Cells commit suicide in a highly regulated manner to sculpt an organism during development, to maintain homeostasis in postnatal tissues, and in response to noxious cues. It was thought until recently that cells in this situation were eliminated solely by apoptosis, a process whereby they shrink, fragment, and undergo phagocytosis—all while maintaining plasma membrane integrity and thereby avoiding inflammation. Necrosis, a nonapoptotic form of cell death characterized by depletion of cellular ATP, plasma membrane rupture, cell swelling, and marked inflammation, has been recognized for centuries but until recently was considered a passive and unregulated process. Work in worms and mammals over the past decade, however, has led to the unexpected discovery that, at least in some instances, necrosis can be deliberate and regulated (1–5). Although intensive work over the past 20 years has produced a mature blueprint for the molecular regulation of apoptosis (6), delineation of programmed necrosis signaling remains a work in progress. Nevertheless, an obvious next step is to begin to elucidate the interface between molecular events that mediate apoptosis and necrosis. As reported in PNAS, Chen et al. (7) manipulated a protein that can induce cell death with either apoptotic or necrotic features to probe the relationship between apoptosis and necrosis mechanisms in the mitochondria and endoplasmic reticulum (ER).
Apoptosis (6) and necrosis (3–5, 8) are each mediated by pathways that involve cell-surface death receptors and the mitochondria/ER. During apoptosis, death signals are relayed via BH3-only proteins [proapoptotic Bcl-2 proteins that share homology only in Bcl-2 homology domain 3 (BH3)] to Bax and Bak (“multidomain” proapoptotic Bcl-2 proteins). Bax and Bak subsequently initiate poorly understood events that lead to the permeabilization of the outer mitochondrial membrane (OMM). The resulting release of cytochrome c into the cytosol triggers apoptosome assembly and subsequent caspase activation and apoptosis. Simultaneous deletion of Bax and Bak renders cells resistant to apoptotic signals transduced via the mitochondrial pathway (9). The mitochondrial events of necrosis are quite different and involve opening of a pore in the inner mitochondrial membrane (IMM) referred to as the mitochondrial permeability transition pore (MPTP). Although the biochemical composition of MPTP is uncertain, it is known to be regulated by cyclophilin D, a peptidyl-prolyl cis-trans isomerase located in the mitochondrial matrix. The cellular consequences of MPTP opening are profound. There is loss of Δψm, the electrical potential across the IMM that is needed to drive ADP→ATP production. Moreover, MPTP opening results in marked mitochondrial swelling and potentially outright rupture of the OMM. OMM rupture during necrosis allows the release of apoptogens that likely engage components of the apoptosis machinery to further enhance cell death. Deletion of ppif, encoding cyclophilin D, confers resistance to necrosis resulting from stimuli that use the mitochondrial pathway without affecting susceptibility to apoptosis (3–5).
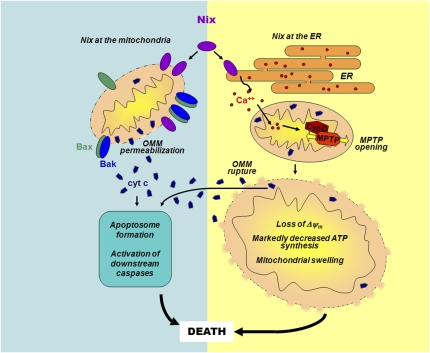
Nix/BNip3L is a “BH3-only-like” protein residing at the OMM and ER that can stimulate apoptotic or necrotic cell death (10). It is referred to as BH3-only-like because, unlike classic BH3-only proteins, its BH3-like domain is dispensable for cell killing. Chen et al. (7) study the differential effects of Nix/BNip3L at the mitochondria vs. ER. The investigators expressed mitochondrially- or ER-targeted versions of Nix/BNip3L in mouse embryo fibroblasts lacking endogenous Nix/BNip3L, Bax/Bak, or cyclophilin D, and in the hearts of transgenic mice. They conclude that Nix/BNip3L induces cell death via two modalities (Fig. 1): (i) Mitochondrially-targeted Nix/BNip3L stimulates classic Bax/Bak-dependent OMM permeabilization, cytochrome c release, caspase activation, and apoptosis; and (ii) ER-targeted Nix/BNip3L induces cyclophilin D-dependent MPTP opening, leading to necrotic-appearing mitochondrial structural changes, and cytochrome c release, which is Bax/Bak independent and presumably involves OMM rupture. Thus, Nix/Bnip3L seems to be capable of inducing apoptotic and necrotic death programs, depending on whether it is located at the OMM or ER membrane.
Fig. 1.
Dual actions of Nix/BNip3L at the mitochondria and ER. Mitochondrial Nix/BNip3L (denoted as Nix) induces traditional Bax/Bak-dependent OMM permeabilization, release of cytochrome c (cyt c), caspase activation, and apoptosis. In contrast, Nix/BNip3L at the ER induces cyclophilin D (CypD)-dependent opening of the MPTP in the IMM, causing loss of the electrical potential difference across the IMM (ΔΨm), marked decreases in ATP production, and changes in mitochondrial morphology, such as swelling, that are typical of necrosis. Cytochrome c release also occurs but is independent of Bax/Bak and likely reflects rupture, as opposed to permeabilization, of the OMM. Ca2+ is postulated to be a connection between ER and mitochondrial events.
An interesting question raised by this study concerns the death signal(s) transmitted to the mitochondria from Nix/BNip3L at the ER and the mechanism by which they are delivered. There is precedent for ER to mitochondrial death signaling involving Ca2+. In classic papers from the Korsmeyer laboratory (11, 12), the absence of Bax and Bak allowed a Bcl-2-mediated ER Ca2+ leak through the type 1 inositol 1, 4, 5-trisphosphate receptor (IP3R-1). The resulting depletion of ER luminal Ca2+ blunted ER Ca2+ release, mitochondrial uptake, and cell death induced by ER-dependent stimuli. Repletion of ER Ca2+ levels by overexpression of sarco/endoplasmic reticulum Ca2+ ATPase 2 bypassed the absence of Bax/Bak to restore cell killing. This work demonstrates that ER-resident Bcl-2 proteins can modulate Ca2+ transfer from ER to mitochondria to regulate cell death.
Interestingly, the role of the proapoptotic proteins, Bax and Bak, in this model is to stop a Bcl-2-induced leak and thereby maintain resting ER Ca2+ levels adequate to respond to an ER death stimulus. This leaves open the question of which mechanism is responsible for the acute release of Ca2+ in response to an ER death stimulus. One possibility is ER-localized BH3-only proteins. In fact, the Pimentel-Muinos laboratory recently showed that Bax/Bak-deficient cells expressing only ER-targeted Bak can be induced to undergo cell death by ER-targeted BimEL or Puma, BH3-only proteins (13). Cell death required the transfer of ER Ca2+ to the mitochondria, although Ca2+ alone was insufficient. In this study, BH3-only proteins were unable to induce cell death in the combined absence of Bax and Bak. This leaves unexplained how ER-localized Nix/BNip3L is able to function in cells globally lacking Bax and Bak, an observation that may reflect fundamental differences in how BH3-only and BH3-only-like proteins work.
These Ca2+-related mechanisms may be relevant to death signals transmitted to the mitochondria by ER-localized Nix/BNip3L. First, Ca2+ is a likely candidate, given that it is a potent stimulus for MPTP opening (14). Second, the authors have previously shown that ER luminal Ca2+ concentrations in Nix/BNip3L null cells are reduced and that restoration of ER Ca2+ in these cells restores killing (15). Thus far, these data are reminiscent of the effects of Bax/Bak in setting resting ER Ca2+ levels. Additional studies are needed to define the extent to which ER-localized Nix/BNip3L controls dynamic ER Ca2+ release and mitochondrial uptake in response to ER death stimuli.
How ER Ca2+ gains access to mitochondria and precisely what it does when it gets there remain unclear. For example, it is unresolved whether Ca2+ is released into the cytosol to bathe all of the mitochondria or whether the transfer occurs through direct connections between the ER and mitochondrial membranes. Indeed, a population of mitochondria is directly tethered to the ER in an anchored complex that allows for more direct communication and exchange of Ca2+(16). These “attached” mitochondria might play a more dedicated role in ER stress-induced cell death through local ER to mitochondrial transfer of Ca2+ and other moieties. Recently Ca2+/calmodulin-dependent protein kinase II has been suggested to facilitate the uptake of Ca2+ into mitochondria (17), but precisely how it fits into these models is presently unclear. It is also poorly understood how ER Ca2+ ends up in specific mitochondrial compartments and how intramitochondrial location influences the cell death program that is activated. Chen et al. (7) show that ER-localized Nix/BNip3L triggered necrosis, as demonstrated by mitochondrial structural changes and cyclophilin D-dependence. But apoptosis is often reported to be the outcome of ER to mitochondrial transfer of Ca2+.
In summary, the results reported by Chen et al. indicate that Nix/BNip3L provides a point of convergence between apoptotic and necrotic signaling determined by the subcellular location of this protein at the mitochondria or ER. More broadly, this study supports an emerging paradigm whereby ER to mitochondrial signaling, often through Ca2+, may serve as a critical link between ER-localized Bcl-2 proteins that traditionally regulate apoptosis, and necrotic cell death.
Acknowledgments
We thank Dr. Elizabeth Tang and Vladimir Kaplinskiy for assistance with figure preparation. R.N.K. is supported by grants from the National Institutes of Health (NIH), the Wilf Family Cardiovascular Research Institute, and the Dr. Gerald and Myra Dorros Chair in Cardiovascular Disease of the Albert Einstein College of Medicine. J.D.M. is supported by grants from the NIH, Fondation Leducq, and Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
See companion article on page 9035.
References
- 1.Xu K, Tavernarakis N, Driscoll M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca(2+) release from the endoplasmic reticulum. Neuron. 2001;31:957–971. doi: 10.1016/s0896-6273(01)00432-9. [DOI] [PubMed] [Google Scholar]
- 2.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 3.Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 4.Nakagawa T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 5.Schinzel AC, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, et al. Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc Natl Acad Sci USA. 2010;107:9035–9042. doi: 10.1073/pnas.0914013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Wei MC, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinnadurai G, Vijayalingam S, Gibson SB. BNIP3 subfamily BH3-only proteins: Mitochondrial stress sensors in normal and pathological functions. Oncogene. 2008;27(Suppl 1):S114–S127. doi: 10.1038/onc.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scorrano L, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 12.Oakes SA, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klee M, Pallauf K, Alcalá S, Fleischer A, Pimentel-Muiños FX. Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. EMBO J. 2009;28:1757–1768. doi: 10.1038/emboj.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Diwan A, et al. Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Invest. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: Structure and signaling dynamics. Trends Cell Biol. 2007;17:511–517. doi: 10.1016/j.tcb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Timmins JM, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]