Abstract
A current paradigm states that non-antigen-specific inflammatory cues attract noncognate, bystander T cell specificities to sites of infection and autoimmune inflammation. Here we show that cues emanating from a tissue undergoing spontaneous autoimmune inflammation cannot recruit naive or activated bystander T cell specificities in the absence of local expression of cognate antigen. We monitored the recruitment of CD8+ T cells specific for the prevalent diabetogenic epitope islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)206–214 in gene-targeted nonobese diabetic (NOD) mice expressing a T cell “invisible” IGRP206–214 sequence. These mice developed islet inflammation and diabetes with normal incidence and kinetics, but their inflammatory lesions could recruit neither naive (endogenous or exogenous) nor ex vivo-activated IGRP206–214-reactive CD8+ T cells. Conversely, IGRP206–214-reactive, but not nonautoreactive CD8+ T cells rapidly homed to and accumulated in the inflamed islets of wild-type NOD mice. Our results indicate that CD8+ T cell recruitment to a site of autoimmune inflammation results from an active process that is strictly dependent on local display of cognate pMHC and suggest that CD8+ T cells contained in extralymphoid autoimmune lesions are largely autoreactive.
Keywords: diabetes, lymphocyte, islet-specific glucose-6-phosphatase catalytic subunit-related protein, islet inflammation, lymphocyte recruitment
Recognition of cognate peptide–major histocompatibility complexes (pMHC) on the surface of dendritic cells (DC) by naive T lymphocytes in lymph nodes draining a site of infection or autoimmune inflammation elicits the lymphocytes’ activation, proliferation, and differentiation into cytolytic effectors. Upon activation, lymphocytes also acquire the ability to survey nonlymphoid tissues for presence of their cognate target antigens, with a preference for inflamed tissues as well as tissues drained by the lymph nodes where activation took place (1 –3). Studies in a number of infection and autoimmune disease models have suggested that recruitment of T lymphocytes into a site of extralymphoid inflammation does not require local expression of cognate (foreign or self) pMHC on tissue cells or tissue-associated antigen-presenting cells (APCs) (4 –7). Accordingly, it is generally thought that non-antigen-specific inflammatory cues such as cytokines and chemokines emanating from the local microenvironment can recruit noncognate (i.e., bystander) T cells to a site of foreign or self antigen-triggered tissue inflammation (8 –19). Notwithstanding that in vitro-activated bystander T cell clones can transiently comigrate with their cognate counterparts into noninflamed tissue in adoptive T cell transfer experiments and that tissue-specific expression of cytokine and/or chemokine transgenes in normal tissues can trigger bystander T cell inflammation, these models do not faithfully mimic the events that take place in spontaneous autoimmune inflammation. Specifically, it is unclear that bystander T cell specificities can effectively compete with their cognate polyclonal counterparts, recognizing pMHC in situ, for occupation of the inflammatory space.
Type 1 diabetes (T1D) in both humans and nonobese diabetic (NOD) mice is a chronic autoimmune disease that results from inflammation of pancreatic islets and destruction of pancreatic β cells by T cells targeting numerous β cell autoantigens (20, 21). A significant fraction of the islet-associated CD8+ T cells in NOD mice recognize the mimotope NRP-V7 in the context of the MHC molecule Kd (22 –25). These CD8+ T cells are diabetogenic (22, 26), target a peptide from islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP206–214, similar to NRP-V7) (27), and circulate in the peripheral blood at a relative high frequency (>1/200 CD8+ T cells), particularly as clinical disease nears (27, 28).
To ascertain whether local expression of cognate pMHC is a sine-qua-non condition for recruitment and/or accumulation of CD8+ T cells to the pancreas during spontaneous autoimmune diabetes, we generated a gene-targeted NOD strain capable of developing islet inflammation but expressing a T cell “invisible” IGRP206–214 epitope. We find that these mice develop insulitis and diabetes essentially like wild-type NOD mice but cannot recruit endogenous or exogenous IGRP206–214-specific CD8+ T cells, regardless of their activation state or degree of islet inflammation.
Results and Discussion
Knock-in NOD Mice Expressing a T cell Invisible IGRP206–214 Epitope.
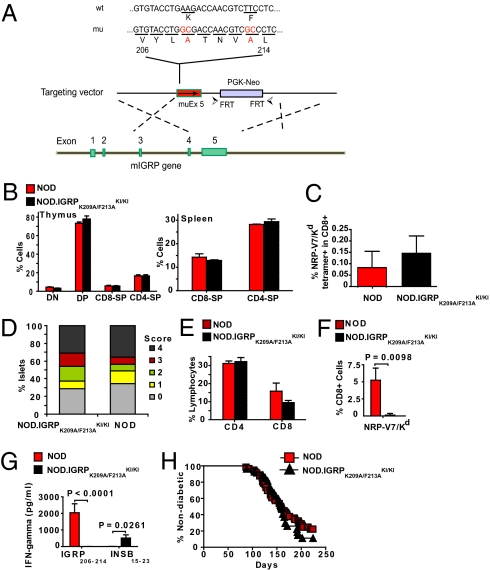
We generated a gene-targeted NOD strain expressing a mutant form of IGRP in which the two T cell receptor (TCR)-contact residues of IGRP206–214 (25) are replaced with alanines (K209A and F213A) (Fig. 1A). The IGRPK209A/F213A peptide cannot trigger the activation or elicit the cytotoxicity of 8.3-CD8+ T cells (25), which express a transgenic IGRP206–214-reactive TCR (22), and does not impair, either in vitro or in vivo, their responsiveness to a subsequent challenge with IGRP206–214 (Fig. S1). As expected, IGRPK209A/F213A-homozygous knock-in NOD mice (NOD.IGRPK209A/F213A KI/KI) and wild-type NOD mice displayed indistinguishable thymic and splenic T cell profiles (Fig. 1B) and exported similar numbers of IGRP206–214-reactive CD8+ cells to the circulation (Fig. 1C). Analyses of pancreata from prediabetic animals indicated that both types of mice developed insulitis lesions of similar severity (Fig. 1D), similar CD4+ T cell content, and slightly different (but not statistically different) CD8+ T cell content (Fig. 1E).
Fig. 1.
NOD.IGRPK209A/F213A KI/KI mice develop insulitis and diabetes without recruiting IGRP206–214-reactive CD8+ T cells into pancreatic islets. (A) Targeting strategy. The FRT-flanked PGK-neo cassette was removed from targeted ES cells by transient transfection of Flp recombinase-encoding cDNA. (B) Distribution of lymphocyte subsets in thymi and spleens from NOD and NOD.IGRPK209A/F213A KI/KI mice (n = 3 and 5, respectively; 3 independent experiments). DN, double negative; DP, double positive; CD8-SP, CD8 single positive; CD4-SP, CD4 single positive. (C) Frequency of NRP-V7–reactive CD8+ T cells in peripheral blood. Peripheral blood mononuclear cells (PBMCs) from 10-week-old mice (NOD, n = 7; NOD.IGRPK209A/F213A KI/KI, n = 8) were stained with NRP-V7/Kd tetramers and anti-CD8 mAb. Data correspond to 4 independent experiments using one to five mice/experiment. (D) Insulitis scores. Pancreata from nondiabetic 32-week-old mice (NOD, n = 3; NOD.IGRPK209A/F213A KI/KI, n = 5) were examined for islet inflammation. Pancreata were from one cohort of NOD mice and two different cohorts of NOD.IGRPK209A/F213A KI/KI mice. (E and F) CD4+ and CD8+ T cell (E) and NRP-V7/Kd tetramer+ CD8+ T cell content (F) in freshly isolated islets of NOD (n = 6; 3 independent experiments) vs. NOD.IGRPK209A/F213A KI/KI mice (n = 7; 4 independent experiments). (G) Absence of IGRP206–214 -reactive CD8+ T cells in the islet infiltrates of NOD.IGRPK209A/F213A KI/KI (n = 10; 10 independent experiments) vs. NOD mice (n = 3; 3 independent experiments). Islet-associated CD8+ T cells were cultured in IL-2 for 7 days and challenged with peptide-pulsed (10 μM) irradiated NOD splenocytes. The IFNγ content in the supernatants (at 48 h) was measured by ELISA. Data correspond to the means ± SEM. (H) Diabetes incidence in female NOD (n = 56) and NOD.IGRPK209A/F213A KI/KI (n = 27) mice. The average blood glucose levels in newly diagnosed diabetic NOD and NOD.IGRPK209A/F213A KI/KI mice are: 22.3 ± 2.5 vs. 23.7 ± 3.3 mM, respectively. In males, the incidence and average age at onset of disease were also similar in both strains (NOD: n = 15; 40% diabetic at 133 ± 33 days; and NOD.IGRPK209A/F213A KI/KI: n = 20; 50% diabetic at 149 ± 36 days). Data in B–F correspond to the means ± SEM. P values in F and G were obtained with Mann-Whitney U-test.
Notably, however, the islet-associated T cells of prediabetic NOD.IGRPK209A/F213A KI/KI mice did not contain IGRP206–214-reactive CD8+ T cells, as determined by NRP-V7/Kd tetramer staining (Fig. 1F), and did not produce IFNγ in response to NRP-V7 peptide-pulsed APCs (Fig. 1G). Impaired recruitment of IGRP206–214-reactive CD8+ T cells was associated with a significant increase in recruitment of other autoreactive T cell specificities that are present at very low precursor frequencies in the islets of prediabetic mice (20, 21, 28), such as insulin-B15–23-reactive CD8+ T cells (29) (Fig. 1G). As a result, NOD and NOD.IGRPK209A/F213A KI/KI mice developed T1D with virtually identical incidence curves (Fig. 1H). These data indicated that (i) IGRP206–214-reactive CD8+ T cells are completely excluded from insulitic lesions in the absence of local expression of IGRP206–214 and that (ii) initiation and progression of spontaneous T1D in NOD mice does not require the accumulation of IGRP206–214-reactive CD8+ T cells into pancreatic islets.
Severely Impaired Recruitment of Naive IGRP206–214-Reactive CD8+ T Cells to the Inflamed Islets of NOD.IGRPK209A/F213A KI/KI Mice.
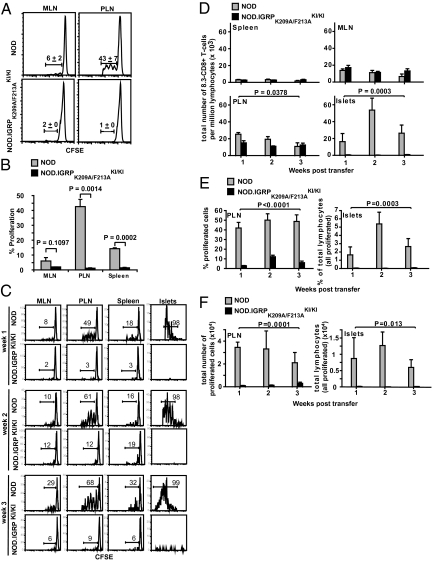
To further investigate the role of local cognate pMHC vs. non-antigen-specific inflammatory cues in the recruitment of CD8+ T cells to pancreatic islets, we ascertained whether naive and in vitro-preactivated IGRP206–214-reactive 8.3-CD8+ T cells could undergo activation in the pancreatic lymph nodes (PLN) and/or home to the inflamed islets of prediabetic 10- to 12-week-old NOD.IGRPK209A/F213A KI/KI hosts (i.e., in response to preexisting local inflammatory cues). Adoptively transferred naive CFSE-labeled 8.3-CD8+ T cells (107) proliferated in the PLNs [and, to a much lesser extent, in the mesenteric lymph nodes (MLNs) and spleen] of insulitic NOD mice within a week after adoptive transfer (Fig. 2 A and B). Analysis of the islet infiltrates of these insulitic NOD hosts 1, 2, and 3 weeks after T cell transfer revealed rapid recruitment (within 1 week) of actively proliferating 8.3-CD8+ T cells (Figs. 2 C–F). Notably, almost all of the 8.3-CD8+ T cells found within islets at this stage had undergone more than two cell divisions, and most of the cells that had only divided fewer than three times were found exclusively in the PLNs (Fig. 2C and Fig. S2), suggesting that recruitment of autoreactive CD8+ T cells into the pancreas is invariably preceded by antigen-induced activation in the PLNs. The islets (but not the PLNs) of hosts analyzed 2 weeks after T cell transfer contained higher percentages of proliferating cells (Fig. 2 C and E and Fig. S2) and total 8.3-CD8+ T cells (Fig. 2 D and F). By the third week, there was a further increase in the extent of cell division in islets (Fig. 2C and Fig. S2) in association with reductions in the percentages and total number of proliferated 8.3-CD8+ T cells, presumably due to attrition by activation-induced cell death (i.e., in response to repetitive stimulation of differentiated CD8+ T cells by cognate pMHC) (Fig. 2 D–F). Thus, accumulation of autoreactive CD8+ T cells in the inflamed islets of prediabetic NOD mice is associated with (i) T cell activation and proliferation in the PLNs, (ii) recruitment of actively proliferating cells into pancreatic islets, and (iii) additional rounds of local (intraislet) proliferation.
Fig. 2.
Naive 8.3-CD8+ T cells are not recruited into the inflamed pancreatic islets of NOD.IGRPK209A/F213A KI/KI mice. (A) Proliferation of naive 8.3-CD8+ T cells in the PLN. CFSE-labeled naive 8.3-CD8+ T cells were transfused into 10- to 12-week-old NOD (n = 3) or NOD.IGRPK209A/F213A KI/KI (n = 3) recipients (three independent experiments, each using both host types). Dilution of CFSE was measured by flow cytometry 7 days posttransfer. Values correspond to the average percentage of proliferated CD8+ T cells ± SEM. (B) Summary of data described in A. P values were obtained with Mann-Whitney U-test. (C) Recruitment and proliferation of naive 8.3-CD8+ T cells from 8.3-NOD.Thy1.1 donor mice in the lymphoid organs and islets of 10- to 12-week-old insulitic NOD and NOD.IGRPK209A/F213A KI/KI hosts 1, 2, and 3 weeks after transfer. CFSE histograms correspond to Thy1.1+CD8+ cells. (D) Mean ± SEM of total numbers of 8.3-CD8+ T cells per million lymphocytes. Data in C and D correspond to three to six experiments/time point and host type (one mouse/time point/host in each experiment). (E) Mean ± SEM of percentages of proliferated cells (for PLNs) or total number of donor lymphocytes (for islet T cell isolates, where all donor-derived T cells were proliferating) (three to six experiments/time point and host type; one mouse/time point/host in each experiment). (F) Absolute numbers of proliferated (for PLNs) or recruited (for islet T cell isolates) 8.3-CD8+ T cells (mean ± SEM) (three to six experiments/time point and host type; one mouse/time point/host in each experiment). P values in D–F were obtained by two-way ANOVA.
A remarkably different outcome was obtained when these experiments were done in age-matched, insulitic NOD.IGRPK209A/F213A KI/KI hosts. Whereas the transfused 8.3-CD8+ T cells readily homed to the spleen, PLNs, and MLNs of insulitic NOD.IGRPK209A/F213A KI/KI hosts (Fig. 2 C and D), they did not proliferate in the PLNs (Fig. 2 A–C and E and F and Figs. S2 and S3), confirming that this event requires cross-presentation of β cell-derived IGRP206–214. There was also a reduction in the proliferation of cognate 8.3-CD8+ T cells in the MLNs and spleens of NOD.IGRPK209A/F213A KI/KI vs. NOD mice (Fig. 2 B and C), suggesting that some of the T cells that are activated in the PLNs and/or islets (or the activating IGRP206–214-loaded APCs) of wild-type NOD mice migrate to distant secondary lymphoid organs during disease progression. Most notably, the adoptively transferred cells failed to home to pancreatic islets of insulitic NOD.IGRPK209A/F213A KI/KI hosts, where they could not be found throughout the 3-week study period (Fig. 2 C–F), despite the presence of severe local inflammation (Fig. 1D). In fact, up to more than 6% (∼104) of all of the islet-associated lymphocytes of NOD hosts were donor derived, compared to virtually none of those isolated from the NOD.IGRPK209A/F213A KI/KI hosts (Fig. 2F). Thus, nonspecific inflammatory cues emanating from insulitic lesions cannot single-handedly (in the absence of local cognate pMHC) recruit naive bystander IGRP206–214-reactive CD8+ T cells to the site.
T1D-Irrelevant CD8+ T cell Specificities Are Not Recruited to the Inflamed Pancreatic Islets of Wild-Type NOD Mice.
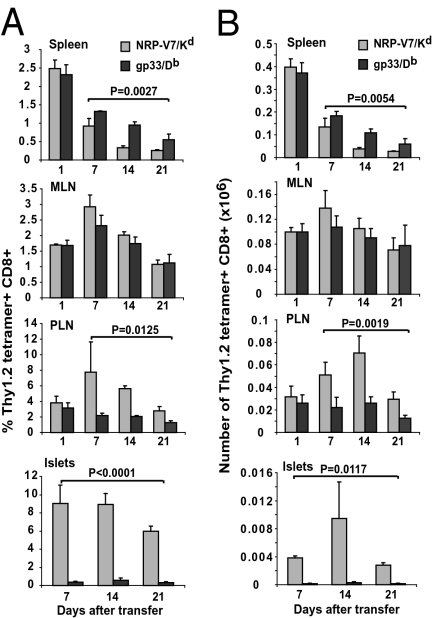
To rule out the possibility that this outcome was a peculiarity of the IGRP206–214-reactive CD8+ T cell population, we tracked the recruitment of adoptively transferred naive Thy1.2+ lymphocytic choriomeningitis virus (LCMV) gp33-specific CD8+ T cells [a nonautoreactive, T1D-irrelevant T cell population from LCMV gp33-TCR-transgenic (P14) NOD donor mice] in insulitic NOD.Thy1.1 hosts. This was done by cotransfusing these cells with an equal number of naive Thy1.2+ 8.3-CD8+ T cells and by analyzing the hosts’ lymphoid organs and pancreatic islets for presence of both T cell pools 1, 7, 14, and 21 days after transfer. Whereas Thy1.2+ NRP-V7/Kd-tetramer+ and gp33/Db-tetramer+ CD8+ T cells were rapidly recruited to the spleen, PLNs, and MLNs within 1 day of transfer, only the former homed to a significant degree to pancreatic islets (Fig. 3 A and B). Accordingly, naive IGRP206–214-reactive CD8+ T cells need to engage cognate pMHC on local APCs and/or on β cells to access and/or accumulate (owing to retention and/or proliferation) in inflamed tissue.
Fig. 3.
Selective recruitment of cognate autoreactive T cells into pancreatic islets of NOD mice. (A) Naive 8.3-CD8+/Thy1.2+ and P14-CD8+/Thy1.2+ T cells were mixed at 1:1 (8 × 106 cells each) and injected i.v. into 10- to 12-week-old NOD.Thy1.1 hosts. Hosts were killed at various time points after transfer and their peripheral lymphoid organs and islet cell suspensions analyzed for presence of NRP-V7/Kd and GP33/Db tetramer+ Thy1.2+ CD8+ T cells. Data correspond to three mice/time point (n = 12 mice) and three independent experiments (one mouse/time point in each experiment) and are shown as mean ± SEM. (B) Data from A presented as absolute numbers of cells (mean ± SEM). P values were obtained with two-way ANOVA.
Preactivated IGRP206–214-Specific Cytotoxic T Lymphocytes Also Fail to Home to the Insulitic Lesions of NOD.IGRPK209A/F213A KI/KI Hosts.
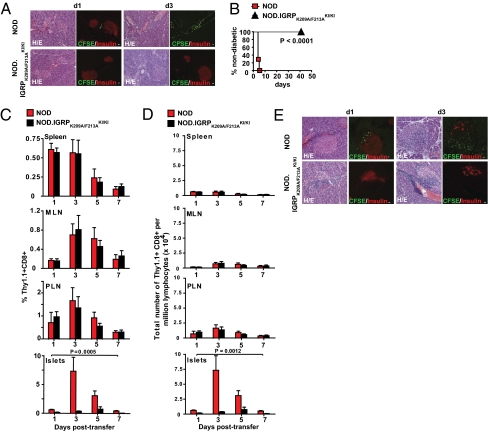
To investigate the role of T cell activation in the recruitment and/or accumulation of bystander T cells to inflamed and noninflamed islets, we transfused CFSE-labeled, in vitro-differentiated Thy1.1+ 8.3-cytotoxic T lymphocytes (CTLs) (1.5 × 107) into noninsulitic (3-week-old) or insulitic (10- to 12-week-old) NOD and NOD.IGRPK209A/F213A KI/KI hosts. Whereas CFSE+ 8.3-CTLs were rapidly recruited into noninsulitic NOD islets (Fig. 4A), leading to rapid loss of insulin-producing β cells and rampant development of diabetes in all hosts within 5 days (Fig. 4B), they were neither recruited to islets nor caused any obvious β cell loss or diabetes in any noninsulitic NOD.IGRPK209A/F213A KI/KI hosts for up to 6 weeks after transfer (Fig. 4 A and B), suggesting that activated CD8+ T cells cannot home to noninflamed tissue in the absence of local cognate pMHC. Similar results were obtained when 8.3-CTLs were transfused into insulitic hosts. The 8.3-CTLs were progressively recruited to, and accumulated in the PLNs and MLNs of both types of mice during the first 3 days after transfer (∼2- to 3-fold on day 3 vs. day 1; Fig. 4 C and D). In contrast, whereas CFSE+ 8.3-CTL accumulated in the islets of insulitic NOD mice (∼7- to 9-fold on day 3 vs. day 1), they did not do so in the insulitic islets of NOD.IGRPK209A/F213A KI/KI hosts (Fig. 4 C and D), confirming a critical role for cognate pMHC on retention and accumulation of IGRP206–214-specific CTL in nonlymphoid tissue. Taken together, these data suggest that T cell occupation of the inflamed islet space in spontaneous autoimmune diabetes is not due to “diffusion” from the periphery in response to inflammatory and chemotactic cues, but rather to an active process that involves local recognition of cognate pMHC.
Fig. 4.
The pancreatic islets of NOD.IGRPK209A/F213A KI/KI mice also fail to recruit activated 8.3-CD8+ T cells, even when inflamed. (A) In vitro-activated, CFSE-labeled 8.3-CD8+ T cells were injected i.v. into 3-week-old NOD or NOD.IGRPK209A/F213A KI/KI hosts. Pancreatic sections were stained with anti-insulin antibodies and examined for presence of CFSE+ T cells by confocal microscopy on days 1 and 3 after transfer (three mice/time point; 10–20 islets/mouse; three independent experiments). Representative images are shown. An adjacent tissue section was stained with H&E (Left). (White scale bars, 20 μm.) On NOD hosts’ day 1 samples, CFSE+ T cells were predominantly found in the peri-insular space. Note the near complete depletion of insulin+ cells in the CFSE+ T cell-containing areas of NOD hosts’ day 3 samples. (B) Incidence of diabetes in 3-week-old NOD (n = 7) or NOD.IGRPK209A/F213A KI/KI recipients (n = 6) of 8.3-CTL (two independent experiments, each including three to four mice/strain type). P values were obtained with log rank test. (C) In vitro-activated, CFSE-labeled 8.3-CD8+ Thy1.1+ T cells were injected i.v. into 10- to 12-week-old NOD or NOD.IGRPK209A/F213A KI/KI hosts. Hosts were analyzed for presence of Thy1.1+ CD8+ T cells in different organs. Data correspond to three to four independent experiments/time point and strain (one mouse/time point/strain in each experiment) and are shown as mean ± SEM. (D) Data from C presented as absolute numbers of cells per million lymphocytes (mean ± SEM). P values in C and D were obtained with two-way ANOVA. (E) Analysis of pancreas sections from 10- to 12-week-old mice transfused with CFSE-labeled in vitro-activated 8.3-CD8+ T cells on days 1 and 3 after transfer, for presence of CFSE+ T cells (three independent experiments; one mouse/time point/strain in each experiment). Images of islets are representative of severe insulitis.
Our observations challenge the generally held assumption that T cell infiltrates in inflamed extralymphoid tissues, such as pancreatic islets in diabetes, contain a mixture of both cognate and noncognate (i.e., bystander) T cell specificities. Our results demonstrate, in a model of highly polyclonal spontaneous autoimmunity, that bystander CD8+ T cells, even after activation, are strongly selected against for retention and accumulation in the target organ. This contention does not argue against the idea that bystander T cells can transiently migrate to a site of inflammation nonspecifically, such as in response to cytokine-induced chemokine receptor ligands like CXCL9, CXCL10, and CXCL11 (30, 31). Rather, our data strongly argue that, in the absence of cognate pMHC, nonspecifically recruited CD8+ T cells cannot effectively compete with cognate T cell specificities for occupation of space. Recent studies in dual TCR retrogenic, sublethally irradiated bone marrow chimeras coexpressing T1D-relevant and irrelevant MHC class II-restricted autoreactive CD4+ T cell specificities in NOD.scid hosts suggest that naive CD4+ T cells may also require local engagement of cognate pMHC for recruitment to noninflamed islets (32). However, whether bystander naive and/or activated CD4+ T cells can home to inflamed islets, particularly in a model of spontaneous polyclonal inflammation such as the one described herein, remains to be determined.
Whichever the case for CD4+ T cells might be, and assuming that this paradigm can be generalized to other antigenic specificities, our data suggest that the majority of all CD8+ T cells that are recruited to sites of autoimmune inflammation, such as pancreatic islets during diabetogenesis, are autoreactive. Autoreactive CD8+ T cells need not have to engage cognate pMHC directly on the β cell surface to be effectively retained at the target site; recognition of pMHC on vascular endothelial cells (2, 33) or on a tissue-resident professional APC population might be sufficient. This would explain why NOD mice expressing a RIP-driven adenoviral E19 transgene, whose β cells express significantly reduced levels of pMHC class I (34), and NOD mice with a β cell-specific disruption of β-2 microglobulin, which cannot display pMHC class I complexes on the surface (35), recruit CD8+ T cells to pancreatic islets.
Materials and Methods
Mice.
The 8.3-TCR-transgenic NOD mice (Thy1.2+) have been described (22). Thy1.1-congenic NOD mice (NOD.Thy1.1) and LCMV-Gp33-specific TCR-transgenic (P14) NOD mice were obtained from the T1D repository (The Jackson Laboratory). To generate IGRPK209A/F213A KI/KI mice, we transfected an igrp targeting construct carrying a mutated exon 5 (encoding an IGRP206–214 epitope in which the two TCR contact residues were replaced by Ala: (VYLATNVAL; K209A/F213A) into CK35 129/Sv-derived murine embryonic stem (ES) cells (Fig. 1). The FRT-flanked PGK-neo cassette was removed from targeted ES cells by transient transfection of Flp recombinase-encoding cDNA. Transfected ES clones were screened by Southern blot analysis using AvrII or ApaI-digested DNA and 5′- and 3′-specific probes differentiating wild-type (11.6 and 11.1 kb for 5′ and 3′ arms, respectively) vs. targeted bands (7.7 and 6.7 kb, respectively). Type II recombinants arising from Flp-mediated deletion of the PGK-neo cassette were identified by Southern blotting using the 3′ probe described above. To produce NOD.IGRPK209A/F213A KI/WT mice, we backcrossed the targeted IGRPK209A/F213A allele from germline-competent 129/Sv chimeras onto the NOD background for at least six generations. Genomewide SNP analyses at the N6 backcross were done to confirm homozygosity for all known NOD diabetes-susceptibility alleles. Mice were intercrossed at the N6 or N7 generations to produce NOD.IGRPK209A/F213A KI/KI homozygotes. These studies were approved by the Faculty of Medicine's Animal Care Committee and followed the guidelines of the Canadian Council of Animal Care.
Peptides and Tetramers.
The peptides IGRP206–214 (VYLKTNVFL), INSB15–23 (LYLVCGERG), NRP-V7 (KYNKANVFL), TUM (KYQAVTTTL), and LCMV GP33 (KAVYNFATM) and the corresponding tetramers (PE labeled) were prepared as described (27, 28). Briefly, the peptides were folded with recombinant human β2m and respective mouse heavy chains and subjected to gel filtration purification, biotinylation, and ion exchange purification using an AKTA FPLC system (GE Healthcare). The final product was verified by both denaturing SDS/PAGE and native PAGE analysis.
Islet Isolation.
Pancreatic islets were isolated by hand-picking after collagenase P digestion of the pancreas, cultured overnight in IL-2-containing media [to avoid additional enzymatic digestion steps and thus enhance T cell recovery and viability, as described (36)], disrupted into single cells, stained, and analyzed by flow cytometry.
Flow Cytometry.
Peripheral blood and islet cell suspensions were stained with tetramers (5 μg/mL) in FACS buffer (0.1% sodium azide and 1% FBS in PBS) for 1 h at 4 °C, washed, and incubated with FITC-conjugated anti-CD8α (5 μg/mL) for 30 min at 4 °C. For other stains, thymi, spleens, and lymph node cell suspensions were analyzed by 3-color flow cytometry using anti-CD8-PerCP (53-6.7), anti-CD4-FITC (IM7), and tetramer-PE, or with anti-CD8-PerCP (53-6.7), tetramer-PE, and FITC-conjugated anti-Thy1.2 mAb, or with anti-CD8-PerCP (53-6.7) and PE-conjugated anti-Thy1.1 mAb. Cells were washed, fixed in 1% PFA/PBS, and analyzed by FACS. All mAbs were from BD Pharmingen. Data were analyzed by FlowJo (Tree Star).
Specificity of Islet-Associated CD8+ T Cells.
Islet-infiltrating cells from NOD or NOD.IGRPK209A/F213A KI/KI mice were cultured for 7 days in the presence of 0.5 units/mL of rIL-2 to expand in vivo-activated islet-associated T cells. Upon washing, CD8+ T cells were cultured, in the absence of exogenous IL-2, with peptide-pulsed (10 μM) irradiated NOD splenocytes for 48 h. The IFNγ content in the supernatants was measured by ELISA (Duoset, R&D Systems). Values obtained with the negative control peptide TUM were subtracted.
Adoptive Transfer.
Splenic CD8+ T cells were purified using iMAG CD8 beads (BD Bioscience) following the manufacturer's protocols, labeled with CFSE (2.5 μM), and injected i.v. (107 CD8+ T cells). In cotransfer experiments employing P14 CD8+ T cells, each mouse received a mixture of 8 × 106 8.3-CD8+ and 8 × 106 P14-CD8+ T cells. To generate in vitro-activated 8.3-CD8+ T cells, splenocytes from 8.3-NOD or 8.3-NOD.Thy1.1 donor mice were cultured in the presence of NRP-V7 peptide (1 μM) for 3 days in the absence of exogenous IL-2. These conditions generate highly diabetogenic CTL (Fig. 4B). The proliferating CD8+ T cells (>95% of the cells) were then labeled with 2.5 μM CFSE and transfused i.v. (15 × 106) into unmanipulated 3-week- or 10- to 12-week-old hosts. Mice were killed 1, 3, 5, 7, 14, or 21 days later and their spleens, PLNs, and MLNs examined for presence of donor CD8+ T cells (Tetramer+ and thy1.2+) or for dilution of CFSE in the CD8+ gate.
Immunopathology.
Formalin-fixed, paraffin-embedded pancreas sections were stained with H&E and scored for insulitis (see below). To examine pancreatic islets for infiltration by transfused CFSE+ T cells, pancreata were embedded in OCT medium and frozen in a dry ice/acetone bath. Cryosections (5 μm) were fixed with 3% paraformaldehyde, stained with guinea pig anti-insulin antibodies and Cy3-conjugated rabbit anti-guinea pig antibodies (Invitrogen). The sections were then mounted with prolong-Gold (Invitrogen) and analyzed with an Olympus FV1000 confocal microscopy system. Immediately adjacent sections were stained with H&E. We analyzed ∼20 islets per mouse.
Insulitis Scores.
Scoring of insulitis lesions was performed as described (26). The degree of mononuclear cell infiltration was scored as: 0, none; 1, peri-insulitis; 2, infiltration covering <25% of the islet; 3, covering 25–50% of the islet; and 4, covering >50% of the islet.
Diabetes.
Diabetes was monitored by measuring urine glucose levels twice weekly. Animals were considered diabetic after two consecutive readings greater than or equal to 3+. The average blood glucose levels in mice diagnosed as diabetic using this criteria are 21.96 ± 3.8 mM, and none of these mice have blood glucose levels below 16 mM.
Statistical Analyses.
Data were compared by two-tailed Mann-Whitney U-test, χ2, or two-way ANOVA tests. Statistical significance was assumed at P < 0.05.
Online Supplemental Material.
Fig. S1 shows that the IGRPK209A/F213A epitope is not recognized by, and does not alter the functional responsiveness of IGRP206–214-reactive CD8+ T cells either in vitro or in vivo. Fig. S2 shows that recruitment of naive 8.3-CD8+ T cells into the pancreatic islets of NOD mice is preceded by antigen-induced proliferation in the PLN. Fig. S3 shows that naive 8.3-CD8+ T cells do not proliferate in the PLN of NOD.IGRPKI/KI hosts.
Supplementary Material
Acknowledgments
We thank N. Ghyselinck for the pFlEx vector; S. Bou, M. DeCrom, M. Foote, B. Han, T. Irvine, H. Metselaar, and S. Thiessen for technical assistance; L. Kennedy and L. Robertson for FACS; and Y. Yang and J. Clemente-Casares for feedback on the manuscript. This work was supported by the Canadian Institutes of Health Research (CIHR), The Natural Sciences and Engineering Research Council of Canada, and the Juvenile Diabetes Research Foundation (JDRF). J.W. was supported by a fellowship from the Canadian Diabetes Association. S.T. and A.S. were supported by a CIHR-training grant (S.T.) and the Alberta Heritage Foundation for Medical Research (AHFMR) (S.T. and A.S.). G.A. was supported by the Dutch Diabetes Research Foundation. P.S. is a scientist of the AHFMR and a JDRF scholar. The authors have no conflicting financial interests.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913835107/-/DCSupplemental.
References
- 1.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 2.Savinov AY, Wong FS, Stonebraker AC, Chervonsky AV. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J Exp Med. 2003;197:643–656. doi: 10.1084/jem.20021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: New concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Stephens R, Randolph DA, Huang G, Holtzman MJ, Chaplin DD. Antigen-nonspecific recruitment of Th2 cells to the lung as a mechanism for viral infection-induced allergic asthma. J Immunol. 2002;169:5458–5467. doi: 10.4049/jimmunol.169.10.5458. [DOI] [PubMed] [Google Scholar]
- 5.Ostler T, Pircher H, Ehl S. “Bystander” recruitment of systemic memory T cells delays the immune response to respiratory virus infection. Eur J Immunol. 2003;33:1839–1848. doi: 10.1002/eji.200323460. [DOI] [PubMed] [Google Scholar]
- 6.Chen AM, Khanna N, Stohlman SA, Bergmann CC. Virus-specific and bystander CD8 T cells recruited during virus-induced encephalomyelitis. J Virol. 2005;79:4700–4708. doi: 10.1128/JVI.79.8.4700-4708.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galkina E, et al. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005;115:3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karin N, Szafer F, Mitchell D, Gold DP, Steinman L. Selective and nonselective stages in homing of T lymphocytes to the central nervous system during experimental allergic encephalomyelitis. J Immunol. 1993;150:4116–4124. [PubMed] [Google Scholar]
- 9.Picarella DE, Kratz A, Li CB, Ruddle NH, Flavell RA. Transgenic tumor necrosis factor (TNF)-alpha production in pancreatic islets leads to insulitis, not diabetes. Distinct patterns of inflammation in TNF-alpha and TNF-beta transgenic mice. J Immunol. 1993;150:4136–4150. [PubMed] [Google Scholar]
- 10.Lee MS, von Herrath M, Reiser H, Oldstone MB, Sarvetnick N. Sensitization to self (virus) antigen by in situ expression of murine interferon-gamma. J Clin Invest. 1995;95:486–492. doi: 10.1172/JCI117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faveeuw C, Gagnerault MC, Kraal G, Lepault F. Homing of lymphocytes into islets of Langerhans in prediabetic non-obese diabetic mice is not restricted to autoreactive T cells. Int Immunol. 1995;7:1905–1913. doi: 10.1093/intimm/7.12.1905. [DOI] [PubMed] [Google Scholar]
- 12.Cantagrel A, Lambert N, Alam A. T cell receptor gene in synovial tissues of rheumatoid arthritis. Int Rev Immunol. 1998;17:323–337. doi: 10.3109/08830189809054409. [DOI] [PubMed] [Google Scholar]
- 13.Peterson JD, Berg R, Piganelli JD, Poulin M, Haskins K. Analysis of leukocytes recruited to the pancreas by diabetogenic T cell clones. Cell Immunol. 1998;189:92–98. doi: 10.1006/cimm.1998.1377. [DOI] [PubMed] [Google Scholar]
- 14.Christen U, et al. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J Clin Invest. 2004;113:74–84. doi: 10.1172/JCI200417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho-Pinto C, et al. Leukocyte attraction through the CCR5 receptor controls progress from insulitis to diabetes in non-obese diabetic mice. Eur J Immunol. 2004;34:548–557. doi: 10.1002/eji.200324285. [DOI] [PubMed] [Google Scholar]
- 16.Rhode A, et al. Islet-specific expression of CXCL10 causes spontaneous islet infiltration and accelerates diabetes development. J Immunol. 2005;175:3516–3524. doi: 10.4049/jimmunol.175.6.3516. [DOI] [PubMed] [Google Scholar]
- 17.Archambault AS, Sim J, Gimenez MA, Russell JH. Defining antigen-dependent stages of T cell migration from the blood to the central nervous system parenchyma. Eur J Immunol. 2005;35:1076–1085. doi: 10.1002/eji.200425864. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, et al. Active participation of antigen-nonspecific lymphoid cells in immune-mediated inflammation. J Immunol. 2006;177:3362–3368. doi: 10.4049/jimmunol.177.5.3362. [DOI] [PubMed] [Google Scholar]
- 19.Smorodchenko A, et al. CNS-irrelevant T-cells enter the brain, cause blood-brain barrier disruption but no glial pathology. Eur J Neurosci. 2007;26:1387–1398. doi: 10.1111/j.1460-9568.2007.05792.x. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman SM, DiLorenzo TP. A comprehensive guide to antibody and T-cell responses in type 1 diabetes. Tissue Antigens. 2003;62:359–377. doi: 10.1034/j.1399-0039.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsai S, Shameli A, Santamaria P. CD8+ T cells in type 1 diabetes. Adv Immunol. 2008;100:79–124. doi: 10.1016/S0065-2776(08)00804-3. [DOI] [PubMed] [Google Scholar]
- 22.Verdaguer J, et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson B, Park BJ, Verdaguer J, Amrani A, Santamaria P. Prevalent CD8(+) T cell response against one peptide/MHC complex in autoimmune diabetes. Proc Natl Acad Sci USA. 1999;96:9311–9316. doi: 10.1073/pnas.96.16.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amrani A, et al. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature. 2000;406:739–742. doi: 10.1038/35021081. [DOI] [PubMed] [Google Scholar]
- 25.Amrani A, et al. Expansion of the antigenic repertoire of a single T cell receptor upon T cell activation. J Immunol. 2001;167:655–666. doi: 10.4049/jimmunol.167.2.655. [DOI] [PubMed] [Google Scholar]
- 26.Verdaguer J, et al. Acceleration of spontaneous diabetes in TCR-beta-transgenic nonobese diabetic mice by beta-cell cytotoxic CD8+ T cells expressing identical endogenous TCR-alpha chains. J Immunol. 1996;157:4726–4735. [PubMed] [Google Scholar]
- 27.Lieberman SM, et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci USA. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trudeau J, et al. Autoreactive T cells in peripheral blood predict development of type 1 diabetes. J Clin Invest. 2003;111:217–223. doi: 10.1172/JCI16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong FS, et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat Med. 1999;5:1026–1031. doi: 10.1038/12465. [DOI] [PubMed] [Google Scholar]
- 30.Loetscher M, et al. Chemokine receptor specific for IP10 and mig: Structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 32.Lennon GP, et al. T cell islet accumulation in type 1 diabetes is a tightly regulated, cell-autonomous event. Immunity. 2009;31:643–653. doi: 10.1016/j.immuni.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marelli-Berg FM, et al. Cognate recognition of the endothelium induces HY-specific CD8+ T-lymphocyte transendothelial migration (diapedesis) in vivo. Blood. 2004;103:3111–3116. doi: 10.1182/blood-2003-08-2717. [DOI] [PubMed] [Google Scholar]
- 34.Yamanouchi J, et al. Cross-priming of diabetogenic T cells dissociated from CTL-induced shedding of beta cell autoantigens. J Immunol. 2003;171:6900–6909. doi: 10.4049/jimmunol.171.12.6900. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton-Williams EE, Palmer SE, Charlton B, Slattery RM. Beta cell MHC class I is a late requirement for diabetes. Proc Natl Acad Sci USA. 2003;100:6688–6693. doi: 10.1073/pnas.1131954100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarchum I, Takaki T, DiLorenzo TP. Efficient culture of CD8(+) T cells from the islets of NOD mice and their use for the study of autoreactive specificities. J Immunol Methods. 2008;339:66–73. doi: 10.1016/j.jim.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.