Abstract
Neutrophils roll on E-selectin in inflamed venules through interactions with cell-surface glycoconjugates. The identification of physiologic E-selectin ligands on neutrophils has been elusive. Current evidence suggests that P-selectin glycoprotein ligand-1 (PSGL-1), E-selectin ligand-1 (ESL-1), and CD44 encompass all glycoprotein ligands for E-selectin; that ESL-1 and CD44 use N-glycans to bind to E-selectin; and that neutrophils lacking core 2 O-glycans have partially defective interactions with E-selectin. These data imply that N-glycans on ESL-1 and CD44 and O-glycans on PSGL-1 constitute all E-selectin ligands, with neither glycan subset having a dominant role. The enzyme T-synthase transfers Gal to GalNAcα1-Ser/Thr to form the core 1 structure Galβ1–3GalNAcα1-Ser/Thr, a precursor for core 2 and extended core 1 O-glycans that might serve as selectin ligands. Here, using mice lacking T-synthase in endothelial and hematopoietic cells, we found that E-selectin bound to CD44 and ESL-1 in lysates of T-synthase–deficient neutrophils. However, the cells exhibited markedly impaired rolling on E-selectin in vitro and in vivo, failed to activate β2 integrins while rolling, and did not emigrate into inflamed tissues. These defects were more severe than those of neutrophils lacking PSGL-1, CD44, and the mucin CD43. Our results demonstrate that core 1-derived O-glycans are essential E-selectin ligands; that some of these O-glycans are on protein(s) other than PSGL-1, CD44, and CD43; and that PSGL-1, CD44, and ESL-1 do not constitute all glycoprotein ligands for E-selectin.
Keywords: cell adhesion, endothelium, inflammation, rolling
Circulating leukocytes emigrate into lymphoid organs or inflamed sites through sequential adhesive and signaling events (1). They tether to and roll on endothelial cells, then decelerate, arrest, and crawl into underlying tissues. Selectin–ligand interactions mediate tethering and rolling, whereas integrin–ligand interactions mediate deceleration, arrest, and crawling. L-selectin, expressed on leukocytes, binds to ligands on other leukocytes and some endothelial cells. P-selectin, expressed on activated platelets and endothelial cells, and E-selectin, expressed on activated endothelial cells, bind to ligands on leukocytes (2, 3).
The C-type lectin domain of each selectin interacts in a Ca2+-dependent manner with α2–3-sialylated and α1–3-fucosylated cell-surface glycoconjugates. The minimal recognition determinant is sialyl Lewis x, or sLex [NeuAcα2–3Galβ1–4(Fucα1–3)GlcNAcβ1-R], a terminal component of some N-glycans and mucin-type O-glycans. P- and L-selectin bind to the leukocyte mucin P-selectin glycoprotein ligand-1 (PSGL-1) through cooperative interactions with N-terminal sulfated tyrosines, other amino acids, and a core 2 O-glycan capped with sLex (4–6). L-selectin also binds to a sulfated form of sLex that caps N- and O-glycans on endothelial cell mucins in lymph nodes (7, 8).
Ligands for E-selectin do not require sulfation. Expression of an α1–3-fucosyltransferase in most cells confers binding to E-selectin (2, 3), suggesting that E-selectin can bind to many proteins bearing sLex-capped glycans. However, E-selectin interacts with only a few glycoproteins on leukocytes. This may reflect the limited α1–3-fucosylation of glycans on these cells, particularly on murine leukocytes (9), and the preferential fucosylation of particular glycoproteins (10, 11). Proteolytic digestion of murine neutrophils removes all binding sites for E-selectin, supporting the key role of glycoproteins as E-selectin ligands (9). Gene knockout studies in mice reveal that PSGL-1 and CD44 are major glycoprotein ligands for E-selectin on neutrophils. PSGL-1–deficient neutrophils tether poorly to E-selectin but roll with normal velocities (12). CD44-deficient neutrophils tether normally to E-selectin and roll with normal or slightly higher velocities. However, neutrophils lacking both PSGL-1 and CD44 roll much faster on E-selectin (13, 14). Knockdown experiments with short hairpin RNA have implicated E-selectin ligand-1 (ESL-1) as a third ligand for E-selectin on murine neutrophils (15). Neutrophils deficient in all three glycoproteins have severe defects in rolling on E-selectin and markedly decreased recruitment to inflamed sites in vivo, leading to the conclusion that PSGL-1, CD44, and ESL-1 constitute all physiologically relevant ligands for E-selectin (15).
CD44 and ESL-1 on neutrophils use N-glycans to interact with E-selectin (13, 16, 17). Core 1-derived O-glycans, which include core 1, extended core 1, and core 2 structures, are the predominant O-glycans on hematopoietic cells (Fig. 1A). The enzyme core 1 β1–3-galactosyltransferase (T-synthase) transfers Gal from UDP-Gal to GalNAcα1-Ser/Thr (Tn antigen) to form the core 1 backbone Galβ1–3GalNAcα1-Ser/Thr (T antigen) (18). Extension of the core 1 backbone or branching to create the core 2 structure is required before sLex can be added (3, 7) (Fig. 1A). One of the seven known β1–3-N-acetylglucosaminyltransferases, β3GlcNAcT-3, extends core 1 structures in lymph node endothelial cells in vivo (19). Two other β3GlcNAcTs exhibit core 1 extension activity in vitro (20). A family of three core 2 β1–6-N-acetylglucosaminyltransferases (C2GnTs) synthesizes core 2 structures (21). Neutrophils from KO mice lacking C2GnT1 have severely impaired rolling on P-selectin in vitro and in vivo (22, 23). C2GnT1-deficient neutrophils tether poorly to E-selectin in inflamed venules but roll with normal velocities (23), which resembles the phenotype observed in PSGL-1–deficient mice (12). These data suggest but do not prove that PSGL-1 uses core 2 O-glycans to interact with E-selectin as well as P-selectin. Some reports (24–26), but not others (27), suggest that the mucin CD43 is a physiologic E-selectin ligand on activated T cells.
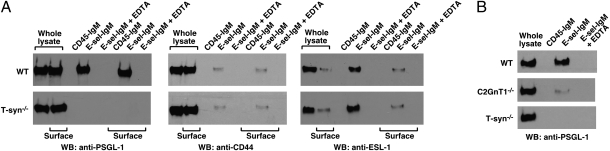
Fig. 1.
E-selectin-IgM binds poorly to T-syn−/− neutrophils. (A) Biosynthesis of core 1-derived O-glycans. The enzyme T-synthase adds Gal to GalNAcα1-Ser/Thr (Tn antigen) to form the core 1 backbone, Galβ1–3GalNAcα1-Ser/Thr (T antigen). Extending the core 1 backbone or branching to create the core 2 structure is required before adding terminal modifications such as sLex, which can interact with selectins. (B) Flow cytometric analysis of Tn antigen on WT and T-syn−/− neutrophils gated by light scatter. (C) Surface expression of the indicated glycoprotein on T-syn−/− neutrophils. The mean fluorescence intensity (MFI) of antibody binding to each glycoprotein on T-syn−/− neutrophils was expressed relative to the value on WT neutrophils, which was normalized to 1. L-selectin is labeled as L-sel. (D) Flow cytometric analysis of binding of E-selectin-IgM to neutrophils of the indicated genotype. The cells were incubated with E-selectin-IgM with or without anti–E-selectin mAb (anti–E-sel). Binding was detected with FITC-conjugated goat anti-human IgM antibodies and is represented as MFI. The inset shows an expanded scale for MFI for the indicated genotypes. The data represent the mean ± SEM from at least three experiments. *P < 0.01.
Importantly, key contributions of O-glycosylated proteins to rolling on E-selectin might not be detected in cells lacking PSGL-1, CD44, and ESL-1. Definitively addressing the role of O-glycans as ligands for E-selectin has been limited by the inability to eliminate all core 1-derived O-glycans on leukocytes. Mice lacking T-synthase specifically in endothelial and hematopoietic cells (EHC T-syn−/− mice) were made by crossing transgenic mice expressing Cre under control of the Tie2 promoter/enhancer with mice bearing the C1galt1 gene flanked with loxP sites (28). EHC T-syn−/− mice have defective lymphangiogenesis with high embryonic and postnatal mortality, primarily because of gastrointestinal bleeding (28). Using EHC T-syn−/− mice that survived to adulthood, we found that core 1-derived O-glycans are essential E-selectin ligands on neutrophils.
Results
E-Selectin-IgM Binds Poorly to T-syn−/− Neutrophils.
Peripheral blood counts in the approximately 10% of EHC T-syn−/− mice that reached adulthood revealed a ninefold elevation in neutrophils (Table S1), similar to that observed in mice lacking P-, E-, and L-selectin (29). In our animal facility, neither EHC T-syn−/− mice nor mice lacking all three selectins had increased rates of infection. All neutrophils from EHC T-syn−/− mice expressed the Tn antigen (Fig. 1B), confirming efficient excision of the C1galt1 gene in hematopoietic cells. Enzymatic desialylation of T-syn−/− neutrophils did not expose more Tn antigen (Fig. 1B), indicating that they expressed little sialylated Tn antigen (NeuAcα2–6GalNAcα1-Ser/Thr). Loss of core 1-derived O-glycans in T-syn−/− neutrophils did not alter the surface density of major membrane glycoproteins, including PSGL-1, CD43, and CD44 (Fig. 1C).
E-selectin-IgM bound to WT neutrophils from peripheral blood (Fig. 1D). A mAb to E-selectin blocked binding, documenting its specificity. E-selectin-IgM binding to neutrophils from PSGL-1−/− mice was significantly reduced, and binding to neutrophils from PSGL-1−/−/CD44−/− mice was further decreased but not eliminated (Fig. 1D), confirming earlier studies (12, 13, 15). No contribution of CD43 to E-selectin-IgM binding was revealed in neutrophils from CD43−/−, PSGL-1−/−/CD43−/−, or PSGL-1−/−/CD43−/−/CD44−/− mice. In contrast, E-selectin-IgM binding to T-syn−/− neutrophils was reduced to near-background levels. No further reduction in binding to T-syn−/−/CD44−/− neutrophils was observed. Thus, E-selectin-IgM binds extremely poorly to neutrophils that lack core 1-derived O-glycans. P- and L-selectin-IgM also failed to bind to T-syn−/− neutrophils (Fig. S1), consistent with their inability to synthesize a core 2 O-glycan near the N terminus of PSGL-1 that is required for binding (4–6).
E-Selectin Binds to CD44 and ESL-1 but Not to PSGL-1 from T-syn−/− Neutrophils.
The conclusion that PSGL-1, CD44, and ESL-1 constitute all E-selectin ligands on neutrophils (15) does not predict the marked decrease in E-selectin-IgM binding to T-syn−/− neutrophils, because E-selectin reportedly binds to N- rather than O-glycans on CD44 and ESL-1 (13, 16, 17). We therefore measured the ability of E-selectin-IgM to precipitate PSGL-1, CD44, or ESL-1 from lysates of surface-biotinylated WT or T-syn−/− leukocytes from bone marrow. Neutrophils account for more than 90% of the selectin-binding cells in this population (14, 30). The portion of proteins on the cell surface was quantified by precipitation with streptavidin. Most of the PSGL-1 and CD44, but only a minority of the ESL-1, was on the cell surface (Fig. 2A), consistent with the predominant distribution of ESL-1 in the Golgi apparatus (31). Total and surface levels of ESL-1 were comparable in WT and T-syn−/− neutrophils. This indicates that loss of core 1-derived O-glycans did not indirectly affect the expression of ESL-1.
Fig. 2.
E-selectin binds to CD44 and ESL-1 but not to PSGL-1 from T-syn−/− neutrophils. (A) Surface-biotinylated WT or T-syn−/− bone marrow leukocytes were lysed (whole lysate). Equal volumes of lysate were precipitated with CD45-IgM, E-selectin-IgM, or E-selectin-IgM in the presence of EDTA and eluted with buffer containing EDTA. Equal volumes of lysate or EDTA eluate were precipitated with streptavidin and eluted to recover cell-surface proteins (surface). The samples were analyzed by Western blotting (WB) with the indicated antibody. (B) WT, T-syn−/−, or C2GnT1−/− leukocytes were lysed. Equal volumes of lysate were precipitated with CD45-IgM, E-selectin-IgM, or E-selectin-IgM in the presence of EDTA and eluted with buffer containing EDTA. The samples were analyzed by Western blotting with the indicated antibody. The data are representative of three independent experiments.
E-selectin-IgM quantitatively precipitated total and surface PSGL-1 from WT but not from T-syn−/− lysates (Fig. 2A), revealing a key role for core 1-derived O-glycans as E-selectin ligands on PSGL-1. E-selectin precipitated only a very small percentage of PSGL-1 from lysates of C2GnT1−/− neutrophils (Fig. 2B). This suggests that core 2 O-glycans constitute most but not all of the E-selectin ligands on PSGL-1.
E-selectin precipitated CD44, although inefficiently, from both WT and T-syn−/− lysates (Fig. 2A). This suggests a low-affinity interaction of E-selectin with CD44 from both genotypes that could not be distinguished in this assay. E-selectin quantitatively precipitated total and surface ESL-1 from both WT and T-syn−/− lysates. These data demonstrate that E-selectin binds to CD44 and ESL-1 from T-syn−/− neutrophils, presumably through interactions with N-glycans. PSGL-1, CD44, and ESL-1 were not precipitated by CD45-IgM or by E-selectin-IgM in the presence of EDTA, confirming the specificities of the observed interactions.
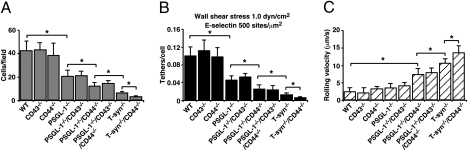
T-syn−/− Neutrophils Have Markedly Impaired Rolling on E-Selectin in Vitro.
We compared the abilities of bone marrow leukocytes to tether to and roll on E-selectin under flow. In our experimental system, more than 90% of the rolling cells are neutrophils (14). We used a high E-selectin density (500 sites/μm2) to detect minor adhesive differences in cells lacking one or more ligands. As reported previously (12), fewer PSGL-1−/− neutrophils rolled on E-selectin (Fig. 3A) because of impaired tethering (Fig. 3B), but those cells that tethered rolled with normal velocities (Fig. 3C). For other genotypes, reductions in the number of cells rolling on E-selectin reflected both decreased tethering and faster rolling. No contribution of CD43 to tethering or rolling was observed. CD44−/− neutrophils rolled normally, but PSGL-1−/−/CD44−/− neutrophils rolled significantly faster than cells lacking either individual glycoprotein. Compared with PSGL-1−/−/CD44−/− neutrophils, tethering and rolling of T-syn−/− neutrophils were even more impaired. The adhesive parameters of T-syn−/−/CD44−/− neutrophils were reduced to near-background levels, suggesting a minor contribution of N-glycans on CD44 to rolling on E-selectin. This contribution was not detected on a lower E-selectin density of 150 sites/μm2 (Fig. S2). We also perfused peripheral blood leukocytes over E-selectin. As for bone marrow leukocytes, greater than 90% of the rolling cells were neutrophils. For each genotype, the rolling parameters of peripheral blood neutrophils on E-selectin were indistinguishable from those of bone marrow neutrophils, confirming their equivalent functions (Fig. S3).
Fig. 3.
T-syn−/− neutrophils have markedly impaired rolling on E-selectin in vitro. Bone marrow leukocytes of the indicated genotype were perfused over E-selectin immobilized at 500 sites/μm2. (A) Number of neutrophils rolling per field of view. (B) Number of neutrophils that tethered to E-selectin during the first 30 s was divided by the number of neutrophils delivered across the field of view in the focal plane. (C) Velocities of neutrophils rolling on E-selectin. The data represent the mean ± SEM from five experiments. *P < 0.01.
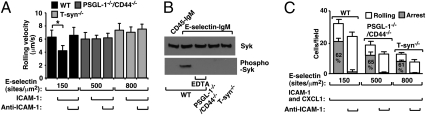
T-syn−/− Neutrophils Rolling on E-Selectin Do Not Trigger Syk-Dependent Activation of Integrin αLβ2 to Slow Rolling on ICAM-1.
WT neutrophils rolling on E-selectin initiate a spleen tyrosine kinase (Syk)–dependent signaling cascade that activates integrin αLβ2 to an intermediate conformation, which slows rolling on intercellular adhesion molecule–1 (ICAM-1) (32). PSGL-1−/− or CD44−/− neutrophils, but not PSGL-1−/−/CD44−/− neutrophils, also activate αLβ2 to slow rolling, demonstrating that E-selectin engagement of either PSGL-1 or CD44 triggers integrin activation in normally glycosylated cells (14). We increased the E-selectin density to 800 sites/μm2 to enable T-syn−/− neutrophils to roll at the same velocity as WT neutrophils rolling on E-selectin at 150 sites/μm2 or PSGL-1−/−/CD44−/− neutrophils rolling on E-selectin at 500 sites/μm2, an indicator of comparable numbers of E-selectin/ligand bonds per unit time (Fig. 4A). WT but not PSGL-1−/−/CD44−/− or T-syn−/− neutrophils rolled slower on coimmobilized ICAM-1. Anti–ICAM-1 mAb prevented slow rolling, confirming the specificity of the interaction. We rotated neutrophils for 5 min on E-selectin at the densities used for rolling and blotted cell lysates with anti–phospho-Syk antibody to measure Syk activation. WT but not PSGL-1−/−/CD44−/− or T-syn−/− neutrophils activated Syk (Fig. 4B). These data demonstrate that E-selectin does not trigger Syk-dependent αLβ2 activation in T-syn−/− neutrophils.
Fig. 4.
T-syn−/− neutrophils rolling on E-selectin do not trigger Syk-dependent activation of integrin αLβ2 to slow rolling on ICAM-1. (A) Velocities of neutrophils from the indicated genotype rolling on E-selectin at the indicated site density with or without coimmobilized ICAM-1 (240 sites/μm2) in the presence or absence of blocking anti–ICAM-1 mAb. (B) Bone marrow leukocytes of the indicated genotype were rotated on control CD45-IgM or on E-selectin-IgM in the presence or absence of EDTA (to prevent selectin binding) for 5 min. Lysates were probed with antibody to Syk or phospho-Syk. (C) Numbers of neutrophils rolling or firmly adherent (arrest) on E-selectin at the indicated density coimmobilized with ICAM-1 and CXCL1 in the presence or absence of blocking anti–ICAM-1 mAb. The percentage of arrested cells is indicated. The wall shear stress was 1 dyn/cm2. The data in A and C represent the mean ± SEM from five experiments. The data in B are representative of three independent experiments. *P < 0.01.
To ensure that loss of O-glycans did not globally impair integrin activation, we immobilized E-selectin with the chemokine CXCL1, which signals through the Gαi protein–coupled receptor CXCR2 to activate αLβ2 to a high-affinity conformation that mediates firm adhesion to ICAM-1 (33). Both WT and T-syn−/− neutrophils rolling on E-selectin rapidly arrested on coimmobilized ICAM-1 (Fig. 4C).
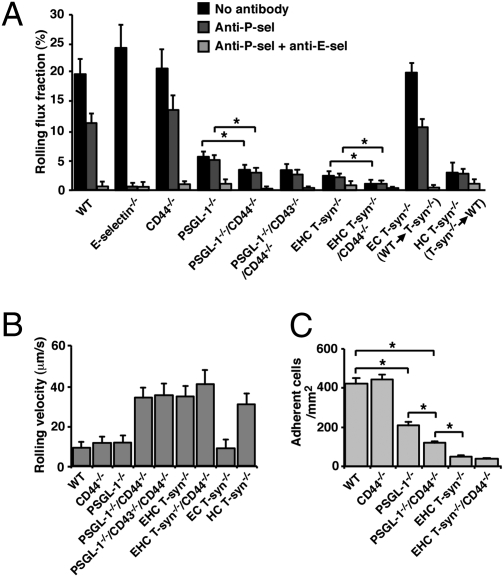
T-syn−/− Neutrophils Have Markedly Impaired Rolling on E-Selectin in Vivo.
We used intravital microscopy of murine cremaster muscle to measure neutrophil rolling in venules after stimulation with TNF-α, which induces expression of P- and E-selectin (34). Neutrophil rolling was defined as the rolling flux fraction, the number of rolling neutrophils divided by the total number of leukocytes passing through the vessel. Rolling was visualized in the same venules before and after sequentially injecting blocking mAbs to P- and E-selectin. In WT mice, rolling was reduced by anti–P-selectin mAb and was subsequently eliminated by anti–E-selectin mAb (Fig. 5A). Anti–P-selectin mAb was sufficient to eliminate rolling in E-selectin−/− mice. Anti–P-selectin mAb did not further reduce neutrophil rolling in PSGL-1−/− and EHC T-syn−/− mice. No contribution of CD43 to rolling was observed. E-selectin–dependent rolling was greatly decreased in PSGL-1−/−/CD44−/− and EHC T-syn−/− mice. Rolling was further reduced to background levels in EHC T-syn−/−/CD44−/− mice, suggesting a modest contribution of N-glycans on CD44 to rolling. To determine the relative contributions of O-glycans on neutrophils and endothelial cells to rolling, we transplanted WT bone marrow cells into irradiated EHC T-syn−/− mice and T-syn−/− bone marrow cells into irradiated WT mice. Rolling of WT neutrophils in T-syn−/− venules was normal, whereas rolling of T-syn−/− neutrophils in WT venules was markedly decreased.
Fig. 5.
T-syn−/− neutrophils have markedly impaired rolling on E-selectin in vivo. (A) Rolling flux fractions of neutrophils of the indicated genotype rolling in TNF-α–stimulated venules, measured before and after injecting a blocking mAb to P-selectin and then a blocking mAb to E-selectin. The panels on the right depict data from bone marrow transplant recipients that lack T-synthase only in endothelial cells (EC) or in hematopoietic cells (HC). (B) Rolling velocities of neutrophils of the indicated genotype. (C) Number of firmly adherent neutrophils of the indicated genotype. The data represent the mean ± SEM from five experiments. *P < 0.01.
We measured velocities of neutrophils rolling on E-selectin after injecting anti–P-selectin mAb (Fig. 5B). The much faster velocities in PSGL-1−/−/CD44−/−, EHC T-syn−/−, and EHC T-syn−/−/CD44−/− mice could not be distinguished. Rolling was highly irregular and included frequent “skipping” whereby neutrophils briefly detached from the venular wall and then reattached downstream in the field of view. Therefore, displacements from skipping were included in the mean rolling velocities. WT neutrophils rolling in TNF-α–stimulated venules encounter the chemokine CXCL1, which stimulates integrin-dependent firm adhesion (33). Firm neutrophil adhesion in venules of PSGL-1−/−/CD44−/− mice was significantly reduced, confirming a previous report (15), but was even lower in EHC T-syn−/− mice (Fig. 5C). No further reduction in adhesion was discerned in EHC T-syn−/−/CD44−/− mice. These data demonstrate that T-syn−/− neutrophils have severely impaired rolling on E-selectin in vivo. As a result, virtually no T-syn−/− neutrophils transition to integrin-dependent arrest on venular surfaces.
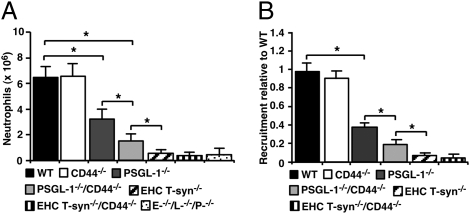
T-syn−/− Neutrophils Have Markedly Impaired Recruitment into Inflamed Tissues.
We measured neutrophil recruitment into the peritoneum 4 h after thioglycollate challenge, a model of selectin-dependent inflammation (29). Neutrophil migration in PSGL-1−/−/CD44−/− mice was significantly reduced, confirming earlier studies (13, 15). Migration in EHC T-syn−/− mice was even lower and was indistinguishable from that observed in mice lacking P-, L-, and E-selectin (Fig. 6A). No further reduction in EHC T-syn−/−/CD44−/− mice was detected. To exclude effects of core 1-derived O-glycans from endothelial cells on recruitment, we used a competitive homing assay in WT mice. A 1:1 mixture of WT and KO leukocytes labeled with different dyes was injected i.v. into WT mice 2 h after i.p. injection of thioglycollate. After another 2 h, blood and peritoneal cells were collected. The ratio of labeled neutrophils in blood remained close to 1 (Fig. S4). Relative to WT neutrophils, migration of PSGL-1−/−/CD44−/− neutrophils into the peritoneum was significantly reduced (Fig. 6B). Migration of T-syn−/− neutrophils was even lower. No further reduction in migration of T-syn−/−/CD44−/− neutrophils was discerned.
Fig. 6.
T-syn−/− neutrophils have markedly impaired recruitment into inflamed tissues. (A) Mice of the indicated genotype were injected with 1 mL of 4% thioglycollate i.p. After 4 h, peritoneal cells were collected, and the number of neutrophils was measured by flow cytometry. (B) WT mice were injected i.p. with thioglycollate. After 2 h, they were injected i.v. with a 1:1 mixture of PKH67-labeled WT leukocytes and PKH26-labeled leukocytes of the indicated genotype. After 2 h, peritoneal cells were collected, and the number of neutrophils labeled with each dye was measured by flow cytometry. Results are plotted as the ratio of neutrophils from the indicated genotype to WT neutrophils. The data represent the mean ± SEM from five experiments. *P < 0.01. Fig. S4 presents the ratios of labeled cells collected from blood at the same time.
Leukocytes Express mRNA for β3GlcNAcTs with Core 1 Extension Activity and for C2GnT1 but Not C2GnT2 or C2GnT3.
Compared with C2GnT1−/− neutrophils (22, 23), T-syn−/− neutrophils interacted much less well with E-selectin. This suggests that neutrophils also express E-selectin ligands on core 2 structures synthesized by C2GnT2 or C2GnT3 or on extended core 1 structures synthesized by β3GlcNAcTs. Using RT-PCR of RNA from WT leukocytes, we detected transcripts for C2GnT1 but not for C2GnT2 or C2GnT3 (Fig. S5). We also detected transcripts for all seven β3GlcNAcTs, including the enzyme with the best-documented core 1 extension activity, β3GlcNAcT3. These data suggest that leukocytes use only C2GnT1 to synthesize core 2 O-glycans and express β3GlcNAcTs that might synthesize extended core 1 O-glycans.
Discussion
Previous studies concluded that the glycoproteins PSGL-1, CD44, and ESL-1 constitute all E-selectin ligands on murine neutrophils (15). E-selectin binds to N-glycans on CD44 and ESL-1 (13, 16, 17), and neutrophils lacking CD44 and ESL-1 have partial defects in rolling on E-selectin (15). C2GnT1−/− neutrophils tether less well to E-selectin but roll with normal velocities (23). Collectively, these data imply that neutrophils use both N- and O-glycans as E-selectin ligands, with neither subset having a dominant role. Here, using T-syn−/− neutrophils lacking all core 1-derived O-glycans, we demonstrated that these structures are essential E-selectin ligands in vitro and in vivo. Our data argue that PSGL-1, CD44, and ESL-1 do not represent all glycoprotein ligands for E-selectin on neutrophils. Furthermore, the N-glycans on CD44 and ESL-1 are not sufficient to support appreciable neutrophil rolling on E-selectin. Although this study focuses on neutrophils, our findings may extend to other leukocyte subsets.
We documented failure of P- and L-selectin to bind to T-syn−/− neutrophils, consistent with the inability of the cells to synthesize a core 2 O-glycan near the N terminus of PSGL-1 that is required for binding (4–6). The additional profound defects in E-selectin binding to T-syn−/− neutrophils were physiologically important. Despite the marked neutrophilia in EHC T-syn−/− mice, very few neutrophils tethered to E-selectin in TNF-α–activated venules. Those that tethered rolled very rapidly, and almost none arrested on the venular surface. Accordingly, migration of T-syn−/− neutrophils into the inflamed peritoneum was as severely reduced as in mice lacking all three selectins.
That E-selectin failed to bind PSGL-1 in lysates of T-syn−/− neutrophils establishes that PSGL-1 uses core 1-derived O-glycans as E-selectin ligands. E-selectin bound indistinguishably to CD44 or ESL-1 from WT and T-syn−/− neutrophils. These results support the importance of N-glycans as E-selectin ligands on these proteins (13, 16, 17), although O-glycans might make minor contributions to binding that our assay did not detect. CD44 expresses both N- and O-glycans (35), but only N-glycans have been observed on ESL-1 (16), and computer algorithms (36) do not predict O-glycans on this protein.
CD43 contributes modestly to rolling of activated T cells on E-selectin (24–26), but we detected no contribution of CD43 to rolling of neutrophils. Our observation is consistent with competitive homing assays that found no contribution of CD43 to neutrophil recruitment to the inflamed peritoneum (27). T-syn−/− neutrophils rolled less well than neutrophils lacking PSGL-1, CD43, and CD44. This strongly suggests that neutrophils use at least one other O-glycosylated protein to mediate rolling. In the absence of O-glycans, the N-glycans on CD44 and ESL-1 supported almost no tethering to or rolling on E-selectin. In the absence of CD44 and ESL-1, the O-glycans on PSGL-1 and other protein(s) mediate tethering to E-selectin, but the neutrophils roll at higher velocities (15). Similar complexity may apply to E-selectin–mediated signaling. Normally glycosylated neutrophils rolling on E-selectin use either PSGL-1 or CD44 to initiate Syk-dependent activation of integrin αLβ2 to slow rolling on ICAM-1 (14). However, even high densities of E-selectin failed to activate Syk or αLβ2 in T-syn−/− neutrophils, even though CD44 on these cells can bind to E-selectin. Signaling may require that E-selectin cooperatively engage CD44 (or PSGL-1) plus another O-glycosylated protein(s).
Interpreted in the context of previous studies, our results argue that neutrophils use both core 2 and extended core 1 O-glycans as E-selectin ligands (Fig. 1A). C2GnT1−/− leukocytes lack measurable C2GnT enzymatic activity and core 2 O-glycans (22). E-selectin binds normally to C2GnT2−/− or C2GnT3−/− leukocytes (37), and we did not detect transcripts for C2GnT2 or C2GnT3 in leukocytes. These data strongly suggest that leukocytes use only C2GnT1 to synthesize core 2 O-glycans. Yet compared with C2GnT1−/− neutrophils (23), T-syn−/− neutrophils interacted much less well with E-selectin. We confirmed that leukocytes express transcripts for β3GlcNAcTs with documented or potential core 1-extension activity. The Consortium for Functional Glycomics (http://www.functionalglycomics.org) provides a partial list of O-glycan structures in murine myeloid cells that were derived by MS. No extended core 1 O-glycans were noted, but some putative core 2 structures could be extended core 1 O-glycans with identical masses. Thus, leukocytes might express extended core 1 O-glycans that are modified to form E-selectin ligands. That we observed markedly decreased E-selectin binding to PSGL-1 from lysates of C2GnT1−/− leukocytes suggests that core 2 O-glycans constitute most E-selectin ligands on PSGL-1. Given the similarities in the E-selectin–binding phenotypes of C2GnT1−/− and PSGL-1−/− mice, many of the core 2–based E-selectin ligands on leukocytes may be on PSGL-1. There is evidence that some glycosyltransferases act preferentially at specific sites on proteins (10, 11, 38, 39). As yet uncharacterized structural features may determine which leukocyte glycoproteins express core 2 and/or extended core 1 O-glycans as E-selectin ligands.
Mucins may have antiadhesive functions that result from repulsion by the negative charges on multiple sialylated O-glycans (40, 41). T-syn−/− neutrophils lack these potential antiadhesive properties because they expressed only the Tn antigen without detectable sialylation. Nevertheless, they still failed to roll on E-selectin in vitro or migrate into inflamed tissues in vivo. Thus, the loss of proadhesive O-glycan ligands for E-selectin negated any loss of antiadhesive functions from diminished cell-surface sialylation. Bone marrow transplants revealed no evidence for augmented rolling or arrest of WT neutrophils in T-syn−/− venules, perhaps because sulfated proteoglycans contribute most negative charges to the glycocalyx of endothelial cells (42).
Unlike murine neutrophils, human neutrophils express sialylated glycosphingolipids with some Galβ1–4GlcNAc units modified with α1–3 fucose (43). Inhibiting glycolipid synthesis in human neutrophils partially impairs rolling on E-selectin (43), but this could result from diminished cell deformability (44). Glycolipids isolated from human neutrophils bind to E-selectin (43). However, glycolipids are much shorter than glycoproteins. Within the glycocalyx of intact cells, they may not be accessible to rapidly interact with E-selectin during rolling. Therefore, core 1-derived O-glycans on proteins may be essential E-selectin ligands on human as well as murine neutrophils.
Previous studies documented both shared and distinct contributions of PSGL-1, CD44, and ESL-1 to E-selectin–mediated adhesion and signaling (12–15). Our demonstration that neutrophils require core 1-derived O-glycans to mediate these events reveals additional complexity during the critical early stages of inflammation. It will be important to define the relative contributions of core 2 and extended core 1 O-glycans as E-selectin ligands and to identify the additional O-glycosylated protein(s) that interact with E-selectin.
Materials and Methods
All mouse experiments were performed in compliance with protocols approved by the Institutional Animal Care and Use Committee of the Oklahoma Medical Research Foundation. The unpaired Student t test was used to determine P values as indicated in the figures. Details including proteins, mice, flow cytometry, precipitation of leukocyte glycoproteins with E-selectin, flow chamber assay, E-selectin–mediated Syk phosphorylation, intravital microscopy, thioglycollate-induced peritonitis, competitive neutrophil recruitment assay, and semiquantitative RT-PCR are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Barry Wolitzky and Dietmar Vestweber for providing antibodies, Paul Kubes and Klaus Ley for providing mice, Yuqing Huo for providing bone marrow cells from C2GnT1−/− mice, and Samuel McGee for technical assistance. This work was supported by National Institutes of Health Grants HL085607 and HL034363.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003110107/-/DCSupplemental.
References
- 1.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2.Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [DOI] [PubMed] [Google Scholar]
- 3.McEver RP. Selectins: Lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- 4.Leppänen A, White SP, Helin J, McEver RP, Cummings RD. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J Biol Chem. 2000;275:39569–39578. doi: 10.1074/jbc.M005005200. [DOI] [PubMed] [Google Scholar]
- 5.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103:467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 6.Leppänen A, Yago T, Otto VI, McEver RP, Cummings RD. Model glycosulfopeptides from P-selectin glycoprotein ligand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin. J Biol Chem. 2003;278:26391–26400. doi: 10.1074/jbc.M303551200. [DOI] [PubMed] [Google Scholar]
- 7.Rosen SD. Ligands for L-selectin: Homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 8.Mitoma J, et al. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat Immunol. 2007;8:409–418. doi: 10.1038/ni1442. [DOI] [PubMed] [Google Scholar]
- 9.Kobzdej MMA, Leppänen A, Ramachandran V, Cummings RD, McEver RP. Discordant expression of selectin ligands and sialyl Lewis x-related epitopes on murine myeloid cells. Blood. 2002;100:4485–4494. doi: 10.1182/blood-2002-06-1799. [DOI] [PubMed] [Google Scholar]
- 10.Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL-60 cells. J Biol Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]
- 11.Huang MC, et al. P-selectin glycoprotein ligand-1 and E-selectin ligand-1 are differentially modified by fucosyltransferases Fuc-TIV and Fuc-TVII in mouse neutrophils. J Biol Chem. 2000;275:31353–31360. doi: 10.1074/jbc.M005449200. [DOI] [PubMed] [Google Scholar]
- 12.Xia L, et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med. 2005;201:1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yago T, et al. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin αLβ2-mediated slow leukocyte rolling. Blood. 2010 doi: 10.1182/blood-2009-12-259556. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenter M, Levinovitz A, Isenmann S, Vestweber D. Monospecific and common glycoprotein ligands for E- and P-selectin on myeloid cells. J Cell Biol. 1994;125:471–481. doi: 10.1083/jcb.125.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153:1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ju T, Brewer K, D'Souza A, Cummings RD, Canfield WM. Cloning and expression of human core 1 β1,3-galactosyltransferase. J Biol Chem. 2002;277:178–186. doi: 10.1074/jbc.M109060200. [DOI] [PubMed] [Google Scholar]
- 19.Yeh JC, et al. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension β 1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 20.Mitoma J, et al. Extended core 1 and core 2 branched O-glycans differentially modulate sialyl Lewis X-type L-selectin ligand activity. J Biol Chem. 2003;278:9953–9961. doi: 10.1074/jbc.M212756200. [DOI] [PubMed] [Google Scholar]
- 21.Bierhuizen MFA, Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal β 1-3-GalNAc-R (GlcNAc to GalNAc) β 1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci USA. 1992;89:9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellies LG, et al. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 23.Sperandio M, et al. Severe impairment of leukocyte rolling in venules of core 2 glucosaminyltransferase-deficient mice. Blood. 2001;97:3812–3819. doi: 10.1182/blood.v97.12.3812. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto M, et al. CD43 functions as a ligand for E-Selectin on activated T cells. J Immunol. 2005;175:8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- 25.Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood. 2006;107:1421–1426. doi: 10.1182/blood-2005-05-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alcaide P, et al. The 130-kDa glycoform of CD43 functions as an E-selectin ligand for activated Th1 cells in vitro and in delayed-type hypersensitivity reactions in vivo. J Invest Dermatol. 2007;127:1964–1972. doi: 10.1038/sj.jid.5700805. [DOI] [PubMed] [Google Scholar]
- 27.Carlow DA, Ziltener HJ. CD43 deficiency has no impact in competitive in vivo assays of neutrophil or activated T cell recruitment efficiency. J Immunol. 2006;177:6450–6459. doi: 10.4049/jimmunol.177.9.6450. [DOI] [PubMed] [Google Scholar]
- 28.Fu J, et al. Endothelial cell O-glycan deficiency causes blood/lymphatic mis-connections and consequent fatty liver disease in mice. J Clin Invest. 2008;118:3725–3737. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson SD, et al. Multiple, targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment. Proc Natl Acad Sci USA. 1999;96:11452–11457. doi: 10.1073/pnas.96.20.11452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miner JJ, et al. Separable requirements for cytoplasmic domain of PSGL-1 in leukocyte rolling and signaling under flow. Blood. 2008;112:2035–2045. doi: 10.1182/blood-2008-04-149468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steegmaier M, Borges E, Berger J, Schwarz H, Vestweber D. The E-selectin-ligand ESL-1 is located in the Golgi as well as on microvilli on the cell surface. J Cell Sci. 1997;110:687–694. doi: 10.1242/jcs.110.6.687. [DOI] [PubMed] [Google Scholar]
- 32.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced α(L)β(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith ML, Olson TS, Ley K. CXCR2- and E-selectin-induced neutrophil arrest during inflammation in vivo. J Exp Med. 2004;200:935–939. doi: 10.1084/jem.20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunkel EJ, Ley K. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 35.Ponta H, Sherman L, Herrlich PA. CD44: From adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 36.Julenius K, Mølgaard A, Gupta R, Brunak S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology. 2005;15:153–164. doi: 10.1093/glycob/cwh151. [DOI] [PubMed] [Google Scholar]
- 37.Stone EL, et al. Glycosyltransferase function in core 2-type protein O glycosylation. Mol Cell Biol. 2009;29:3770–3782. doi: 10.1128/MCB.00204-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zöllner O, Vestweber D. The E-selectin ligand-1 is selectively activated in Chinese hamster ovary cells by the α(1,3)-fucosyltransferases IV and VII. J Biol Chem. 1996;271:33002–33008. doi: 10.1074/jbc.271.51.33002. [DOI] [PubMed] [Google Scholar]
- 39.Miller E, et al. A necessary and sufficient determinant for protein-selective glycosylation in vivo. J Biol Chem. 2008;283:1985–1991. doi: 10.1074/jbc.M708160200. [DOI] [PubMed] [Google Scholar]
- 40.Stockton BM, Cheng G, Manjunath N, Ardman B, von Andrian UH. Negative regulation of T cell homing by CD43. Immunity. 1998;8:373–381. doi: 10.1016/s1074-7613(00)80542-7. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto M, Shigeta A, Miyasaka M, Hirata T. CD43 plays both antiadhesive and proadhesive roles in neutrophil rolling in a context-dependent manner. J Immunol. 2008;181:3628–3635. doi: 10.4049/jimmunol.181.5.3628. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Fuster M, Sriramarao P, Esko JD. Endothelial heparan sulfate deficiency impairs L-selectin- and chemokine-mediated neutrophil trafficking during inflammatory responses. Nat Immunol. 2005;6:902–910. doi: 10.1038/ni1233. [DOI] [PubMed] [Google Scholar]
- 43.Nimrichter L, et al. E-selectin receptors on human leukocytes. Blood. 2008;112:3744–3752. doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yago T, et al. Distinct molecular and cellular contributions to stabilizing selectin-mediated rolling under flow. J Cell Biol. 2002;158:787–799. doi: 10.1083/jcb.200204041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.