Abstract
Dysregulation of programmed cell death due to abnormal expression of Bcl-2 proteins is implicated in cancer, neurodegenerative diseases, and heart failure. Among Bcl-2 family members, BNip proteins uniquely stimulate cell death with features of both apoptosis and necrosis. Localization of these factors to mitochondria and endoplasmic reticulum (ER) provides additional complexity. Previously, we observed regulation of intracellular calcium stores by reticular Nix. Here, we report effects of Nix targeting to mitochondria or ER on cell death pathways and heart failure progression. Nix-deficient fibroblasts expressing mitochondrial-directed or ER-directed Nix mutants exhibited similar cytochrome c release, caspase activation, annexin V and TUNEL labeling, and cell death. ER-Nix cells, but not mitochondrial-Nix cells, showed dissipation of mitochondrial inner membrane potential, Δψm, and were protected from cell death by cyclosporine A or ppif ablation, implicating the mitochondrial permeability transition pore (MPTP). ER-Nix cells were not protected from death by caspase inhibition or combined ablation of Bax and Bak. Combined inhibition of caspases and the MPTP fully protected against Nix-mediated cell death. To determine the role of the dual pathways in heart failure, mice conditionally overexpressing Nix or Nix mutants in hearts were created. Cardiomyocte death caused by mitochondrial- and ER-directed Nix was equivalent, but ppif ablation fully protected only ER-Nix. Thus, Nix stimulates dual autonomous death pathways, determined by its subcellular localization. Mitochondrial Nix activates Bax/Bak- and caspase-dependent apoptosis, whereas ER-Nix activates Bax/Bak-independent, MPTP-dependent necrosis. Complete protection against programmed cell death mediated by Nix and related factors can be achieved by simultaneous inhibition of both pathways.
Keywords: apoptosis, heart failure, mitochondrial permeability transition pore
Programmed cell death is necessary for life. Mitochondria play essential roles in both cell maintenance and programmed death, and disturbances in mitochondrial pathways regulating cell elimination have been causally linked to cancer, Alzheimer's disease, and heart failure (1–3). Recent advances have delineated different forms and mechanisms of programmed cell death: Caspase-dependent apoptosis is mediated through coordinated effects of multiple Bcl-2 family members (4). In contrast, programmed necrosis is mediated by opening of the mitochondrial permeability transition pore (MPTP) (5, 6). Thus, there appear to be at least two independent mitochondrial pathways to programmed cell death.
Interactions between proapoptotic Bcl-2 family proteins are numerous and complex. The “BNip” subgroup (BNip1, BNip2, BNip3, and Nix/BNip3L) is distinguished structurally by an atypical BH3-only domain that is dispensable for death-promoting effects, and functionally by induction of programmed cell death having features of both apoptosis and necrosis (7). One of these proteins, Nix/BNip3L (Nix), limits survival of, and mediates mitochondrial clearance from, erythroid cells (8–10) and is responsible for cardiomyocyte dropout and heart failure after chronic pressure overload (11, 12). We have postulated that functional diversity of Nix may relate to dual localization at mitochondria and endoplasmic reticulum (13).
It is thought that Bcl2 factors stimulate cell death by activating Bax and Bak, the multidomain pore-forming Bcl2 proteins considered to be the “mitochondrial gatekeepers” for apoptosis. Bax and Bak permeabilize mitochondrial outer membranes, releasing cytochrome c that activates caspases (14). Bax/Bak-mediated mitochondrial outer membrane permeabilization is restrained by antiapoptotic Bcl2 proteins that bind and sequester BH3-only and BNip proteins (4). This mechanism does not explain atypical cell death stimulated by BNip family members important in heart failure (11, 12, 15–17). Some have suggested that Nix directly activates the mitochondrial permeability transition (10, 18), but we have shown that recombinant Nix cannot open MPTP in isolated mitochondria (8). Thus, the mechanism for Nix-mediated programmed necrosis remains unknown, and the involvement of mitochondrial permeability transition is uncertain.
Here, we show that Nix-mediated necrotic cell death involves ER-mitochondrial crosstalk and is uniquely cyclophilin D-dependent, whereas mitochondrial Nix induces only caspase-dependent apoptosis. We also show that reticular Nix-mediated programmed necrosis does not require Bax or Bak, establishing a unique regulatory pathway for “proapoptotic” Bcl2 proteins.
Results
Mitochondrial- and Reticular-Directed Nix Activate Apoptosis Pathways and Are Equally Lethal.
Nix is found in mitochondria and ER, and Nix that is up-regulated in response to pressure overload cardiac hypertrophy preferentially localizes to sarcoplasmic reticulum (13). Because Nix gene ablation prevents ventricular remodeling in response to pressure overload (12), we considered that reticular Nix may have a special role in inducing reactive programmed cardiac myocyte death. To dissociate the organelle-specific effects of Nix, we generated Nix mutants specific to mitochondria or ER (13) and used adenoviral-mediated expression in primary fibroblasts derived from BNip3L/Nix null mouse embryos (MEFs) to evaluate Nix-dependent events leading to cell death. As described (13, 19, 20), wild-type (WT) Nix is visualized on SDS/PAGE as both a monomer and a dimmer (Fig. 1A). The majority of WT Nix expressed in Nix null MEFs cofractionates with the mitochondrial membrane marker, cytochrome oxidase IV (Cox-IV), whereas a minor amount is detected in ER-enriched membranes labeled by calnexin (Fig. 1A). In contrast, Nix-ActA (which migrates as a monomer) is found exclusively in the mitochondria-enriched fraction and Nix cb5 (also seen in monomeric form) cofractionates with ER (Fig. 1A). Confocal studies confirmed this localization (Fig. 1B). The consequences of Nix localization on programmed cell death were assessed by fluorescence of calcein-AM and ethidium homodimer-1 (which label live and dead cells, respectively) (Fig. 1 C and D Bottom), and by TUNEL staining (Fig. 1 D Upper and E). Compared with control adenovirus expressing β-galactosidase, WT Nix and both organelle-specific Nix mutants increased cell death and TUNEL to a similar degree.
Fig. 1.
Mitochondrial and reticular Nix are equally effective in causing in vitro cell death. (A) Immunoblot analysis of subcellular fractions: 10,000 × g pellet (10p), 100,000 × g pellet (100p), and 100,000 × g supernatant (S). (B) Confocal colocalization of Nix (green) with MitoFluor Red 589 (mito; Upper) or ER calnexin (ER; Lower) (both red). (Original magnification: ×600.) (C) Quantitative analysis of cell live/dead assay (n = 6 per group). (D) Representative TUNEL (green, with blue DAPI nuclear counterstain) and live (green) dead (red) images. (E) Quantitative TUNEL analysis (n = 6 per group). (Scale bars: 50 μm.) *, P < 0.05 vs β-gal control.
The in vivo consequences of organelle-specific Nix-mediated cell death were evaluated in cardiac-specific transgenic mice conditionally overexpressing WT, mitochondrial-, or reticular-Nix. Multiple independent lines were produced, each exhibiting similar phenotypes; those with comparable levels of protein expression were examined (Fig. 2A). Subcellular localization of Nix and its mutants in cardiomyocytes was identical to MEFs (Fig. 2B). Cardiomyocyte TUNEL positivity in all three mouse lines was increased to similar levels (Fig. 2C), and paralleled cardiac dilation (Fig. 2D), impaired contractile performance at baseline (Fig. 2E) and in response to β-adrenergic stimulation (Fig. 2F), and altered expression of heart failure-associated fetal genes (Fig. S1). Thus, differential localization of Nix to mitochondria or ER does not impact its lethality or ability to activate apoptosis signaling.
Fig. 2.
Mitochondrial and reticular Nix are equally effective in causing in vivo cardiomyopathy. (A) Nix immunoblot analysis of heart homogenates. (B) Mutant Nix localization to cardiac subcellular fractions. (C) TUNEL assays (n = 5 hearts per group). (D) Echocardiographic left ventricular end-diastolic dimension. (E) Echocardiographic left ventricular fractional shortening (n = 26 tet off controls, n = 6 Nix-WT, n = 12 Nix-ActA, n = 10 Nix cb5). (F) Left ventricular peak positive dP/dt as a function of dobutamine infusion dose (n = 9 tet off controls, n = 5 Nix-WT, n = 5 Nix-ActA and n = 4 Nix cb5). *, P < 0.05 compared with tet off control.
Cell Death Induced by Reticular-Directed Nix Is Caspase Independent and Mediated Through the MPTP.
We further interrogated mechanisms of cell death induced by mitochondrial and reticular Nix by measuring activation of components within the intrinsic apoptosis pathway. Mitochondrial-directed and ER-directed Nix MEFs exhibited cytochrome c release, caspase activation, and phosphatidylserine exposure (annexin V staining) indistinguishable from MEFs expressing WT Nix (Fig. 3A). Caspase inhibition with zVAD.fmk had little protective effect on annexin V (Fig. 3B) or TUNEL positivity (Fig. 3C) in WT or Nix cb5-expressing cells, and only partially prevented their cell death (Fig. 3D, black bars). In striking contrast, caspase inhibition completely prevented apoptosis and cell death in mitochondrial Nix ActA-expressing cells (Fig. 3 B–D, black bars). Thus, cell death stimulated by mitochondrial Nix occurs exclusively via activation of the mitochondrial apoptosis pathway. In contrast, cell death mediated by reticular Nix can proceed independently of caspases.
Fig. 3.
Mitochondrial and reticular Nix cause cell death by different mechanisms. (A) Confocal imaging of: cytochrome c (green) and mitochondrial (red) colocalization (Top), caspase activity (Middle) and annexin V labeling (Bottom). (B–D) Group analysis of Annexin V labeling (B), TUNEL staining (C), and cell death (D) as a function of ZVAD-FMK (black bars) or cyclosporin A (gray bars) treatment (n = 6 per group). (E) Confocal imaging of annexin V (green) and TMRE (red) staining (Top), and effects of ZVAD-FMK (Middle) or cyclosporin A (Bottom). (F) Group analysis of live/dead, Annexin V, and TUNEL labeling in Nix-WT expressing MEFS and effects of combined caspase and MPTP inhibition. (Scale bars: 50 μm.)
Programmed cell death involving the mitochondrial permeability transition might explain the above findings, but we previously observed that recombinant Nix applied directly to isolated mitochondria does not open MPTPs (8). To determine whether Nix has an indirect effect on MPTP opening seen only in intact cells, we monitored the inner mitochondrial membrane potential, Δψm, with the voltage-sensitive dye tetramethylrhodamine ethyl ester (TMRE). Annexin V labeling was assessed simultaneously as a measure of apoptosis activation. Nix null MEFs expressing β-galactosidase exhibited robust TMRE fluorescence and no annexin V staining, whereas WT Nix expressing MEFs showed dissipation of Δψm (loss of TMRE fluorescence) and positive annexin V labeling (Fig. 3E Top). Cells expressing mitochondrial Nix ActA did not show dissipation of Δψm, but were nevertheless positive for annexin V (Fig. 3E Top), showing that mitochondrial Nix activates apoptotic pathways without opening the MPTP. An indirect effect of reticular Nix on the MPTP was revealed by dissipation of Δψm and annexin V positivity in Nix null MEFs expressing ER Nix cb5 (Fig. 3E Top). MPTP opening in Nix-expressing cells was not a secondary consequence of apoptosis, as caspase inhibition with ZVAD-fmk attenuated annexin V labeling in cells expressing all three forms of Nix, but had no effect on dissipation of Δψm by WT and Nix cb5 (Fig. 3E Middle). It is likely that WT or Nix cb5 indirectly stimulate the mitochondrial permeability transition via ER-mitochondrial crosstalk (13, 21).
We next determined the role of MPTP opening in cell death mediated by reticular Nix. Opening of the MPTP depends on cyclophilin D, which is encoded by the ppif gene and pharmacologically blocked by cyclosporin A. Cyclosporin A prevented dissipation of Δψm in WT and Nix cb5-expressing cells (Fig. 3E Bottom), but only reticular Nix cb5-expressing MEFs were completely protected from apoptosis activation (Fig. 3 B and C, gray bars) and cell death (Fig. 3D, gray bars). Taken together, the above data show that mitochondrial Nix does not open MPTP, and cells expressing Nix-ActA are protected from death by caspase inhibition, but not cyclosporin A. In contrast, reticular Nix activates the MPTP, and cells expressing Nix cb5 are protected by cyclosporin A, but not caspase inhibition. Cells expressing WT Nix were incompletely protected by either cyclosporine A or the caspase inhibitor (Figs. 3 B–D), but were completely protected by concomitant application of both agents (Fig. 3F).
Cell Death Induced by Reticular-Directed Nix Does Not Require Bax and Bak.
Apoptosis induced by BH3-only factors is activated by cytochrome c released through pores formed by Bax and Bak in mitochondrial outer membranes (22). It is not known whether MPTP-dependent death induced by reticular-Nix shares a requirement for these multidomain proteins. To examine this notion, we expressed WT Nix, Nix-ActA, and Nix cb5 in MEFs lacking both Bax and Bak (23) and assayed events leading to apoptotic and MPTP-dependent cell death. In contrast to their effects in cells expressing Bax and Bak (see Figs. 1 and 3), expression of WT Nix and mitochondrial Nix-ActA in cells lacking Bax and Bak failed to induce cytochrome c release (Fig. 4A Upper) and annexin V labeling (Fig. 4B Upper). Strikingly, absence of Bax and Bak had no effect on dissipation of Δψm, activation of apoptosis, or promotion of cell death by reticular Nix cb5 (Fig. 4 B Upper and Fig. 4C). Cyclosporin A protected Nix cb5 bax/bak double null cells from dissipation of Δψm and activation of apoptosis (Fig. 4 A Bottom and B Bottom). These data reveal that ER-associated Nix stimulates MPTP-dependent death that does not require Bax and Bak.
Fig. 4.
Combined bax/bak ablation protects mitochondrial-, but not reticular-, Nix-expressing cells from death. (A) Confocal imaging of cytochrome c (green) and mitochondrial (red) colocalization in bax-bak double null MEFs expressing Nix and Nix mutants. (Top) Vehicle. (Bottom) Cyclosporin A-treated. (B) Confocal imaging of annexin V staining (green) and TMRE (red) in identically treated cells. (C) Group data for cell death (white bars), annexin V labeling (black bars), and TUNEL positivity (gray bars) in bax-bak double null MEFs expressing Nix mutants (n = 6 per group). (Scale bars: 50 μm.) *, P < 0.05 vs. identically treated β-gal controls.
Cyclophilin D Ablation Prevents in Vitro Cell Death and in Vivo Cardiomyopathy Induced by Reticular-Directed Nix.
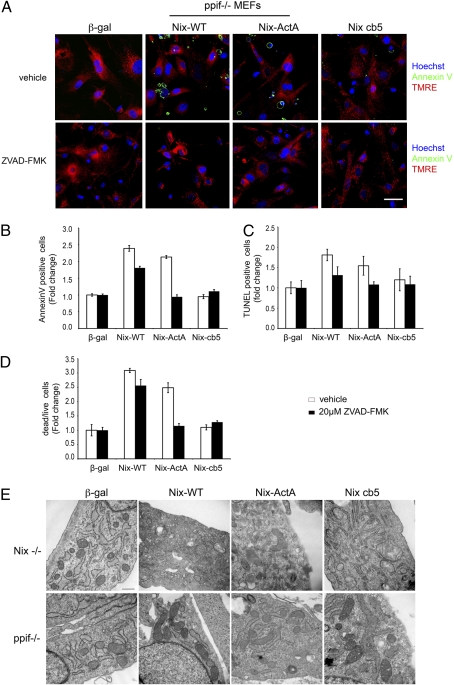
Cyclosporin A has effects that are not specific for cyclophilin D. Therefore, we also used a genetic approach to MPTP inhibition. Ppif ablation preserved Δψm in cells expressing all three forms of Nix (Fig. 5A Upper), but only reticular Nix cb5-expressing cells were protected from activation of apoptosis and cell death (Fig. 5 A Upper and B–D, open bars). Addition of ZVAD-fmk protected mitochondrial Nix-ActA cells (Fig. 5 A Lower and B–D, filled bars). These studies indicate that MPTP opening is not required for cell death by apoptosis induced by mitochondrial Nix but is indispensible for cell death mediated by reticular Nix.
Fig. 5.
Ppif ablation protects against death induced by reticular Nix. (A) Confocal imaging of annexin V (green) and TMRE (red) in ppif null MEFs expressing Nix and Nix mutants (Upper) and in cells treated with caspase inhibitor ZVAD-FMK (Lower). (B–D) Group analysis of annexin V staining (B), TUNEL labeling (C), and live/dead assay (D) in Nix-expressiing ppif null MEFs with or without ZVAD-FMK treatment. (Scale bars: 50 μm.) (E) Transmission electron microscopy demonstrating mitochondrial degeneration in Nix and Nix-cb5 expressing Nix null MEFs (Upper), which did not occur in ppif null MEFs (Lower).
Changes in mitochondrial structure induced by the Nix mutants paralleled those of death signaling. Transmission electron microscopy revealed that WT Nix and Nix cb5, i.e., the two forms of Nix that could localize to endoplasmic reticulum, induced mitochondrial swelling, outer membrane disruption, and lamellar degeneration, whereas mitochondria appeared more normal in Nix ActA-expressing cells (Fig. 5E Upper). Mitochondrial degeneration induced by reticular Nix was prevented by ablation of ppif (Fig. 5E Lower).
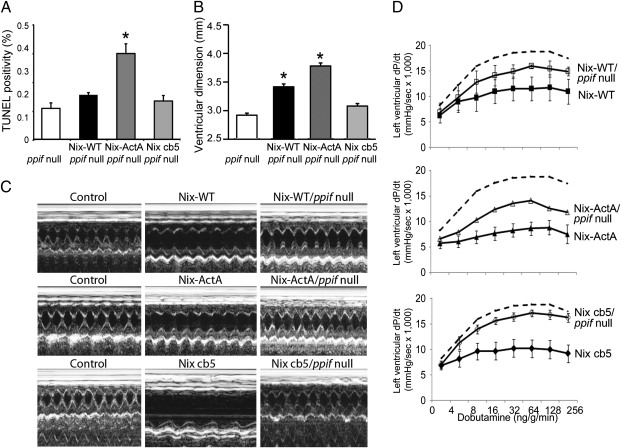
We used genetic complementation to examine apoptotic and MPTP-dependent cell death in the Nix cardiomyopathies by breeding the Nix and Nix mutant cardiac transgenic mice onto the ppif null background. Absence of ppif did not significantly reduce TUNEL positivity in mitochondrial Nix-ActA hearts, whereas TUNEL labeling was normalized in the reticular Nix cb5 hearts (Fig. 6A). Likewise, cardiac dilation was not improved in mitochondrial Nix mice but was prevented in reticular Nix mice (Fig. 6 B and C). Absence of ppif normalized contractility and adrenergic responsiveness only in reticular-Nix mice (Fig. 6D). Indeed, ppif ablation eliminated all signs of heart failure in reticular-directed Nix cb5 transgenic mice. For each of these endpoints, ppif ablation was partially protective in WT Nix-expressing hearts.
Fig. 6.
Cyclophilin D (ppif) ablation rescues the cardiomyopathy induced by reticular, but not mitochondrial, Nix. (A) Cardiomyocyte TUNEL positivity in mice expressing WT Nix, Nix-ActA, or Nix cb5 on a ppif null background. (B) Cardiac enlargement measured as echocardiographic left ventricular end-diastolic diameter. (C) Representative m-mode echocardiograms of control. Nix mutant mice, and Nix mutant mice on Cyclophilin D null background. (D) Left ventricular peak positive dP/dt response to i.v. dobutamine in the same groups of mice. Control data (dotted line) are replotted from Fig. 2. *, P < 0.05 vs. ppif null.
Our cell culture studies suggested that the mechanism for differential cardiac effects of mitochondrial and reticular localized Nix should be activation of separate apoptotic and programmed necrotic death pathways by Nix ActA and Nix cb5, respectively. However, because TUNEL labeling is positive in both processes (due to release of cytochrome c by both Nix ActA-mediated mitochondrial outer membrane permeabilization and Nix cb5-mediated opening of the permeability transition pore), this assay is not discriminatory. Therefore, we stained myocardial sections for complement 9 (C5b9), a component of the membrane attack complex that is more specific for cell and tissue necrosis (24). Cardiomyocyte C5b9 labeling was prominent in WT Nix- and Nix cb5-expressing hearts, but was rare in Nix ActA hearts (Fig. 7A). Strikingly, ppif ablation completely eliminated cardiomyocyte C5b9 labeling in WT Nix and Nix cb5 hearts (Fig. 7A).
Fig. 7.
Cyclophilin D (ppif) ablation prevents cardiomoycyte necrosis and mitochondrial degeneration induced by reticular Nix. (A) Anti-C5b-9 staining shows prevalent cardiomyocyte necrosis in Nix, and Nix-cb5 expressing myocardium (Upper Left) that is prevented by concomitant ppif ablation (Lower Left). Quantitative group data are shown at Right. (B) Transmission electron microscopy showing mitochondrial abnormalities in Nix and Nix-cb5 cardiomyocytes (Upper) that are prevented by ppif ablation (Lower). (Scale bar: 300 μm.)
If the proposed mechanism by which differential Nix subcellular targeting induces distinct forms of programmed cell death is correct, then myocardial mitochondrial morphology should reflect the distinct actions and consequences of Nix and its organelle-specific mutants, as shown in the cell culture studies (Fig. 5E). Indeed, ultrastructural studies showed markedly more severe mitochondrial degeneration and outer membrane disruption in WT Nix and Nix cb5 hearts than Nix ActA myocardium, which exhibited only mild dilation of cristae (Fig. 7B Upper). Furthermore, concomitant ppif ablation corrected the mitochondrial abnormalities in WT Nix and Nix cb5 hearts without improving the appearance of Nix ActA cardiac mitochondria (Fig. 7B Lower).
Discussion
Here, we describe a mitochondrial pathway to programmed cell death initiated by Bcl2 family members that does not require Bax or Bak, but instead is induced by ER-mitochondrial crosstalk and activation of an alternate “mitochondrial gateway”, the mitochondrial permeability transition pore. Partitioning of Bcl-2 proteins to ER in addition to mitochondria was first recognized by Scorrano et al (23). Identification of other Bcl-2 proteins in the ER was subsequently described, and ER-mitochondrial crosstalk mediated by BH3-only proteins PUMA and Bim was recently reported to activate the mitochondrial apoptosis machinery via ER stress pathways (25). However, the observation that ER-localized Nix activates a cell death pathway that does not depend on Bax and Bak is, we believe, unique, and adds another layer of complexity to regulation of programmed cell death by Bcl-2 family proteins: (i) Apoptosis can be stimulated directly by Bax/Bak-dependent mitochondrial outer membrane permeabilization (14); (ii) apoptosis can be stimulated indirectly through a recently described ER stress, unfolded protein response mechanism that also requires Bax and Bak (25, 26); and (iii) necrosis can be induced through Bax/Bak-independent, ER-directed opening of the mitochondrial permeability transition pore.
The original clinical observations of increased cardiomyoyte apoptosis in heart failure were made more than a decade ago (27, 28). Subsequent experimental models identified the mechanisms that regulate apoptosis in stressed hearts and demonstrated that cardiomyocyte apoptosis can be sufficient to produce heart failure (29–31). In these and other studies, caspase activation and positive TUNEL staining are too often interpreted as evidence for apoptotic cell death; the observation of apoptosis pathway activity was assumed to show that apoptotic death was the primary mechanism. However, caspase inhibition has been incompletely effective in preventing heart failure, even in a Nix-dependent model wherein “apoptosis” is known to be the primary mechanism (32). Greater understanding of multiple mechanisms for programmed cell death mandates a re-evaluation of these findings. Published data have suggested both involvement and independence of the MPTP in cytochrome c-dependent caspase activation (6, 33–35). The current results clearly demonstrate bidirectional crosstalk between apoptotic and necrotic programmed death pathways and describe two autonomous pathways. Conventional Bax/Bak-dependent apoptosis is mediated by cytochrome c release and caspase activation. In contrast, MPTP-dependent necrosis can proceed independently of Bax/Bak and caspases but is nevertheless associated with secondary caspase activation due to physical mitochondrial disruption.
Nix is up-regulated during cardiac hypertrophy (11, 16) and is both necessary and sufficient to cause the transition from pressure overload hypertrophy to dilated cardiomyopathy (12, 15). Nix is closely related to, and shares the atypical functional characteristics of, BNip3, which is transcriptionally increased by cardiac ischemia and mediates postinfarction cardiac remodeling (16, 17). It is therefore likely that mechanisms of Nix-mediated cell death apply also to BNip3 and other members of the BNip family. However, Nix-mediated cell death is independent of BNip3, as shown in by lethal effects of Nix in MEFs derived from BNip3 null mice (ref. 17) (Fig. S2). Our findings that Nix autonomously activates independent apoptosis and necrosis pathways, and that Nix-induced programmed cell death therefore progresses despite inhibition of either individual pathway, explain previously observed incomplete effects of caspase inhibition alone (32) or MPTP inhibition alone (this study) in Nix-mediated cardiac disease. Because reticular localization and atypical death are features of each of the BNip family members, similar dual death pathway stimulation should be considered in any disease caused by programmed cell death attributable to dysregulation of these factors.
Materials and Methods
Studies of Mouse Embryonic Fibroblasts.
MEFs were isolated from 15.5- to 16.5-day embryos of germ-line Nix and ppif knockout mice (6, 8). bax/bak double null MEFs were described (14). For confocal studies, MEFs (5 × 104 cells per well) were plated on chamber slides and infected with recombinant adenoviri (100 PFUs per cell) and studied at 36 or 48 h.
For live cell studies, Δψm was monitored with TMRE (200 nM; Invitrogen) and caspase-3 activity with caspase-3 substrate (5 μM, NucViewTM 488 Caspase-3 assay; Biotium) with Hoechst 33342 dye (Invitrogen) nuclear staining. Cyclosporin A (20 μM; Sigma) or the general caspase inhibitor Z-VAD-FMK (20 μM; BD Pharmingen) were added concomitant with adenoviral infection. Cell death was analyzed with the Live/Dead Assay from Invitrogen.
Fixed cell studies used anti-BNip3L (1:1,000 dilution; Abcam), cytochrome c (1:200 dilution; Abcam), and rabbit polyclonal anti-calnexin (1:100 dilution, Santa Cruz Biotechnology) labeled with either Alexa Fluor 488-labeled goat anti-mouse IgG (1:500 dilution, Invitrogen) or Alexa Fluor 546-labeled goat anti-rabbit IgG (1:500 dilution, Invitrogen). MitoTracker RedCMRos (Invitrogen) labeled mitochondria and DAPI (Vector Laboratories) labeled cell nuclei, respectively. TUNEL staining used the DeadEnd Fluorometric TUNEL assay (Promega). Myocardial necrosis was visualized with anti-C5b-9 (Abcam) and costained with Alexa Fluor 546-phalloidin (Invotrogen). Fluorescent images were obtained on a Nikon C1si D- eclipse Confocal microscope system and camera (Nikon Instruments) with a Nikon plan Apro VC ×60/1.40 oil objective.
For high throughput analysis of cell death, Annexin V staining, caspase activation, and TUNEL positivity, MEFs were plated on Microtest 96-well assay plates at density of 1 × 104 cells overnight and infected with adenoviri for 36 h. Fluorescence was monitored on a Spectra MAX M5 (Molecular Devices). Ex/Em settings for various assays were as follows: Live/Dead Assay (Invitrogen) 494/517 nm for Calcein AM and 528/617 nm for EthD-1, 495/519 nm for Annexin V Alexa Fluor 488 (Invitrogen) staining, 488/520 nm for NucView 488 Caspase-3 assay (Invitrogen), and 350/461 nm for Hoechst 33342 (Invitrogen).
Ultrastructural Studies.
Myocardial or cell specimens were immersed overnight in Karnovsky's fixative, embedded, ultra-thin sectioned (900 A), and stained with uranyl acetate and lead citrate. Sections were viewed on a Philips/FEI Morgagni electron microscope at ×8,900 or ×14,000 direct magnification.
Subcellular Fractionation Studies.
Homogenized MEFs or ventricular tissues were centrifuged at 3,800 × g for 10 min to remove nuclei and myofibrils. The supernatant was centrifuged at 10,000 × g for 10 min (4 °C), to obtain a mitochondrial-enriched pellet, designated 10p. The supernatant was centrifuged at 100,000 × g for 1 h (4 °C). The supernatant from this spin was labeled the S fraction. To obtain an ER-rich and mitochondrial-free membrane fraction, the pellet was resuspended and separated on a discontinuous sucrose gradient (15, 30, and 35%) at 132,000 × g for 16.5 h (4 °C). The microsomal enriched fraction was in the 15%/35% interface. Proteins from the fractions were size-separated on SDS/PAGE gels and immunoblotted with antibodies against BNip3L (Abcam), COX IV (Abcam), and calnexin (Santa Cruz Biotechnology).
Generation and Characterization of Nix Transgenic Mice.
Mice conditionally expressing cardiomyocyte Nix (wild type: Nix-WT) have been described (15). Nix-ActA and Nix cb5 mice used the same doxycycline-suppressible α-myosin heavy chain-driven system. Two founder lines for Nix-ActA and three founder lines for Nix cb5 were obtained, with similar phenotypes (Table S1 and Fig. S3). Mice were housed and studied according to procedures approved by Animal Studies Committee at Washington University School of Medicine. M-mode echocardiography, invasive hemodynamic studies, and TUNEL analyses were performed as described (12).
Statistical Analysis.
Data are mean ± SEM. Student's t test was used for paired comparisons, and ANOVA was used with Tukey's post hoc test for multiple comparisons. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We acknowledge Robert A. Santoianni for the electron microscopy. This work was supported by grants from the National Institutes of Health (to G.W.D.), American Heart Association Scientist Development (to A.D.), and the Veterans Administration (to A.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary article on page 9031.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914013107/DCSupplemental.
References
- 1.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 2.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–558. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- 4.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 5.Newmeyer DD, Ferguson-Miller S. Mitochondria: Releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 6.Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 7.Chinnadurai G, Vijayalingam S, Gibson SB. BNIP3 subfamily BH3-only proteins: Mitochondrial stress sensors in normal and pathological functions. Oncogene. 2008;27(Suppl 1):S114–S127. doi: 10.1038/onc.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diwan A, et al. Unrestrained erythroblast development in Nix-/- mice reveals a mechanism for apoptotic modulation of erythropoiesis. Proc Natl Acad Sci USA. 2007;104:6794–6799. doi: 10.1073/pnas.0610666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweers RL, et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci USA. 2007;104:19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandoval H, et al. Essential role for Nix in autophagic maturation of erythroid cells. Nature. 2008;454:232–235. doi: 10.1038/nature07006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yussman MG, et al. Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat Med. 2002;8:725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 12.Diwan A, et al. Nix-mediated apoptosis links myocardial fibrosis, cardiac remodeling, and hypertrophy decompensation. Circulation. 2008;117:396–404. doi: 10.1161/CIRCULATIONAHA.107.727073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diwan A, et al. Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Invest. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei MC, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syed F, et al. Physiological growth synergizes with pathological genes in experimental cardiomyopathy. Circ Res. 2004;95:1200–1206. doi: 10.1161/01.RES.0000150366.08972.7f. [DOI] [PubMed] [Google Scholar]
- 16.Gálvez AS, et al. Distinct pathways regulate proapoptotic Nix and BNip3 in cardiac stress. J Biol Chem. 2006;281:1442–1448. doi: 10.1074/jbc.M509056200. [DOI] [PubMed] [Google Scholar]
- 17.Diwan A, et al. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007;117:2825–2833. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vande Velde C, et al. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20:5454–5468. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen G, et al. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J Exp Med. 1997;186:1975–1983. doi: 10.1084/jem.186.12.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novak I, et al. Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 2010;11:45–51. doi: 10.1038/embor.2009.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 22.Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304:437–444. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- 23.Scorrano L, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 24.Doran JP, Howie AJ, Townend JN, Bonser RS. Detection of myocardial infarction by immunohistological staining for C9 on formalin fixed, paraffin wax embedded sections. J Clin Pathol. 1996;49:34–37. doi: 10.1136/jcp.49.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klee M, Pallauf K, Alcalá S, Fleischer A, Pimentel-Muiños FX. Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. EMBO J. 2009;28:1757–1768. doi: 10.1038/emboj.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamarca V, Scorrano L. When separation means death: Killing through the mitochondria, but starting from the endoplasmic reticulum. EMBO J. 2009;28:1681–1683. doi: 10.1038/emboj.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narula J, et al. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 28.Olivetti G, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 29.Adams JW, et al. Enhanced Galphaq signaling: A common pathway mediates cardiac hypertrophy and apoptotic heart failure. Proc Natl Acad Sci USA. 1998;95:10140–10145. doi: 10.1073/pnas.95.17.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirota H, et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999;97:189–198. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 31.Wencker D, et al. A mechanistic role for cardiac myocyte apoptosis in heart failure. J Clin Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayakawa Y, et al. Inhibition of cardiac myocyte apoptosis improves cardiac function and abolishes mortality in the peripartum cardiomyopathy of Galpha(q) transgenic mice. Circulation. 2003;108:3036–3041. doi: 10.1161/01.CIR.0000101920.72665.58. [DOI] [PubMed] [Google Scholar]
- 33.Marzo I, et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 34.Eskes R, et al. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Ahsen O, et al. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J Cell Biol. 2000;150:1027–1036. doi: 10.1083/jcb.150.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.