Abstract
The mechanism by which the intrinsic pathway of coagulation contributes to physiological hemostasis is enigmatic. Thrombin activates factor XI, a key zymogen in this pathway, which leads to increased thrombin generation. As thrombin-dependent activation of factor XI in vitro is relatively inefficient, we hypothesized that a physiological cofactor supports this reaction in a plasma environment. We therefore investigated whether the cofactors of coagulation, activated factor V, activated factor VIII, high-molecular weight kininogen, or protein S, influenced activation of factor XI by thrombin. Only activated factor V stimulated activation of factor XI by thrombin in a purified system. Binding studies demonstrated that factor XI specifically interacts with both factor V and factor Va through multiple binding sites. We further investigated this cofactor function of activated factor V in plasma. Depletion of factor V, or the addition of activated protein C, decreased the activation of the intrinsic pathway by thrombin in plasma. However, activated protein C did not exert this effect in the plasma of a homozygous carrier of the prothrombotic factor V Leiden mutation. In conclusion, we propose a role for (activated) factor V as a cofactor in the activation of factor XI by thrombin. These findings offer insights into the coagulation system in both health and disease.
Keywords: coagulation, coagulation factor V, coagulation factor XI, feedback activation
The mechanism behind the activation of the intrinsic pathway of coagulation in vivo has been subject to discussion since the introduction of the model of coagulation (1, 2). The importance of the intrinsic pathway for physiological hemostasis is apparent from the bleeding phenotypes that are seen in factor VIII (FVIII) deficiency, factor IX (FIX) deficiency and, to a lesser extent, in factor XI (FXI) deficiency. In vitro, activation of the intrinsic pathway can be initiated by factor XII (FXII) on negatively charged surfaces, which results in clotting. However, the role of FXII-dependent coagulation in physiological hemostasis is controversial. FXII deficiency, for instance, protects against thrombosis in murine models (3), which conflicts with the protective role of high FXII antigen levels in epidemiological studies on thrombosis in humans (4), as well as with the absence of bleeding symptoms in FXII-deficient individuals. Additionally, FXII hyperactivity leads to angioedema, rather than to thrombosis (5), which corresponds with evidence that activation of the kallikrein-kinin system by FXII can take place independent of coagulation (6).
As FXI deficiency, unlike FXII deficiency, is associated with mild bleeding phenomena, a FXI activator other than FXII is likely. Thrombin, the final and central enzyme of the coagulation cascade, can directly activate FXI, which leads to downstream formation of more thrombin (7, 8). This feedback activation mechanism offered an alternative explanation for the existence of the intrinsic pathway: The amplification of minute amounts of thrombin, which leads to the thrombin levels required to protect clots by activation of thrombin-activatable fibrinolysis inhibitor (TAFI) (9, 10).
Recently, a number of publications have debated the existence of feedback activation in plasma, providing both evidence against (11) and in favor (12, 13) of its existence. Here, we aimed to obtain insight into the mechanism that underlies the activation of FXI by thrombin in plasma. Because the activation of FXI by thrombin is an inefficient process in vitro (7, 8), but occurs in plasma nonetheless, we hypothesized that a plasma cofactor mediates efficient feedback activation of FXI by thrombin. The hemostatic system has many cofactors, such as activated coagulation factors V (FVa) and VIII (FVIIIa), high-molecular weight kininogen (HK), and protein S. This study shows that, out of all the cofactors that were tested, FVa is a phospholipid-dependent cofactor for thrombin-dependent FXI activation, in both a purified system and in plasma.
Results
The Activation of FXI by Thrombin Is Stimulated by Activated Factor V.
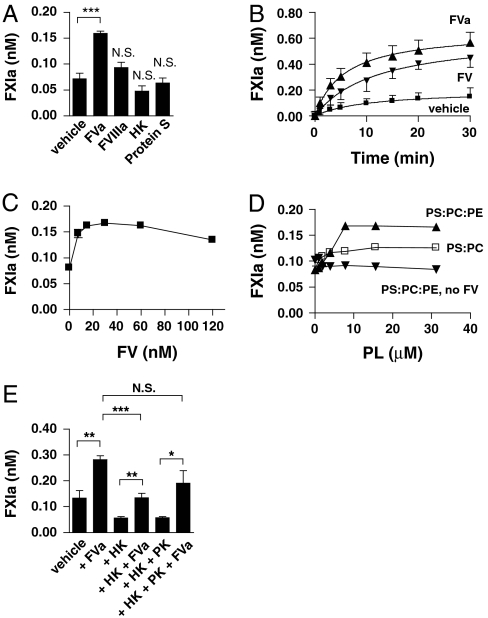
The activation of FXI by thrombin is a rather inefficient process in vitro, but anionic surfaces such as dextran sulphate are known to enhance this reaction tremendously. Since feedback activation of FXI occurs in plasma regardless of the presence of an anionic surface, we investigated the possibility of a plasma cofactor for this mechanism. We therefore studied the effects of the cofactors of coagulation, FVa, FVIIIa, HK, and protein S, on the activation of FXI by thrombin with purified coagulation factors (Fig. 1A). FXI was incubated with α-thrombin in the presence of each cofactor and samples were taken in time. Thrombin activity was neutralized by the addition of hirudin, which inhibited further FXIa formation and conversion of the chromogenic substrate by thrombin. Addition of FVIIIa, HK, or protein S did not influence the formation of FXIa. However, the presence of FVa stimulated FXIa generation by thrombin. This finding was further assessed in time-dependent experiments; the amounts of FXIa that formed over time increased in the presence of FVa (Fig. 1B). Addition of FV resulted in a similar enhancement of FXI activation by thrombin. However, it is likely that FV is rapidly converted into FVa by thrombin under these conditions. The cofactor activity of FV on FXI activation by thrombin was dose-dependent with an optimal concentration around the plasma concentration of 30 nM (Fig. 1C). FV(a) influenced neither the catalytic activity of FXIa towards the chromogenic substrate for FXIa, nor the activation of FIX. The effect of FV(a) on thrombin-mediated FXIa generation depended on the presence, concentration, and composition of phospholipid vesicles (Fig. 1D). Whereas FV increased FXIa formation by thrombin in the presence of phospholipid vesicles that contain phosphatidylserine (PS) and phosphatidylcholine (PC), the addition of phospholipid vesicles that contain PS, PC, and phosphatidylethanolamine (PE) increased the activation rate of FXIa even further. Although addition of dextran sulphate significantly increased the activation of FXI by thrombin, it did not support the cofactor activity of FV(a). Because neither phospholipid vesicles, nor FV, influenced thrombin-dependent FXIa formation on their own, both are required to enhance FXI activation.
Fig. 1.
FV(a) is a cofactor for the activation of FXI by thrombin. (A) FXI (30 nM) was activated with thrombin (10 nM) in the presence 10 μM PS:PC:PE and vehicle, FVa (30 nM), FVIIIa (30 nM), HK (630 nM), or Protein S (150 nM) for 10 min at 37 °C. Reactions were stopped with hirudin (10 U/mL). The amount of formed FXIa was measured with Pefachrome XIa3371 as described in Materials and Methods. (B) Factor XI (30 nM) was activated with thrombin (10 nM) in the presence of 10 μM PS:PC:PE and vehicle, FV, or FVa. Samples were taken in time and reactions were stopped with hirudin (10 U/mL). The amount of formed FXIa was determined with Pefachrome XIa3371. (C) Factor XI (30 nM) was activated with thrombin (10 nM) in the presence of 10 μM PS:PC:PE and increasing amounts of FVa for 10 min at 37 °C. Reactions were stopped with hirudin (10 U/mL). The amount of formed FXIa was determined with Pefachrome XIa3371. (D) Factor XI (30 nM) was activated with thrombin (10 nM) in the presence or absence of FV (30 nM) and increasing concentrations of phospholipid vesicles for 10 min at 37 °C. The amount of formed FXIa was determined with Pefachrome XIa3371. (E) FXI (30 nM) was activated with thrombin (10 nM) in the presence of 10 μM PS:PC:PE and vehicle, FVa (30 nM), HK (630 nM), or PK (580 nM) for 20 min at 37 °C, as indicated. Reactions were stopped with hirudin (10 U/mL) and amounts of formed FXIa were determined indirectly, as described in Materials and Methods. Data are shown as mean ± SD for data from at least three experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
FXI circulates in complex with HK in human plasma (14, 15) and HK was reported to inhibit the activation of FXI by thrombin (7). We therefore investigated the effects of HK on the cofactor activity of FV(a) on thrombin-dependent FXI activation (Fig. 1E). At plasma concentration, HK decreased the cofactor activity of FVa. However, in the presence of PK, which also circulates in a 1∶1 complex with HK, the cofactor activity of FVa was restored to normal.
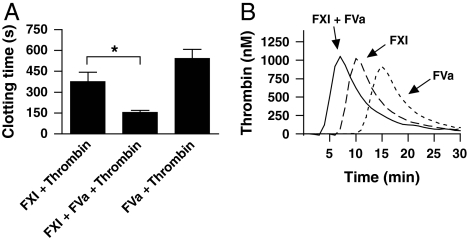
The nonphysiological polysaccharide dextran sulphate reportedly enhances the activation of FXI by thrombin 1,000-fold (7, 8). In comparison, the combined cofactor activity of FV and phospholipids is modest, with only a 2- to 4-fold increase in FXIa formation. We therefore tested whether the differences in the amount of FXIa generated in the presence or absence of FVa has an effect on the coagulation reaction. Indeed, the coagulation time was reduced by 60% when FXIa was formed by thrombin in the presence of FVa (Fig. 2A). When FXIa was formed in the absence of FVa, there was no significant difference with the background coagulation time. When monitoring thrombin activity in these plasmas, we observed a corresponding reduction in the lag time of thrombin generation (Fig. 2B).
Fig. 2.
The FV(a)-dependent increase in the activation of FXI by thrombin enhances the coagulation reaction. FXI (30 nM) was activated with thrombin in the presence of 10 μM PS:PC:PE and vehicle or FVa (30 nM) for 10 min at 37 °C, after which reactions were stopped with hirudin (10 U/mL). Thrombin and FVa alone served as control. (A) Coagulation was initiated with diluted samples as described in Materials and Methods. Data are shown as mean ± SD for data from at least three experiments. (B) Thrombin generation was measured with the Technothrombin TGA kit as described in Materials and Methods. Graph shows a representative experiment. *P < 0.05.
Factor XI Binds to Factor V with Multiple Binding Sites.
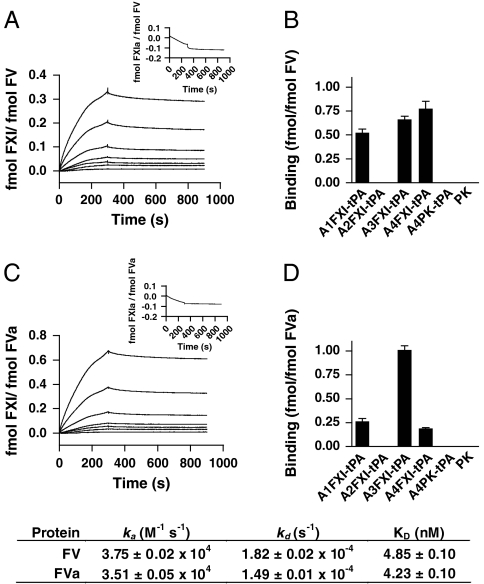
The experiments above suggest a binding interaction between FXI and FV(a). We therefore determined whether binding interactions take place between FXI and FV or FVa with surface plasmon resonance analysis. FXI bound to immobilized FV with high affinity (Fig. 3A). We obtained a similar value for the interaction between FXI and immobilized FVa (Fig. 3C). No binding was observed with FXIa (insets in Fig. 3 A and C). Although there is a structural similarity between FV and FVIII, FVIII was not able to bind FXI.
Fig. 3.
Factor XI binds to factor V(a) with high affinity via multiple binding sites. Surface plasmon resonance studies were performed with either FV or FVa immobilized on C1 sensor chips. Binding of FXI (0, 3.1, 6.3, 12.5, 25, 50, 100 and 200 nM) to immobilized FV (A) or FVa (C) was assessed at a flow rate of 50 μL/ min at 25 °C. No binding of FXIa (100 nM) to either FV or FVa was observed (insets). Domains responsible for the interaction between FXI and FV (B) or FVa (D) were mapped with individual tPA-tagged apple domains of FXI (25 nM), designated A1FXI-tPa to A4FXI-tPA. Binding of constructs to immobilized FV or FVa was assessed as described in Materials and Methods. PK and its tPA-tagged apple4 domain (A4PK-tPA) served as controls. Data are expressed as mean ± SD in fmol bound analyte (FXI, PK, or construct) per fmol immobilized ligand (FV or FVa). Inset shows the obtained association (ka) and dissociation (kd) rates ± SD, as well as the affinity constants (KD) ± SD for the interaction between FXI and FV or FVa. All binding experiments were performed at least three times. Representative SPR traces are shown.
Next, we investigated the binding sites on FXI for FV and FVa in more detail. We analyzed the binding of recombinant tPA-tagged constructs of individual FXI apple domains to immobilized FV and FVa. The apple1, apple3, and apple4 domains of FXI bound to immobilized FV, whereas the apple2 domain was unable to bind to FV (Fig. 3B). Likewise, apple1, apple3, and apple4 of FXI interacted with FVa, with the strongest interaction between apple3 and FVa (Fig. 3D). No binding of apple2 to FVa could be observed. FXI is highly homologous to PK. No binding of PK could be observed to either FV or FVa. Similarly, no binding was observed with a tPA-tagged construct of the apple4 domain of prekallikrein.
Activated Factor V Contributes to Feedback Activation of the Intrinsic Pathway in Plasma.
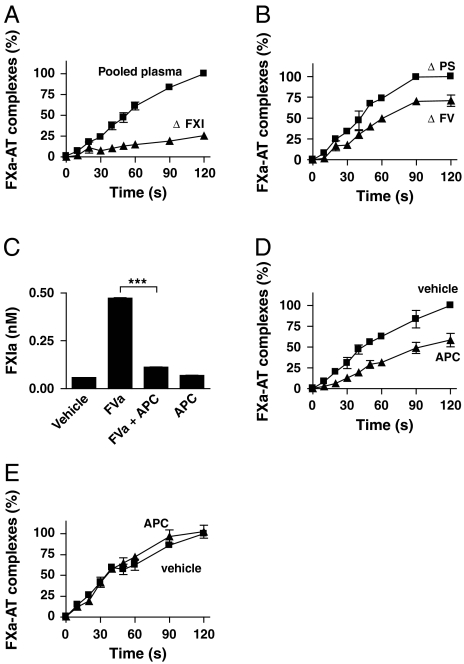
It was demonstrated that the activation of FXI by thrombin mediates the propagation of the coagulation reaction in plasma (7, 8, 13). We investigated whether FV(a) contributes to this mechanism. Activation of the intrinsic pathway was evaluated by quantification of formed complexes of FXa and its natural inhibitor antithrombin (AT). Because FV also takes part in the prothrombinase complex, we performed our experiments in plasmas that were immuno-depleted of prothrombin (< 1%). In this way, we prevented the formation of additional thrombin by the prothrombinase complex and excluded a dual role for FV in these experiments. In a first experiment, we confirmed the vital role of FXI in activation of the intrinsic pathway by thrombin. Absence of FXI (< 1%) strongly reduced the formation of FXa-AT complexes in response to thrombin (Fig. 4A). No FXa-AT complexes were detected in the absence of thrombin. In a second experiment, FV was immuno-depleted from plasma. Decreased amounts of FXa-AT complexes were observed in FV depleted plasma upon activation with thrombin, as compared with control depletion of protein S (P < 0.0001) (Fig. 4B). FXI was not co-depleted with FV.
Fig. 4.
FVa influences feedback activation of FXI in plasma. The role of FV in the activation of FXI by thrombin in plasma was investigated after depletion of prothrombin to prevent feedback activation. All plasmas were activated with 20 nM thrombin in the presence of 10 μM PS:PC:PE vesicles. Samples were taken in time and reactions were stopped with 10 U/mL hirudin. Factor Xa-AT complexes were measured as described in Materials and Methods. The generation of FXa-AT complexes after 120 s in control plasma was normalized to 100%. Under these conditions, 100% represents the formation of 0.27 ± 0.12% of the FXa-AT complex levels present in serum obtained after the full coagulation of recalcified normal plasma in glass tubes. The results displayed are the combined data of four separate experiments, each performed in triplicate. (A) Prothrombin-depleted pooled normal plasma and factor XI deficient plasma were activated with thrombin. (B) The role of FV was investigated in pooled normal plasma that was depleted of either protein S (ΔPS) or factor V (ΔFV). (C) FXI (30 nM) was activated with thrombin (10 nM) in the presence of vehicle, FVa (30 nM), or APC (5 nM) as indicated for 20 min at 37 °C. The amount of formed FXIa was determined indirectly as described in Materials and Methods. (D) Prothrombin-depleted pooled normal plasma was activated with thrombin in the presence or absence of APC (20 nM). (E) Prothrombin-depleted plasma of an individual homozygous for the G1691A mutation in the FV gene was activated with thrombin in the presence or absence of APC (20 nM). Data are depicted as mean ± SD. ***P < 0.001.
Activated protein C (APC) strongly inhibits the cofactor function of FVa during prothrombin activation by FXa. APC exerts its function by degradation of the heavy chain of FVa. The importance of this natural anticoagulant is emphasized by the increased risk of thrombosis in persons with protein C deficiency or APC resistance. APC resistance is most often caused by a mutation in FV (G1691A) that results in insensitivity towards proteolytic degradation by APC. In a purified system, APC completely abolished the cofactor function of FVa on thrombin-mediated FXIa formation (Fig. 4C). In plasma, APC strongly inhibited the formation of FXa-AT complexes upon activation with thrombin (P < 0.0001) (Fig. 4D). When the same experiment was performed in plasma of a person that was homozygous for the FV G1691A mutation, APC had no effect on the formation of FXa-AT complexes (P = 0.46) after activation with thrombin. These data indicate a role for FVa in the activation of the intrinsic pathway by thrombin, which can be modulated by APC.
Discussion
This study demonstrates a function of FVa as a cofactor in the activation of FXI by thrombin, both in a purified system and in plasma. This cofactor activity of FVa enhances the activation of FXI by thrombin 2- to 4-fold and requires phospholipid surfaces. FXI binds to FV via its apple1, apple3, and apple4 domains, whereas the binding of FXI to FVa is principally mediated via its apple3 domains. APC attenuates the cofactor activity of FVa on thrombin-mediated FXIa formation in normal plasma, but not in plasma of an individual homozygous for the factor V Leiden G1691A mutation.
Kravtsov and colleagues recently confirmed older publications that feedback activation of FXI by thrombin takes place in plasma (13), a mechanism that was disputed earlier (11). Our findings add to the understanding of this process in plasma by the identification of a plasma cofactor for FXI activation by thrombin. FXI binds both FV and FVa, but our data indicate that it is FVa that functions as a cofactor. Because FV is immediately activated by thrombin, all of our experiments were essentially performed in the presence of FVa. Moreover, the cofactor activity we observed was strongly inhibited in the presence of APC, which preferentially degrades FVa. APC also degrades FVIIIa, thereby inhibiting intrinsic tenase activity. The lack of an effect of APC in the plasma of an individual with the FV Leiden mutation indicates that the effects of APC on FXa-AT complex formation are based on FVa degradation and not on inhibition of the intrinsic tenase complex. Based on our findings, we propose that feedback activation of the intrinsic pathway of coagulation takes place on a phospholipid surface. Although FXI itself cannot bind to phospholipid bilayers, FVa can (16). Our data therefore suggest that FXI is sequestered to phospholipid surfaces by FVa.
FXI binds to immobilized FV(a) with high affinity, which suggests that these molecules could circulate as a complex in human plasma. However, we were unable to show the presence of such complexes. Further studies will have to determine whether these complexes exist in plasma or are formed during coagulation.
The activation of FXI by thrombin is a relatively inefficient process, which is potently stimulated by anionic polysaccharides such as dextran sulphate. Besides their contribution to feedback activation, anionic compounds like dextran sulphate also induce coagulation by activation of FXII. To our knowledge, FVa is the only reported cofactor that enhances feedback activation, without inducing FXII activation.
Factor Va enhances the activation of prothrombin by FXa more than 1,000-fold (17, 18). We here describe that the activation of FXI by thrombin is 2–4 times more efficient in the presence of FVa and a phospholipid surface, which is moderate compared to the role of FVa in the prothrombinase complex. However, small increases in the activation of FXI are amplified greatly by the intrinsic pathway and result in a shortening of the clotting time (9), as we show in plasma. Thus, a moderate increase in FXIa formation may have significant consequences for hemostasis.
In human plasma, most of the FXI circulates in a complex with high-molecular weight kininogen (HK) due to a strong binding interaction (14, 15). Similar to our findings with FV and FVa, this binding involves multiple apple domains of FXI. However, the light chain of HK is mainly bound by the apple2 domain of FXI (19), which is the only apple domain of FXI that did not display affinity for FVa. In a purified system, plasma concentrations of HK partially inhibit the cofactor activity of FVa. The presence of plasma concentrations of PK, which also circulates in complex with HK (19, 20), reverses this inhibition. Moreover, the cofactor role of FVa in the intrinsic pathway was demonstrated in plasma that contained normal levels of HK and PK.
What do our findings contribute to the understanding of the coagulation mechanism? Feedback activation of FXI leads to thrombin levels that exceed the amounts required for fibrin formation. At high thrombin concentrations, thrombin is able to activate TAFI. This carboxypeptidase helps to protect fibrin polymers from enzymatic degradation through removal of C-terminal lysine residues that are important in tPA-mediated plasminogen activation (21). Our studies suggest that the cofactor function of FVa in feedback activation of the intrinsic pathway plays a role in this mechanism by stimulating thrombin formation to levels that promote TAFI activation.
The most prevalent genetic risk factor for venous thrombosis is found in the gene that codes for FV (22), also known as the factor V Leiden mutation. Because of an Arginine to Glutamine substitution at position 506 in the FV heavy chain, the protein becomes resistant against degradation by APC (23). This causes loss of regulation of FV cofactor activity. Until now, the role of FV as a procoagulant protein was limited to direct enhancement of thrombin formation by FXa. We here show that FV is also indirectly important for thrombin formation, as it enhances the activation of FXI by thrombin. Similar to its role in the prothrombinase complex, this new function of FV is regulated by APC. Thus, in carriers of the factor V Leiden mutation, diminished regulation of intrinsic pathway activation can contribute to excess thrombin formation. This enhanced thrombin formation will ultimately lead to increased TAFI activation, and therefore to increased clot stability. Our findings offer an additional explanation for the elevated risk of thrombosis associated with the factor V Leiden mutation.
Materials and Methods
Proteins, Plasmas, Antibodies, and Chemicals.
Purified α-thrombin was purchased from Kordia (Leiden, The Netherlands), purified factors HK, Prekallikrein (PK), XI, XIa, and IXa were purchased from Calbiochem (San Diego, CA). Coagulation factors VIII (AaFact, Sanquin, CLB, Amsterdam, The Netherlands), B-domain-deleted FVIII (Refacto, Wyeth Pharmaceuticals, Maidenhead, UK) and FIX (Nonafact, Sanquin, CLB, Amsterdam, The Netherlands), tissue-type plasminogen activator (tPA [Actilyse], Boehringer Ingelheim Pharma GmbH & Co, Germany), pentasaccharide (Fondaparinux [Arixtra], GlaxoSmithKline, Zeist, The Netherlands), and hirudin (lepirudin [Refludan], Pharmion, Tiel, The Netherlands) were obtained from the local hospital pharmacy as pharmaceutical formulations. Factor V, factor Va and protein S were purchased from Haemtech Laboratories (Essex, VT). FX was purified from fresh-frozen plasma by immunoaffinity chromatography, followed by Q-Sepharose chromatography as described previously (24). Protein C was purified from fresh-frozen plasma by barium sulphate precipitation, followed by immunoaffinity chromatography and activated as published (25, 26). Citrated FXI-deficient plasma was purchased from George King Biomedical Inc. (Kansas, KS). Prothrombin-depleted plasmas were prepared by immunoaffinity chromatography, after which prothrombin levels were determined on a Behring Coagulation System (BCS) XP analyzer (Siemens Healthcare Diagnostics, Germany), or purchased from Haemtech Laboratories (Essex, VT). H-Gly-Pro-Arg-Pro-Amide (GPRP) was from Sigma-Aldrich (St. Louis, MO), Pefachrome XIa3371 and Pefachrome Xa were from Pentapharm (Basel, Switzerland). Monoclonal antibody RU-FX10F1 directed against FX, RU-PS7E9 directed against protein S, and RU-FV3B1 directed against FV were produced in-house. Polyclonal antibodies against antithrombin were from Cedarlane (Hornby, Canada). HRP-conjugated affinity purified donkey-anti-goat antibodies were from AbD Serotec (Düsseldorf, Germany). Individual apple domains of FXI and PK with a tPA-tag were produced recombinantly and purified as described earlier (27, 19). 1,2-Dioleyl-sn-glycero-3-phospho-L-serine (PS), 1,2-dioleyl-sn-glycero-3-phosphocholine (PC), and 1,2-dioleyl-sn-glycero-3 phosphoethanolamine (PE) were purchased from Sigma-Aldrich (St. Louis, MO). Phospholipid vesicles were prepared in a PS:PC:PE ratio of 20∶40∶40 and PS:PC vesicles were prepared in a ratio of 20∶80.
Activation of Factor XI by Thrombin in a Purified System.
The activation of FXI by thrombin was assayed by incubation of 30 nM FXI with 10 nM α-thrombin. Samples were taken and the reactions were terminated by inactivation of thrombin with 10 U/mL of hirudin. Effects of activated protein C were evaluated at a concentration of 5 nM. All assays were performed in 25 mM Hepes, 137 mM NaCl, 3.5 mM KCl, 3 mM CaCl2, 0.1% bovine serum albumin (m/v), pH 7.4 (coagulation buffer). The amount of formed FXIa was subsequently determined either directly by conversion of 0.5 mM Pefachrome XIa3371, or indirectly by the addition of FXIa-containing samples (diluted 1∶300) to a mixture with phospholipids (10 μM), FVIII (0.15 nM), FIX (45 nM), and FX (68 nM) and subsequent conversion of 0.4 mM Pefachrome Xa, a substrate for FXa. The FXIa concentration in each sample was deduced from a FXIa calibration series. Substrate conversion was measured in a Spectramax 340 microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 405 nm and 37 °C.
Thrombin Generation and Plasma Clotting Assay.
Thirty nM FXI was activated with 10 nM thrombin and 10 μM PS:PC:PE vesicles in the presence or absence of 30 nM FVa for 20 min, after which reactions were terminated with 10 U/mL hirudin. FVa incubated with thrombin served as a control. Samples were supplemented with FXI, FVa, or both, so each sample contained the same concentrations of coagulation factors. All reactions were performed in coagulation buffer.
Thrombin generation was assayed on a Fluoroskan Ascent microplate reader (Thermo Fisher Scientific, MA) with the Technothrombin Thrombin Generation Assay (TGA) kit and software, according to the manufacturer’s instructions (Technoclone GmbH, Vienna, Austria). Thrombin generation was initiated by addition of 33 μL diluted sample (1∶10 in-assay dilution) to 33 μL pooled normal plasma containing 1.5 mM thrombin substrate (z-Gly-Gly-Arg-AMC) and 33 μL coagulation buffer with 30 mM CaCl2 and 30 μM PS:PC:PE. The formation of thrombin was followed for 30 min and analyzed by comparison with a thrombin calibrator, according to the manufacturer’s instructions.
Coagulation times were recorded on KC10 coagulometer (Amelung, Lemgo, Germany). Coagulation was initiated by addition of 33 μL diluted sample (in-assay dilution 1∶10) to 33 μL pooled normal plasma and 33 μL coagulation buffer with 30 mM CaCl2 and 30 μM PS:PC:PE.
Surface Plasmon Resonance Analysis.
Surface plasmon resonance (SPR) binding assays were performed on a Biacore T100 system (GE Healthcare Europe, Diegem, Belgium). FV and FVa were immobilized on a Series-S C1 sensor chip (GE Healthcare Europe) with the amine-coupling kit in 10 mM sodium acetate (pH 5.0) according to the supplier’s instructions. A reference channel was activated and blocked in the absence of protein. Signals obtained from this channel were used to correct for residual binding to the chip. Binding was assessed in 10 mM Hepes, 137 mM NaCl, 5 mM CaCl2, 0.005% Tween-20, pH 7.4, with a flow rate of 50 μL/ min. Prior to each injection, hydrophobic interactions of analyte with the surface of the chip were prevented with a BSA injection, according to the instructions of the manufacturer. Bound proteins were eluted with a 30 μL injection of 1 mM NaOH in a 137 mM NaCl solution. Binding kinetics were analyzed with the Biacore T100 evaluation software, according to the 1∶1 Langmuir binding model. tPA-tagged apple domain constructs and PK were injected at a concentration of 25 nM until binding equilibrium was reached. Binding of tPA-tagged constructs was normalized to the molecular weight of individual apple domains. Signals obtained with tPA alone were considered background and were subtracted from all data.
Activation of the Intrinsic Pathway in Plasma.
Pooled normal plasma, prothrombin-deficient plasma, or FXI-deficient plasma was activated with 20 nM α-thrombin in the presence of 10 μM PS:PC:PE vesicles and 10 mM CaCl2. Fibrin polymerization was inhibited by addition of 3 mM GPRP, while inactivation of activated FX by antithrombin was stimulated with 20 μg/mL of pentasaccharide. All reagents were dissolved or diluted in coagulation buffer. Samples were taken in time series and thrombin activity was neutralized by four times dilution of the samples in 250 U/mL of hirudin in coagulation buffer. The role of FV was studied by depletion of FV from prothrombin-deficient plasma with a monoclonal antibody directed against FV (RU-FV3B1) that was covalently coupled to cyanogen bromide-activated Sepharose beads. Beads with a monoclonal antibody against protein S (RU-PS7E9) were used as a control. Plasma was incubated with these beads at room temperature under constant rotation for 1 h, after which the beads were removed by centrifugation. The effects of APC were studied in plasma at a concentration of 20 nM, a concentration that is commonly used in APC resistance tests for diagnostic purposes.
Activated Factor X—Antithrombin Complex Enzyme-Linked Immuno-Sorbent Assay (ELISA).
A murine monoclonal antibody that recognizes both FX and FXa (RU-FX10F1) was coated at 2 μg/mL in 10 mM NaHCO3 (pH 9.6) in 96-wells Nunc Maxisorp round bottom plates (Thermo Fisher, Rochester, NY), while shaking at room temperature for 1 h. Plates were blocked with 100 μL per well of 4% (m/v) bovine serum albumin in 50 mM Tris-Cl, 150 mM NaCl, pH 7.4 (TBS). Afterwards, plates were washed three times with TBS, 0.1% Tween-20. Wells were incubated in triplicate for 1 h at room temperature with 50 μL per well of the samples taken from the plasma activation procedure, described above. After incubation, the plates were washed three times and incubated with 50 μL/well of affinity-purified polyclonal sheep antibodies against antithrombin in TBS, 0.05% Tween-20. Plates were washed again and incubated with peroxidase-labeled polyclonal donkey-anti-sheep antibody in TBS with 0.05% Tween-20. Plates were stained with 100 μL/well of 3, 3′, 5, 5′-tetramethylbenzidine (TMB; Tebu-Bio, Le Perray-en-Yvelines, France), whereafter the reactions were stopped by addition of 50 μL/well of 2M H2SO4. Plates were read at 450 nm in a Spectramax 340 microplate reader. FXa-AT complex levels were deduced from a dilution series of human serum and expressed as a percentage of serum FXa-AT complex levels. To correct for interassay variability, levels of FXa-AT complexes in normal pooled plasma at 120 s after addition of thrombin were normalized to 100%.
Statistical Analysis.
Data were analyzed with Graphpad software v4.03 for Windows. Differences between conditions were analyzed with Student’s t test. Differences in FXa-AT complex levels were analyzed by two-way ANOVA.
Footnotes
The authors declare no conflict of interest.
References
- 1.Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 2.MacFarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 3.Renne T, et al. Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doggen CJ, Rosendaal FR, Meijers JC. Levels of intrinsic coagulation factors and the risk of myocardial infarction among men: Opposite and synergistic effects of factors XI and XII. Blood. 2006;108:4045–4051. doi: 10.1182/blood-2005-12-023697. [DOI] [PubMed] [Google Scholar]
- 5.Cichon S, et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am J Hum Genet. 2006;79:1098–1104. doi: 10.1086/509899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maas C, et al. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118:3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naito K, Fujikawa K. Activation of human blood coagulation factor XI independent of factor XII. Factor XI is activated by thrombin and factor XIa in the presence of negatively charged surfaces. J Biol Chem. 1991;266:7353–7358. [PubMed] [Google Scholar]
- 8.Gailani D, Broze GJ., Jr Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 9.von dem Borne PA, Meijers JC, Bouma BN. Feedback activation of factor XI by thrombin in plasma results in additional formation of thrombin that protects fibrin clots from fibrinolysis. Blood. 1995;86:3035–3042. [PubMed] [Google Scholar]
- 10.von dem Borne PA, et al. Thrombin-mediated activation of factor XI results in a thrombin-activatable fibrinolysis inhibitor-dependent inhibition of fibrinolysis. J Clin Invest. 1997;99:2323–2327. doi: 10.1172/JCI119412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedicord DL, Seiffert D, Blat Y. Feedback activation of factor XI by thrombin does not occur in plasma. Proc Natl Acad Sci USA. 2007;104:12855–12860. doi: 10.1073/pnas.0705566104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wielders SJ, Beguin S, Hemker HC, Lindhout T. Factor XI-dependent reciprocal thrombin generation consolidates blood coagulation when tissue factor is not available. Arterioscler Thromb Vasc Biol. 2004;24:1138–1142. doi: 10.1161/01.ATV.0000128125.80559.9c. [DOI] [PubMed] [Google Scholar]
- 13.Kravtsov DV, et al. Factor XI contributes to thrombin generation in the absence of factor XII. Blood. 2009;114:452–458. doi: 10.1182/blood-2009-02-203604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson RE, Mandle R, Jr, Kaplan AP. Association of factor XI and high molecular weight kininogen in human plasma. J Clin Invest. 1977;60:1376–1380. doi: 10.1172/JCI108898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson RE, Mandle R, Jr, Kaplan AP. Studies of binding of prekallikrein and Factor XI to high molecular weight kininogen and its light chain. Proc Natl Acad Sci USA. 1979;76:4862–4866. doi: 10.1073/pnas.76.10.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnaswamy S, Mann KG. The binding of factor Va to phospholipid vesicles. J Biol Chem. 1988;263:5714–5723. [PubMed] [Google Scholar]
- 17.Nesheim ME, Taswell JB, Mann KG. The contribution of bovine Factor V and Factor Va to the activity of prothrombinase. J Biol Chem. 1979;254:10952–10962. [PubMed] [Google Scholar]
- 18.Rosing J, et al. The role of phospholipids and factor Va in the prothrombinase complex. J Biol Chem. 1980;255:274–283. [PubMed] [Google Scholar]
- 19.Renne T, Gailani D, Meijers JC, Muller-Esterl W. Characterization of the H-kininogen-binding site on factor XI: a comparison of factor XI and plasma prekallikrein. J Biol Chem. 2002;277:4892–4899. doi: 10.1074/jbc.M105221200. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson VH, Kleniewski J, Saito H, Sayed JK. Prekallikrein deficiency in a kindred with kininogen deficiency and Fitzgerald trait clotting defect. Evidence that high molecular weight kininogen and prekallikrein exist as a complex in normal human plasma. J Clin Invest. 1977;60:571–583. doi: 10.1172/JCI108809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosnier LO, Bouma BN. Regulation of fibrinolysis by thrombin activatable fibrinolysis inhibitor, an unstable carboxypeptidase B that unites the pathways of coagulation and fibrinolysis. Arterioscler Thromb Vasc Biol. 2006;26:2445–2453. doi: 10.1161/01.ATV.0000244680.14653.9a. [DOI] [PubMed] [Google Scholar]
- 22.Vandenbroucke JP, et al. Increased risk of venous thrombosis in oral-contraceptive users who are carriers of factor V Leiden mutation. Lancet. 1994;344:1453–1457. doi: 10.1016/s0140-6736(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 23.Bertina RM, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64–67. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- 24.Hackeng TM, van ’t Veer C, Meijers JC, Bouma BN. Human protein S inhibits prothrombinase complex activity on endothelial cells and platelets via direct interactions with factors Va and Xa. J Biol Chem. 1994;269:21051–21058. [PubMed] [Google Scholar]
- 25.Kisiel W. Human plasma protein C: Isolation, characterization, and mechanism of activation by alpha-thrombin. J Clin Invest. 1979;64:761–769. doi: 10.1172/JCI109521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koedam JA, Meijers JC, Sixma JJ, Bouma BN. Inactivation of human factor VIII by activated protein C. Cofactor activity of protein S and protective effect of von Willebrand factor. J Clin Invest. 1988;82:1236–1243. doi: 10.1172/JCI113721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meijers JC, Mulvihill ER, Davie EW, Chung DW. Apple four in human blood coagulation factor XI mediates dimer formation. Biochemistry. 1992;31:4680–4684. doi: 10.1021/bi00134a021. [DOI] [PubMed] [Google Scholar]