Abstract
Secretion of glucocorticoid hormones during stress produces an array of physiological changes that are adaptive and beneficial in the short term. In the face of repeated stress exposure, however, habituation of the glucocorticoid response is essential as prolonged glucocorticoid secretion can produce deleterious effects on metabolic, immune, cardiovascular, and neurobiological function. Endocannabinoid signaling responds to and regulates the activity of the hypothalamic–pituitary–adrenal (HPA) axis that governs the secretion of glucocorticoids; however, the role this system plays in adaptation of the neuroendocrine response to repeated stress is not well characterized. Herein, we demonstrate a divergent regulation of the two endocannabinoid ligands, N-arachidonylethanolamine (anandamide; AEA) and 2-arachidonoylglycerol (2-AG), following repeated stress such that AEA content is persistently decreased throughout the corticolimbic stress circuit, whereas 2-AG is exclusively elevated within the amygdala in a stress-dependent manner. Pharmacological studies demonstrate that this divergent regulation of AEA and 2-AG contribute to distinct forms of HPA axis habituation. Inhibition of AEA hydrolysis prevented the development of basal hypersecretion of corticosterone following repeated stress. In contrast, systemic or intra-amygdalar administration of a CB1 receptor antagonist before the final stress exposure prevented the repeated stress-induced decline in corticosterone responses. The present findings demonstrate an important role for endocannabinoid signaling in the process of stress HPA habituation, and suggest that AEA and 2-AG modulate different components of the adrenocortical response to repeated stressor exposure.
Keywords: corticosterone, endocannabinoid, habituation, hypothalamic–pituitary–adrenal axis, amygdala
The release of glucocorticoids in response to stressful stimuli is necessary for an organism to respond appropriately to a threat at hand and subsequently to restore homeostasis. Persistent glucocorticoid secretion, however, can result in deleterious effects on cardiovascular, immune, metabolic, and neural systems (1). Therefore, it is beneficial for an individual to avoid inappropriate or unnecessary glucocorticoid secretion. For example, reduced glucocorticoid mobilization in response to stressors that have been previously experienced, but not to novel stressors, is an essential form of plasticity (called habituation) that avoids needless secretion but maintains the ability to mount a hormonal response. An inability to appropriately adapt to chronic stress is associated with a plethora of disease states, such as depression, cardiovascular disease, and metabolic syndrome (1–3).
Preclinical studies have helped to elucidate the neurobiological mechanisms involved in stress adaptation. Relative to novel presentation of an acute stressor, repeated exposure to the same (homotypic) stimulus has been found to result in a decrement in both neuronal activation of the corticolimbic circuit regulating activation of the hypothalamic–pituitary–adrenal (HPA) axis and the synthesis and release of glucocorticoid hormones from the adrenals (4–6). As such, current theories of stress adaptation suggest that a systems-level attenuation of neuronal responses to stressful stimuli within sensory–limbic regions of the brain produces the resultant habituation of HPA axis activation and glucocorticoid secretion (4, 5). However, the central mechanisms involved in stress habituation remain largely enigmatic.
In recent years, the endocannabinoid system has emerged as an important regulator of the stress response and as a candidate mediator of the process of stress adaptation (7, 8). The endocannabinoid system is a neuroactive lipid signaling system, which is composed of two arachidonate derived ligands, anandamide (AEA) and 2-arachidonoylglycerol (2-AG; an extensive review of the biochemical properties of endocannabinoid signaling is provided in ref. 9). These ligands primarily signal through cannabinoid CB1 receptors, which are localized on axonal processes to inhibit terminal calcium influx and neurotransmitter release (10). Although the distinct functional roles of AEA and 2-AG have yet to be fully elucidated, we and others have hypothesized that, based on their differential biosynthetic/catabolic pathways and pharmacodynamic properties, AEA may subserve more of a “tonic-like” mechanism, whereas 2-AG more a “burst-like” mechanism for CB1 receptor activation (9, 11). CB1 receptors are largely distributed on GABAergic and glutamatergic terminals, further underscoring endocannabinoid signaling as an important regulator of synaptic weighting to modulate the axonal release of both excitatory and inhibitory neurotransmitters (10).
The endocannabinoid system has been elucidated as a critical regulator of the stress response through its ability to modulate the sensitivity and activation of the HPA axis (11, 12). Disruption of endocannabinoid signaling increases the activity of the HPA axis (13, 14), which could involve a loss of inhibitory regulation of excitatory neurotransmission within the neural stress circuit (15). A growing body of evidence suggests that endocannabinoid signaling also contributes to the process of stress habituation (8). CB1 receptor blockade has been found to reverse the decline in neuronal activation within corticostriatal regions in response to repeated stress exposure (16), whereas CB1 receptor knockout mice display inappropriate behavioral adaptation to repeatedly presented stressors (17, 18).
To date, no studies have conclusively demonstrated the sites and requirements for AEA and 2-AG to mediate the expression of stress HPA habituation. We combined systemic and central pharmacological manipulations of endocannabinoid signaling in animals exposed to repeated restraint stress. The results demonstrate that repeated stress differentially alters AEA and 2-AG signaling in the brain, and that both of these endocannabinoids are critical adaptation of the HPA axis to repeated stress to occur.
Results
Stress HPA Habituation.
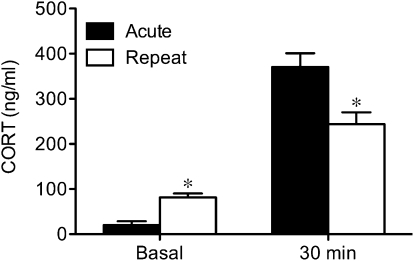
In a preliminary study, we compared the decline in plasma corticosterone responses to 30 min of repeated restraint on days 3, 5, 7, or 9 of exposure. Relative to the acute response on day 1, the absolute decline in the corticosterone response was most reliable and maximal by day 9 (P < 0.01). Thus, all subsequent studies used 9 daily episodes of 30-min restraint. As illustrated in Fig. 1, this repeated restraint paradigm produced a significant decline in plasma corticosterone responses to restraint relative to acute stress (P < 0.01), as well as a significant increase in basal (time 0) plasma corticosterone concentrations (P < 0.03) relative to unstressed animals.
Fig. 1.
Repeated restraint causes increase in basal corticosterone secretion and decrease in magnitude of corticosterone response. Mean (±SEM) corticosterone (CORT; ng/mL) concentrations in circulation on first (Acute) and last (Repeat) day of restraint under basal conditions and at 30 min of exposure. *P < 0.05 vs. acute condition (n = 5/group).
Differential Influence of Repeated Restraint on AEA and 2-AG Content.
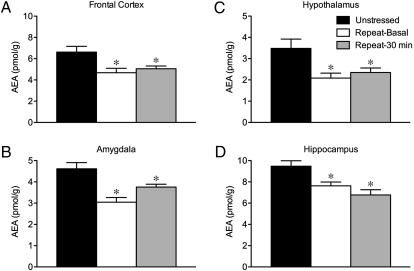
To determine whether adaptations in the HPA axis following repeated stress are associated with alterations in endocannabinoid signaling throughout the corticolimbic brain regions implicated in stress adaptation, we compared AEA and 2-AG content within this circuit (composed of the amygdala, hypothalamus, prefrontal cortex, hippocampus, and thalamus) under conditions of no stress, immediately after the last bout of restraint, or under basal conditions 24 h after final stress exposure. With respect to AEA (Fig. 2), significant effects of stress were seen in the amygdala (F2,23 = 11.99, P < 0.005), hypothalamus (F2,23 = 5.70, P < 0.02), prefrontal cortex (F2,23 = 5.73, P < 0.02), and hippocampus (F2,23 = 8.97, P < 0.005). However, there was no significant effect of stress on AEA content in the thalamus (F2,23 = 0.83, P > 0.05). Mean ± SEM tissue content levels (pmol/g protein) for AEA in the thalamus of unstressed animals, and at 30 min and 24 h after the last bout of repeated restraint were 3.7 ± 0.4, 3.2 ± 0.3, and 3.3 ± 0.4, respectively.
Fig. 2.
Repeated restraint causes decrease in anandamide signaling throughout corticolimbic circuit implicated in stress adaptation. Mean (± SEM) tissue content levels (pmol/g protein) for the endocannabinoid ligand anandamide (AEA) in frontal cortex (A), amygdala (B), hypothalamus (C), and hippocampus (D) in unstressed animals and in response to repeated restraint under basal conditions and at 30 min of exposure. *P < 0.05 vs. unstressed condition (n = 7–8/group).
As shown in Fig. 2, all of the structures showing changes in AEA content exhibited a common trend such that AEA content was reduced after repeated restraint, under both basal and stress conditions (P < 0.05 for all comparisons relative to the nonstress condition). Regression analyses of stress-induced corticosterone as a function of AEA levels during the last bout of repeated restraint failed to yield any significant correlations (P > 0.5 in all cases). However, analyses of basal corticosterone and AEA levels across unstressed and repeatedly stressed animals revealed a significant negative correlation in the prefrontal cortex (r = −0.64, P < 0.01), but not for any other brain region (P > 0.5, in all cases). This suggests that the increase in basal corticosterone secretion during repeated restraint could be accounted for by a decrease in AEA content within the prefrontal cortex.
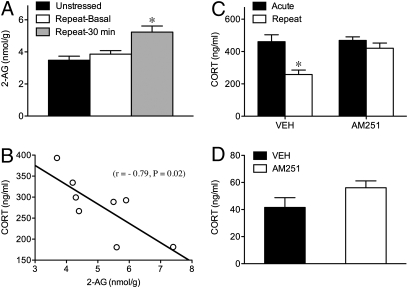
2-AG content, on the other hand, was unaffected by stress (Table S1) in the hypothalamus (F2,23 = 1.61, P > 0.05), prefrontal cortex (F2,23 = 0.03, P > 0.05), hippocampus (F2,23 = 0.63, P > 0.05), or thalamus (F2,23 = 0.13, P > 0.05). However, there was a pronounced effect of stress on 2-AG content in the amygdala (F2,23 = 7.55, P < 0.005). As illustrated in Fig. 3A, repeated stress produced a robust elevation in amygdalar 2-AG content immediately after the last episode of repeated restraint (P < 0.004), but not under basal conditions 24 h after repeated restraint exposure (P > 0.05). Furthermore, there was a significant negative correlation between 2-AG content in the amygdala and the magnitude of the corticosterone response to the last episode of restraint within individual animals (Fig. 3B). Thus, the decline in corticosterone responses to repeated restraint could be accounted for by an increase in 2-AG content in the amygdala.
Fig. 3.
Repeated restraint causes situation-specific increase in 2-AG signaling in the amygdala that is functionally related to the decline in corticosterone responses. Mean (±SEM) tissue content levels (nmol/g protein) for endocannabinoid ligand 2-arachidonoylglycerol (2-AG) in amygdala in unstressed animals and in response to repeated restraint under basal conditions and at 30 min of exposure (A). *P < 0.05 vs. unstressed and repeat basal conditions (n = 7–8/group). Simple regression analysis (B) of corticosterone (CORT) response on last day of restraint as a function of 2-AG content at 30 min of exposure to show a strong negative relationship between these variables. Mean (±SEM) corticosterone (ng/mL) concentrations in circulation on first (Acute) and last (Repeat) day of restraint at 30 min of exposure (C) and under basal conditions (D) in animals bearing acute injections of vehicle or AM251 into basolateral amygdala. P < 0.05 vs. acute condition (n = 6/group).
Endocannabinoid Signaling and Adaptation of the HPA Axis to Repeated Stress.
Based on the differential influence of repeated restraint on AEA and 2-AG content, as well as the opposing relations observed between these ligands and basal and stress-induced corticosterone secretion, respectively, we then examined HPA habituation in animals bearing systemic injections of compounds that inhibit AEA hydrolysis (URB597) and/or reuptake (AM404), or that block CB1 receptors (AM251). These pharmacological agents were used at doses sufficient to modulate endocannabinoid signaling (19, 20) but not to modulate HPA responses to acute stress exposure (SI Text and Fig. S1).
Basal corticosterone secretion.
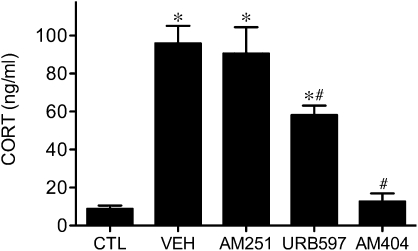
There was a significant interaction between repeated restraint and drug treatment (F4,20 = 26.72, P < 0.001) on basal corticosterone secretion. Relative to unstressed controls, animals that received vehicle or AM251 treatment daily before stress exposure showed a significant increase in basal corticosterone levels after 9 days of repeated restraint (Fig. 4). Conversely, the repeated stress induced increase in basal corticosterone was attenuated in animals treated with daily with URB597 and completely abolished in those treated with AM404. Taken together with the global decrease in AEA content observed after repeated restraint exposure (Fig. 2), the results suggest that the increased basal drive to the HPA axis requires a reduction in AEA signaling.
Fig. 4.
Facilitation of anandamine signaling, but not antagonism of CB1 receptors, attenuates repeated restraint-induced increase in basal corticosterone secretion. Mean (±SEM) corticosterone (CORT; ng/mL) concentrations in circulation under basal conditions in unstressed controls (CTL), and after 9 days of repeated restraint in animals bearing daily systemic injections of vehicle (VEH), AM251, URB597, or AM404. *P < 0.05 vs. unstressed controls; #P < 0.05 vs. vehicle- and AM251-treated animals (n = 5/group).
Stress-induced corticosterone secretion.
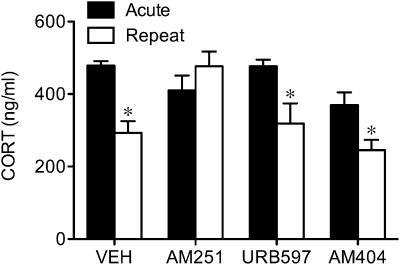
There was a significant interaction between repeated restraint and drug treatment (F3,19 = 3.71, P < 0.04) on the magnitude of the corticosterone response to repeated restraint exposure. As illustrated in Fig. 5, post hoc analysis confirmed that animals receiving a vehicle injection before the last bout of repeated restraint displayed a significant reduction in corticosterone secretion compared with the first (acute) exposure (P < 0.005), as did the animals acutely treated with AM404 (P < 0.03) or URB597 (P < 0.05) before the final bout of restraint. Animals receiving daily injections of AM404 and URB597 exhibited a decline in stress-induced corticosterone secretion comparable to that in vehicle-treated animals on the last day of restraint (P < 0.05 in both cases; Fig. S2). Thus, unlike the regulation of basal corticosterone secretion, AEA reuptake and its metabolism is not involved in the decline in the magnitude of the HPA response to repeated restraint. Remarkably, a single injection of AM251 before the last restraint completely abolished the repeated stress-induced decline in corticosterone release. In fact, rats injected with AM251 before the final restraint exhibited a near-significant sensitization (P = 0.053) in the magnitude of the corticosterone response. Animals bearing daily injections of AM251, administered before each bout of restraint, likewise failed to show a decline in stress-induced corticosterone secretion on the last day (Fig. S2), further reinforcing a role for the CB1 receptor for this component of stress HPA habituation.
Fig. 5.
Antagonism of CB1 receptors, but not facilitation of anandamide signaling, blocks repeated restraint-induced decline in corticosterone responses. Mean (±SEM) corticosterone (CORT; ng/mL) concentrations in circulation on first (Acute) and last (Repeat) day of restraint at 30 min of exposure in animals bearing acute systemic injections of vehicle (VEH), CB1 receptor antagonist AM251, anandamide hydrolysis inhibitor URB597, or anandamide uptake/hydrolysis inhibitor AM404. *P < 0.05 vs. acute counterpart (n = 5/group).
CB1 receptors in the basolateral amygdala.
Based on the strength of the negative association observed between 2-AG content in the amygdala and the magnitude of the corticosterone response on the last day of restraint (Fig. 3B), and our previous experiments showing that the local administration of a CB1 receptor agonist into the basolateral nucleus of the amygdala (BLA) reduces the corticosterone response to acute stress (21), we examined HPA habituation in animals receiving injections of the CB1 receptor antagonist AM251 into the BLA before the final episode of restraint. A significant interaction was found between repeated restraint and AM251 treatment (F1,12 = 10.05, P < 0.05). Post hoc analysis confirmed that animals receiving vehicle infusions into the BLA before the last bout of repeated restraint showed a significant decline in corticosterone responses compared with the first restraint exposure (P < 0.01), whereas those with AM251 infusions showed no such decline in response to repeated restraint (Fig. 3C and Fig. S3 for schematic of cannula placement within the BLA). With respect to basal corticosterone secretion, there was no significant difference between animals treated with vehicle and animals infused with AM251 in the amygdala (t8 = 0.25, P > 0.05; Fig. 3D). Taken together, the data suggest that increased 2-AG–mediated activation of CB1 receptors signaling within the basolateral amygdala regulates the decline in corticosterone responses in response to repeated restraint.
Discussion
Consistent with previous reports (4, 5, 22, 23), repeated exposure to restraint stress resulted in an adaptation of the HPA axis that was manifested as a habituation to stress-induced corticosterone secretion, as well as a mild but reliable hypersecretion of basal corticosterone (24, 25). Our data demonstrate that AEA and 2-AG are divergently regulated by repeated stress, and independently contribute to distinct forms of HPA axis adaptation. Specifically, the steady-state reductions in AEA that occur following repeated stress contribute to the basal hypersecretion of corticosterone, whereas the stress-specific increase in 2-AG signaling within the amygdala following repeated stress induces habituation of HPA axis activation. These data represent unique evidence that a common neurochemical system can contribute to distinct elements of stress HPA habituation, as well as demonstrate functionally dissociable roles for AEA and 2-AG within a common physiological process.
Systemic administration of a CB1 receptor antagonist before the last of nine daily exposures to restraint stress abrogated the habituation of repeated stress-induced corticosterone secretion. These data demonstrate that endocannabinoid signaling is essential for the expression of stress HPA habituation and complement previous reports demonstrating that endocannabinoid signaling is required for the adaptation of neuronal activation and behavioral responses to repeated aversive stimuli (16–18). At this point, whether endocannabinoids assist in the process of HPA habituation in response to other types of repeated stimuli remains an important question. Furthermore, a hallmark response of animals repeatedly exposed to the same (homotypic) stimulus is to show facilitated and/or maintained HPA responses to subsequent introduction of a novel (heterotypic) stressor (26). Thus, also worthy of pursuit is whether endocannabinoid signaling contributes to other complex forms of stress adaptation.
These data suggest that repeated exposure to restraint stress recruits endocannabinoid signaling. As endocannabinoids are synthesized on demand, we can assume that alterations in tissue content reflect the concentrations of ligands available to activate the CB1 receptor. To test the hypothesis that stress habituation is associated with increased endocannabinoid signaling, we examined the effects of repeated restraint stress on AEA and 2-AG tissue contents throughout the corticolimbic circuit implicated in stress habituation. We did not detect global changes in 2-AG signaling throughout the proposed stress regulatory circuit, as has been previously reported in mice (16), but rather a selective increase in 2-AG content within the amygdala. This increase was transient and occurred in a stress-specific manner, as 2-AG content in the amygdala returned to unstressed control levels 24 h after the last episode of restraint. Because acute stress causes little if any effect on 2-AG content within the amygdala (16, 21), we conclude that the ability of stress to augment 2-AG signaling within the amygdala is a progressive phenomenon that requires repeated exposure to the same stimulus. In support of this, a recent report has physiologically demonstrated an augmentation of 2-AG–mediated signaling within the BLA after 10 days of repeated restraint, but not after a single restraint exposure (27).
The 2-AG increase within the amygdala was negatively correlated with the magnitude of the corticosterone response to the last restraint exposure, suggesting that 2-AG in the amygdala contributes to the decline in HPA axis activation. In support of this argument, habituation of the corticosterone response to repeated restraint was blocked by the infusion of AM251 directly into the BLA. We have previously proposed that CB1 receptors within the BLA inhibit excitatory afferent activation of projection neurons, and thus reduce activation of efferent targets of the BLA (21). BLA efferents target the paraventricular nucleus (PVN) of the hypothalamus via a multisynaptic pathway likely involving the bed nucleus of the stria terminalis (28). Therefore, we hypothesize that 2-AG–mediated activation of CB1 receptors in the BLA dampens the firing activity of output neurons of the BLA, including those that ultimately activate the HPA axis. The BLA communicates with many forebrain regions, including the prefrontal cortex, striatum, hippocampus, and hypothalamus (28–31). Thus, a reduction in neuronal activity within the BLA could also mediate generalized dampening of neural circuits resulting in a lack of response to a stimulus that the animal has learned is nonthreatening.
The ability of CB1 receptor antagonism to prevent the expression of stress HPA habituation is reminiscent of previous reports showing comparable reversals in stress habituation following pharmacological blockade of glucocorticoid and mineralocorticoid receptors (5, 23). As previous studies have demonstrated that glucocorticoid feedback inhibition within the PVN is also CB1 receptor–dependent (32, 33), the current data offer further support for the overarching hypothesis that endocannabinoid signaling is fundamental to the intrinsic regulation of the HPA axis.
In contrast to the region-specific and transient response for 2-AG in the amygdala, repeated stress produced a widespread and sustained reduction in tissue AEA content throughout the corticolimbic stress circuit. This result is similar to the reduction in AEA content seen after acute stress (16, 21), save for the fact that the decline in AEA content persisted for up to 24 h after the final bout of repeated restraint. Furthermore, chronic inhibition of AEA clearance blocked the development of basal corticosterone hypersecretion, but not the habituation of the corticosterone response to the final restraint episode. On the basis of these findings, we propose that a reduction in AEA-mediated signaling contributes to the increase in basal HPA tone that accompanies the expression of stress HPA habituation. A strong and negative relationship was found for basal corticosterone and AEA levels unique to the prefrontal cortex across unstressed and repeatedly stressed animals. However, in light of the widespread reduction of AEA content following repeated stress, it remains unclear whether the prefrontal cortex represents the seat of, or an obligatory relay for, the central effects of endocannabinoid signaling on basal HPA function. In the case of the latter possibility, the BLA (21) and PVN of the hypothalamus (32, 33) continue to stand out as important candidates.
We propose that the diminution in AEA signaling to increase basal corticosterone secretion involves, at least in part, a decrease in endocannabinoid signaling at the level of the CB1 receptor. Thus, unlike the failure of the CB1 receptor antagonist AM251 to alter basal corticosterone levels under repeated restraint conditions in the current study, we have previously shown that AM251 increases basal corticosterone secretion in stress-naive animals (13, 14). Furthermore, the capacity of the FAAH inhibitor URB597 to alter HPA activity, at least under acute stress conditions, is reversible by coadministration of AM251 (21). Nevertheless, although both URB597 and AM404 are potent inhibitors of AEA degradation and uptake, we cannot rule out the possibility that AEA may act not only via CB1 but also via other signaling pathways, including those mediated by GPR55 (34) or the vanilloid type 1 receptor (35).
The current data demonstrate that stress habituation is met by both a decrease in activation of the HPA axis by the homotypic stressor and increased basal HPA tone, and that AEA and 2-AG signaling individually contribute to these changes. On one hand, increased 2-AG and CB1 receptor signaling in the BLA contribute to the decline in HPA responses to stress, whereas the development of basal increases in corticosterone secretion involves a reduction in corticolimbic AEA. These distinct forms of HPA axis regulation could confer differential benefits, whereby habituation of stress-induced HPA axis activation prevents excessive secretion of glucocorticoids to stimuli proved to be nonthreatening, whereas the increase in basal corticosterone secretion would allow for normal or facilitated responses of the HPA axis to novel stimuli (6). As such, the current study provides compelling evidence for a dual component role for the endocannabinoid system and positions it as a critical requirement for the central nervous system to respond to and function in the face of repeated and unexpected threats to homeostasis.
Independent studies have shown that both endocannabinoids and glucocorticoids are integral for the consolidation of emotionally aversive stimuli (36–38). Taken together with our current findings, stress-induced alterations in CB1 and 2-AG signaling within the amygdala could form an essential link between the HPA axis and associative learning, affording greater behavioral flexibility and adaptation to aversive environmental stimuli (17, 18). Given that deficits in adaptation and habituation to stress are coupled to vulnerability to affective illnesses, particularly depression and posttraumatic stress disorder (3), it is not surprising that deficits in endocannabinoid signaling are thought to contribute to the pathophysiology of these diseases (11). Understanding the mechanisms by which endocannabinoid signaling contributes to stress adaptation may assist in the determination of novel therapeutic treatments for these mental illnesses.
Methods
Subjects.
Seventy-day-old male Sprague-Dawley rats (300 g; University of British Columbia Breeding Center) were used in this study. The rats were pair housed in standard maternity bins lined with contact bedding. Colony rooms were maintained at 21 °C, and on a 12-h light/dark cycle, with lights on at 9:00 AM. All rats were given ad libitum access to Purina Rat Chow and tap water. All protocols were approved by the Canadian Council for Animal Care and the Animal Care Committee of the University of British Columbia. All studies occurred during the first third of the light cycle, during the daily nadir of HPA axis activity.
Stress Paradigm and Corticosterone RIA.
Animals were randomly assigned to unstressed conditions or exposed to a 30-min episode of restraint, in which rats were put into a polystyrene tube (21) each day, for 9 consecutive days. Unstressed animals were treated comparably with their repeatedly stressed counterparts (daily handling and transport), save that they never experienced restraint. For all studies, blood samples were obtained via tail nick either at the beginning (time 0) or at the termination of 30-min restraint. Samples were centrifuged at 3,000 × g for 20 min, after which serum was removed and stored at −80 °C. Corticosterone (5 μL) was measured in duplicate using commercial RIA kits (MP Biomedicals), as previously described (39). Samples were diluted 1:100 and 1:200 for basal and stress conditions, respectively, to render hormone detection within the linear part of the corticosterone standard curve. [125I]-Labeled corticosterone was used as tracer; the corticosterone antibody cross-reacts slightly with desoxycorticosterone (0.34%) and testosterone and cortisol (0.10%).
Pharmacological Studies.
To determine a role for endocannabinoid signaling on the process of HPA habituation, we used the CB1 receptor antagonist AM251 (Tocris-Cookson) dissolved in dimethyl sulfoxide (DMSO), Tween 80, and physiological (0.9%) saline (1:1:8, respectively); the AEA hydrolysis inhibitor URB597 (Cayman Chemical) dissolved in DMSO, Tween 80, and saline (1:1:18, respectively), and the AEA hydrolysis/uptake inhibitor AM404 (Tocris-Cookson) commercially prepared in water-soluble emulsion (Tocrisolve) containing soya oil and water (1:4, respectively).
To obtain dosages suitable for detecting repeated stress-dependent shifts in CB1 receptor requirements and AEA metabolism, we first tested a range of doses encompassing concentrations shown to be functionally active with respect to acute stress and/or basal HPA function (SI Text and Fig. S1). Piloted under basal conditions and in response to acute stress, the doses arrived at for all subsequent tests of repeated restraint were 1 mg/kg for AM251, 0.3 mg/kg for URB597, and 2 mg/kg for AM404. Systemic i.p.injections of these drugs and their respective vehicles were made either daily or on the last day of stress testing, from 30 min (AM251) to 90 min (URB597 and AM404) before restraint exposure.
Amygdala AM251 Infusion.
As previously described (21), rats were anesthetized with 100 mg/kg of ketamine hydrochloride and 7 mg/kg xylazine, and received stereotaxically guided, bilateral implants of 23-gauge stainless-steel guide cannulae directed at the basolateral amygdala (AP = −3.1 mm from bregma; ML = ± 5.0 mm from midline; and DV = −6.1 mm from dura) (40). All rats were given 1 week of recovery before the initiation of repeated stress testing and were exposed to daily mock injections over this interval. On the final day of repeated restraint, animals received bilateral infusions of the CB1 receptor antagonist AM251 (500 ng/side) or vehicle (50:50 of 0.9% saline:dimethyl sulfoxide) using 30-gauge injection cannulae extending 0.8 mm below the tips of the guide cannulae, delivered at a flow rate of 0.5 μL/72 s. Injection cannulae were left in place for an additional 1 min to allow for drug diffusion, after which the animals were returned to their home cages for 10 min before restraint exposure. Brains were removed 24 h later, and processed for Thionin staining to verify proper cannulae placement (Fig. S3).
Biochemical Studies.
Brain tissue was obtained via decapitation from stress-naive animals and from animals exposed to repeated restraint. With respect to the latter group, tissue was obtained either immediately or 24 h after the last bout of repeated restraint. Trunk blood collected at the same time was analyzed for corticosterone. Regions of interest included the prefrontal cortex, amygdala, thalamus, hypothalamus, and hippocampus, which were rapidly dissected and frozen on dry ice (41). Brain regions were subjected to a lipid extraction process (21), in which the contents of the two primary endocannabinoids, AEA and 2-AG, were obtained from lipid extracts in methanol using isotope-dilution, liquid chromatography−mass spectrometry as previously described (42).
Statistical Analysis.
Grouped data from the endocannabinoid biochemical analyses and corticosterone responses to daily drug treatments were compared with one-way ANOVA for restraint condition. A two-way mixed (between restraint conditions, within-subject) design ANOVA was used to analyze corticosterone responses to repeated stress and/or acute drug treatments using day of restraint as the repeated measure. When appropriate, post hoc pairwise comparisons were made using Tukey's HSD test. Correlational analyses were performed using simple regression. Statistical comparisons were made observer-blind by assigning coded designations to the tissue and data sets in advance.
Supplementary Material
Acknowledgments
This research was supported by the Canadian Institutes of Health Research (V.V. and B.B.G.), National Institutes of Health Grants DA022439 and DA09155 (to C.J.H.), Canadian Graduate Student Scholarships Doctoral Award from the Canadian Institutes of Health Research (to R.J.M., B.B., T.T.L., and J.M.G.), Natural Sciences and Engineering Research Council Canadian Graduate Scholarship (to M.N.H.), and Michael Smith Foundation for Health Research Trainee Award (to B.B. and M.N.H.) and Senior Scholarship Award (V.V.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0914661107/-/DCSupplemental.
References
- 1.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Glucocorticoids, depression, and mood disorders: Structural remodeling in the brain. Metabolism. 2005;54(5, Suppl 1):20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girotti M, et al. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147:4917–4930. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- 6.Dallman MF. Modulation of stress responses: How we cope with excess glucocorticoids. Exp Neurol. 2007;206:179–182. doi: 10.1016/j.expneurol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2009 doi: 10.1016/j.pnpbp.2009.11.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel S, Hillard CJ. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: A novel mechanism for stress habituation. Eur J Neurosci. 2008;27:2821–2829. doi: 10.1111/j.1460-9568.2008.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endo-cannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- 11.Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: Implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog Brain Res. 2008;170:397–432. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- 13.Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- 14.Cota D, et al. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology. 2007;148:1574–1581. doi: 10.1210/en.2005-1649. [DOI] [PubMed] [Google Scholar]
- 15.Steiner MA, Marsicano G, Wotjak CT, Lutz B. Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral and neuroendocrine stress responses. Psychoneuroendocrinology. 2008;33:1165–1170. doi: 10.1016/j.psyneuen.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- 17.Kamprath K, et al. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fride E, Suris R, Weidenfeld J, Mechoulam R. Differential response to acute and repeated stress in cannabinoid CB1 receptor knockout newborn and adult mice. Behav Pharmacol. 2005;16:431–440. doi: 10.1097/00008877-200509000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Kathuria S, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Jaimes L, Stouffer DG, Parsons LH. Chronic ethanol treatment potentiates ethanol-induced increases in interstitial nucleus accumbens endocannabinoid levels in rats. J Neurochem. 2009;111:37–48. doi: 10.1111/j.1471-4159.2009.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill MN, et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsycho-pharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress—comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- 23.Cole MA, et al. Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. J Neuroendocrinol. 2000;12:1034–1042. doi: 10.1046/j.1365-2826.2000.00555.x. [DOI] [PubMed] [Google Scholar]
- 24.Bechtold AG, Patel G, Hochhaus G, Scheuer DA. Chronic blockade of hindbrain glucocorticoid receptors reduces blood pressure responses to novel stress and attenuates adaptation to repeated stress. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1445–R1454. doi: 10.1152/ajpregu.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ottenweller JE, Servatius RJ, Natelson BH. Repeated stress persistently elevates morning, but not evening, plasma corticosterone levels in male rats. Physiol Behav. 1994;55:337–340. doi: 10.1016/0031-9384(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 26.Bhatnagar S, et al. A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J Neurosci. 2000;20:5564–5573. doi: 10.1523/JNEUROSCI.20-14-05564.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.French SJ, Hailstone JC, Totterdell S. Basolateral amygdala efferents to the ventral subiculum preferentially innervate pyramidal cell dendritic spines. Brain Res. 2003;981:160–167. doi: 10.1016/s0006-8993(03)03017-8. [DOI] [PubMed] [Google Scholar]
- 30.Wright CI, Beijer AV, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci. 1996;16:1877–1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- 32.Evanson NK, Ulrich-Lai YM, Furay AR, Tasker JG, Herman JP. Hypothalamic paraventricular cannabinoid receptor signaling in fast feedback inhibition of the hypothalamus-pituitary-adrenal axis response to acute restraint stress. Soc Neurosci Abs. 2007;197:195. [Google Scholar]
- 33.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown AJ, Robin Hiley C. Is GPR55 an anandamide receptor? Vitam Horm. 2009;81:111–137. doi: 10.1016/S0083-6729(09)81005-4. [DOI] [PubMed] [Google Scholar]
- 35.Tóth A, Blumberg PM, Boczán J. Anandamide and the vanilloid receptor (TRPV1) Vitam Horm. 2009;81:389–419. doi: 10.1016/S0083-6729(09)81015-7. [DOI] [PubMed] [Google Scholar]
- 36.McIntyre CK, Power AE, Roozendaal B, McGaugh JL. Role of the basolateral amygdala in memory consolidation. Ann N Y Acad Sci. 2003;985:273–293. doi: 10.1111/j.1749-6632.2003.tb07088.x. [DOI] [PubMed] [Google Scholar]
- 37.Campolongo P, et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci USA. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganon-Elazar E, Akirav I. Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J Neurosci. 2009;29:11078–11088. doi: 10.1523/JNEUROSCI.1223-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bingham B, Viau V. Neonatal gonadectomy and adult testosterone replacement suggest an involvement of limbic arginine vasopressin and androgen receptors in the organization of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2008;149:3581–3591. doi: 10.1210/en.2007-1796. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1998. 4th Ed. [DOI] [PubMed] [Google Scholar]
- 41.Hill MN, et al. Involvement of the endocannabinoid system in the ability of long-term tricyclic antidepressant treatment to suppress stress-induced activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2006;31:2591–2599. doi: 10.1038/sj.npp.1301092. [DOI] [PubMed] [Google Scholar]
- 42.Patel S, et al. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.