Abstract
Male moths are endowed with odorant receptors (ORs) to detect species-specific sex pheromones with remarkable sensitivity and selectivity. We serendipitously discovered that an endogenous OR in the fruit fly, Drosophila melanogaster, is highly sensitive to the sex pheromone of the silkworm moth, bombykol. Intriguingly, the fruit fly detectors are more sensitive than the receptors of the silkworm moth, although its ecological significance is unknown. By expression in the “empty neuron” system, we identified the fruit fly bombykol-sensitive OR as DmelOR7a (= DmOR7a). The profiles of this receptor in response to bombykol in the native sensilla (ab4) or expressed in the empty neuron system (ab3 sensilla) are indistinguishable. Both WT and transgenic flies responded with high sensitivity, in a dose-dependent manner, and with rapid signal termination. In contrast, the same empty neuron expressing the moth bombykol receptor, BmorOR1, demonstrated low sensitivity and slow signal inactivation. When expressed in the trichoid sensilla T1 of the fruit fly, the neuron housing BmorOR1 responded with sensitivity comparable to that of the native trichoid sensilla in the silkworm moth. By challenging the native bombykol receptor in the fruit fly with high doses of another odorant to which the receptor responds with the highest sensitivity, we demonstrate that slow signal termination is induced by overdose of a stimulus. As opposed to the empty neuron system in the basiconic sensilla, the structural, biochemical, and/or biophysical features of the sensilla make the T1 trichoid system of the fly a better surrogate for the moth receptor.
Keywords: BmorOR1, Bombyx mori, DmelOR7a, Drosophila melanogaster, signal inactivation
Since the identification of bombykol (IUPAC name (10E,12Z)-hexadeca-10,12-dien-1-ol) as a sex pheromone for the silkworm moth, Bombyx mori (1), hundreds of sex pheromones have been identified from lepidopteran species (2). In general, female-produced sex pheromones in moths are complex mixtures of straight-chain acetates, aldehydes, and alcohols, with 10–12 carbon atoms and up to three unsaturations (3). This fatty acid–derived semiochemistry (4, 5) is unique to chemical communication in moths, although (7Z)-dodec-7-en-1-yl acetate also has been identified as a sex pheromone for the Asian elephant, Elephas maximus (6). Male moths detect these sex pheromones with such high selectivity and sensitivity that minimal structural modifications render them inactive (7), whereas a single molecule of bombykol is estimated to be sufficient to activate olfactory receptor neurons (ORNs) in male antennae of the silkworm moth (8). These species-specific communication channels seem to have evolved under a strong evolutionary pressure into a sophisticated signal receptor system. In contrast, we have serendipitously identified an ORN housed in the ab4 basiconic sensilla in the antennae of the fruit fly, Drosophila melanogaster, which responds to bombykol with high sensitivity (9), although the ecological significance of this bombykol detector in the fruit fly remains enigmatic. The OR housed in the ab4a neuron was previously identified as DmelOR7a (= DmOr7a) (10) and tested against a panel of compounds that, understandably, did not include bombykol.
Previously, we expressed the bombykol receptor from the silkworm moth, BmorOR1, in the “empty neuron” housed in the ab3 basiconic sensilla of a D. melanogaster mutant—an ORN that lacks a native odorant receptor (OR) but retains the biochemical machinery necessary for detecting odorants (11). Single-sensillum recordings from the BmorOR1-expressing neurons in the ab3 basiconic sensilla of the transgenic flies responded to bombykol, but with lower sensitivity than the endogenous bombykol receptor in the ab4 sensilla of the fruit fly, and showed slow signal termination (9). In the silkworm moth, BmorOR1-expressing neurons are housed in trichoid sensilla, with 17,000 of them dedicated to bombykol reception in male moths (12). In D. melanogaster, basiconic sensilla outnumber the trichoid sensilla classes (T1, T2, and T3) by ∼2-fold (13). These findings prompted us to address questions regarding sensitivity to bombykol and signal termination when the native bombykol receptor from the fruit fly is expressed in the empty neuron and the silkworm moth receptor, BmorOR1, is expressed in trichoid sensilla in the fruit fly. Here we report a systematic comparison between the responses of the fruit fly and the silkworm moth to bombykol and demonstrate that under the same conditions, the bombykol-detecting ORNs in the fruit fly are more sensitive than the moth counterparts. By comparing flies expressing DmelOR7a in the empty neuron of the ab3 sensilla with those containing the native bombykol receptor in the ab4 sensilla, we show a mirror image of the native receptor regarding bombykol-induced excitation and inactivation. We then demonstrate that—in marked contrast to flies expressing the moth OR in ab3 sensilla—the physiological responses to bombykol by flies containing BmorOR1 in the T1 trichoid sensilla are almost indistinguishable from those recorded from the trichoid sensilla of the silkworm moth.
Results and Discussion
Bombykol Elicits Significantly Higher Responses in Flies than in Moths.
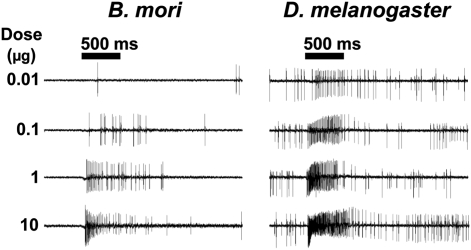
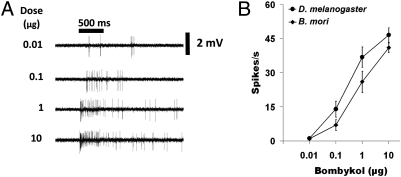
Previously we identified an ORN sensitive to bombykol in the ab4 sensilla of the D. melanogaster antennae (9), which seemed more sensitive than the moth detectors reported in the literature (7), although comparison from different electrophysiological settings might be misleading. Here, using single-unit recordings under the same conditions, we compared the sensitivity of the ORNs housed in long trichoid sensilla of the silkworm moth with those in the ab4 sensilla in fruit fly antennae. Whereas bombykol elicited dose-dependent responses from the moth and fly ORNs, the fruit fly ORNs were indeed more sensitive (Fig. 1). For example, with bombykol delivered from a source loaded with 10 μg of the odorant, the fruit flies demonstrated at least a 5-fold higher response; that is, ORNs in the long trichoids of the moth elicited only a maximum of ∼40 spikes/s, whereas >200 spikes/s were recorded from the ab4A ORNs in the basiconic sensilla of the fruit fly. Although individual bombykol detectors in the fruit fly are more sensitive than those in the silkworm moth, the fruit fly antennae has fewer than 40 basiconic ab4 sensilla (13), whereas the silkworm moth is endowed with 17,000 bombykol-detecting trichoid sensilla (12), explaining the remarkable overall sensitivity of pheromone detection in the silkworm moth (8).
Fig. 1.
Bombykol elicited excitatory responses from trichoid sensilla in B. mori and basiconic sensilla in D. melanogaster. Action potential traces from the trichoid sensilla of B. mori and ab4 basiconic sensilla of D. melanogaster in response to increasing doses of bombykol (from top to bottom). Significantly higher action potentials were generated from the ORN housed in the basiconic sensillum of fruit flies compared with the long trichoid sensillum of silkworm moths.
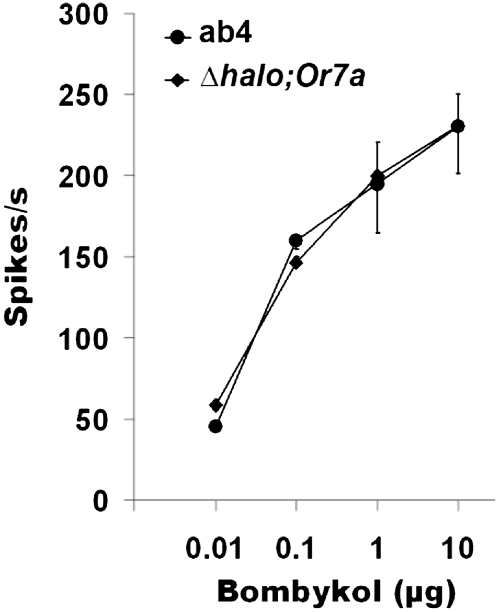
The ab4 sensilla in the fruit fly house two ORNs, termed ab4A and ab4B on the basis of their spike amplitudes (14). The large–spike amplitude ab4A ORN, which is sensitive to bombykol (Fig. 1), expresses the odorant receptor DmelOR7a (10). We obtained flies expressing DmelOR7a in the empty neuron and compared their physiological responses to bombykol with those from the ab4A neuron of WT flies. The dose–response curves obtained by single-sensillum recordings from the ORNs housing DmelOR7a in the endogenous neuron (ab4A) and in the empty neuron (ab3A) were remarkably similar (Fig. 2), as is the case for other D. melanogaster ORs expressed in the empty neuron (10, 11). Thus, we concluded that the native bombykol receptor in the fruit fly is indeed DmelOR7a.
Fig. 2.
D. melanogaster odorant receptor DmelOR7a was stimulated by bombykol in its native environment of the ab4 sensilla and in the empty neuron of the ab3 sensilla in a dose-dependent manner. The bombykol-induced excitatory responses in the ab3 and ab4 sensilla (n = 7) were comparable at all doses tested.
It is reasonable to expect that ORs sensitive to the same odorant in different species might show high amino acid identity. Indeed, we identified an oviposition attractant-sensitive OR from the Southern house mosquito, Culex quinquefasciatus (16), by mining the Culex genome for putative receptors with amino acid sequences related to an OR identified from the malaria mosquito, Anopheles gambiae, which detected related semiochemicals (16, 17). Likewise, AaegOR8 from the yellow fever mosquito, Aedes aegypti, showed high amino acid identity to the malaria mosquito counterpart, AgamOR8, and both receptors were highly sensitive to 1-octen-3-ol (16–18). This assumption might not hold true for species from different orders, however, as suggested by the low amino acid identity between BmorOR1 and DmelOR7a (17%). In short, these two ORs with very low amino acid identity are sensitive to the same ligand.
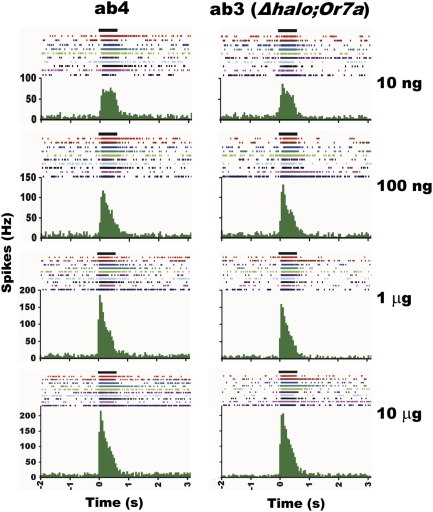
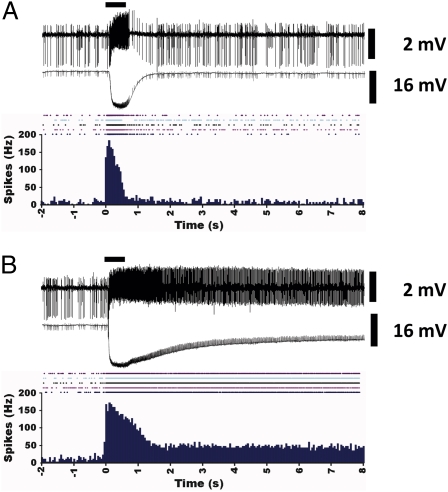
In marked contrast to flies expressing DmelOR7a in the empty neuron, those containing BmorOR1 in ab3 sensilla elicited lower responses to bombykol even when sensitivity was enhanced by coexpression of a moth pheromone-binding protein, BmorPBP1 (9). In addition, termination of bombykol signal in the BmorOR1-expressing flies continued far beyond the duration of the stimulation (9). We compared the dynamics of bombykol-induced excitation and signal inactivation in the fruit flies carrying DmelOR7a in the native biochemical milieu of ab4 sensilla with those expressing the fly bombykol receptor in the empty neuron. Electrophysiological responses recorded from the ab3 and ab4 sensilla were remarkably comparable (Fig. 3). Higher doses of bombykol sharpened the phasic response in both types of flies, but signal termination was rapid and comparable in both cases (Fig. 3). We reasoned that the slow signal termination in BmorOR1-expressing flies (9) could be due, at least in part, to the low sensitivity of the system, which in turn required high doses of bombykol to stimulate the receptor. Alternatively, signal termination of physiologically high doses of pheromones might be achieved through a combination of a hypothesized deactivation mechanism coupled with pheromone degradation (19). At physiological doses of bombykol, endogenous enzymes from the fruit fly might suffice for signal termination. We tested the hypothesis that an overdose of an odorant leads to a delayed signal termination (20) by challenging ab4A ORN with its “best ligand,” (E)-2-hexenal (i.e., the ligand to which the receptor responded with the highest sensitivity) (14). Signal termination was indeed rapid when the sensillum was stimulated with a physiologically relevant dose (10 ng) of this odorant (Fig. 4A), but a three orders of magnitude higher dose (10 μg) led to delayed signal termination (Fig. 4B), with a profile resembling that seen in BmorOR1 flies (Fig. S1B).
Fig. 3.
Response kinetics recorded from ORNs in D. melanogaster expressing DmelOR7a in its native environment of ab4 sensillum and in the empty neuron of the ab3 sensilla. Data from the WT flies (Left) and transgenic flies (Right), with increasing decadic doses from 10 ng to 10 μg from top to bottom. The duration of stimulation (500 ms) is indicated at the top of each plot. Individual spikes are shown at the top of each plot (each line representing one neuron), and their peristimulus time histograms are displayed in the lower part. Each plot indicates excitatory responses pattern of 10 different ORNs to bombykol. The peristimulus time histograms were obtained by averaging firing rates in 50-ms bins for each dose. Note that bombykol-induced excitatory responses in ab3 and ab4 sensilla terminated approximately at the end of the stimulus period.
Fig. 4.
Kinetics of signal termination in the ab4 sensilla when stimulated with optimal and high concentrations of the same ligand, (E)-2-hexenal. (A) A source dose of 10 ng of the odorant elicited a near-maximal response from the ab4A neuron. (Top) The spiking pattern. (Middle) The corresponding receptor potential. (Bottom) Five traces of extracted spikes and their summed response (details as in Fig. 3). (B) An overdose stimulus, 10 μg of (E)-2-hexenal, induced comparable excitation (∼200 spikes/s) but the neuronal activity extended far beyond the duration of stimulation (500 ms).
In conclusion, our findings demonstrate that the empty neuron system in the ab3 basiconic sensilla of D. melanogaster has some limitations for testing ORs from species of a distant taxon (e.g., Lepidoptera vs. Diptera), although this mutant fruit fly was an enormously successful surrogate for D. melanogaster (10, 11) as well as mosquito ORs (16), probably because flies and mosquitoes, both in the order Diptera, harbor related odorant-binding proteins (21, 22). We then tested BmorOR1 in the trichoid sensilla of the fruit fly, which already has a biochemical machinery for the detection of the fruit fly sex pheromone (23, 24).
Responses to Bombykol by BmorOR1 Receptors in the Trichoid Sensilla of the Fruit Fly and the Silkworm Moth.
Typically, pheromone receptors in moths are expressed in trichoid sensilla (25) along with pheromone-binding proteins (26). In the fruit fly, this type of sensilla houses ORNs sensitive to the sex pheromone (Z)-octadec-11-enyl acetate (also known as 11-cis-vacenyl acetate) (27, 28). Previously, BmorOR1 was expressed in trichoid T1 sensilla of the fruit fly by using a mutant knock-in allele in which the ORF of the native OR67d was replaced with that of the yeast transcriptional activator GAL4 (24). In this earlier study, the generated flies did not seem very sensitive to bombykol and required stimulation with pure pheromone to elicit the highest spike activity (100% pheromone, ∼60 spikes/s) (24). In contrast, our flies expressing BmorOR1 in the trichoid T1 sensilla responded to bombykol in a dose-dependent manner and with high sensitivity (Fig. 5 A and B). Because we used the same genetic strategy and used the mutant knock-in allele (a gift from Barry J. Dickson, The Research Institute of Molecular Pathology, Vienna) to generate OR67dGAL4, UAS-BmorOR1 flies, we assume that the differences in electrophysiological response might be due to different stimulus delivery/recording systems. The electrophysiological responses recorded from the BmorOR1-housing trichoid sensilla in the fruit fly were comparable to those obtained from the long trichoid sensilla in the antennae of the silkworm moth (Fig. 5B). In addition, termination of bombykol signal in the trichoid sensilla of the fruit fly was rapid (Fig. S1A), in marked contrast to what we found with the same receptor expressed in the ab3 basiconic sensilla (Fig. S1B).
Fig. 5.
Response patterns from trichoid sensilla expressing BmorOR1. (A) Spiking pattern of an ORN from T1 type trichoid sensillum stimulated with increasing doses of bombykol. (B) Dose-dependent excitatory responses are comparable between trichoid sensilla in the moth and the T1 trichoid sensilla in the fruit fly expressing BmorOR1 (n = 11 for B. mori; n = 6 for D. melanogaster).
In conclusion, the electrophysiological profile obtained using a modified trichoid system of D. melanogaster mirrored that recorded from the native sensilla in the silkworm moth, with similar sensitivity, magnitude, and kinetics of signal inactivation. This response profile recorded from trichoid sensilla differed significantly from that obtained when the same receptor was expressed in the basiconic ab3 sensilla. Interestingly, both trichoid and basiconic sensilla have a single wall with a smooth surface with multiple pores, but they differ in terms of dendrite structure. T1 trichoid sensilla are innervated by one ORN, whose dendrites extend unbranched into the sensillum lumen (29), thus resembling the structure of pheromone-detecting sensilla in moths (30). In contrast, basiconic sensilla display a dendritic branching pattern, with their dendrites split into as few as 5 to as many as 150 parallel terminal branches (29). As with pheromone-detecting sensilla in moths, which house species-specific pheromone-binding proteins (26), an OBP from D. melanogaster, termed LUSH, is specifically expressed in trichoid sensilla (31) and seems to be essential for pheromone reception (23). In addition, a sensory membrane protein (SNMP), originally isolated from moth antennae (32), seems essential for pheromone detection in the trichoid sensilla of the fruit fly, but it is not involved in odorant reception by basiconic sensilla (33). Whether structural, biochemical, and/or biophysical features of the trichoid sensilla contribute to a faithful reproduction of the moth signal reported here remains an exciting area for future research.
Materials and Methods
Insects.
Strains carrying Δhalo (11) and OR22a-Gal4 or UAS-OR7a were a gift from John Carlson (Yale University, New Haven, CT) (10). Flies were crossed, as reported previously (9), to produce transgenic test flies with OR7a expressed in ab3A empty neurons (w;Δhalo;OR22a-Gal4/UAS-OR7a). Oregon-R flies were used as the standard WT strain. Flies expressing BmOR1 in the trichoid sensilla of D. melanogaster were generated by crossing flies with OR67dGAL4, a mutant allele in which the ORF of OR67d was replaced with that of the yeast transcriptional activator GAL4 (a gift from Barry Dickson) (24), to a UAS-BmOR1 line generated with the BmorOR1 P-element as reported previously (9). The resulting w;UAS-BmOR1/CyO;OR67d-Gal4/MKRS strain was selected to obtain homozygous flies for electrophysiologic recordings. Bombyx mori (Nishitari strain) adult males that emerged from individually segregated pupae were used for electrophysiological recordings.
Stimuli.
Bombykol (purchased from Plant Research International) was dissolved in dichloromethane (DCM) to make a stock solution of 10 μg/μL, from which decadic dilutions were made. (E)-2-hexenal (98%; Sigma-Aldrich) solutions were prepared similarly. A 10-μL aliquot of a stimulus dissolved in DCM in the desired dose was loaded on a filter paper strip, the solvent was evaporated for 30 s, and the strip was placed in a disposable Pasteur pipettes. DCM alone and an empty Pasteur pipette served as controls.
Single-Sensillum Recordings.
Recordings were performed essentially as described previously (9). In brief, a fly was restrained, a glass reference electrode was placed in the eye, and the recording electrode was brought into contact with the base of the sensillum. Recorded extracellular action potentials were amplified, fed into an IDAC4-USB box (Syntech), recorded on the hard disk of a PC, and analyzed with Auto Spike version 3.7 (Syntech). AC signals (action potentials or spikes) were bandpass-filtered between 100 and 10,000 Hz, whereas a high filter of 3 kHz was used for the DC signals (receptor potentials/sensillum potentials). The preparation was held in a humidified air stream delivered at 20 mL/s, to which a stimulus pulse of 2 mL/s was added for 500 ms. (For Fig. S1, the duration of stimulation was 2 s). Unless specified otherwise, signals were recorded for 10 s starting 2 s before stimulation, and spikes were counted off-line in a 500-ms period before and during the stimulation. Responses from individual neurons were calculated as the increase in spike frequency (spikes/s) relative to the prestimulus frequency. At least three flies of each genotype were recorded, and up to five sensilla from each fly were tested. Data were pooled because no significant differences between sensilla or sexes or age groups (1- to 5-day-old flies) were observed. For the silkworm moth, we essentially followed the recording methods described previously (8), in which the tips of long trichoid sensilla are clipped and inserted into a glass electrode filled with a physiological solution.
Supplementary Material
Acknowledgments
We thank Dr. Julien Pelletier for enlightening discussions and suggestions for improving the manuscript and Afsoon Badiei and Dr. Yuko Ishida for rearing the fruit flies and silkworm moths, respectively. This work was supported in part by National Science Foundation Grant 0918177 (to W.S.L.) and National Institutes of Health Grant GM82843-01A2 (to A.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003881107/-/DCSupplemental.
References
- 1.Butenandt A, Beckmann R, Stamm D, Hecker E. Uber den sexual lockstoff des seidenspinners Bombyx mori: Reindarstellung und konstitution. Z Naturforschung B. 1959;14:283–284. [Google Scholar]
- 2.El-Sayed AM. The Pherobase: Database of insect pheromones and semiochemicals. 2008. Available at http://www.pherobase.com. Accessed March 20, 2010.
- 3.Ando T, Inomata SI, Yamamoto M. Lepidopteran sex pheromones. Top Curr Chem. 2004;239:51–96. doi: 10.1007/b95449. [DOI] [PubMed] [Google Scholar]
- 4.Roelofs WL, Rooney AP. Molecular genetics and evolution of pheromone biosynthesis in Lepidoptera. Proc Natl Acad Sci USA. 2003;100:9179–9184. doi: 10.1073/pnas.1233767100a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roelofs WL. Chemistry of sex attraction. Proc Natl Acad Sci USA. 1995;92:44–49. doi: 10.1073/pnas.92.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen LE, Lee TD, Roelofs WL, Zhang A, Daves GD., Jr Insect pheromone in elephants. Nature. 1996;379:684. doi: 10.1038/379684a0. [DOI] [PubMed] [Google Scholar]
- 7.Kaissling KE. Wright Lectures on Insect Olfaction. Burnaby, BC, Canada: Simon Fraser Univ; 1987. [Google Scholar]
- 8.Kaissling KE, Priesner E. Olfactory threshold of silk moths. Naturwissenschaften. 1970;57:23–28. doi: 10.1007/BF00593550. [DOI] [PubMed] [Google Scholar]
- 9.Syed Z, Ishida Y, Taylor K, Kimbrell DA, Leal WS. Pheromone reception in fruit flies expressing a moth's odorant receptor. Proc Natl Acad Sci USA. 2006;103:16538–16543. doi: 10.1073/pnas.0607874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 12.Steinbrecht RA. Morphometric studies on antenna of silk moth, Bombyx mori L.: Number and distribution of olfactory sensilla. Zeitschrift Fur Morphologie Der Tiere. 1970;68:93–126. [Google Scholar]
- 13.Shanbhag SR, Muller B, Steinbrecht RA. Atlas of olfactory organs of Drosophila melanogaster, 1: Types, external organization, innervation and distribution of olfactory sensilla. Int J Insect Morphol Embryol. 1999;28:377–397. [Google Scholar]
- 14.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 15.Pelletier J, Hughes DT, Luetje CW, Leal WS. An odorant receptor from the southern house mosquito, Culex pipiens quinquefasciatus, sensitive to oviposition attractants. PLoS One. 2010;5:e10090. doi: 10.1371/journal.pone.0010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey AF, Wang G, Su CY, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito, Anopheles gambiae. Nature. 2010;464:66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, Carey AF, Carlson JR, Zwiebel LJ. Molecular basis of odor coding in the malaria vector mosquito, Anopheles gambiae. Proc Natl Acad Sci USA. 2010;107:4418–4423. doi: 10.1073/pnas.0913392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohbot JD, Dickens JC. Characterization of an enantioselective odorant receptor in the yellow fever mosquito, Aedes aegypti. PLoS One. 2009;4:e7032. doi: 10.1371/journal.pone.0007032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaissling KE. Olfactory perireceptor and receptor events in moths: A kinetic model revised. J Comp Physiol A. 2009;195:895–922. doi: 10.1007/s00359-009-0461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaissling KE. In: Mechanism in Insect Olfaction. Payne TL, Birch MC, Kennedy CEJ, editors. Oxford, UK: Clarendon Press; 1986. pp. 189–199. [Google Scholar]
- 21.Leal WS. Behavioural neurobiology: The treacherous scent of a human. Nature. 2010;464:37–38. doi: 10.1038/464037a. [DOI] [PubMed] [Google Scholar]
- 22.Pelletier J, Leal WS. Genome analysis and expression patterns of odorant-binding proteins from the southern house mosquito, Culex pipiens quinquefasciatus. PLoS One. 2009;4:e6237. doi: 10.1371/journal.pone.0006237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate–induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 25.Christensen TA, Hildebrand JG. Pheromonal and host-odor processing in the insect antennal lobe: How different? Curr Opin Neurobiol. 2002;12:393–399. doi: 10.1016/s0959-4388(02)00336-7. [DOI] [PubMed] [Google Scholar]
- 26.Steinbrecht RA, Laue M, Ziegelberger G. Immunolocalization of pheromone-binding proteins and general odorant-binding proteins in olfactory sensilla of the silk moths Antheraea and Bombyx. Cell Tissue Res. 1995;282:203–217. [Google Scholar]
- 27.Clyne P, Grant A, O'Connell R, Carlson JR. Odorant response of individual sensilla on the Drosophila antenna. Invert Neurosci. 1997;3:127–135. doi: 10.1007/BF02480367. [DOI] [PubMed] [Google Scholar]
- 28.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stocker RF. Drosophila as a focus in olfactory research: Mapping of olfactory sensilla by fine structure, odor specificity, odorant receptor expression, and central connectivity. Microsc Res Tech. 2001;55:284–296. doi: 10.1002/jemt.1178. [DOI] [PubMed] [Google Scholar]
- 30.Steinbrecht RA. In: Atlas of Arthropod Sensory Receptors. Eguchi E, Tominaga Y, editors. New York: Springer; 1998. [Google Scholar]
- 31.Shanbhag SR, et al. Expression mosaic of odorant-binding proteins in Drosophila olfactory organs. Microsc Res Tech. 2001;55:297–306. doi: 10.1002/jemt.1179. [DOI] [PubMed] [Google Scholar]
- 32.Rogers ME, Sun M, Lerner MR, Vogt RG. Snmp-1, a novel membrane protein of olfactory neurons of the silk moth Antheraea polyphemus with homology to the CD36 family of membrane proteins. J Biol Chem. 1997;272:14792–14799. doi: 10.1074/jbc.272.23.14792. [DOI] [PubMed] [Google Scholar]
- 33.Benton R, Vannice KS, Vosshall LB. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 2007;450:289–293. doi: 10.1038/nature06328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.