Abstract
Human and mouse embryonic stem cells (ESCs) are derived from blastocyst-stage embryos but have very different biological properties, and molecular analyses suggest that the pluripotent state of human ESCs isolated so far corresponds to that of mouse-derived epiblast stem cells (EpiSCs). Here we rewire the identity of conventional human ESCs into a more immature state that extensively shares defining features with pluripotent mouse ESCs. This was achieved by ectopic induction of Oct4, Klf4, and Klf2 factors combined with LIF and inhibitors of glycogen synthase kinase 3β (GSK3β) and mitogen-activated protein kinase (ERK1/2) pathway. Forskolin, a protein kinase A pathway agonist which can induce Klf4 and Klf2 expression, transiently substitutes for the requirement for ectopic transgene expression. In contrast to conventional human ESCs, these epigenetically converted cells have growth properties, an X-chromosome activation state (XaXa), a gene expression profile, and a signaling pathway dependence that are highly similar to those of mouse ESCs. Finally, the same growth conditions allow the derivation of human induced pluripotent stem (iPS) cells with similar properties as mouse iPS cells. The generation of validated “naïve” human ESCs will allow the molecular dissection of a previously undefined pluripotent state in humans and may open up new opportunities for patient-specific, disease-relevant research.
Keywords: pluripotency, reprogramming
Mouse embryonic stem cells (mESCs) are derived from the inner cell mass (ICM) of developing blastocysts (1) and can be maintained indefinitely in vitro in a pluripotent state, as they retain the capacity to contribute to somatic cell lineages when injected into host embryos (2). Recently, an additional type of pluripotent cells was derived from the postimplantation epiblast of murine embryos, termed epiblast stem cells (mEpiSCs) (3, 4). mESCs and mEpiSCs are molecularly and epigenetically distinct and therefore represent discrete pluripotent states recently termed naïve and primed pluripotent states, respectively (5). ICM-derived naïve pluripotent stem cells efficiently contribute to chimeric embryos, maintain both X chromosomes in an active state (XaXa) in female cells, and are relatively refractory in their potential to differentiate into primordial germ cells (PGCs) in vitro (6). mEpiSCs, or primed pluripotent cells, can give rise to differentiated teratomas, but are highly inefficient in repopulating the ICM upon aggregation or injection into host blastocysts, have predominantly undergone X-chromosome inactivation (XiXa), and are poised for differentiation into PGC precursors in vitro (3, 4, 7). Naïve mESCs can be cloned with high efficiency, grow as packed dome colonies, and are stabilized by LIF/Stat3 and destabilized by bFGF and TGFβ/Activin signaling. In contrast, primed mEpiSCs are characterized by a flattened morphology, intolerance to passaging as single cells, and a dependence on bFGF and TGFβ/Activin signaling rather than LIF/Stat3. EpiSCs from the 129 mouse strain can be reverted to naïve state upon exposure to LIF/Stat3 signaling, and this reversion can be boosted by expression of pluripotency factors including Klf4, Klf2, Nanog, or c-Myc (8–11).
The derivation of naïve pluripotent stem cells from nonobese diabetic (NOD) mouse strains and from rats, previously considered “nonpermissive” for ESC derivation, has been achieved by culturing the cells in medium supplemented with small molecules or growth factors that alleviate inhibitory differentiation cues and/or reinforce key signaling pathways that stabilize the core transcriptional circuitry of naïve pluripotency [e.g., inhibition of glycogen synthase kinase 3β (GSK3β) and mitogen-activated protein kinase pathway (ERK1/2) in addition to LIF/Stat3 stimulation or exogenous constitutive expression of Klf4 or c-Myc] (5, 11–13). These conditions compensate for genetic determinants, which may be unique to each strain or species but preclude the in vitro propagation of naïve pluripotency. Notably, these genetic determinants do not interfere with the maintenance of the primed pluripotent state, as EpiSCs from both rats and NOD mice can be readily isolated (4, 11). NOD naïve pluripotent cells when isolated from preimplantation blastocysts or generated through in vitro reprogramming are highly metastable and adopt a primed pluripotent state in vitro upon withdrawal of the exogenous supporting factors and addition of bFGF/Activin (11).
Although not identical, human ES cells (hESCs) share several defining features with primed mEpiSCs and are distinct from naïve mESCs (14). mEpiSCs and hESCs share a flattened morphology, intolerance to passaging as single cells, dependence on TGFβ/Activin signaling (15), inactivation of the X chromosome in most female cell lines isolated (16), and a high propensity to differentiate into PGCs in response to BMP4 in vitro (17). The similarities between hESCs and mEpiSCs in addition to the aforementioned highly “metastable” naïve NOD pluripotent cells have underscored the possibility that the establishment and maintenance of the primed pluripotent state in human cells may reflect an inherent instability of naïve pluripotency that cannot be stabilized by the conventional culture conditions used to propagate hESCs (11). These observations have provoked further questions relating to the nature of in vitro isolated hESCs and human induced pluripotent stem cells (hiPSCs) (5, 11, 18): Can the same exogenous factors used for the isolation of NOD mESCs help establish and maintain the naïve or “mouse ESC-like” pluripotent state in human cells? Or, alternatively, are human cells even less permissive and require manipulation of additional or different signaling pathways? Here we sought to define the factors that stabilize a human pluripotent state in vitro which shares defining features with the naïve pluripotent cells of mice by molecular and functional criteria. The conditions described herein allow the isolation of naïve hiPSCs and epigenetic reversion of conventional hESCs toward a naïve pluripotent state. Our findings provide evidence for a validated and previously unidentified naïve state of pluripotency in humans.
Results
Stabilization of the Naïve Pluripotent State in Human Cells.
To test whether conditions devised to stabilize mouse NOD and rat ESCs (5, 11, 12) influence the properties of human pluripotent stem cells in vitro, we utilized previously described C1 secondary human female fibroblasts (19) to derive hiPSCs under various culture conditions (Fig. 1A). The C1 secondary fibroblast line harbors doxycycline (DOX) inducible lentiviral vectors encoding OCT4, SOX2, and KLF4 reprogramming factors and a constitutively active lentivirus encoding the reverse tetracycline transactivator. After 14–25 days of culture in serum-free N2B27 medium and a combination of the ERK1/2 inhibitor PD0325901 (PD), the GSK3 inhibitor CHIR99021 (CH), and LIF (abbreviated PD/CH/LIF), colonies with naïve mESC-like morphology appeared that could be maintained in media with DOX and PD/CH/LIF (Fig. 1A and Figs. S1A and S2A). Similar colonies were observed after transferring DOX-independent C1 hiPSC lines grown in bFGF/serum-containing growth conditions into N2B27 PD/CH/LIF + DOX for 5–7 days (Fig. 1B). Individual C1 hiPSC naïve-like clones were picked and further passaged with trypsin in the presence of DOX and inhibitors for over 50 passages. These lines were nearly indistinguishable morphologically from mESCs (Fig. 1A), remained karyotypically normal after extended passaging (Fig. S1B), expressed human pluripotency markers (Fig. S1C), displayed a pre-X inactivation epigenetic state (XaXa) as evidenced by lack of XIST clouds (Fig. S3A), generated differentiated teratomas (Fig. S1D), were insensitive to inhibition of bFGF and Activin/Nodal signaling or addition of BMP4 similar to mouse ESCs (Fig. 1C), and could be stably grown in DOX + PD/CH/LIF in the absence of serum or feeder cells (Fig. S2B). However, unlike NOD murine iPSCs that could be maintained under these conditions independent of transgene induction by DOX (11), these C1 naïve-like hiPSCs rapidly differentiated upon withdrawal of DOX (Fig. 1 A and E and Fig. S2A). Constitutive transgene-mediated expression of Klf4 and Oct4 or Klf4 and Klf2 was required to propagate C1 hiPSCs in the PD/CH/LIF condition independent of DOX (Fig. 1D). Similarly, BGO1 or WIBR3 hESCs were stably transfected with the Oct4 and Klf4 transgenes and transferred into growth condition of N2B27 medium with PD/CH/LIF (Fig. S4). Stable pluripotent lines were established that had a normal karyotype (Fig. S4 A and B), maintained endogenous pluripotency genes in a hypomethylated state (Fig. S4C), generated mature differentiated teratomas (Fig. S4D), and required continuous expression of ectopic transgenes and PD/CH/LIF to remain stable in vitro (Fig. S4E). These results indicate that human cells may be less “permissive” than that of the NOD mouse strain and thus may require additional exogenous factors to allow stabilization of naïve pluripotent cells in vitro.
Fig. 1.
Derivation of naïve mouse ESC-like induced pluripotent stem cells. (A) Strategy and representative images of C1 cultures and subcloned cell line C1.2 observed at different stages during reprogramming. p, passage number. NOD mESCs and C1.2 hiPSCs after DOX withdrawal are also shown. (B) C1 hiPSC line maintained in conventional bFGF/serum-supplemented human ES growth conditions (hESM) was transferred into N2B27 PD/CH/LIF + DOX, and emerging colonies were subcloned. Representative C1.10 hiPSC clone is shown. (C) Signaling dependence of pluripotent cell lines. Pluripotent cells were equally divided and plated on feeders in the indicated growth medium in which these cell lines are normally maintained, and 36 h later the wells were supplemented with the indicated inhibitors or growth factors. After 6 days, wells were fixed and stained for Nanog to determine the relative percentage of pluripotent colonies. Colony formation is normalized to an internal control growth medium only without inhibitors. (D) C1.2 hiPSC line was electroporated with mammalian expression vectors expressing the indicated reprogramming factors and cells were subjected to puromycin selection and passaged in PD/CH/LIF without DOX. Values indicate relative percentage of SSEA4+ colonies obtained in comparison with control cells that were transfected with an Oct4/Klf4/Sox2-encoding polycistronic construct. (E) Screening of factors that allow propagation of transgene-independent (i.e., DOX-independent) C1 hiPSCs in PD/CH/LIF-supplemented media. Effect of the removal of individual factors from the pool of 13 indicated small molecules or cytokines on the stabilization of pluripotent C1 hiPSCs independent of DOX. C1 cells were plated on feeders in N2B27 media with the indicated factors. P values using Student's t test indicate significant change in comparison with cells grown in DOX/PD/CH/LIF conditions, which were defined as a control (100% survival).
The DOX-dependent naïve C1.2 hiPSC line was next used to screen for other compounds and growth factors that could stabilize C1.2 hiPSCs upon DOX withdrawal in N2B27 PD/CH/LIF medium (Fig. 1E). We included additional compounds that have previously been shown to support the pluripotency of naïve mESCs, such as inhibitors of TGFβ (SB431542, RepSox, A83-01) and FGF tyrosine kinase receptor (PD173074) signaling pathways, BMP4, IL-6, or molecules that substitute for Klf4/Klf2 in reprogramming or regulate their expression (Kenpaullone, BIX01294, BayK8644, Forskolin, and AICAR) (20–23). The combined action of the perturbations (termed 13i + LIF conditions) allowed the stabilization of C1.2 hiPSCs in the absence of DOX. By removing one inhibitor at a time, we identified PD, CH, and Forskolin (FK) as crucial components in this mixture of inhibitors (Fig. 1E). FK, which was previously used for the propagation of human embryonic germ (EG) cells (24), activates the enzyme adenylate cyclase, which increases the intracellular levels of cyclic adenosine monophosphate (cAMP) and subsequently activates the protein kinase A (PKA) signaling pathway (25). FK stabilizes C1 hiPSCs independently of DOX, at least partly through induction of Klf2 and Klf4 expression (Fig. S5). We tested whether PD/CH/LIF/FK in combination with transient transgene induction could revert the established WIBR3 hESC line to a naïve pluripotent state. WIBR3 hESCs were transiently transfected with Oct4 and Klf4 or Klf4 and Klf2 and then grown in PD/CH/LIF/FK (Fig. 2A). After 8–12 days, dome-shaped colonies with packed round-cell morphology, typical of naïve mESCs, appeared (Fig. 2A and Fig. S6A). Colonies were picked, trypsinized, and passaged in PD/CH/LIF/FK. Because these cell lines were morphologically similar to naïve mESCs, we refer to the selected cells as naïve hESCs (naïve WIBR3.1–3.5) and naïve hiPSCs.
Fig. 2.
Characteristics of naïve hESC lines. (A) Scheme for reverting hESCs to generate naïve hESCs. Representative images of WIBR3 hESCs at different stages of the reversion process in PD/CH/LIF/FK. p, passage number. Magnifications of captured images are indicated. (B) Single-cell cloning efficiency of different pluripotent stem cell lines as determined by the number of wells containing Nanog+ colonies after 7 days. (C) Estimated cell doubling time. Plated cells were plated in triplicates and counted at 1, 4, and 7 days after plating, and increase in cell number was used to extrapolate average doubling time. Error bars represent SD, and P values using Student's t test indicate significant difference in the average of hESC/hiPSC lines in comparison with the average of naïve hESC/hiPSC lines.
Naïve hESCs and hiPSCs Are Pluripotent.
Naïve hESC/hiPSC lines were grown on feeder cells in PD/CH/LIF/FK, passaged using trypsin, and demonstrated a normal karyotype (Fig. S6B). Naïve hESCs and hiPSCs displayed >85% single-cell cloning efficiency after trypsinization, comparable to the high clonogenicity typical for mESCs (Fig. 2B). In contrast, conventional hESCs and hiPSCs have a low clonogenicity (<5%), or ~20% in the presence of Rho kinase (ROCK) inhibitor (Fig. 2B). The average doubling time for naïve hESCs was slightly decreased by ~20% (Fig. 2C). The cells stained positive for pluripotency markers (Fig. S6C), and Oct4 and Nanog promoters were hypomethylated in naïve hESCs in comparison with somatic fibroblasts (Fig. S6D). To determine the differentiation ability of naïve hESCs/hiPSCs in vitro, we used suspension culture to generate embryoid bodies (EBs). After 7 days in suspension, RT-PCR confirmed that these EB differentiated cells expressed markers of all three developmental lineages (Fig. S7A). The naïve hESCs/hiPSCs formed teratomas with somatic tissues representative of the three germ layers (Fig. S7B). Naïve hiPSCs could also be directed to differentiate into neuronal cells in vitro (Fig. S7C). Unlike the naïve pluripotent stem cells ectopically expressing the factor transgenes that could be maintained for more than 50 passages in PD/CH/LIF (Fig. S1B), the genetically unmodified Forskolin-dependent naïve hESCs could not be maintained for longer than 15–20 passages, at which point they stopped proliferating and differentiated. An appreciable difficulty and restriction in passaging pluripotent cells have been previously described for human EG cells propagated in the presence of Forskolin (24), and may be due to toxicity associated with this compound or its inability to fully substitute for ectopic transgene expression (Fig. 1).
Naïve Human Pluripotent Cells Share Signaling and Functional Features with Mouse ESCs.
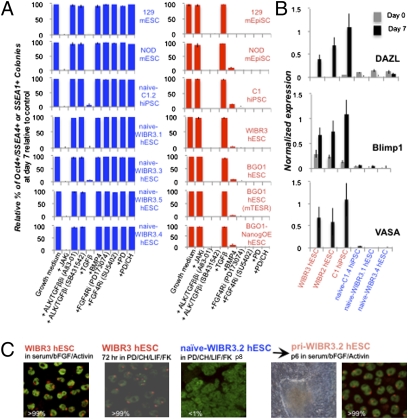
We investigated whether naïve hESCs share defining features with mESCs and thus would constitute a pluripotent state which is distinct from the previously described hESCs. ICM-derived mESCs are stabilized upon inhibition of the ERK1/2 pathway, in contrast to hESCs and mEpiSCs, which are induced to differentiate by ERK inhibition (5, 26). Consistent with previously described observations, genetically unmodified conventional hESCs could not be propagated in the presence of the ERK1/2-specific inhibitor PD (Fig. 3A). Similarly to NOD mESCs, the stability of naïve hESCs was dependent on the continuous presence of ERK1 inhibition (Fig. 3A and Figs. S2A and S8A) (11). Also, the naïve hESCs depended on LIF signaling, displayed high levels of pStat3 (Fig. S8B), and readily differentiated when exposed to a JAK inhibitor (JAKi) that blocks Stat3 phosphorylation. This behavior is similar to mESCs and contrasts with conventional hESCs, which were resistant to JAKi (Fig. 3A). Consistent with this observation, naïve hESCs that were stably transfected with a dominant-negative Stat3 encoding transgene rapidly differentiated and could not be maintained, whereas cells transgenic for a constitutively active Stat3 mutant could be propagated in the absence of exogenous LIF (Fig. S8C). In addition, hESCs and mEpiSCs rapidly differentiated upon inhibition of the TGFβ/Activin signaling pathway by SB431542 or A83-01, whereas mESCs and naïve hESCs/hiPSCs maintained their pluripotent state in response to SB431542 or A83-01 but differentiated upon addition of recombinant TGFβ (Fig. 3A). Finally, addition of BMP4 growth factor or inhibition of bFGF signaling by two different FGF4-receptor inhibitors (PD173074 and SU5401) resulted in the differentiation of hESCs and mEpiSCs, but not of naïve hESCs/hiPSCs or mESCs (Fig. 3A).
Fig. 3.
Naïve hESCs share defining signaling and epigenetic features with mESCs. (A) Signaling dependence of pluripotent cell lines conducted as in Fig. 1C. After 7 days, wells were fixed and stained to determine the relative percentage of colonies positive for pluripotency markers. SSEA1 staining was used for mouse stem cells. Colony formation was normalized to an internal control growth medium without inhibitors (first left column). Normalized percentages lower than 5% are defined as “sensitivity” to the presence of the supplemented inhibitor. (B) RT-PCR expression of early germ-cell markers in the presence or absence of BMP4/7/8 cytokines. (C) Representative fluorescence in situ hybridization (FISH) analysis for XIST RNA (red) and Cot1 nuclear RNA (green). Pri-WIBR3.2 cell line was analyzed after passaging in conventional bFGF/serum-containing human ESC growth conditions. Numbers indicate average percentage of XIST-positive nuclei counted.
It has been shown that mEpiSCs as well as hESCs are primed for differentiation into PGC-like cells and readily activate germ-cell markers such as DAZL, BLIMP1, and VASA upon exposure to BMP4. This is in contrast to mESCs, which are inefficient in activating these markers and require prior EB formation (7, 17, 27). We tested whether the naïve ESCs would resist activation of PGC markers similarly to mESCs (3). Fig. 3B shows that VASA, BLIMP1, and DAZL were readily activated in hESCs upon culture in BMP4, in contrast to naïve hESCs, which showed no up-regulation of these markers. Similar results were obtained when using a VASA-EGFP reporter transgene to measure efficiency of early PGC differentiation (Fig. S9A). In summary, mESCs and naïve hESCs have comparable biological characteristics and depend on similar signaling pathways. However, these pathways are different from those operating in hESCs and mEpiSCs, suggesting that the two states of pluripotency can be controlled, at least in part, by similar mechanisms in the human and mouse species.
Epigenetic Reversion and Maintenance of the Pre-X Inactivation State in Female Naïve hESCs.
X-chromosome inactivation represents an important epigenetic difference between the two states of pluripotency: Whereas female mESCs are in a pre-X inactivation state with two active X chromosomes (XaXa), mEpiSCs and most if not all hESCs have already undergone X inactivation (XiXa) (10, 28). Recently, we have demonstrated that environmental conditions such as oxidative stress induce precocious and irreversible X inactivation during early stages of hESC derivation (28). X inactivation is reversible in mouse cells, as reprogramming of XiXa somatic cells to pluripotency has been shown to reactivate the silent X chromosome (10, 29–31). Therefore, we tested whether the conversion of hESCs into naïve hESCs would reactivate the inactive X chromosome. Fig. 3C shows that WIBR3 hESCs grown in conventional bFGF/serum containing hESC medium and in atmospheric oxygen concentration (20% pO2) or exposed to PD/CH/LIF/FK for 72 h expressed an XIST cloud in all cells, which is indicative of X inactivation. However, no XIST clouds were seen in any of eight independently derived naïve WIBR3 hESC and naïve hiPSC lines tested between p8-p20 (Fig. 3C and Fig. S3A), consistent with the observed changes in the methylation of the XIST promoter region (Fig. S3D). When naïve hESCs were transferred to bFGF/serum-containing hESC growth conditions, the cells adapted the flattened morphology of conventional hESCs. These reverted cells, designated primed pluripotent cell lines, had initiated X inactivation, as evident by the acquisition of XIST clouds and changes in XIST promoter methylation (e.g., pri-WIBR3.2 in Fig. 3C and Fig. S3D). Similar results were obtained when analyzing XIST clouds and XIST promoter methylation in naïve and primed C1 hiPSC lines (Fig. S3 A, B, and D). Overall, these results indicate that, similar to mouse cells, X inactivation is reversible and sustainable in naïve human stem cells after epigenetic reversion of the primed pluripotent state.
Naïve Human Pluripotent Cells Are Transcriptionally Similar to Mouse ESCs.
To define molecular signatures of the naïve hESCs, we compared their global gene expression pattern with that of hESCs, mESCs, and mEpiSCs. Fig. S9B shows that four naïve hESC lines and two naïve hiPSC lines cluster together and are different from a large number of hESCs and hiPSCs, including hESCs grown in mTESR1-defined culture conditions and Nanog-overexpressing hESCs, which can grow in a feeder-independent manner (32, 33). Gene array analysis and confirmation by RT-PCR showed that Oct3/4 and Sox2 were expressed at equivalent levels in hESCs/hiPSCs and naïve hESCs/hiPSCs, whereas transcripts associated with naïve mESCs such as Klf4, Klf2, Tbx3, Gbx2, Lin28, and SOCS3 were significantly up-regulated in naïve hESCs/hiPSCs (Fig. 4A and Fig. S9 C and D). In contrast, transcripts associated with genes expressed in the epiblast and early germ layers as well as in hESCs (3, 4) were significantly down-regulated in the naïve hESCs/hiPSCs. This set of genes included Otx2, Sox17, Cer1, Foxa2, Zic1, Lhx2, and XIST (Fig. 4A and Fig. S9C). These expression differences between hESCs/hiPSCs and naïve hESCs/hiPSCs are consistent with previously described differences in gene expression between mESCs and mEpiSCs (3, 4). Finally, fluorescence-activated cell sorter (FACS) analysis showed that hESCs and mEpiSCs had initiated surface expression of MHC class I proteins, which are normally expressed on somatic cells (34), in contrast to mESCs and naïve hESCs/hiPSCs, which had residual surface expression consistent with a more immature phenotype (Fig. 4B).
Fig. 4.
Naïve hESCs/hiPSCs share a global transcriptional profile with mESCs. (A) Bar chart showing median expression ratio of pluripotency and lineage-specific marker genes in hESCs and naïve hESCs. Asterisks delineate genes in which the false discovery rate was <0.1 between the naïve and primed group of samples. (B) FACS analysis for surface expression of human and mouse MHC class I alleles. Black graph indicates isotype match control. MFI, median fluorescence intensity. P values using Student's t test indicate significant change (P < 0.01). (C) Cross-species gene expression clustering where mESCs and naïve hESCs formed a distinct group apart from mEpiSCs and hESCs. Legend shown on right with yellow and blue indicating positive and negative correlation, respectively. Gene expression relative abundance was clustered by Spearman correlation and average linkage. Mouse samples are labeled in purple and human samples are labeled in brown.
An unbiased cross-species hierarchical clustering (35) was performed to assess whether the naïve and primed state of pluripotency in human cells globally corresponded to those characterized in mouse cells. For this, 24 different pluripotent stem cell lines were compared (Fig. 4C), including hESCs/hiPSCs; naïve hESCs/hiPSCs; 129 mESCs and EpiSCs; NOD mESCs; NOD mEpiSC-like cells which were generated from NOD mESCs and miPSCs, which were grown in bFGF/Activin after withdrawal of exogenous inhibitors that stabilize their naïve pluripotency (11). Cross-species gene expression analysis on 9,949 mouse-human orthologous genes in the gene expression datasets clustered the samples into two main groups as indicated by the top bifurcation in Fig. 4C representing two distinct pluripotent states. All naïve hESCs/hiPSCs clustered with mESCs and miPSCs independent of genetic background, species differences, or growth conditions (Fig. 4C). Notably, hESCs and hiPSCs clustered with mouse EpiSCs and NOD EpiSC-like cells, and marked anticorrelation was apparent between the naïve hiPSCs/ESCs and the hESCs/hiPSCs, as indicated by the blue blocks in the correlation matrix (Fig. 4C and Fig. S9D). Finally, we measured the activity of the distal and proximal enhancer regions of Oct4 genes that are reciprocally regulated in the pre- and postimplantation mouse embryo (3) as well as in mESCs and mEpiSCs. Predominant utilization of the highly conserved Oct4 distal enhancer (36) was observed in naïve hESCs/hiPSCs as measured by luciferase reporter constructs (Fig. S9E), indicating that the gene expression network active in naïve hESCs enhances utilization of the distal Oct4 enhancer as typically observed in mESCs.
Discussion
Our results demonstrate that naïve hESCs/hiPSCs are distinct from conventional hESCs/hiPSCs and mEpiSCs and closely resemble the naïve pluripotent state of ICM-derived mESCs (5, 11) by numerous molecular and biological criteria including growth properties, signaling pathway dependency, state of X-chromosome inactivation, and transcriptional characteristics. These findings support the notion that distinct states of pluripotency can be specified by culture conditions but that genetic background, as well as species differences, determine the requirements and threshold for exogenous factors that establish and maintain the naïve pluripotent state. As schematically shown in Fig. 5, the 129 mouse strain is the most permissive genetic background, as active LIF/Stat3 signaling is sufficient to stabilize the naïve state of ICM-derived mESCs in the presence of feeder cells, whereas the NOD genetic background requires constitutive expression of Klf4 or c-Myc or simultaneous enhancement of Wnt signaling and inhibition of the ERK1/2 pathway to stabilize the ICM-derived ESCs in the naïve pluripotent state (11, 13). Human cells seem to be the least permissive, as naïve hESCs are obtained at relatively low efficiencies and require perturbation of additional molecular pathways in order to achieve stochastic epigenetic reversion and stabilization of the naïve state of pluripotency (Fig. 5) (37). In the absence of such exogenous culture and transcription factors, naïve hESCs adopt an EpiSC-like or primed pluripotent state in vitro that is stabilized by bFGF/Activin signaling (Fig. 5). It would be important to delineate whether the latter effect underlies the relative increased heterogeneity in gene expression and differentiation characteristics observed between different conventional hESCs and hiPSCs (Fig. S9B) (38). Moreover, the clonal relationship demonstrated by Southern blot analysis between naïve and primed C1 hiPSC lines (Fig. S3) demonstrates that the conversions between the primed and naïve pluripotent states are not due to simple selection for preexisting cells continuously present in hESC polyclonal in vitro cultures.
Fig. 5.
Metastable states of pluripotency. Model describing relationships between genetic background and requirements for exogenous factors to achieve in vitro stabilization of the naïve (ICM-like or ESC-like) and primed (epiblast-like or EpiSC-like) pluripotent states. “Metastability” pertains to describing a system with two or more in vitro stable states that can interconvert by defined signals. The transcription factors and culture supplements minimally required for interconversion and stabilization of the respective pluripotent states in the different strains and species are highlighted.
Our findings further support previous observations implicating regulation of the KLF transcriptional circuitry in establishing and maintaining naïve pluripotency (10, 11, 39, 40). It will be of interest to define the genetic determinants underlying the different requirements for propagation of the naïve pluripotent state in different strains and species, and whether strain and species differences in the regulatory regions of KLF genes or their transcriptional target genes may be important and reflect differences observed in early development. Moreover, it is possible that the origin of pluripotent cells explanted in vitro for propagation may influence the requirement for exogenous factors to stabilize naïve pluripotency. This hypothesis is supported by the observation that NOD-derived iPSCs and ESCs required exogenous supplementation of PD/CH or KP/CH in addition to LIF, whereas NOD germ-line stem cells remained stable in LIF alone (41).
It should be emphasized that undefined differences may exist between mESCs and the naïve human pluripotent cells described in this work. Also, further optimization of growth conditions need to be conducted to enhance the stability of naïve human pluripotency and to permanently stabilize this pluripotent state in the absence of genetic manipulations and to test whether such conditions can be applicable for other species. Further enhancement of the naïve core transcriptional circuitry by shielding Activin/Nodal signaling, FGF4-receptor autophosphorylation and p38 signaling inhibition, or hypoxia is likely to stabilize naïve pluripotency (8, 28). Finally, the definition and characterization of a naïve pluripotent state in human cells may expand the capabilities for using human ESCs and iPSCs in regenerative medicine and disease modeling both in vitro and in vivo (42, 43).
Materials and Methods
Culture of Naïve Human Pluripotent Cells.
Naïve human pluripotent cells were grown in serum-free N2B27-based media. Five hundred microliters of media was generated by including: 240 mL DMEM/F12 (Invitrogen; 11320), 240 mL Neurobasal (Invitrogen; 21103), 5 mL N2 supplement (Invitrogen; 17502048), 10 mL B27 supplement (Invitrogen; 17504044), 10 μg recombinant human LIF (Millipore; LIF1005), 1 mM glutamine (Invitrogen), 1% nonessential amino acids (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma), penicillin-streptomycin (Invitrogen), 5 mg/mL BSA (Sigma), and small-molecule inhibitors as below. Naïve hESCs/hiPSCs were kept on mitomycin C-inactivated mouse embryonic fibroblast feeder cells unless indicated otherwise, and were passaged by single-cell trypsinization every 5–10 days. Passage numbers of naïve hiPSCs/hESCs indicate number of passages counted after induction of the naïve state. Small molecules and cytokines were purchased from Tocris, Calbiochem, Stemgent, or Sigma, and were supplemented as indicated at the following final concentrations: JAK inhibitor (JAKi, 6 μM), Kenpaullone (KP, 5 μM), PD0325901 (PD, 1 μM), CHIR99021 (CH, 3 μM), forskolin (FK, 10 μM), FGF4-receptor inhibitors PD173074 (0.1 μM) and SU5401 (2 μM), TGFβ/ALK inhibitors A83-01 (1 μM), RepSox (1 μM), and ALK inhibitor (ALKi; SB431542, 2 μM), AICAR (0.5 mM), BixO1294 (1 μM), BayK8644 (1 μM), BMP4 (10 ng/mL), IL-6 (10 ng/mL), and recombinant human TGFβ (500 ng/mL). Detailed experimental procedures are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Hillel and Liliana Bachrach and Susan Whitehead for their generous gifts enabling this research. This research was also supported in part by a gift from Landon Clay. J.H. is supported by Helen Hay Whitney and Genzyme fellowships. K.S. is supported by the Society in Science: The Branco-Weiss Fellowship. A.W.C. is supported by a Croucher scholarship.
Footnotes
Conflict of interest statement: R.J. is a cofounder of Fate Therapeutics and an adviser to Stemgent. R.J. and J.H. have filed a patent application describing the results and concepts presented herein.
Data deposition: The gene array datasets reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE 21222).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004584107/-/DCSupplemental.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tesar PJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 4.Brons IG, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 5.Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Rossant J. Stem cells and early lineage development. Cell. 2008;132:527–531. doi: 10.1016/j.cell.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi K, Surani MA. Self-renewing epiblast stem cells exhibit continual delineation of germ cells with epigenetic reprogramming in vitro. Development. 2009;136:3549–3556. doi: 10.1242/dev.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greber B, et al. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell. 2010;6:215–226. doi: 10.1016/j.stem.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Bao S, et al. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature. 2009;461:1292–1295. doi: 10.1038/nature08534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo G, et al. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna J, et al. Metastable pluripotent states in NOD-mouse-derived ESCs. Cell Stem Cell. 2009;4:513–524. doi: 10.1016/j.stem.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buehr M, et al. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Nichols J, et al. Validated germline-competent embryonic stem cell lines from nonobese diabetic mice. Nat Med. 2009;15:814–818. doi: 10.1038/nm.1996. [DOI] [PubMed] [Google Scholar]
- 14.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 15.Xu RH, et al. NANOG is a direct target of TGFβ/Activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva SS, Rowntree RK, Mekhoubad S, Lee JT. X-chromosome inactivation and epigenetic fluidity in human embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:4820–4825. doi: 10.1073/pnas.0712136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovell-Badge R. Many ways to pluripotency. Nat Biotechnol. 2007;25:1114–1116. doi: 10.1038/nbt1007-1114. [DOI] [PubMed] [Google Scholar]
- 19.Hockemeyer D, et al. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 2008;3:346–353. doi: 10.1016/j.stem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Impey S, et al. Defining the CREB regulon: A genome-wide analysis of transcription factor regulatory regions. Cell. 2004;119:1041–1054. doi: 10.1016/j.cell.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–574. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Ying QL, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichida JK, et al. A small-molecule inhibitor of Tgf-β signaling replaces Sox2 in reprogramming by inducing Nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shamblott MJ, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delghandi MP, Johannessen M, Moens U. The cAMP signalling pathway activates CREB through PKA, p38 and MSK1 in NIH 3T3 cells. Cell Signal. 2005;17:1343–1351. doi: 10.1016/j.cellsig.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Nichols J, Silva J, Roode M, Smith A. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geijsen N, et al. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 28.Lengner CJ. Derivation of pre-X inactivation human embryonic stem cell line in physiological oxygen conditions. Cell. 2010 doi: 10.1016/j.cell.2010.04.010. in press. [DOI] [PubMed] [Google Scholar]
- 29.Eggan K, et al. X-chromosome inactivation in cloned mouse embryos. Science. 2000;290:1578–1581. doi: 10.1126/science.290.5496.1578. [DOI] [PubMed] [Google Scholar]
- 30.Maherali N, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Darr H, Mayshar Y, Benvenisty N. Overexpression of NANOG in human ES cells enables feeder-free growth while inducing primitive ectoderm features. Development. 2006;133:1193–1201. doi: 10.1242/dev.02286. [DOI] [PubMed] [Google Scholar]
- 33.Ludwig TE, et al. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–646. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 34.Drukker M, et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki R, Shimodaira H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 36.Nordhoff V, et al. Comparative analysis of human, bovine, and murine Oct-4 upstream promoter sequences. Mamm Genome. 2001;12:309–317. doi: 10.1007/s003350010279. [DOI] [PubMed] [Google Scholar]
- 37.Hanna J, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osafune K, et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 39.Hall J, et al. Oct4 and LIF/Stat3 additively induce Krüppel factors to sustain embryonic stem cell self-renewal. Cell Stem Cell. 2009;5:597–609. doi: 10.1016/j.stem.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Jiang J, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10:353–360. doi: 10.1038/ncb1698. [DOI] [PubMed] [Google Scholar]
- 41.Ohta H, et al. Male germline and embryonic stem cell lines from NOD mice: Efficient derivation of GS cells from a nonpermissive strain for ES cell derivation. Biol Reprod. 2009;81:1147–1153. doi: 10.1095/biolreprod.109.079368. [DOI] [PubMed] [Google Scholar]
- 42.Hanna J, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 43.Saha K, Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5:584–595. doi: 10.1016/j.stem.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.