed several electrophysiological experiments in acute hippocampal slices. We chose to focus on CA1 synapses, as they are the canonical excitatory synapse and have been extensively characterized. Input output curves (I/O) were constructed by measuring the slope of the field excitatory postsynaptic potential (fEPSP) and the amplitude of the presynaptic fiber volley. We found no difference in the I/O curves between WT mice and mutant littermates, suggesting there are no gross alterations in synaptic transmission in area CA1 of the hippocampus (Fig. 4A). We also found that the mean amplitude of miniature excitatory postsynaptic currents (mEPSCs) in recordings from GluA2wt/wt mice was 14 ± 1 pA (n = 7), and was not different from the amplitude of mEPSCs recorded in GluA2L483Y/wt mice of the same age (15 ± 0.8 pA, n = 10, P > 0.05) (Fig. 4 B and C). These results suggest that the density of AMPA receptors at hippocampal synapses is largely unaltered despite a significant decrease in total expression of the two primary hippocampal AMPA receptor subunits.
Fig. 4.
Basal synaptic transmission in hippocampal CA1 synapses in GluA2L483Y/wt mice. (A) Input/output curve for synaptic transmission in CA1. (B) Representative traces of mEPSCs recorded from CA1 pyramidal neurons in GluA2wt/wt (top) and GluA2L483Y/wt mice (bottom). Calibration: 20pA, 500 ms. (C) Cumulative probability distribution of mEPSC amplitudes from all recordings (Left) and mean mEPSC amplitude (Right). (D) Representative EPSC traces recorded at −60 mV and +40mV in GluA2wt/wt (top) and GluA2L483Y/wt mice (bottom). The NMDA component was measured at +40 mV, 60 ms after the onset of the evoked outward current (shaded area). Calibration: 50 pA, 100 ms. (E) Current/voltage curves for AMPA EPSCs in GluA2wt/wt and GluA2L483Y/wt mice. Representative AMPA EPSC traces from WT and mutant animals recorded at −60 mV and +40 mV (in D-APV). Calibration: 50 pA, 10 ms. (F) Rectification index of AMPA EPSCs.
The relative proportion of the two major glutamate receptor types, NMDA and AMPA, is strongly correlated with the developmental maturity of excitatory synapses, and the potential ability of synapses to increase or decrease their efficacy (16). By measuring the AMPA component at hyperpolarized membrane potentials (−60 mV) and the NMDA component at depolarized membrane potentials (+40 mV) (Materials and Methods), we determined a mean NMDA/AMPA (N/A) ratio of 0.33 ± 0.03 (n = 10) in GluA2wt/wt mice (Fig. 4D). In recordings from GluA2L483Y/wt there was a small but significant reduction in the N/A ratio of CA1 synapses, (0.25 ± 0.03, n = 10, P < 0.05) (Fig. 4D). Viewed in light of the biochemical analysis and the mEPSC data, it seems likely that there is little alteration in synaptic AMPA receptor distribution at hippocampal synapses, but there is a small reduction in NMDA receptors.
The presence of edited GluA2 subunits in a heteromeric AMPA receptor complex confers a reduction in Ca2+ permeability and single-channel conductance upon AMPA receptors. GluA2-lacking receptors exhibit inwardly rectifying current–voltage (I/V) relationships (17) because outward current flow at depolarized membrane potentials is blocked by intracellular polyamines. GluA2 protein is decreased in GluA2L483Y/wt mice; therefore we sought to determine if there might be an abundance of synaptic receptors lacking the GluA2 subunit. AMPA receptor mediated EPSCs in WT mice exhibited linear I/V curves (Fig. 4E). To quantify the amount of rectification, we calculated the rectification index (RI) (Materials and Methods) of AMPA EPSCs in GluA2wt/wt as 1.0 ± 0.08 (n = 15) (Fig. 4 E and F). In interleaved recordings from littermate GluA2L483Y/wt mice the calculated RI was significantly reduced (0.7 ± 0.07, n = 12, P < 0.001) (Fig. 4 E and F). A closer look at the grouped data revealed a subset of recordings in which the RIs were closer to 0.5 (and well below the group mean). In these five recordings, the RI of AMPA EPSCs was 0.4 ± 0.02 (Fig. 4F). Thus it seems likely that there is an increase in the proportion of Ca2+-permeable AMPA receptors in GluA2L483Y/wt mice at some hippocampal CA1 synapses.
Extrasynaptic AMPA Receptor Density Is Reduced in GluA2L483Y/wt Mice.
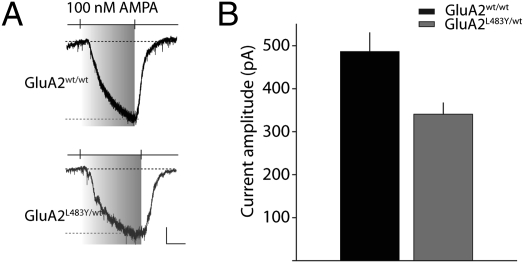
The electrophysiological analysis of hippocampal synaptic transmission found moderate alterations in synaptic glutamate receptors in GluA2L483Y/wt mice. In prior studies, it was noted that disrupting glutamate receptor expression by knockout of one of the AMPA receptor subunits (18), or by ablation of one of the accessory proteins associated with AMPA receptors (19), did not drastically alter synaptic AMPA receptor localization, but reduced the extrasynaptic pool of receptors. Although our biochemical analyses was consistent with a preferential redistribution of glutamate receptors to synaptic sites, we wanted to determine whether there was an overall reduction in the surface expression of AMPA receptors that would also support this model for a normalization of synaptic receptors. Application of the agonist AMPA (100 nM) elicited a current of amplitude 480 ± 44 pA (n = 15) in GluA2wt/wt mice (Fig. 5). In similar recordings from GluA2L483Y/wt mice the amplitude of the elicited current was smaller by ≈30% (340 ± 26 pA, n = 14, P < 0.001). Therefore, although the density of synaptic receptors is largely unaltered, there is a reduction in extrasynaptic AMPA receptors in GluA2L483Y/wt mice.
Fig. 5.
Agonist-induced AMPA receptor currents are reduced in GluA2L483Y/wt mice. (A) Representative traces from whole-cell recordings of CA1 pyramidal neurons. AMPA receptors are activated by bath application of 100 nM AMPA in the presence of other receptor antagonist and cyclothiazide. Calibration: 100 pA, 200 s. (B) Grouped data from all recordings.
Synaptic Plasticity in GluA2L483Y/wt Mice.
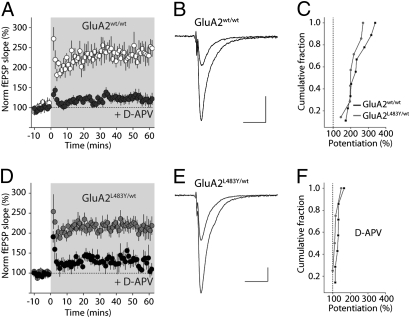
Previous work demonstrated that when the extrasynaptic pool of AMPA receptors was depleted in knockout mice, LTP in the CA1 region of the hippocampus was impaired (19, 18). This is likely due to the expression mechanisms of LTP, which involve the insertion of new receptors into synapses either by lateral diffusion along the membrane, or from intracellular compartments. Because of the reduced extrasynaptic receptor pool in GluA2L483Y/wt we tested whether the expression of LTP might be reduced in mutant mice. We recorded fEPSP in the CA1 and induced LTP using a tetanic stimulation (two trains at 100 Hz for 1 s, each train separated by 20 s). In GluA2wt/wt mice, the slope of the fEPSPs was potentiated on average by 240 ± 20%, n = 9, between 50 and 60 min posttetanus (Fig. 6 A and B). As expected, in interleaved experiments, inclusion of the NMDA receptor antagonist D-APV in the ACSF significantly blocked LTP (130 ± 6.3%, n = 7).
Fig. 6.
No impairment of hippocampal LTP in GluA2L483Y/wt mice. (A) Time-course of LTP in GluA2wt/wt mice. LTP is induced at time 0 using tetanic stimulation (shaded area). In interleaved experiments, LTP was blocked by addition of NMDA receptor antagonist D-APV. (B) Representative fEPSP traces before and 50 min after LTP induction. Calibration: 0.2 mV, 20 ms. (C) Cumulative probability distribution of LTP in all recordings from GluA2wt/wt and GluA2L483Y/wt mice. (D) Time-course of LTP in GluA2L483Y/wt. (E) Representative fEPSP traces from one experiment before and 50 min after LTP induction. (F) Cumulative probability distribution of all LTP recording in the presence of NMDA receptor antagonist D-APV.
Surprisingly, in recordings from littermate GluA2L483Y/wt, the same tetanic induction protocol resulted in LTP, which was not different in magnitude from that observed in WT recordings (210 ± 16%, n = 7, P > 0.05) (Fig. 6 D and E). When GluA2 is ablated in knockout mice, LTP is enhanced and a small NMDA receptor independent form of plasticity is observed in CA1 (20). To determine whether a similar LTP, presumably triggered by Ca2+-permeable receptors, was present in GluA2L483Y/wt mice, we performed further LTP experiments in the presence of D-APV. At 50–60 min posttetanus, the fEPSP was 120 ± 10% (n = 4) of control, which is not different from what we observed when NMDA receptors were inhibited in WT animals (n = 4, P > 0.05) (Fig. 6F). Therefore, NMDA receptor dependent LTP is unaffected in GluA2L483Y/wt mice despite a reduction in the extrasynaptic pool of AMPA receptors. Similarly, the small increase in Ca2+-permeable AMPA receptors in hippocampal synapses had no effect on NMDA receptor–independent synaptic plasticity.
Paired Pulse Facilitation Is Enhanced in GluA2L483Y/wt Mice.
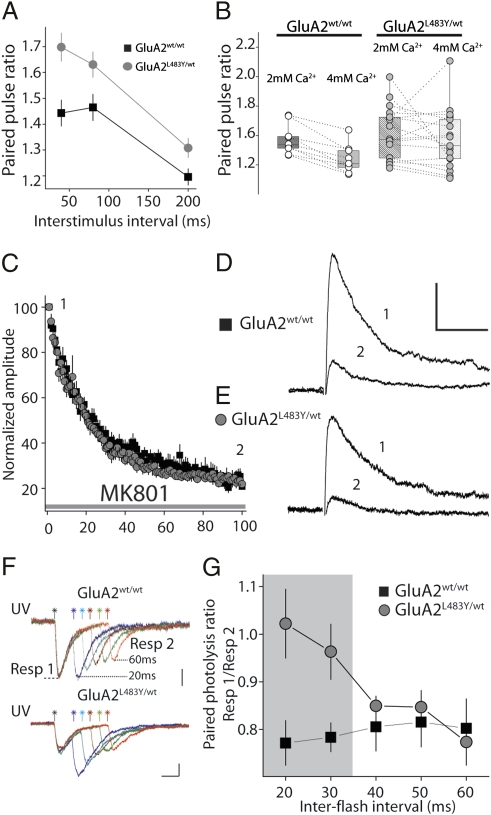
Repetitive sequential presynaptic activity at several synapses results in desensitization of postsynaptic AMPA receptors and contributes to short-term plasticity (4, 21, 5, 7). In hippocampal synapses prior work has demonstrated either a clear effect (22), or little evidence for postsynaptic desensitization during paired presynaptic stimuli (10). We performed paired pulse recordings at several interstimulus intervals in CA1 neurons to readdress this issue. In recordings from GluA2L483Y/wt mice, we found that the paired pulse ratio (PPR) was higher at all of the intervals tested (up to 200 ms) (Fig. 7A). In a subset of recordings, PPR (40 ms interstimulus interval) measured under conditions of increased release probability (4 mM Ca2+) was also higher in GluA2L483Y/wt (GluA2wt/wt: 1.3 ± 0.06, n = 9; GluA2L483Y/wt: 1.5 ± 0.1, n = 17, P < 0.05) (Fig. 7B). An alteration in PPR is generally interpreted as an altered initial release probability; however, postsynaptic receptor desensitization could also play a role in determining the degree of paired pulse facilitation (23). To distinguish between these two possibilities, we made comparison of the rate of block of synaptic NMDA receptors by the open channel blocker MK801, a common proxy for determining changes in glutamate release (24). In interleaved experiments, we found no difference in the progressive block of synaptic NMDA receptors in the CA1 of GluA2L483Y/wt mice and littermate controls (Fig. 7 C–E). Therefore, from this analysis, it appears that there is no evidence for altered release probability of excitatory synapses in the CA1 region of the hippocampus of mutant mice.
Fig. 7.
Receptor desensitization contributes to paired pulse ratio of synaptic and extrasynaptic receptors. (A) Paired pulse ratio of AMPA receptor EPSCs in hippocampus from GluA2wt/wt and GluA2L483Y/wt recordings. (B) Paired pulse ratio at 40 ms interval in low (2 mM) and high (4 mM) external Ca2+. (C) Progressive block of NMDA receptors by MK801 is not different in GluA2L483Y/wt mice. (D and E) Representative NMDA receptors EPSCs before and after block by MK801. Calibration: GluA2wt/wt: 50 pA, 100 ms; GluA2L483Y/wt: 25 pA, 100 ms. (F) Representative traces of direct responses from CA1 pyramidal neurons elicited by paired UV photolysis of caged glutamate. Calibration: GluA2wt/wt: 50 pA, 20 ms; GluA2L483Y/wt: 100 pA, 20 ms. (G) Summary of all experiments.
To directly test for alterations in desensitization of postsynaptic receptors without the complicating variable of synaptic release, we probed AMPA receptor depression during activation by UV photolysis of caged glutamate. We used pairs of flashes from an UV laser to uncage glutamate over the same area of a neuron. We found that, at the shortest intervals (<40 ms), there was a clear difference in the paired photolysis ratio in GluA2L483Y/wt mice (Fig. 7 F and G). At both 20 ms and 30 ms intervals, the AMPA receptor response in WT littermate mice demonstrated depression, whereas little depression was observed in GluA2L483Y/wt (n = 6), suggesting that the presence of nondesensitizing AMPA receptors increased this ratio when receptors were activated repetitively over a short time window. However, at intervals of ≥40 ms, there was no difference in paired photolysis ratios, suggesting that receptor desensitization plays a significant role only when AMPA receptors are activated at the shortest intervals.
Discussion
In this study, we generated a mutant mouse in which a single codon mutation produced an amino acid switch in the S1 domain of the GluA2 AMPA receptor subunit. Although heterozygous mice survived past birth, they displayed developmental deficits, a progressive proclivity for seizures, and early postnatal mortality. The overall effect of this single amino acid change was greater than that observed when GluA2 was completely ablated in GluA2 knockout mice (20) or even when two of the major AMPA receptor subunits were ablated in GluA2/3 double knockout mice (25). Interestingly, a superficially similar gross phenotype was observed in mutant mice with a deletion of the intronic editing complementary sequence in the Gria2 gene (GluA2ΔECS) (26), although the cellular and synaptic phenotype seemed to differ in this case (13). A recent study reported that a novel polypeptide snail toxin that inhibits AMPA receptor desensitization caused profound excitotoxicity, highlighting the importance of desensitization for neuronal viability (27). The striking phenotype engendered in GluA2L483Y/wt mice clearly demonstrates that AMPA receptor desensitization is critical for viability of the animal.
Preferential Distribution of Receptors to Synaptic Sites.
Both GluA1 and GluA2 expression was decreased in hippocampal homogenates, whereas GluN1 expression was elevated. Despite this, we found only small differences in basal synaptic transmission in GluA2L483Y/wt mice. I/O curves in the CA1 of the hippocampus were not altered, and mEPSC amplitudes were unaffected, suggesting that AMPA receptors are preferentially targeted to synaptic sites. In agreement with this, we observed a significant re-duction in extrasynaptic receptors on CA1 neurons. Previous studies in GluA1 knockout mice reported similar effects on the distribution of AMPA receptors (18); when GluA1 was ablated synaptic AMPA receptors are not significantly altered, but extrasynaptic receptor density is reduced. Similarly, knockout of the primary hippocampal TARP (transmembrane AMPA receptor regulatory protein) γ-8 resulted in a relatively small reduction in the synaptic distribution of AMPA receptors, but a significant alteration in extrasynaptic receptors (19). Therefore, our data are consistent with a preferential targeting of AMPA receptors to synapses at the expense of extrasynaptic receptor density.
AMPA Receptors Do Not Accumulate in the ER.
The L483Y mutation lies at the dimer interface between adjacent subunits in the receptor complex. Stabilization of this dimer interface caused by the mutation at this site eliminates the ability of the receptor to desensitize (28). Expression studies have determined that GluA2(L483Y) mutant receptors can assemble efficiently, yet their exit from the ER is considerably reduced, suggesting that conformational changes are used by ER quality control mechanisms for further processing of AMPA receptors (15). We postulated that a similar retention of nondesensitizing GluA2(L483Y) receptor subunits could cause retention of AMPA receptors in the ER in the knock-in mice. We found there was no increase in the immature glycosylated form of the receptor subunit and no enhancement of the UPR in GluA2L483Y/wt, which might be expected to be engaged if misfolded proteins were stressing the ER. Furthermore, we found no enhancement of the interaction between GluA2 AMPA receptor subunits and the ER resident chaperone molecule calnexin, an interaction that we might also expect to be enhanced if misfolded GluA2 receptors were being improperly processed in neurons. This suggests that introduction of this mutation in vivo does not cause accumulation of AMPA receptors in intracellular compartments, unlike when GluA2(L483Y) is overexpressed in neurons. This is likely because heterotetrameric assemblies of mutant and WT receptors assemble and traffic differently from homomeric GluA2(L483Y) receptors, which are in large abundance when introduced exogenously (29).
Synaptic AMPA Receptor Desensitization.
The extent of desensitization of AMPA receptors at synapses and the role that this plays in the fidelity of synaptic transmission and plasticity has been studied in various regions of the brain. Several studies have determined that postsynaptic receptor desensitization plays a role in shaping the response to repeated stimuli in the retinogeniculate synapse (4), the mossy fiber to granule cell synapse in the cerebellum (7), and calyceal synapses in the auditory brainstem (30). These synapses are all specialized structures with multiple release sites and delayed glutamate clearance mechanisms, which undoubtedly contribute significantly to AMPA receptor desensitization. In hipp-ocampal synapses early work had suggested that here, too, AMPA receptors desensitized significantly during bursts of presynaptic activity (22). However, a later study found that under conditions of high-release probability, cyclothiazide, which blocks AMPA receptor desensitization, had no effect on paired pulse responses of CA1 synapses. We found that paired pulse ratios were increased in GluA2L483Y/wt mice. An independent measure of release probability suggested that there was no measurable difference in glutamate release at CA1 synapses, leaving us to conclude that the enhanced ratio resulted from postsynaptic mechanisms. A recent interesting study demonstrated that rapid lateral diffusion of AMPA receptors out of synapses played a significant role in responding to multiple closely timed presynaptic release events. In particular exchange of desensitized receptors could contribute to the paired pulse ratio (23). Our findings suggest that the amount of receptor desensitization contributes to the changes in paired pulse ratio in GluR2L483Y/wt CA1 synapses. Therefore, given that in vivo, particularly during early development in the neonate, CA1 synapses receive bursts of synaptic activity, it is likely that repetitive activation of receptors will result in an enhancement of the postsynaptic response (31). In complementary experiments, the paired ratio to uncaged glutamate over a small area of the somatodendritic region of pyramidal neurons was also larger in mutant mice. This demonstrated that reduced receptor desensitization was apparent in GluA2L483Y/wt mice. We also expected to see changes in receptor deactivation that might be apparent in the slowing of quantal EPSCs. Surprisingly, we did not see any change in the kinetics of mEPSCs in the CA1 region. Analysis of these events can be complicated by dendritic filtering; therefore we also recorded desynchronized quantal events at mossy fiber synapses (Fig. S2). However, again there was no observed difference in deactivation kinetics. The degree of deactivation introduced by the L483Y mutation will be dependent on the stoichiometry of heteromeric channels (32), possible association with auxiliary proteins (33, 34), and the number of receptors containing mutant subunits; therefore, it is possible that the lack of observed effect on deactivation reflects the fact that there are likely many receptors containing only a single or no mutant subunits in GluR2L483Y/wt mice. In conclusion, we demonstrate here that AMPA receptor desensitization is a critical mechanism for proper development and, ultimately, for the survival of the organism.
Materials and Methods
Slice Preparation and Electrophysiology.
Transverse hippocampal slices (350 μM) were prepared from postnatal day 14 (P14) to P21 GluR2L483Y/wt (detailed in SI Text). Voltage clamp recordings were made from visually identified CA1 pyramidal neurons and synaptic currents were evoked in the Schaffer collateral pathway. For UV photolysis of caged glutamate, direct current responses were measured by uncaging glutamate directly over the pyramidal cell body UV power was calibrated to give an initial current amplitude of between 150 and 200 pA.
Biochemical Analysis.
Biochemical analysis is detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Stephanie Bennett and Diya Zhang for mouse ES cell culture, Lynn Doglio for blastocyst injections, Wensheng Liu and Lennell Reynolds for help with EM, Herman Fernandes for comments, and Geoffrey Swanson and Yael Stern-Bach for valuable discussion. Support was provided by the Fragile X research foundation (to A.C.) and the National Institutes of Health, National Institute of Neurological Disorders and Stroke (R01NS058894 to A.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0908206107/-/DCSupplemental.
References
- 1.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 2.Hansen KB, Yuan H, Traynelis SF. Structural aspects of AMPA receptor activation, desensitization and deactivation. Curr Opin Neurobiol. 2007;17:281–288. doi: 10.1016/j.conb.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Arai AC, Kessler M. Pharmacology of ampakine modulators: From AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Blitz DM, Regehr WG. Contributions of receptor desensitization and saturation to plasticity at the retinogeniculate synapse. Neuron. 2002;33:779–788. doi: 10.1016/s0896-6273(02)00611-6. [DOI] [PubMed] [Google Scholar]
- 5.Rozov A, Jerecic J, Sakmann B, Burnashev N. AMPA receptor channels with long-lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex. J Neurosci. 2001;21:8062–8071. doi: 10.1523/JNEUROSCI.21-20-08062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trussell LO, Zhang S, Raman IM. Desensitization of AMPA receptors upon multi-quantal neurotransmitter release. Neuron. 1993;10:1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]
- 7.Xu-Friedman MA, Regehr WG. Ultrastructural contributions to desensitization at cerebellar mossy fiber to granule cell synapses. J Neurosci. 2003;23:2182–2192. doi: 10.1523/JNEUROSCI.23-06-02182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: Quantal fluctuation affects subsequent release. J Physiol. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiGregorio DA, Rothman JS, Nielsen TA, Silver RA. Desensitization properties of AMPA receptors at the cerebellar mossy fiber granule cell synapse. J Neurosci. 2007;27:8344–8357. doi: 10.1523/JNEUROSCI.2399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hjelmstad GO, Isaac JT, Nicoll RA, Malenka RC. Lack of AMPA receptor desensitization during basal synaptic transmission in the hippocampal slice. J Neurophysiol. 1999;81:3096–3099. doi: 10.1152/jn.1999.81.6.3096. [DOI] [PubMed] [Google Scholar]
- 11.Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 12.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldmeyer D, et al. Neurological dysfunctions in mice expressing different levels of the Q/R site-unedited AMPAR subunit GluR-B. Nat Neurosci. 1999;2:57–64. doi: 10.1038/4561. [DOI] [PubMed] [Google Scholar]
- 14.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 15.Greger IH, Akamine P, Khatri L, Ziff EB. Developmentally regulated, combinatorial RNA processing modulates AMPA receptor biogenesis. Neuron. 2006;51:85–97. doi: 10.1016/j.neuron.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 17.Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol. 1995;486:297–303. doi: 10.1113/jphysiol.1995.sp020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamanillo D, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 19.Rouach N, et al. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- 20.Jia Z, et al. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron. 1996;17:945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 21.Koike-Tani M, Kanda T, Saitoh N, Yamashita T, Takahashi T. Involvement of AMPA receptor desensitization in short-term synaptic depression at the calyx of Held in developing rats. J Physiol. 2008;586:2263–2275. doi: 10.1113/jphysiol.2007.142547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai A, Lynch G. AMPA receptor desensitization modulates synaptic responses induced by repetitive afferent stimulation in hippocampal slices. Brain Res. 1998;799:235–242. doi: 10.1016/s0006-8993(98)00447-8. [DOI] [PubMed] [Google Scholar]
- 23.Heine M, et al. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 25.Meng Y, Zhang Y, Jia Z. Synaptic transmission and plasticity in the absence of AMPA glutamate receptor GluR2 and GluR3. Neuron. 2003;39:163–176. doi: 10.1016/s0896-6273(03)00368-4. [DOI] [PubMed] [Google Scholar]
- 26.Brusa R, et al. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–1680. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- 27.Walker CS, et al. A novel Conus snail polypeptide causes excitotoxicity by blocking desensitization of AMPA receptors. Curr Biol. 2009;19:900–908. doi: 10.1016/j.cub.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, et al. Mechanism of glutamate receptor desensitization. Nature. 2002;417:245–253. doi: 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- 29.Shanks NF, Maruo T, Farina AN, Ellisman MH, Nakagawa T. Contribution of the global subunit structure and stargazin on the maturation of AMPA receptors. J Neurosci. 2010;30:2728–2740. doi: 10.1523/JNEUROSCI.5146-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otis T, Zhang S, Trussell LO. Direct measurement of AMPA receptor desensitization induced by glutamatergic synaptic transmission. J Neurosci. 1996;16:7496–7504. doi: 10.1523/JNEUROSCI.16-23-07496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menendez de la Prida L, Sanchez-Andres JV. Heterogeneous populations of cells mediate spontaneous synchronous bursting in the developing hippocampus through a frequency-dependent mechanism. Neuroscience. 2000;97:227–241. doi: 10.1016/s0306-4522(00)00029-4. [DOI] [PubMed] [Google Scholar]
- 32.Robert A, Irizarry SN, Hughes TE, Howe JR. Subunit interactions and AMPA receptor desensitization. J Neurosci. 2001;21:5574–5586. doi: 10.1523/JNEUROSCI.21-15-05574.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Priel A, et al. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwenk J, et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science. 2009;323:1313–1319. doi: 10.1126/science.1167852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.