Abstract
Mutations in mitochondrial oxidative phosphorylation complex I are associated with multiple pathologies, and complex I has been proposed as a crucial regulator of animal longevity. In yeast, the single-subunit NADH dehydrogenase Ndi1 serves as a non-proton-translocating alternative enzyme that replaces complex I, bringing about the reoxidation of intramitochondrial NADH. We have created transgenic strains of Drosophila that express yeast NDI1 ubiquitously. Mitochondrial extracts from NDI1-expressing flies displayed a rotenone-insensitive NADH dehydrogenase activity, and functionality of the enzyme in vivo was confirmed by the rescue of lethality resulting from RNAi knockdown of complex I. NDI1 expression increased median, mean, and maximum lifespan independently of dietary restriction, and with no change in sirtuin activity. NDI1 expression mitigated the aging associated decline in respiratory capacity and the accompanying increase in mitochondrial reactive oxygen species production, and resulted in decreased accumulation of markers of oxidative damage in aged flies. Our results support a central role of mitochondrial oxidative phosphorylation complex I in influencing longevity via oxidative stress, independently of pathways connected to nutrition and growth signaling.
Keywords: aging, mitochondria, respiratory chain, free radicals
Mitochondria are key metabolic organelles whose oxidative phosphorylation (OXPHOS) system is considered to be one of the most efficient producers of bioenergy. When OXPHOS function is compromized (e.g., by mutations or toxins), bioenergy supply and cellular homeostasis are seriously disrupted, which can be lethal.
OXPHOS complex I plays a central role in the regulation of ATP production, intermediary metabolism, and apoptosis (1, 2), and mutations affecting it cause many human pathologies (3). It has also been proposed as a pacemaker of the aging process (4). Treatments inferred to decrease the production of reactive oxygen species (ROS) at the level of complex I can prolong lifespan in Drosophila (5). All these characteristics make it essential to understand better the role of complex I in vivo and its involvement in aging.
Many organisms possess alternative enzymes that can bypass or replace the proton-translocating complexes of the mitochondrial respiratory chain. These include the alternative oxidases (AOX) and the NADH dehydrogenases of the Ndi and Nde families. Together these enzymes provide an alternative respiratory chain that potentially allows the maintenance of redox homeostasis and intermediary metabolism under conditions where flux through the “standard” respiratory chain is limited by high ATP levels, the action of toxins or other physiological restraints (6, 7). AOX acts as a bypass of complexes III and IV, whereas Nde or Ndi can bypass complex I.
In previous studies these bypass enzymes were shown to be active when introduced into the mitochondria of higher metazoans such as mammals (8–12), arthropods (13), or nematodes (14), all of which lack endogenous alternative enzymes. Furthermore, their expression appears largely benign, and confers resistance against respiratory chain inhibition by toxins or functional gene knockdown. Specifically, expression of AOX from the sea-squirt Ciona intestinalis was able to protect both flies (13) and mammalian cells (11) from the toxicity of cyanide or antimycin, or from functional depletion of cytochrome oxidase (12, 13). Similarly, yeast Ndi1 was able to protect rat neurons from rotenone toxicity in vivo (15), and functionally to replace complex I in both Caenorhabditis elegans (14) and cultured human cells (8–10). An internal alternative NADH:ubiquinone oxidoreductase can also substitute for complex I when introduced into Yarrowia lipolytica (16).
To study the role of complex I in vivo, we engineered the yeast NDI1 gene for expression in Drosophila melanogaster. We here demonstrate that that enzyme is active when expressed in Drosophila mitochondria, that its ubiquitous expression confers protection against toxins and the lethality induced by complex I knockdown, and that it increases lifespan independently of the effects of diet. NDI1 expression mitigated mitochondrial ROS production in aging flies and limited the accumulation of markers of oxidative damage. Our findings implicate ROS production at complex I, resulting from disturbances in redox homeostasis, as a key determinant of aging.
Results
We established transgenic Drosophila lines carrying insertions of yeast NDI1 under the control of a GAL4-dependent promoter. In crosses to flies bearing the ubiquitously acting da-GAL4 driver to induce expression, they showed no loss of viability, eclosing in comparable numbers (Fig. S1 A and B) and with similar developmental timing (Fig. 1A and Fig. S1C) as control flies from the crosses. NDI1-expressing flies were viable and fertile, with no morphological, behavioural, or reproductive abnormalities, and were able to tolerate two copies of the NDI1 transgene (Fig. S1D). However, coexpression, under standard conditions, of NDI1 and the AOX gene from Ciona, was lethal (Fig. S1E).
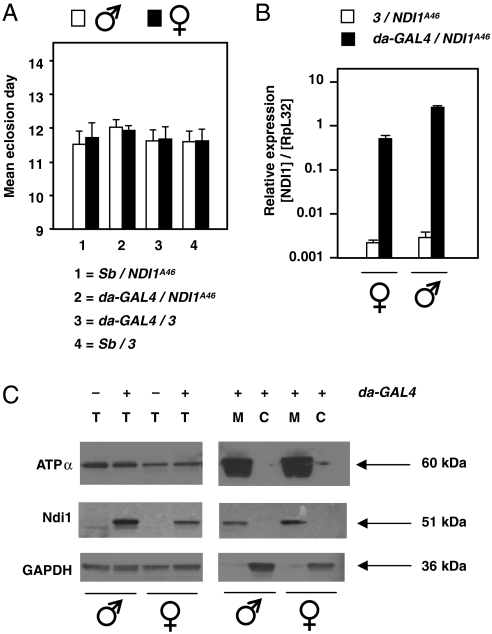
Fig. 1.
Expression of NDI1 in Drosophila is benign. (A) Mean eclosion day ± SEM (4 replicate experiments) of progeny from cross between da-GAL4/Sb females and males hemizygous for the NDI1A46 transgene. n = 1827 males, 1978 females. There were no significant differences between classes (ANOVA, p > 0.05). For similar experiments using transgenic line NDI1B20 (see Fig. S1). (B) Q-RT-PCR of NDI1 RNA from flies as indicated, normalized to that of endogenous RpL32. (C) Western blots of total (T) mitochondrial (M) and cytosolic (C) protein extracts from NDI1-expressing and nonexpressing flies, probed with antibodies as indicated: ATPα—ATP synthase subunit α.
Expression of the NDI1 transgene at the RNA and protein levels was confirmed by quantitative RT-PCR (Q-RT-PCR) (Fig. 1B and Fig. S1F) and by Western blotting (Fig. 1C). Expression at the RNA level was higher in males, but approximately equal at the protein level in the two sexes. Based on mitochondrial and cytosolic markers, the protein was localized to mitochondria and appeared to have been processed to the expected mature size (approximately 51 kDa). Expression at the RNA and protein levels was maintained in aging flies (Fig. S1 G and H) and did not interfere with the expression, assembly, or activity of complex I or the other OXPHOS complexes (Fig. S1 I, J, and K).
Mitochondrial extracts from NDI1-expressing flies showed a rotenone-resistant NADH dehydrogenase activity (approximately 25% of the uninhibited activity), whereas mitochondria from control flies did not (Fig. 2A, B). The uninhibited activity appeared slightly higher in extracts from NDI1-expressing flies than nonexpressors (Fig. 2A), but this was not statistically significant. Two independent transgenic lines gave similar results (Fig. 2 A and B). The rotenone-resistant NADH dehydrogenase activity conferred by NDI1 was fully sensitive to cyanide in such extracts (Fig. S2G), indicating functional coupling to the downstream portion of the respiratory chain. Although we were unable to measure a significant effect on substrate oxidation by mitochondrial suspensions (Fig. S2 A and B), we were able to observe a significantly increased rotenone-resistant respiration (substrate-driven oxygen consumption) in crude homogenates from NDI1-expressing flies, supplied with pyruvate plus proline (Fig. S2H).
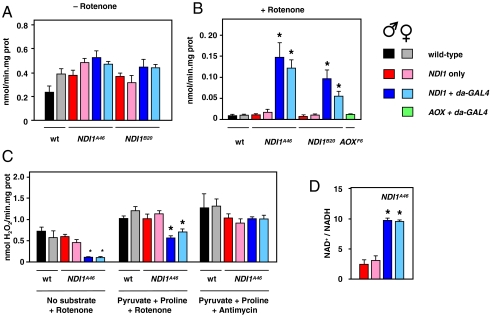
Fig. 2.
NDI1 creates rotenone-resistant NADH dehydrogenase activity and decreases ROS production. NADH dehydrogenase activity in mitochondrial extracts from flies as indicated, in absence (A) or presence (B) of 5 μM rotenone. * indicates significantly different data classes (ANOVA, p < 0.001). (C) ROS production from mitochondrial suspensions as indicated, in presence of substrates and inhibitors as shown. * indicates significantly different data classes (ANOVA, p < 0.001). See also Fig. S2. (D) NAD+/NADH ratio in fly homogenates (means ± SD, t test comparing expressors and nonexpressors of the same sex, p < 0.001 indicated by *). “NDI1 only” indicates presence of the transgene without driver.
NDI1 expression did produce a clear suppressive effect on mitochondrial ROS (mtROS) production in vitro, in the presence of rotenone, plus complex I-linked substrates or no added substrate (Fig. 2C and Fig. S2D). No such effect was seen in mitochondria from young flies, when rotenone was absent (Fig. S2C). Unlike the situation with NADH oxidation, the effect on mtROS production was not dependent on electron flow to oxygen, because it was not inhibited by KCN (Fig. S2F), but myxothiazol abolished it (Fig. S2F). The suppression of mtROS production in the presence of rotenone was maintained in aging flies (Fig. S2E). The NAD+/NADH ratio was significantly increased in homogenates of NDI1 expressors compared with nonexpressors (Fig. 2D), although total nicotinamide adenine dinucleotide levels were not affected (Fig. S2I).
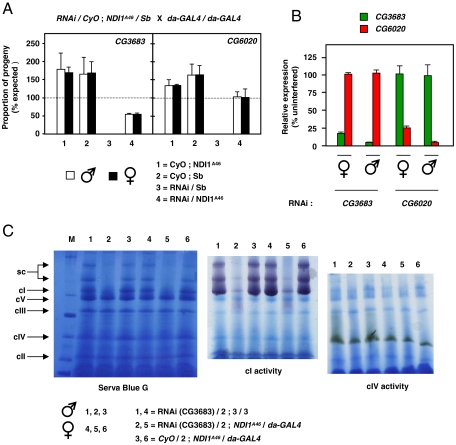
NDI1-expressing flies exhibited a significant resistance against rotenone and paraquat, but not against antimycin, in comparison with otherwise isogenic nonexpressing flies tested alongside (Fig. S3 A–C). The range of drug concentrations at which rotenone resistance was observed was narrow: At concentrations of 1 mM or less no toxicity was seen, but at ≥6 mM all flies succumbed rapidly. Resistance to both rotenone and paraquat also differed between the sexes. Expression of NDI1, driven by da-GAL4, was also able to overcome the lethality of knockdown of either of two subunits of complex I: CG3683, homologue of human NDUFA8 and CG6020, homologue of human NDUFA9 (Fig. 3A and Fig. S3D), indicating that NDI1 can compensate for a substantial deficiency of complex I in vivo. Knockdown of the targeted subunit in each case was verified at the RNA level by Q-RT-PCR (Fig. 3B and Fig. S3F), at the protein level by Western blots using an antibody against NDUFS3 (Fig. S3G), and at the level of assembled, active complex I by blue native-polyacrylamide gel electrophoresis gels combined with in-gel histochemistry (Fig. 3C and Fig. S3H). The other OXPHOS complexes were not affected by complex I knockdown. The rescued flies eclosed with a developmental delay of 3–5 d. Rescue was not due to a promoter dilution effect, because AOX transgenic lines tested in parallel did not rescue the lethality (Fig. S3E).
Fig. 3.
NDI1 can compensate for complex I deficiency in Drosophila in vivo. (A) Mean proportions of each progeny class (± SD, 2 replicate experiments) expressed as percentages of expected proportion eclosing from crosses as shown. RNAi knockdown targets were CG3683 (n = 423 total progeny) and CG6020 (n = 673 total progeny). In absence of NDI1 knockdown was lethal in both cases (class3). See also Fig. S3 D and E. (B) Verification of knockdown at RNA level by Q-RT-PCR. Data normalized to target gene expression level in progeny from the same cross lacking interfering RNA (for full data, see Fig. S3F). (C) Verification of knockdown at the protein level. BNE gels of mitochondrial protein extracts, stained as indicated (cI-V—complex I-V, sc—supercomplexes). Knockdown of CG3683 (here) or CG6020 (Fig. S3H) in NDI1-rescued flies gave almost complete absence of assembled, active complex I.
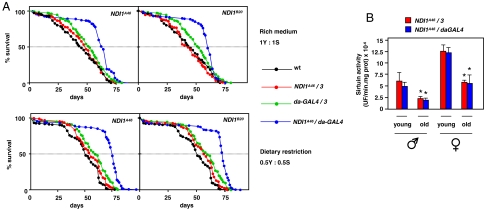
NDI1 expression had a clear effect on lifespan, both in the heterozygous (w1118/Dahomey w-) background (Fig. S4A) and after backcrossing over 11 generations to the Dahomey w- reference strain (Fig. S4B). Median, mean, and maximum lifespan were substantially increased. The increase was more pronounced in males (30–40%) than females (10–20%), and was highly significant when NDI1-expressors were compared with all nonexpressor control groups (Fig. S4B). Importantly, it was independent, in both sexes, of the lifespan enhancement caused by dietary restriction (Fig. 4A and Fig. S4C). Consistent with this, and despite the altered steady-state ratio of NAD+/NADH, NDI1 expression caused no alteration in sirtuin activity (Fig. 4B).
Fig. 4.
NDI1 expression increases lifespan independently of dietary restriction. (A) Lifespan curves for males of indicated genotypes, in Dahomey w- background, on media containing full (1Y∶1S) or half (0.5Y∶0.5S) normal content of yeast and sugar. Dotted lines indicate extrapolation of median lifespan. In all cases, dietary restriction increased median (log-rank test, p < 0.001), mean and maximum (ANOVA, p < 0.01) lifespan. See also Fig. S4. (B) Sirtuin activity in homogenates of young (1–5 d) and old (30 d males, 50 d females) flies as indicated. * indicates significantly different data classes (means ± SEM, ANOVA, p < 0.05). UF—units of fluorescence at 460 nm.
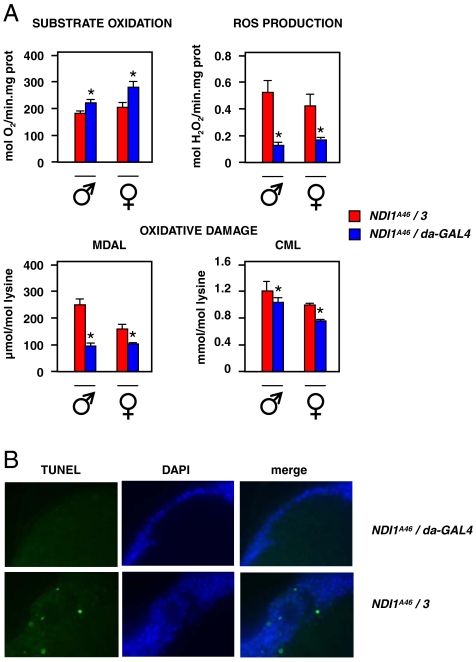
To gain insight into the mechanism promoting lifespan extension by NDI1, we analyzed respiratory functions in aging flies, at approximately 2/3 of the median lifespan of nonexpressors. In contrast to young flies (Fig. S5), isolated mitochondria from aging NDI1-expressing flies showed clearly increased substrate oxidation and decreased mitochondrial ROS production compared with nonexpressors, when supplied with a complex I-linked substrate mix (Fig. 5A). Comparing young and old flies, NDI1 expression appeared to block completely the age-associated increase in mtROS production. Consistent with this, NDI1 expression was associated with a substantial and significant decrease in the steady-state levels of several markers of oxidative damage in aging flies (Fig. 5), notably malondialdehyde-lysine and carboxymethyl-lysine [plus aminoadipic semialdehyde only in males (Fig. S5)]. Levels of these markers were indistinguishable in young expressors versus nonexpressors (Fig. S5).
Fig. 5.
NDI1 expression mitigates mitochondrial ROS production, oxidative damage and brain apoptosis in aging flies. (A) Mitochondrial substrate oxidation (pyruvate + proline + sn-glycerol-3-phosphate, state 3), ROS production (pyruvate + proline substrate mix) and markers of oxidative damage in aging (30 d male, 50 d female) flies. MDAL—malondialdehyde-lysine, CML—carboxymethyl-lysine. * indicates statistically significantly differences between expressing and nonexpressing flies of the same sex (means ± SEM, t test, p < 0.05 for substrate oxidation and oxidative damage, p < 0.01 for ROS production). See also Fig. S5. (C). Apoptosis by TUNEL staining of brain sections from aged females (77 d) of the indicated genotypes, counterstained by DAPI. See also Table S1.
TUNEL staining revealed extensive apoptosis in brain sections of nonexpressor females (Fig. 5B), but only at around 77 d (80% of the maximum lifespan), when most of the population had already died. NDI1-expressing females of the same chronological age showed no such apoptosis (Fig. 5B). However, there was also no brain apoptosis detected in males at the corresponding point in the lifespan curve, regardless of NDI1 expression (Table S1). Thus, we found no compelling evidence that NDI1 extends lifespan by restraining apoptosis in the brain.
Discussion
In this paper we demonstrate that the yeast single-subunit NADH dehydrogenase NDI1 can be successfully expressed ubiquitously in Drosophila throughout life. Such expression produces subtle effects on mitochondrial biochemistry and increased resistance to toxins, but no other discernable effects on development or behavior. However, it rescues the lethality caused by complex I knockdown and extends lifespan in both sexes, independently of dietary restriction and without affecting sirtuin activity. Studies on isolated mitochondria showed that NDI1 expression mitigated the age-associated decline in respiratory function and the corresponding increase in mitochondrial ROS production using complex I-linked substrates. Importantly, this was associated with a substantially decreased level of several key markers of oxidative damage, indicating that the mechanism by which NDI1 extends lifespan is by protecting flies against oxidative stress emanating from excess ROS production at mitochondrial complex I.
Our results demonstrate that metazoan longevity can be regulated by at least two separable pathways: the well-established sirtuin pathway, responding to nutritional and growth regulatory signals, and a second pathway involving oxidative damage resulting from ROS production at complex I.
Functionality of NDI1 in Drosophila.
Although the effects of NDI1 expression on flies were subtle, and did not compromise mitochondrial ATP production to the point of affecting viability or inducing other phenotypes seen in OXPHOS mutants (17–19), several lines of evidence indicate that the enzyme was constitutively active in vivo, and able functionally to replace complex I, as seen earlier in nematodes, human cultured cells and fungi (8–10, 14, 16). NDI1 expression produced a clear shift in the steady-state ratio of the reduced and oxidized forms of NAD, which provides a possible explanation for its ability to confer significant resistance against paraquat in vivo, as shown earlier in cultured cells (20). Paraquat is believed to act by using NADPH as an electron donor to generate superoxide (21). Thus, the change in intracellular redox homeostasis promoted by NDI1 should be protective.
NDI1 was able to rescue the lethality caused by complex I knockdown in the whole fly, leaving only a small residual amount of active, fully assembled enzyme. The implication is, surprisingly, that flies require rather little proton-pumping at complex I for survival, at least under laboratory conditions. The fact that NDI1 is able to substitute for complex I to produce a largely normal fly indicates that maintenance of redox homeostasis and channeling of electrons to the downstream portion of the respiratory chain must be more important functions of complex I in vivo. This ability of NDI1 to replace complex I should prove a useful tool in elucidating the molecular pathology of complex I defects, using Drosophila as a model for human disease.
Redox Homeostasis, Sirtuins, and Aging.
The NAD+/NADH ratio, which is altered by NDI1 expression, is known to influence sirtuin activity (22, 23). Nevertheless, we detected no change in total sirtuin activity consequent upon NDI1 expression, although sirtuin levels were higher in younger than older flies and also in longer lived females than shorter lived males, supporting the role of Sir2 as a longevity gene (24–26). This implies that the regulation of sirtuin activity in vivo is more complex than hitherto supposed, and does not depend only on the relative levels of the reduced and oxidized forms of NAD.
The fact that NDI1 produced no change in sirtuin activity is consistent with its acting independently of dietary restriction. The modulation of sirtuin activity appears to be a key downstream mediator of dietary restriction (23, 25, 27, 28) and also of growth regulation via the insulin signaling pathway, with which dietary restriction interacts at various levels (29, 30). The lifespan-extending effect of NDI1 is thus independent of these pathways, and a different mechanistic explanation must be invoked.
Oxidative Damage and Aging.
A wealth of previous circumstantial data supports the view that loss of respiratory competence at complex I during aging is accompanied by an increase in mtROS production arising from the oxidation of complex I-linked substrates. In Drosophila, these changes are well documented (31, 32). Our finding that NDI1 mitigates both of these outcomes while decreasing oxidative damage in aging flies provides a mechanistic basis for understanding its effects on lifespan.
A possible effect on mitochondrial membrane potential may also be considered. This could be hypothesized to act by altering the global susceptibility to apoptosis, which in turn would affect the rate of aging by influencing the loss of vital or irreplaceable cells, such as in the brain. However, the effects of NDI1 expression on brain apoptosis were not congruent between the sexes, and even in females did not manifest until most flies were already dead. Conversely, ROS production at complex I is most likely independent of membrane potential, at least for electron flow in the forward direction, as shown recently for isolated complex I from Yarrowia (33).
The effect of NDI1 on ROS production in aging flies is more likely mediated by its ability to act as a complex I bypass and by its effects on NAD+/NADH levels (34, 35), although an indirect effect cannot be ruled out (e.g., in which NDI1 promotes increased reduction of ubiquinol or cytochrome c, which then act locally as antioxidants) (36, 37). The decline in respiratory capacity with age, whatever its basis, is expected to exacerbate ROS production due to the inappropriate passage of electrons to oxygen, such as occurs when complex I is inhibited by toxins such as rotenone. Mitochondrial ROS production in aging NDI1-expressing flies was decreased to the level seen in young flies, and was associated with a significant decrease in the levels of key markers of oxidative damage to biomolecules, whose levels have been shown in many other studies to be related to longevity (38–41). Mitigation of complex I-derived ROS has also been suggested to be the mechanism of lifespan increase obtained by manipulation of NF1 (5).
In other systems (e.g., nematodes), insulin signaling has been shown to regulate proteins involve in resistance to oxidative stress that also influence lifespan (42). Moreover, dietary restriction in both mammals (40) and flies (43) also impinges on the steady-state levels of markers of oxidative stress, including those influenced by NDI1 expression in flies. Thus, the two pathways of longevity regulation inferred from the present study to operate independently in flies, namely the sirtuin/insulin signaling/dietary restriction pathway on the one hand, and ROS generation at complex I on the other, should eventually converge on a single-readout of oxidative damage leading to physiological impairment. Our findings indicate that the readouts from these pathways are additive: One gives rise to agents of damage, and the other determines the ability to the organism to resist such agents. The combination of the two, acting independently, determines the final outcome, as measured by the amount of cumulative damage, and consistent with the classical free radical theory of aging (44).
Mitochondria are clearly not the only source of ROS-induced damage influencing longevity. For example, long-lived mice deficient in the p66Shc signaling molecule were recently shown to produce less superoxide in macrophages (45). However, modulation of ROS production at complex I, by means of NDI1 or other approaches, now offers a way to manipulate metazoan lifespan by mitigating a key source of age-associated damage.
Materials and Methods
Full details are presented in SI Text and Table S2.
Drosophila Stocks and Maintenance.
w1118, balancer, and da-GAL4 driver lines were obtained from stock centers, and maintained in standard medium (13). AOX transgenic lines were as described previously (13).
Generation of NDI1 Transgenic Lines and Genetic Nomenclature.
Yeast NDI1, amplified from genomic DNA, was cloned into a Drosophila P-element vector under the control of a GAL4-dependent promoter, flanked by insulator elements. Following microinjection (VANEDIS Drosophila Injection Service), transgenic progeny were established as independent lines NDI1A46 and NDI1B20, with genomic insertion sites (on chromosomes 3 and 2, respectively) determined by inverse PCR (13). NDI1A46/3 and NDI1B20/2 denote these transgenes in combination with the corresponding wild-type chromosomes. CyO and Sb denote the standard markers present on balancer chromosomes 2 and 3, respectively. For lifespan experiments NDI1 transgenic lines were backcrossed over 11 generations to the Dahomey w- reference strain.
Toxin Resistance and Lifespan Curves.
Resistance to antimycin, rotenone, or paraquat was assayed essentially as described by Fridell et al. (46). Lifespan curves were derived in standard medium or under dietary restriction as indicated.
RNA and Protein Analysis.
RNA was isolated and analyzed by quantitative RT-PCR as described previously (13), using gene-specific primers, with RpL32 as reference gene. Subcellular fractions were analyzed by Western blots (13) and blue native-polyacrylamide gel electrophoresis combined with in-gel histochemistry (47).
Enzymatic Assays.
Standard assays were used to measure NADH dehydrogenase and sirtuin activity, mitochondrial H2O2 production and NAD+/NADH levels. Respiration was measured by polarography and apoptosis by TUNEL staining. Markers of oxidative damage were analyzed by mass spectrometry as previously (40).
Supplementary Material
Acknowledgments.
We thank Outi Kurronen for technical assistance. This work was supported by the Academy of Finland, Tampere University Hospital Medical Research Fund, Sigrid Juselius Foundation, European Research Council, European Molecular Biology Organization, National Institutes of Health Grant R01 DK053244, the Spanish Ministries of Health and of Science and Innovation, and the Generalitat of Catalonia.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0911539107/-/DCSupplemental.
References
- 1.Ventura B, Genova ML, Bovina C, Formiggini G, Lenaz G. Control of oxidative phosphorylation by Complex I in rat liver mitochondria: Implications for aging. Biochim Biophys Acta. 2002;1553:249–260. doi: 10.1016/s0005-2728(01)00246-8. [DOI] [PubMed] [Google Scholar]
- 2.Perier C, et al. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci USA. 2005;102:19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smeitink JA, Zeviani M, Turnbull DM, Jacobs HT. Mitochondrial medicine: A metabolic perspective on the pathology of oxidative phosphorylation disorders. Cell Metab. 2006;3:9–13. doi: 10.1016/j.cmet.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antioxid Redox Sign. 2006;8:582–599. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- 5.Tong JJ, Schriner SE, McCleary D, Day BJ, Wallace DC. Life extension through neurofibromin mitochondrial regulation and antioxidant therapy for neurofibromatosis-1 in Drosophila melanogaster. Nat Genet. 2007;39:476–485. doi: 10.1038/ng2004. [DOI] [PubMed] [Google Scholar]
- 6.Rhoads DM, Subbaiah CC. Mitochondrial retrograde regulation in plants. Mitochondrion. 2007;7:177–194. doi: 10.1016/j.mito.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Borecký J, Vercesi AE. Plant uncoupling mitochondrial protein and alternative oxidase: Energy metabolism and stress. Biosci Rep. 2005;25:271–286. doi: 10.1007/s10540-005-2889-2. [DOI] [PubMed] [Google Scholar]
- 8.Seo BB, et al. Molecular remedy of complex I defects: Rotenone-insensitive internal NADH-quinone oxidoreductase of Saccharomyces cerevisiae mitochondria restores the NADH oxidase activity of complex I-deficient mammalian cells. Proc Natl Acad Sci USA. 1998;95:9167–9171. doi: 10.1073/pnas.95.16.9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo BB, Wang J, Flotte TR, Yagi T, Matsuno-Yagi A. Use of the NADH-quinone oxidoreductase (NDI1) gene of Saccharomyces cerevisiae as a possible cure for complex I defects in human cells. J Biol Chem. 2000;275:37774–37778. doi: 10.1074/jbc.M007033200. [DOI] [PubMed] [Google Scholar]
- 10.Bai Y, et al. Lack of complex I activity in human cells carrying a mutation in MtDNA-encoded ND4 subunit is corrected by the Saccharomyces cerevisiae NADH-quinone oxidoreductase (NDI1) gene. J Biol Chem. 2001;276:38808–38813. doi: 10.1074/jbc.M106363200. [DOI] [PubMed] [Google Scholar]
- 11.Hakkaart GA, Dassa EP, Jacobs HT, Rustin P. Allotopic expression of a mitochondrial alternative oxidase confers cyanide resistance to human cell respiration. EMBO Rep. 2006;7:341–345. doi: 10.1038/sj.embor.7400601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dassa EP, et al. Expressing the Alternative Oxidase complements cytochrome c oxidase deficiency in human cells. EMBO Mol Med. 2009;1:30–36. doi: 10.1002/emmm.200900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Ayala DJM, et al. Expression of the Ciona intestinalis alternative oxidase (AOX) in Drosophila complements defects in mitochondrial oxidative phosphorylation. Cell Metab. 2009;9:449–460. doi: 10.1016/j.cmet.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 14.DeCorby A, Gásková D, Sayles LC, Lemire BD. Expression of Ndi1p, an alternative NADH:ubiquinone oxidoreductase, increases mitochondrial membrane potential in a C. elegans model of mitochondrial disease. Biochim Biophys Acta. 2007;1767:1157–1163. doi: 10.1016/j.bbabio.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Marella M, et al. Protection by the NDI1 gene against neurodegeneration in a rotenone rat model of Parkinson’s disease. PLoS ONE. 2008;3:e1433. doi: 10.1371/journal.pone.0001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garofano A, Eschemann A, Brandt U, Kerscher S. Substrate-inducible versions of internal alternative NADH: Ubiquinone oxidoreductase from Yarrowia lipolytica. Yeast. 2006;23:1129–1136. doi: 10.1002/yea.1426. [DOI] [PubMed] [Google Scholar]
- 17.Zhang YQ, et al. Stress sensitive B encodes an adenine nucleotide translocase in Drosophila melanogaster. Genetics. 1999;153:891–903. doi: 10.1093/genetics/153.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toivonen JM, et al. Technical knockout, a Drosophila model of mitochondrial deafness. Genetics. 2001;159:241–254. doi: 10.1093/genetics/159.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fergestad T, Bostwick B, Ganetzky B. Metabolic disruption in Drosophila bang-sensitive seizure mutants. Genetics. 2006;173:1357–1364. doi: 10.1534/genetics.106.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JS, Li YF, Bai Y. Yeast NDI1 improves oxidative phosphorylation capacity and increases protection against oxidative stress and cell death in cells carrying a Leber’s hereditary optic neuropathy mutation. Biochim Biophys Acta. 2007;1772:533–542. doi: 10.1016/j.bbadis.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 24.Finkel T, Deng C, Mostoslavsky R. Recent progress in the biology and physiology of sirutins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balan V, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283:27810–27819. doi: 10.1074/jbc.M804681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood JG, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 28.Rogina B, Helfand SL, Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;289:2126–2128. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- 29.Clancy DJ, Gems D, Hafen E, Leevers SJ, Partridge L. Dietary restriction in long-lived dwarf flies. Science. 2002;296:319. doi: 10.1126/science.1069366. [DOI] [PubMed] [Google Scholar]
- 30.Wenzel U. Nutrition, sirtuins and aging. Genes Nutr. 2006;1:85–93. doi: 10.1007/BF02829950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melvin RG, Ballard JW. Intraspecific variation in survival and mitochondrial oxidative phosphorylation in wild-caught Drosophila simulans. Aging Cell. 2006;5:225–533. doi: 10.1111/j.1474-9726.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem J. 2005;390:501–511. doi: 10.1042/BJ20042130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dröse S, Galkin A, Brandt U. Measurement of superoxide formation by mitochondrial complex I of Yarrowia lipolytica. Methods Enzymol. 2009;456:475–90. doi: 10.1016/S0076-6879(08)04426-1. [DOI] [PubMed] [Google Scholar]
- 34.Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639–645. doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Marella M, Seo BB, Matsuno-Yagi A, Yagi T. Mechanism of cell death caused by complex I defects in a rat dopaminergic cell line. J Biol Chem. 2007;282:24146–24156. doi: 10.1074/jbc.M701819200. [DOI] [PubMed] [Google Scholar]
- 36.Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 37.Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: Modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz MC, et al. Protein methionine content and MDA-lysine adducts are inversely related to maximum life span in the heart of mammals. Mech Ageing Dev. 2005;126:1106–1114. doi: 10.1016/j.mad.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Sanz A, et al. Methionine restriction decreases mitochondrial oxygen radical generation and leak as well as oxidative damage to mitochondrial DNA and proteins. FASEB J. 2006;20:1064–1073. doi: 10.1096/fj.05-5568com. [DOI] [PubMed] [Google Scholar]
- 40.Magwere T, et al. Flight activity, mortality rates, and lipoxidative damage in Drosophila. J Gerontol A-Biol. 2006;61A:136–145. doi: 10.1093/gerona/61.2.136. [DOI] [PubMed] [Google Scholar]
- 41.Barja G. Rate of generation of oxidative stress-related damage and animal longevity. Free Radical Biol Med. 2002;33:1167–1172. doi: 10.1016/s0891-5849(02)00910-3. [DOI] [PubMed] [Google Scholar]
- 42.Kondo M, et al. Effect of oxidative stress on translocation of DAF-16 in oxygen-sensitive mutants, mev-1 and gas-1 of Caenorhabditis elegans. Mech Ageing Dev. 2005;126:637–641. doi: 10.1016/j.mad.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Zheng J, Mutcherson R, II, Helfand SL. Calorie restriction delays lipid oxidative damage in Drosophila melanogaster. Aging Cell. 2005;4:209–216. doi: 10.1111/j.1474-9726.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 44.Harmann D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 45.Tomilov AA, et al. Decreased superoxide production in macrophages of long-lived p66Shc knock-out mice. J Biol Chem. 2010;285:1153–1165. doi: 10.1074/jbc.M109.017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fridell YW, Sanchez-Blanco A, Silvia BA, Helfand SL. Targeted expression of the human uncoupling protein 2 (hUCP2) to adult neurons extends lifespan in the fly. Cell Metab. 2005;1:145–152. doi: 10.1016/j.cmet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Nijtmans LG, Henderson NS, Holt IJ. Blue Native electrophoresis to study mitochondrial and other protein complexes. Methods. 2002;26:327–334. doi: 10.1016/S1046-2023(02)00038-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.