Abstract
Sauropods were the largest terrestrial tetrapods (>105 kg) in Earth's history and grew at rates that rival those of extant mammals. Magyarosaurus dacus, a titanosaurian sauropod from the Upper Cretaceous (Maastrichtian) of Romania, is known exclusively from small individuals (<103 kg) and conflicts with the idea that all sauropods were massive. The diminutive M. dacus was a classical example of island dwarfism (phyletic nanism) in dinosaurs, but a recent study suggested that the small Romanian titanosaurs actually represent juveniles of a larger-bodied taxon. Here we present strong histological evidence that M. dacus was indeed a dwarf (phyletic nanoid). Bone histological analysis of an ontogenetic series of Magyarosaurus limb bones indicates that even the smallest Magyarosaurus specimens exhibit a bone microstructure identical to fully mature or old individuals of other sauropod taxa. Comparison of histologies with large-bodied sauropods suggests that Magyarosaurus had an extremely reduced growth rate, but had retained high basal metabolic rates typical for sauropods. The uniquely decreased growth rate and diminutive body size in Magyarosaurus were adaptations to life on a Cretaceous island and show that sauropod dinosaurs were not exempt from general ecological principles limiting body size.
Keywords: bone histology, Sauropoda, secondary osteon, nanism, island fauna
Sauropod dinosaurs were the largest animals that ever roamed the surface of the Earth (1, 2). Gigantic size was acquired early in the evolutionary history of the group, in the Late Triassic (3). Recent studies of bone histology have shown that sauropods attained their gargantuan sizes by an evolutionary increase in their growth rate to levels comparable to those of extant endothermic mammals (4, 5). However, not all sauropods were multi-ton animals. Some titanosaurs are known to have had relatively small body sizes by sauropod standards; e.g., the South American Neuquensaurus australis reached a body length of about 7–9 m (6, 7), and its body mass is estimated at 3,500 kg. The recently described basal macronarian Europasaurus holgeri from the Late Jurassic of Germany (8) was even smaller, with a total estimated adult body length of approximately 6.2 m and a body mass of 800 kg.
Another small-bodied titanosaurian sauropod, Magyarosaurus dacus, is known from the Upper Cretaceous (Maastrichtian) continental formations of the Haţeg Basin of Romania (9, 10). These strata contain an array of relatively small-bodied dinosaur taxa, including the basal hadrosaurid Telmatosaurus (11), and two species of the noniguanodontian euornithopod Zalmoxes (12). In a famous early evolutionary hypothesis involving dinosaurs, the small body size of these taxa prompted the brilliant Hungarian paleontologist Franz Baron Nopcsa to hypothesize that, like Mediterranean dwarf proboscideans (13), the Haţeg dinosaurs evolved their diminutive body size on a paleo-island (14, 15). Later, however, rare larger titanosaur bones were recovered from the Haţeg Basin as well and described (16) as “M.” hungaricus.
At present, all titanosaur bones from the Haţeg basin are tacitly grouped together as M. dacus (9, 17). Morphological work (by Z.C.) suggests that the larger taxon is different from M. dacus, we will hence use the name M. dacus to the exclusion of these large specimens. A full redescription, however, is beyond the scope of this paper. Today, M. dacus is known form numerous but mostly isolated bones of different-sized individuals, representing a growth series (Fig. 1). Magyarosaurus has been incorporated in only one phylogenetic analysis (18), in which the position of Magyarosaurus is resolved relatively high within the Titanosauria, inside the lithostrotian Rapetosaurus clade. This suggests that Magyarosaurus is closely related to taxa such as Rapetosaurus, Nemegtosaurus, Malawisaurus, and Trigonosaurus. Neither of these taxa shows any significant size reduction compared with members of less derived outgroups (SI Text). Small body size in M. dacus would thus represent an autapomorphic feature.
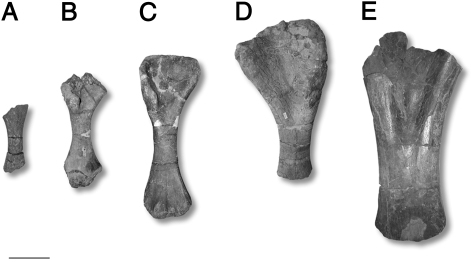
Fig. 1.
Photographs of some of the sampled titanosaur bones from the Maastrichtian of Romania. (A–D) Magyarosaurus dacus humeri, specimens (A) MAFI Ob. 3092 (smallest recorded body size, 45% maximum size), (B) FGGUB R.1246 (65% maximum size), (C) MAFI v.13492 (76% maximum size), (D) FGGUB R.1048 (largest known specimen), and (E) “Magyarosaurus” hungaricus, MAFI Ob.3104. (Scale bar, 100 mm.)
Morphological and Morphometric Evidence for a Nanoid Fauna.
Modern work on this classical dinosaur fauna suggests that phylogenetic size reduction [nanism sensu (19)] through paedomorphosis (20) had occurred in Telmatosaurus (11) and Zalmoxes robustus (12), based on patterns of heterochronic shifts in the morphology and morphometry in these taxa. Similarly, morphometric analysis of a wide range of sauropod humeri indicated that M. dacus bones were more similar to the bones of subadult than adult representatives of other, more typical sauropod taxa. These results were considered consistent with the interpretation of M. dacus as a heterochronic dwarf (21).
Alternative Hypothesis: Small Size Reflects Juvenile Status.
The co-occurrence of the rare large-bodied titanosaurian elements (M. hungaricus) (16) with M. dacus (22) and uncertainty about the paleogeographic setting of the Haţeg Basin (23), have drawn the insular nanism interpretation for Magyarosaurus into question. This has led to the suggestion that the small titanosaurian remains collected in the Haţeg Basin are not dwarfs at all, but represent merely juveniles of a sauropod with a more typical, massive adult body size (22) such as M. hungaricus. Historically, the ontogenetic status of dinosaurs has been difficult to resolve based on bone morphology alone because, unlike mammalian long bones, dinosaur long bones lack morphological indicators of full size having been attained. However, fossil bone histology has evolved into a powerful tool for detecting the ontogenetic status of nonmammalian tetrapods (reviewed in refs. 24 and 25; recent applications discussed in refs. 26–28). Because sauropod dinosaurs are one of the two histologically best sampled clades of dinosaurs (the other being Theropoda), we use long bone histology to resolve the controversy surrounding Magyarosaurus dacus and test the competing hypotheses of insular nanism vs. juveniles of a large-bodied species. We sampled a growth series of the small M. dacus, as well as one of the two long bones of M. hungaricus, available for study (Table 1).
Table 1.
List of sampled titanosaur specimens, with dimensions
| Specimen | Collection | Locality | Taxon | Bone type | Side | Length (mm) | Minimal shaft circumference (mm) | Percentage maximum size | Standardized length (to humerus) | Standardized percentage maximum size | HOS |
| R.1220 | FGGUB | Groapa | Magyarosaurus | Femur | R | (346) | 176 | 64 | 266 | 54.5 | 13 |
| R.1511 | FGGUB | Groapa | Magyarosaurus | Femur | L | (466) | 179 | 86 | 358 | 73 | 13 |
| R.1046 | FGGUB | Ciula | Magyarosaurus | Femur | L | 525 | 193 | 97 | 403.5 | 82.5 | 14 |
| R.1992 | FGGUB | Ciula | Magyarosaurus | Femur | R | (540) | 195 | 100 | 414.5 | 85 | 14 |
| Ob.3092 | MAFI | Vălioara | Magyarosaurus | Humerus | L | (222) | 115 | 46 | 222.5 | 46 | 12 |
| R.1246 | FGGUB | Groapa | Magyarosaurus | Humerus | R | (320) | 122 | 65.5 | 320 | 65.5 | 14 |
| R.1195 | FGGUB | Scoaba | Titanosauria indet. (?Mag-yarosaurus) | Humerus | L | (346) | 150 | 71 | 346 | 71 | 13 |
| Ob.3089 | MAFI | Vălioara | Magyarosaurus | Humerus | L | (365) | 136 | 75 | 365 | 75 | 14 |
| v.13492 | MAFI | Vălioara | Magyarosaurus | Humerus | R | 372 | 140 | 76 | 372 | 76 | 13 |
| Ob.3128 | MAFI | Vălioara | Magyarosaurus | Humerus | L | (432) | 151 | 88 | 432 | 88 | 14 |
| R.1047 | FGGUB | Ciula | Magyarosaurus | Humerus | R | 403 | 183 | 82.5 | 403 | 82.5 | 13 |
| R.1048 | FGGUB | Sînpetru | Magyarosaurus | Humerus | L | (488) | 194 | 100 | 488 | 100 | 14 |
| Ob.3104 | MAFI | Vălioara - Budurone | “M.” hung-aricus | Humerus | R | (914) | 365 | 914 | — | 12 | |
| R.1252 | FGGUB | Groapa | Magyarosaurus | Tibia | L | (354) | 105 | 79 | — | — | 12 |
| Ob.4212 | MAFI | Vălioara | Magyarosaurus | Tibia | L | (323) | 109 | 72 | — | — | 12.5 |
| R.1380 | FGGUB | Cărare | Magyarosaurus | Tibia | L | (402) | 134 | 89 | — | — | 13 |
| R.1045 | FGGUB | unknown | Magyarosaurus | Tibia | R | 450 | 181 | 100 | — | — | 14 |
| Ob.3087 | MAFI | Vălioara | Magyarosaurus | Tibia | L | (858) | 260 | — | — | 14 | |
| Ob.3086a | MAFI | Vălioara | Magyarosaurus | Fibula | L | (388) | 100 | — | — | 14 | |
| Ob.3086b | MAFI | Vălioara | Magyarosaurus | Fibula | L | (384) | 101 | — | — | 14 | |
| R.1598 | FGGUB | Groapa | Magyarosaurus | Ulna | L | (219) | 95 | 65 | — | — | 14 |
| Ob.3099 | MAFI | Vălioara | Magyarosaurus | Ulna | R | 337 | 128 | 100 | — | — | 14 |
Data in parentheses indicate estimated total length, provenance, relative size, and histologic ontogenetic stage (HOS). L, left; R, right.
Size and Age in Dinosaurs.
Sauropods as well as theropods [and ornithischian dinosaurs, where sample size is sufficient (29–31)] follow a narrow growth trajectory, i.e., they lack developmental plasticity. In sauropods, this is documented by a close correlation between histologic ontogenetic stage (HOS) and body size (32–38). In theropods, which commonly show good quantifiable growth records, growth curves vary little between individuals (39–45). This indicates that dinosaurs, like mammals, showed little intraspecific variation in asymptotic body size. Hence, large differences in adult size, in otherwise morphologically similar fossils, suggests that these individuals represent different biological species.
Results
M. dacus Long Bone Histology.
Like those of other sauropods (34, 35, 38, 46), M. dacus long bones are characterized by a small medullary cavity and relatively thick cortex (Fig. 2A). The medullary cavity merges into the cortex via cancellous bone that surrounds large erosion cavities. The cancellous bone is secondary in origin, and the erosion cavities become smaller as they grade into the innermost cortex. The cortical bone histology, however, represents a radical departure from that seen in any other sauropod, with the exception of the very largest and oldest of normal-sized sauropods. In all but the smallest individuals of M. dacus and irrespective of type of skeletal element, the primary bone of the cortex is completely replaced by dense secondary osteons or Haversian bone (Fig. 2 A, B, E, and F). The smallest individual (MAFI Ob.3092, less than 46% the length of FGGUB R.1048, the largest M. dacus humerus and 24% the length of the M. hungaricus humerus) retains primary bone in the outer cortex that, however, is also disrupted by numerous secondary osteons (Fig. 2 C and D). This primary bone is of the laminar fibrolamellar type with circumferential vascular canals and primary osteons. Unlike in typical laminar fibrolamellar bone of large mammals and other dinosaurs, the bone matrix between the vascular canals in M. dacus consists mostly of parallel-fibered and lamellar bone, with a minimal amount of woven bone. This well-organized primary bone matrix suggests that primary bone deposition rates were relatively slow (47–49), although the bone retained the extensive network of vascular canals typical of fibrolamellar bone seen in other sauropods and fast-growing extant vertebrates (48–50).
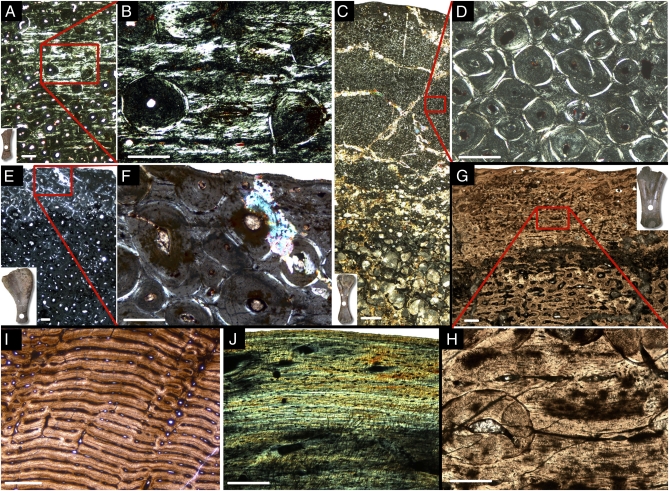
Fig. 2.
Micrographs of long bone histology. (A–E) Long bone histology of Magyarosaurus dacus under crossed polarizers. (A) Micrograph of a midshaft section of the smallest available specimen of Magyarosaurus dacus (MAFI Ob.3092, 46% max size). (B) Close-up of A: largely interstitial laminar primary bone in the outermost cortex. The vascular canals are oriented circumferentially as in laminar fibrolamellar bone, but the bone matrix between the vascular canals consists largely of parallel-fibered and lamellar bone, with only a minute fraction of fibrous (or woven) bone tissue. (C) Micrograph of a midshaft section of MAFI v.13492 (76% max. size). The cortex is completely remodeled, in some areas several generations of secondary osteons can be seen crosscutting each other. (D) Closeup of C: cortex dominated by several generations of secondary remodeling. (E) Micrograph of a midshaft section of the largest available M. dacus humerus (FGGUB R.1048). (F) Close-up of E: Note the secondary osteons of the third generation, and truncated secondary osteons at the outer bone surface. (G and H) Long bone histology of ‘M.’ hungaricus under polarized light. (G) Micrograph of a midshaft section of ‘M.’ hungaricus (MAFI Ob.3104). The specimen is strongly remodeled, but the interstitial primary tissue is of the highly vascularized laminar fibrolamellar kind, with well developed primary osteons in the middle cortex, and poorly developed primary osteons with no lamellar bone infilling in the outermost cortex. Note that secondary osteons of the first generation are less well developed than in the largest M. dacus specimens. (H) Close-up of G: Secondary osteons crosscutting well developed primary osteons in the middle cortex. (I) Laminar fibrolamellar bone of Apatosaurus (BYU 72517014). (J) Alligator (SMNS 10481) long bone histology showing lamellar-zonal bone. (Scale bars: A, B, and D–H, 200 μm; C, 1,000 μm; I and J, 500 μm).
An external fundamental system (EFS, outer circumferential lamellae sensu 51) was not observed in any of the Magyarosaurus dacus individuals in this study. In the smallest individuals, those that retain some primary bone in their outermost cortex, an EFS could have been observed, if present. In the larger, completely remodeled specimens (HOS 13 or more), an EFS, if present, would have been obscured by this remodeling. An additional agent of destruction of an EFS is preparation. Some specimens showing secondary osteons truncated by the outer bone surface (Fig. 2 E and F) suggest that bone has been removed by rough preparation methods, possibly leading to the loss of the micrometer-thin EFS.
Histologic Ontogenetic Stages in the M. dacus Sample.
We emphasize again that, in its extreme degree of cortical remodeling even in very small individuals, the long bone histology of Magyarosaurus is unique among sauropods. In some larger individuals, three to four generations of secondary osteons can be observed (FGGUB R.1048; Fig. 2 E and F). However, the Magyarosaurus dacus sample is still amenable to relative age determination of individuals using histologic ontogentic stages (HOS) (35). The smallest individual, represented by specimen MAFI Ob.3092, records HOS 12 (Fig. 3). The bone microstructure of specimens FGGUB R.1220, FGGUB R.1511, FGGUB R.1246, FGGUB R.1195, MAFI v.13492, and FGGUB R.1047 corresponds to HOS 13, where the cortex is completely or almost completely remodeled. In a number of femora and humeri (FGGUB R.1046, FGGUB R.1992, MAFI Ob.3089, MAFI Ob.3128, FGGUB R.1048), at least one additional generation of secondary osteons, crosscutting secondary osteons of the first or subsequent generations are present in the outer cortex. The microstructure of these specimens corresponds to tissue type H, and is thus assigned to HOS 14 (35) (Fig. 3, Materials and Methods, and SI Text).
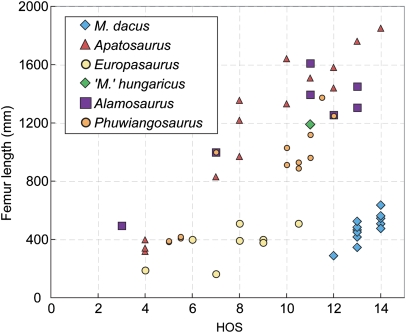
Fig. 3.
Plot of histologic ontogenetic stage (HOS) (35) vs. body size as expressed by femur length in Magyarosaurus dacus, compared with Europasaurus, Apatosaurus, Alamosaurus, and Phuwiangosaurus. The samples of Magyarosaurus dacus derive from humeri that were normalized to femur length. The single “M”' hungaricus sample is also included. Data for Alamosaurus were obtained from a previous report (38), supported by own data. Data for Phuwiangosaurus were obtained from another report (37).
Titanosaur long bone histology has received only limited study so far. However, specimens of the basal titanosaur Phuwiangosaurus and the advanced titanosaur Alamosaurus that are the size of the smallest M. dacus show HOS 3–5 (35, 37, 38) (Fig. 3). HOS 14 has not been observed in Alamosaurus and Phuwiangosaurus, but fully remodeled specimens (HOS 13) have femur lengths of 1,400 mm, nearly 2.5 times the size of the largest M. dacus (Fig. 3).
Long Bone Histology and HOS of M. hungaricus.
The histology of the large titanosaurian bone (MAFI Ob.3104) is different from the Magyarosaurus dacus bones (Fig. 2 G and H). The inner and outer cortex are dominated by secondary osteons, but with laminar primary bone still present in the outermost cortex. The primary bone is of the fibrolamellar kind, with a thick lining of lamellar bone in the vascular canals. These vascular canals, however, are not as narrow as in the M. dacus bones. Erosion cavities, but also mature secondary osteons, are visible in the outermost primary cortex. The outer bone surface is intact in MAFI Ob.3104, but there is no EFS, indicating that the animal was not fully grown. The bone microstructure of MAFI Ob.3104 corresponds to bone tissue type F (35) and is assigned to HOS 11 (Fig. 3). This is a lower stage than in the smallest bones in the M. dacus sample. However, this MAFI Ob.3104 is more than four times larger than the M. dacus specimens showing a later HOS (Figs. 1 and 3 and Table 1).
The histological sample of M. hungaricus shows a bone microstucture identical to that of Phuwiangosaurus, and a histology similar to that of the advanced titanosaur Alamosaurus (refs. 35, 37, 38) (Fig. 3). M. hungaricus thus displays a typical titanosaur long bone microstructure. A general observation of these titanosaur taxa compared to more basal neosauropods (e.g., Apatosaurus), is their accelerated remodeling rates (37, 38), which may be a result of continued peramorphic processes in Sauropodomorpha (34, 38, 51).
Interpretation.
Both, the comparison of bone tissue types and of HOS (Fig. 3) indicates that the small (M. dacus) and large titanosaur bones (M. hungaricus) cannot be placed on the same growth trajectory. This suggests that two distinct titanosaur taxa are present in the Haţeg Basin, with the great majority of bones belonging to a growth series of the diminutive M. dacus. We therefore reject the hypothesis (22) that the small titanosaur bones from the Haţeg Basin are merely juveniles of the large-bodied sauropod taxon, and we conclude that M. dacus is a dwarf taxon.
Discussion
Potential Problems: Lack of EFS.
The lack of an EFS in any of the studied long bones represents a weakness in our argument for M. dacus having been a dwarf taxon. An EFS would most convincingly indicate that growth had terminated (24, 25). However, we see the evidence as conclusive that the M. dacus sample does not represent juveniles of the larger M. hungaricus. First, as noted earlier, the advanced remodeling reaching the outer bone surface in the larger specimens of M. dacus would have obliterated any EFS, the lack of which thus cannot be cited as evidence for the M. dacus specimens being juveniles. Second, M. hungaricus, having been the adult of M. dacus would mean that an earlier HOS is present in specimens differing 4-fold in size. Such an extreme variability in size at a given HOS is not seen in any other sauropod (35, 37, 38) and runs counter to the general observation of a close correlation between body size and histology in dinosaurs in general (32–36, 38). The only known exception to this pattern appears to be the Triassic basal sauropodomorph Plateosaurus (53), but this taxon is much more basal in the saurischian phylogeny than Magyarosaurus. Third, the completely remodeled cortex of the larger M. dacus specimens is wholly inconsistent with a juvenile status, not only in comparison with other sauropods (as seen in the HOS comparisons) but also with amniotes in general. Even in slow-growing mammals such a humans, complete remodeling of the long bones is a sign that full size has been reached (25, 47, 54–57).
Co-occurrence of Large and Small Titanosaurs on an Island.
The very rare fossils of the larger titanosaur M. hungaricus in the Haţeg fauna are an interesting exception to the general dwarfing of other dinosaurs on Haţeg island. The presence of a few individuals of a larger titanosaurian species might relate to a time of lower sea level, for example, when the effective island size increased and allowed the survival of a larger-sized subsequent immigrant population, or they represent the remains of stray animals from nearby larger land masses. A similar example comes from the Pliocene–Pleistocene from Sulawesi, where the presence of the large Stegodon among smaller proboscideans was explained as the result of a late immigration event (58). Alternatively, the large bones may represent an early immigrant population before it reduced in size or went extinct. Nanism is known to occur very rapidly (59), at a time scale of 103 years, which is well below the time resolution in terrestrial sedimentary deposits, potentially making early colonists and later dwarfs seem comtemporaneous.
However, determining the most likely scenario is beyond the scope of this contribution, and will ultimately rely on future paleobiogeographic and phylogenetic work on the Haţeg dinosaur assemblage.
Significance of the Unique Long Bone Histology of M. dacus.
The nanoid status of M. dacus is unique among titanosaurs, all of which have body masses an order of magnitude greater (1, 60). The only other island nanoid sauropod known is Europasaurus from the Upper Jurassic of Germany (8). At 900 kg, M. dacus had a similar adult body mass as Europasaurus, but the two taxa show distinctive histologies and ontogenetic growth trajectories (Fig. 3). Europasaurus does not have as intensely remodeled bone cortices as M. dacus, even in the largest known individual, which shows a clear EFS (8). The fully grown Europasaurus individuals are HOS 10.5, and the smallest ones (34% maximum size) are only HOS 4. Europasaurus, like large-bodied sauropods, also shows fibrolamellar bone in its long bone cortex (Fig. 2I), and only late in its ontogeny, growth marks and Haversian remodeling started to appear (8). The primary bone in the smallest individual of M. dacus (46% maximum size) shows a large proportion of parallel-fibered bone, and our sample of M. dacus exhibits HOS ranging from 12–14. These observations suggest a reduced growth rate of M. dacus, in comparison not only with large sauropods but also with Europasaurus (Fig. 3).
Implications for Metabolic Rate.
The highly vascularized fibrolamellar tissue in the long bones of M. dacus, albeit with a strong lamellar component, suggests that the high metabolic rate of sauropods (5, 53, 61) has been retained in Magyarosaurus, because the phyletic nanism did not result in the reversal to a bone histology seen in similar-sized ectothermic vertebrates (62). In ectotherms such as crocodiles (Fig. 2J) and large pseudosuchians (63), lamellar-zonal bone predominates, and these ectotherms lack strongly vascularized primary bone and Haversian bone of the kind observed in Magyarosaurus. That this is an evolutionary option for endothermic amniotes in a resource-limited habitat is shown by the Neogene dwarf goat Myotragus from the Balearic islands, which shows typical lamellar–zonal bone (ref. 64). Instead, Magyarosaurus reduced both adult body size and overall ontogenetic growth rate, presumably to adapt to island dwelling with its resource limitations.
Materials and Methods
Materials.
Since Nopcsa's time, much new material has been recovered, and M. dacus is now known from numerous small-sized long bones and vertebrae. We sampled limb bone material (humeri, ulnae, femora, tibiae, fibulae) (Table 1 and Fig. 1) from the collections at the Faculty of Geology and Geophysics of the University of Bucharest, Romania (FGGUB) and the Geological Survey of Hungary in Budapest (MAFI). A total of 21 specimens were sampled, representing 18 M. dacus individuals, and one M. hungaricus. The humeral growth series of the diminutive M. dacus covers a size range from ≈22 cm to 49 cm in humerus length, whereas the large specimens are twice this large, with a sampled M. hungaricus humerus having an estimated length of 91 cm. For comparative purposes, we also sampled 5 individuals of Alamosaurus sanjuanensis to augment previous data (38). We sampled the specimens with a histological coring technique (34, 65). Samples were processed into thin sections, which were then studied histologically under a Leica DMLP polarized light microscope. Images were acquired with a Leica DFC420 digital camera and processed with Imagic Imageaccess software.
Mass Estimates.
Most of the bones were found in isolation and come from a number of different localities within the Haţeg Basin. However, in a few cases, associated material allowed the sampling of multiple appendicular elements from the same skeleton (FGGUB R.1046, FGGUB R.1047, FGGUB R.1992). The length of the fragmentary femora was estimated from FGGUB R.1046; the length of fragmentary humeri from FGGUB R.1047; that of the tibiae from FGGUB R.1045; all ulnae and fibulae are virtually complete. We used a bone size estimation method based on identification of morphological landmarks and estimation of the preserved percentage of total length. Size standardization was performed for femora relative to humeral length. The humerus to femur ratio (0.768) is calculated from associated specimens FGGUB R.1046 and FGGUB R.1047. Unlike in most other studies, the humerus was chosen because it represents the largest subset of our samples and histology is better preserved than in femora. Note, however, that humerus length was scaled to femur length in the HOS diagram (Fig. 3).
The masses of Neuquensaurus and Magyarosaurus were estimated using an equation for calculating large quadrupedal animal masses based on humerus and femur circumference (66, 67). Humerus and femur data for Neuquensaurus were obtained from the literature (7). For M. dacus, measurements were directly taken from an associated humerus (FGGUB R.1047) and femur (FGGUB R.1046) (Table 1).
Aging Sauropod Long Bones Using Histologic Ontogenetic Stages.
Hundreds of individuals of different ontogenetic stages from close to 20 taxa accross the entire sauropod phylogeny have been sampled so far [reviewed in (46)]. This breadth of sampling has led to the identification of histologic indicators of ontogenetic stage (32–34), formalized in the histologic ontogenetic stage (HOS) scheme (35). This scheme allows qualitative ontogenetic comparisons between humeri and femora of different sauropod taxa (37, 38). The previously used HOS scale ranges from HOS 1, representing embryonic bone, to HOS 13, representing individuals with a completely or almost completely remodeled long bone cortex (35) (SI Text). Histologic ontogenetic stages 12 and 13 are only seen in very old and large sauropod individuals that had lived for many years after reaching asymptotic body size, such as in the 1.58 and 1.76 m femora (BYU 601–17328, OMNH 01991) of Apatosaurus (Fig. 3). Some sauropod individuals, however, are characterized by a completely remodeled cortex, displaying successive crosscutting relations of secondary osteons in the outer bone cortex, with the inner and middle cortex displaying this feature anyway, as remodeling progresses from the medullary region outward (32–35). This feature is seen only in the largest and oldest sauropod individuals, such as in a 1.8-m femur (OMNH 4020) of Apatosaurus. Degrees of remodeling in large sauropod individuals have not previously been distinguished (35), but we believe that it is necessary to make this distinction for comparative histological purposes, as is done in forensic science (54, 55, 57). Therefore, we define a tissue type with a completely remodeled cortex and at least two generations of crosscutting secondary osteons in the outer cortex as tissue type H, representing HOS 14. Although it is tempting to define additional HOSs for every generation of secondary osteons, this is problematic. Eventually, as remodeling continues, it will have obscured earlier generations of secondary osteons, making it impossible to detect the precise number of generations of secondary osteons. The introduction of HOS 14 thus serves to refine the histologic ontogenetic staging of sauropods.
Supplementary Material
Acknowledgments
We thank M. Benton for catalyzing this project, L. Kordos (Geological Survey of Hungary, Budapest) for permission to sample Magyarosaurus specimens in his care. We also thank T. Rowe (University of Texas at Austin, Department of Geological Sciences) for permission to sample Alamosaurus specimens in his care. Technical help with photographing specimens and thin sectioning samples was provided by O. Dülfer and G. Oleschinski (University of Bonn, Steinmann Institut für Geologie, Mineralogie und Paläontologie). K.S. and P.M.S. gratefully acknowledge financial support by the Deutsche Forschungsgemeinschaft (DFG). Z.C. was supported by grants from the Royal Society and the Synthesys Programme (GB-TAF 3417-2007), as well as the CNCSIS-UEFISCSU (Project PNII-IDEI 1930/2008). This is Contribution 92 of the DFG Research Unit “Biology of the Sauropod Dinosaurs.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000781107/-/DCSupplemental.
References
- 1.Mazzetta GV, Christiansen P, Farina RA. Giants and bizarres: Body size of some southern South American Cretaceous dinosaurs. Hist Biol. 2004;2004:1–13. [Google Scholar]
- 2.Upchurch P, Barret P, Dodson P. Sauropoda. In: Weishampel DB, Dodson P, Osmolska H, editors. The Dinosauria. 2nd Ed. Berkeley: University of California Press; 2004. pp. 259–322. [Google Scholar]
- 3.Buffetaut E, et al. The first giant dinosaurs: A large sauropod from the Late Triassic of Thailand. C R Palevol. 2002;1:103–109. [Google Scholar]
- 4.Erickson GM, Rogers KC, Yerby SA. Dinosaurian growth patterns and rapid avian growth rates. Nature. 2001;412:429–433. doi: 10.1038/35086558. [DOI] [PubMed] [Google Scholar]
- 5.Sander PM, et al. Adaptive radiation in sauropod dinosaurs: Bone histology indicates rapid evolution of giant body size through acceleration. Org Divers Evol. 2004;4:165–173. [Google Scholar]
- 6.Salgado L, Apesteguia S, Heredia SE. A new specimen of Neuquensaurus australis, a Late Cretaceous saltasaurine from North Patagonia. J Vert Paleontol. 2005;25:623–634. [Google Scholar]
- 7.Wilson JA. An overview of titanosaur evolution and phylogeny. In: Salense CA-P, editor. Actas de las III Jornados sobre Dinosaurios y su Entorno. España: Salas de los Infantes, Burgos; 2006. pp. 169–190. [Google Scholar]
- 8.Sander PM, Mateus O, Laven T, Knötschke N. Bone histology indicates insular dwarfism in a new Late Jurassic sauropod dinosaur. Nature. 2006;441:739–741. doi: 10.1038/nature04633. [DOI] [PubMed] [Google Scholar]
- 9.Weishampel D, Grigorescu D, Norman DB. The dinosaurs of Transylvania. Natl Geogr Res. 1991;7:196–215. [Google Scholar]
- 10.Grigorescu D. Nonmarine Cretaceous formations of Romania. In: Mateer NJ, Chen P-J, editors. Aspects of Nonmarine Cretaceous Geology. Beijing: China Ocean Press; 1992. pp. 142–164. [Google Scholar]
- 11.Weishampel DB, Norman DB, Grigorescu D. Telmatosaurus transsylvanicus from the Late Cretaceous of Romania: The most basal hadrosaurid dinosaur. Palaeontology. 1993;36:361–385. [Google Scholar]
- 12.Weishampel DB, Jianu C-M, Csiki Z, Norman DB. Osteology and phylogeny of Zalmoxes (n.g.), an unusual euornithopod dinosaur from the latest Cretaceous of Romania. J Syst Palaeontology. 2003;1:1–56. [Google Scholar]
- 13.Bate DM. Further note on the remains of Elephas cypriotes from a cave-deposit in Cyprus. Philos Trans R Soc Lond B Biol Sci. 1905;197:347–360. [Google Scholar]
- 14.Nopcsa F. On the occurrence of dinosaurs in Siebenbürgen (translated from German) Verhandlungen der Zoologisch-Botanischen Gesellschaft. 1914;54:12–14. [Google Scholar]
- 15.Nopcsa F. On the geological importance of the primitive reptilian fauna of the uppermost Cretaceous of Hungary; with a description of a new tortoise (Kallokibotium) Q J Geol Soc Lond. 1923;79:100–116. [Google Scholar]
- 16.Huene FFv. The Fossil Reptile Order Saurischia, Their Development and History (translated from German) Leipzig: Gebrueder Borntraeger; 1932. [Google Scholar]
- 17.Le Loeuff J. European titanosaurids. Revue de Paléobiologie. Volume Spéciale. 1993;7:105–117. [Google Scholar]
- 18.Curry Rogers K. Titanosauria: A phylogenetic overview. In: Curry Rogers K, Wilson JA, editors. The Sauropods: Evolution and Paleobiology. Berkeley: University of California Press; 2005. pp. 50–103. [Google Scholar]
- 19.Gould GC, MacFadden BJ. Gigantism, dwarfism, and Cope's rule: ‘‘Nothing in evolution makes sense without a phylogeny’’. Bull Am Mus Nat Hist. 2004;285:219–237. [Google Scholar]
- 20.Alberch P, Gould SJ, Oster GF, Wake DB. Size and shape in ontogeny and phylogeny. Paleobiology. 1979;5:296–317. [Google Scholar]
- 21.Jianu CM, Weishampel DB. The smallest of the largest: A new look at possible dwarfing in sauropod dinosaurs. Geol Mijnb. 1999;78:335–343. [Google Scholar]
- 22.Le Loeuff J. Romanian Late Cretaceous dinosaurs: Big dwarfs or small giants? Hist Biol. 2005;17:15–17. [Google Scholar]
- 23.Jianu C-M, Boekschoten GJ. The Haţeg—island or outpost? Deinsea. 1999;7:195–198. [Google Scholar]
- 24.Erickson G. Assessing dinosaur growth patterns: A microscopic revolution. Trends Ecol Evol. 2005;20:677–684. doi: 10.1016/j.tree.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Chinsamy-Turan A. The Microstructure of Dinosaur Bone. Baltimore: Johns Hopkins University Press; 2005. [Google Scholar]
- 26.Xu X, et al. A basal tyrannosauroid dinosaur from the Late Jurassic of China. Nature. 2006;439:715–718. doi: 10.1038/nature04511. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Tan Q, Wang J, Zhao X, Tan L. A gigantic bird-like dinosaur from the Late Cretaceous of China. Nature. 2007;447:884–847. doi: 10.1038/nature05849. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, et al. A Jurassic ceratosaur from China helps clarify avian digital homologies. Nature. 2009;459:940–944. doi: 10.1038/nature08124. [DOI] [PubMed] [Google Scholar]
- 29.Erickson GM, Tumanova TA. Growth curve of Psittacosaurus mongoliensis Osborn (Ceratopsia: Psittacosauridae) inferred from long bone histology. Zool J Linn Soc. 2000;130:551–566. [Google Scholar]
- 30.Erickson G, et al. Was dinosaurian physiology inherited by birds? Slow growth in Archaeopteryx. PLoS One. 2009;4:e7390. doi: 10.1371/journal.pone.0007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee AH, Werning S. Sexual maturity in growing dinosaurs does not fit reptilian growth models. Proc Natl Acad Sci USA. 2008;105:582–587. doi: 10.1073/pnas.0708903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curry KA. Ontogenetic histology of Apatosaurus (Dinosauria: Sauropoda): New insights on growth rates and longevity. J Vert Paleontol. 1999;19:654–665. [Google Scholar]
- 33.Sander PM. Life history of the Tendaguru sauropods as inferred from long bone histology. Mitt Mus Natk Humboldt-Univ Berlin, Geowiss Reihe. 1999;2:103–112. [Google Scholar]
- 34.Sander PM. Long bone histology of the Tendaguru sauropods: Implications for growth and biology. Paleobiology. 2000;26:466–488. [Google Scholar]
- 35.Klein N, Sander PM. Ontogenetic stages in the long bone histology of sauropod dinosaurs. Paleobiology. 2008;34:248–264. [Google Scholar]
- 36.Lehman T, Woodward H. Modeling growth rates for sauropod dinosaurs. Paleobiology. 2008;34:264–281. [Google Scholar]
- 37.Klein N, Sander PM, Suteethorn V. Bone histology and its implications for the life history and growth of the Early Cretaceous titanosaur Phuwiangosaurus sirindhornae. Geol Soc Lond Spec Publ. 2009;315:217–228. [Google Scholar]
- 38.Woodward H, Lehman T. Bone histology and microanatomy of Alamosaurus sanjuanensis (Sauropoda: Titanosauria) from the Maastrichtian of Big Bend National Park, Texas. J Vert Paleont. 2009;29:807–821. [Google Scholar]
- 39.Erickson G, et al. Gigantism and comparative life-history parameters of tyrannosaurid dinosaurs. Nature. 2004;430:772–775. doi: 10.1038/nature02699. [DOI] [PubMed] [Google Scholar]
- 40.Erickson G, Currie PJ, Inouye BD, Winn AA. Tyrannosaur life tables: An example of nonavian dinosaur population biology. Science. 2006;313:213–217. doi: 10.1126/science.1125721. [DOI] [PubMed] [Google Scholar]
- 41.Erickson GM, Curry Rogers K, Varricchio D, Norell MA, Xu X. Growth patterns in brooding dinosaurs reveals the timing of sexual maturity in non-avian dinosaurs and genesis of the avian condition. Biol Lett. 2007;3:558–561. doi: 10.1098/rsbl.2007.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bybee PJ, Lee AH, Lamm E-T. Sizing the Jurassic theropod dinosaur Allosaurus: Assessing growth strategy and evolution of ontogenetic scaling of limbs. J Morphol. 2006;267:347–359. doi: 10.1002/jmor.10406. [DOI] [PubMed] [Google Scholar]
- 43.Horner JR, Padian K. Age and growth dynamics of Tyrannosaurus rex. Proc R Soc Lond B Biol Sci. 2004;271:1875–1880. doi: 10.1098/rspb.2004.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper LN, Lee AH, Taper ML, Horner JR. Relative growth rates of predator and prey dinosaurs reflect effect of predation. Proc R Soc Lond B Biol Sci. 2008;275:2609–2615. doi: 10.1098/rspb.2008.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varricchio D, et al. Avian paternal care had dinosaur origin. Science. 2008;322:1826–1828. doi: 10.1126/science.1163245. [DOI] [PubMed] [Google Scholar]
- 46.Sander PM, Klein N, Stein K, Wings O. Sauropod bone histology and its implications for sauropod biology. In: Klein N, Remes K, Gee CT, Sander PM, editors. Biology of the Sauropod Dinosaurs. Bloomington: Indiana University Press; [Google Scholar]
- 47.Francillon-Vieillot H, et al. Microstructure and mineralization of vertebrate skeletal tissues. In: Carter JG, editor. Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends. Vol. 1. New York: Van Nostrand Reinhold; 1990. pp. 471–530. [Google Scholar]
- 48.Castanet J, Rogers KC, Cubo J, Boisard J-J. Periosteal bone growth rates in extant ratites (ostrich and emu). Implications for assessing growth in dinosaurs. C R Acad Sci Paris Sci Terre. 2000;323:543–550. doi: 10.1016/s0764-4469(00)00181-5. [DOI] [PubMed] [Google Scholar]
- 49.Margerie Ed, Cubo J, Castanet J. Bone typology and growth rate: Testing and quantifying “Amprino's rule” in the mallard (Anas platyrhynchos) C R Acad Sci Paris Biol. 2002;325:221–230. doi: 10.1016/s1631-0691(02)01429-4. [DOI] [PubMed] [Google Scholar]
- 50.de Margerie E, et al. Assessing a relationship between bone microstructure and growth rate: A fluorescent labelling study in the king penguin chick (Aptenodytes patagonicus) J Exp Biol. 2004;207:869–879. doi: 10.1242/jeb.00841. [DOI] [PubMed] [Google Scholar]
- 51.Ham AW. Histology. 2nd Ed. Philadelphia: Lippincot; 1953. [Google Scholar]
- 52.McNamara KJ. Shapes of Time. Baltimore: Johns Hopkins Univ Press; 1997. [Google Scholar]
- 53.Sander PM, Klein N. Developmental plasticity in the life history of a prosauropod dinosaur. Science. 2005;310:1800–1802. doi: 10.1126/science.1120125. [DOI] [PubMed] [Google Scholar]
- 54.Kerley ER. The microscopic determination of age in human bone. Am J Phys Anthropol. 1965;23:149–164. doi: 10.1002/ajpa.1330230215. [DOI] [PubMed] [Google Scholar]
- 55.Kerley ER, Ubelakker DH. Revisions in the microscopic method of estimating age at death in human cortical bone. Am J Phys Anthropol. 1978;49:545–546. doi: 10.1002/ajpa.1330490414. [DOI] [PubMed] [Google Scholar]
- 56.Castanet J, Francillon-Vieillot H, Meunier FJ, Ricqlès Ad. Bone and individual aging. In: Hall BK, editor. Bone. Volume 7: Bone Growth—B. Boca Raton: CRC Press; 1993. pp. 245–283. [Google Scholar]
- 57.Thomas CDL, Stein MS, Feik SA, Wark JD, Clement JG. Determination of age at death using combined morphology and histology of the femur. J Anat. 2000;196:463–473. doi: 10.1046/j.1469-7580.2000.19630463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Vos J, Van den Hoek Ostende L, Van den Bergh G. Patterns in insular evolution of mammals: A key to island palaeogeography. In: Renema W, editor. Biogeography, Time, and Place: Distributions, Barriers, and Islands. Amsterdam: Springer; 2007. Top-ics in Geobiology, Vol 29, pp 315–345. [Google Scholar]
- 59.Millien V. Morphological evolution is accelerated among island mammals. PLoS Biol. 2006;4:1863–1868. doi: 10.1371/journal.pbio.0040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seebacher F. A new method to calculate allometric length-mass relationships of dinosaurs. J Vert Paleont. 2001;21:51–60. [Google Scholar]
- 61.Sander PM, Clauss M. Sauropod Gigantism. Science. 2008;322:200–201. doi: 10.1126/science.1160904. [DOI] [PubMed] [Google Scholar]
- 62.Erickson GM, Brochu CA. How the “terror crocodile” grew so big. Nature. 1999;398:205–206. [Google Scholar]
- 63.Ricqlès Ad, Padian K, Horner JR. On the bone histology of some Triassic pseudosuchian archosaurs and related taxa. Ann Paleontol. 2003;89:67–101. [Google Scholar]
- 64.Köhler M, Moya-Sola S. Physiological and life history strategies of a fossil large mammal in a resource-limited environment. Proc Natl Acad Sci USA. 2009;106:20354–20358. doi: 10.1073/pnas.0813385106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stein K, Sander PM. Histological core drilling: A less destructive method for studying bone histology. In: Brown MA, Kane JF, Parker WG, editors. Methods in Fossil Preparation: Proceedings of the First Annual Fossil Preparation and Collections Symposium. 2009. pp. 69–80. [Google Scholar]
- 66.Anderson JF, Hall-Martin A, Russell DA. Long bone circumference and weight in mammals, birds and dinosaurs. J Zool A. 1985;207:53–61. [Google Scholar]
- 67.McNeil AR. Dynamics of Dinosaurs and Other Extinct Giants. New York: Columbia University Press; 1989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.