Abstract
The recognition of virus infected or malignantly transformed cells by cytotoxic T lymphocytes critically depends on the transporter associated with antigen processing (TAP), which delivers proteasomal degradation products into the endoplasmic reticulum lumen for subsequent loading of major histocompatibility complex class I molecules. Here we have identified a single cysteinyl residue in the TAP complex that modulates peptide binding and translocation, thereby restricting the epitope repertoire. Cysteine 213 in human TAP2 was found to be part of a newly uncovered substrate-binding site crucial for peptide recognition. This residue contacts the peptide in the binding pocket in an orientated manner. The translocation complex can be reversibly inactivated by thiol modification of this cysteinyl residue. As part of an unexpected mechanism, this residue is crucial in complementing the binding pocket for a given subset of epitopes as well as in maintaining a substrate-receptive conformation of the translocation complex.
Keywords: ATP-binding cassette transporter, cysteine scanning mutagenesis, membrane proteins, molecular recognition, substrate specificity
Adaptive immunity plays an essential role protecting vertebrates against a broad range of pathogens and cancer. The major histocompatibility complex (MHC) class-I-dependent pathway of antigen presentation represents a sophisticated strategy to recognize and eliminate infected or malignantly transformed cells, taking advantage of the constant protein turnover by the proteasome pathway (1–3). The endoplasmic reticulum (ER) resident transporter associated with antigen processing (TAP1/2, ABCB2/3) is a crucial component of this pathway because it delivers proteasomal degradation products into the ER, thereby catalyzing the assembly of peptide-MHC I complexes for presentation and recognition by cytotoxic T lymphocytes at the cell surface (4, 5).
The ATP-binding cassette (ABC) transport complex is composed of two subunits, TAP1 and TAP2, each containing a transmembrane domain (TMD) followed by a cytosolic nucleotide-binding domain (NBD) (6). A core complex of 6 + 6 transmembrane segments (TM) has been identified to be essential and sufficient for ER targeting, membrane insertion, complex formation, peptide binding, and ER translocation, whereas a unique N-terminal domain (TMD0) at each subunit is crucial for tapasin binding and thus for assembly of a macromolecular MHC-I-loading complex (7). The translocation mechanism can be dissected into an ATP-independent peptide binding and an ATP-dependent translocation step (8). TAP1 and TAP2 are both essential and sufficient for these processes (8, 9). The peptide-binding pocket of the core TAP complex has been mapped to the cytosolic loop 2 and a stretch of 15 amino acids following TM6 of the core TAP complex (10, 11). Although TAP preferentially binds peptides with a length of 8–16 amino acids, peptides of 8–12 amino acids are most efficiently translocated (8). By using combinatorial peptide libraries, the binding motif of the human TAP complex was systematically deciphered. Apart from the amino acid and carboxy termini, the three N-terminal and the last C-terminal residues of the peptide are critical for binding to TAP (12–14). It has been shown that Cys-less TAP1 or TAP2 can restore MHC I antigen presentation in TAP1- or TAP2-negative cells (15). However, functional details of a Cys-less TAP complex have not been investigated.
Here we have identified a single residue in the TAP2 subunit (Cys 213) that is crucial for recognition of a subset of peptides by the TAP complex. This residue interacts directly with the peptide in a defined orientation within the binding pocket. As part of an unexpected mechanism, Cys 213 is crucial to complement the substrate-binding pocket of the TAP complex by stabilizing a peptide-receptive conformation.
Results
Cys-Less TAP Displays an Altered Substrate Specificity.
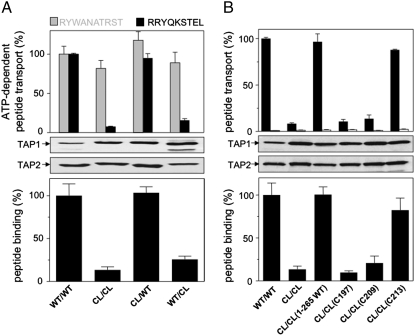
To investigate the impact of the 10 and 9 cysteines in TAP1 and TAP2, respectively, we combined the Cys-less (CL) and wild-type (WT) subunits and analyzed the effect of peptide recognition and ER translocation. TAP-specific and ATP-dependent peptide transport was examined by incubating microsomes with the fluorescently labeled, high-affinity epitope RRYQNSTC(F)L (R9L-F) for 3 min at 32 °C. N-core glycosylated, thus translocated, peptides were recovered on ConA beads, eluted with methyl-α-D-mannopyranoside, and quantified. Notably, all constructs showed a TAP-specific, ATP-dependent peptide transport activity (Fig. 1A). Each subunit was expressed at the same level as shown by immunoblotting. Slight changes in electrophoretic mobility of the TAP variants are due to a C-terminal His10- and Strep-tag of TAP1 and TAP2, respectively. Based on the epitope R10T-A (RYWANATRC(A)T labeled with ATTO565), which was transported with the same efficiency by all complexes (gray bars) and therefore serves as reference, we surprisingly found that the CL/CL or WT/CL TAP1/2 complexes displayed a significantly lower transport activity for the epitope R9L-F compared to WT/WT or CL/WT (black bars). We conclude that the Cys-less TAP complex is functional with respect to peptide transport, however, displaying a different substrate specificity, which is caused by Cys-less TAP2.
Fig. 1.
Function of cysteines in the human TAP complex. (A) Wild-type and Cys-less TAP subunits have a different impact on peptide binding and translocation. ATP-dependent transport assays were performed with microsomes (normalized to TAP2 expression) for 3 min at 32 °C using 1 μM of R9L-F (RRYQNSTC(F)L, black bars) and R10T-A (RYWANATRC(A)T, gray bars). After lysis, N-core glycosylated peptides were bound to ConA beads, eluted with methyl-D-mannopyranoside, and quantified by fluorescence detection. Transport of R9L-F by wild-type TAP was set to 100%. TAP-containing microsomes (20 μg protein per lane) were analyzed by SDS-PAGE (10%) followed by immunoblotting against TAP1 and TAP2 (mAb148.3 and mAb435.3, respectively). Peptide-binding studies were performed with microsomes (normalized to TAP2 expression) and 1 μM radiolabeled RR(125I)YQKSTEL for 20 min on ice and corrected for background binding. Peptide binding of wild-type TAP was set to 100%. All experiments were performed as triplicates. (B) C213 in TAP2 is critical for substrate binding and transport. Transport of R9L-F in the presence (black bar) or absence of MgATP (3 mM, open bar) was carried as described in A. Expression of and peptide binding to TAP mutants were analyzed as described in A. R9L-F binding of wild-type TAP was set to 100%. All experiments were performed as triplicates.
Peptide translocation into the ER lumen is a multistep process, accompanied by structural rearrangements in the TMDs and NBDs (16, 17). We therefore examined if the altered substrate specificity is caused by a different binding mode. TAP-containing microsomes were incubated with the two radiolabeled epitopes on ice. In case of the epitope R9L, a drastically reduced binding activity was observed for the CL/CL or WT/CL complex in comparison with WT/WT or CL/WT (Fig. 1A, Lower), while binding of the epitope R10T is not affected (Table 1). These data demonstrate that one or more of the 10 intrinsic cysteines of TAP2 play a key role in modulating the substrate specificity of the TAP complex.
Table 1.
Peptide dissociation constants Kd and maximal binding Bmax (Eq. S1) of TAP variants for the different epitopes R9L and *R10T (in bold)
| TAP1/TAP2 | Bmax, % | Kd, nM |
| WT/WT | 100.0 ± 5.1 | 493 ± 78 |
| CL/CL | 8.0 ± 1.5 | 688 ± 371 |
| CL/CL(C197) | 3.7 ± 0.8 | 861 ± 516 |
| CL/CL(C209) | 7.6 ± 1.1 | 913 ± 350 |
| CL/CL(C213) | 130.7 ± 6.9 | 536 ± 85 |
| WT/WT* | 100.0 ± 5.1 | 1,578 ± 450 |
| CL/CL* | 85.0 ± 8.5 | 1,700 ± 400 |
Single Cysteinyl Residue Modulates TAP Specificity.
The peptide-binding region of TAP2 has been previously mapped to residues 330–452 (10). Notably, 3 out of 10 of the exchanged cysteines (C353, C362, and C394) are located within this region and thus might be critical for peptide binding. To address the impact of these residues, we reintroduced single cysteines at position 353, 362, and 394 in Cys-less TAP2 and combined those with Cys-less TAP1. Surprisingly, CL/CL(S353C), CL/CL(S362C), and CL/CL(V394C) show the same peptide binding and transport activity as the CL/CL complex (Fig. 1B). In addition, combinations of double and triple mutations of these three cysteines display an identical functional fingerprint as Cys-less TAP. These results together indicate that the cysteines within the previously identified peptide-binding region are not involved in the change of the substrate specificity.
We next introduced a combination of four cysteines within the TMD, which are located outside of the putative peptide-binding region (residues 70, 197, 209, and 213 of TAP2). Strikingly, this CL/CL(1265WT) complex displayed wild-type activity (Fig. 1B). We thus conclude that either one or combinations of the four cysteines located outside of the previously identified peptide-binding region of TAP2 are critical for substrate selection of the antigen translocation machinery.
Because the 6 + 6 TM core TAP complex is sufficient for peptide binding and transport (7), C70 located outside of core TAP2 should not be responsible for the altered peptide-binding activity of the CL/CL complex. Therefore, the remaining three cysteines at positions 197, 209, and 213 were individually reintroduced into Cys-less TAP2. Equal expression levels of all mutants were confirmed by immunoblotting. Strikingly, reinsertion of a single cysteine into TAP2 at position 213, CL/CL(C213), fully restores the binding and transport activity of wild-type TAP. By contrast, translocation complexes with single cysteines at position 197 or 209 in TAP2, very close to C213, resembled the Cys-less phenotype. In conclusion, a single cysteine in the TAP complex is crucial to control the substrate specificity of the antigen translocation machinery.
Cysteine 213 of TAP2 is Directly Involved in Substrate Binding.
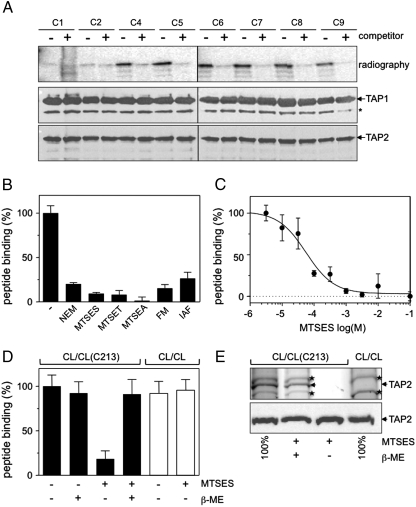
To investigate whether C213 of TAP2 is localized in the peptide-binding pocket, we performed cysteine cross-linking experiments with the single-cysteine CL/CL(C213) complex. Single-cysteine peptides can form a disulfide crosslink in the presence of copper phenanthroline only if two cysteines are in very close proximity. After quenching of free cysteines by N-ethylmaleimide (NEM), TAP complexes were purified and analyzed by nonreducing SDS-PAGE and autoradiography. Cross-linking was observed for peptides containing a cysteine at positions 4–9 (Fig. 2A). Notably, an excess of competitor peptide blocks the cross-linking, confirming the specificity of the reaction. These results demonstrate that C213 of TAP2 is in direct contact with the bound peptide and therefore part of the substrate-binding pocket of the TAP complex. Because the peptide positions 1 and 2 are not cross-linked, we conclude that the peptide is bound to TAP in an oriented fashion.
Fig. 2.
Functional importance of the C213 in TAP2. (A) Oxidative cross-linking of CL/CL(C213) in microsomes (0.5 mg of total protein) with radiolabeled peptides containing a single cysteine (1.25 μM, 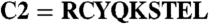 ). Cross-linking was induced by adding 1 mM of copper phenanthroline in the presence or absence of the competitor RRYQKSTEL (0.25 mM). After metal affinity purification of the TAP complex, cross-linked products were analyzed by nonreducing SDS-PAGE (10%) and autoradiography. The TAP expression was confirmed by SDS-PAGE and immunoblotting against TAP1 and TAP2 (mAb148.3 and mAb435.3, respectively). The asterisk indicates a TAP1 degradation product. (B) Modification of C213 blocks peptide binding of TAP. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with various thiol-specific reagents [0.5 mM NEM; 10 mM MTSES; 1 mM [2-{trimethylammonium)ethyl]methanethiosulfonate; 2.5 mM (2-aminoethyl)methanethiosulfonate; 0.25 mM fluorescein-5-maleimide (F-Mal); 0.25 mM 5-iodoacetamidofluorescein (5-IAF)] for 15 min on ice. Subsequently, peptide-binding assays were performed using radiolabeled RR(125I)YQKSTEL (1 μM) as a reporter. (C) Inhibition of peptide binding by MTSES. To determine the half-maximum inhibition value (IC50) of MTSES, CL/CL(C213) containing microsomes were incubated with an increasing concentration of MTSES followed by peptide-binding studies under conditions as described in Fig. 2A. The IC50 was determined to be 52 ± 7 μM (Eq. S2). (D) Reversible TAP inhibition by thiol-specific modification. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with 10 mM of MTSES for 15 min on ice and subsequently incubated with or without β-ME (100 mM) for 30 min on ice. Peptide binding was assayed with 1 μM of radiolabeled RR(125I)YQKSTEL. (E) Efficiency of MTS labeling was determined by means of accessibility for alkylation by 5-IAF. After MTS labeling, samples were incubated with 5-IAF (250 μM) for 15 min on ice and the relative amounts of 5-IAF-modified TAP were determined by in-gel fluorescence. To determine the maximal labeling capacity by 5-IAF (shown as 100%), TAP was denatured by SDS (2%) for 20 min at room temperature and then labeled with 250 μM of 5-IAF for 3 min before SDS-PAGE (10%) and immunoblotting. Asterisks indicate nonspecific labeling.
). Cross-linking was induced by adding 1 mM of copper phenanthroline in the presence or absence of the competitor RRYQKSTEL (0.25 mM). After metal affinity purification of the TAP complex, cross-linked products were analyzed by nonreducing SDS-PAGE (10%) and autoradiography. The TAP expression was confirmed by SDS-PAGE and immunoblotting against TAP1 and TAP2 (mAb148.3 and mAb435.3, respectively). The asterisk indicates a TAP1 degradation product. (B) Modification of C213 blocks peptide binding of TAP. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with various thiol-specific reagents [0.5 mM NEM; 10 mM MTSES; 1 mM [2-{trimethylammonium)ethyl]methanethiosulfonate; 2.5 mM (2-aminoethyl)methanethiosulfonate; 0.25 mM fluorescein-5-maleimide (F-Mal); 0.25 mM 5-iodoacetamidofluorescein (5-IAF)] for 15 min on ice. Subsequently, peptide-binding assays were performed using radiolabeled RR(125I)YQKSTEL (1 μM) as a reporter. (C) Inhibition of peptide binding by MTSES. To determine the half-maximum inhibition value (IC50) of MTSES, CL/CL(C213) containing microsomes were incubated with an increasing concentration of MTSES followed by peptide-binding studies under conditions as described in Fig. 2A. The IC50 was determined to be 52 ± 7 μM (Eq. S2). (D) Reversible TAP inhibition by thiol-specific modification. CL/CL(C213) containing microsomes (20 μg of total protein) were incubated with 10 mM of MTSES for 15 min on ice and subsequently incubated with or without β-ME (100 mM) for 30 min on ice. Peptide binding was assayed with 1 μM of radiolabeled RR(125I)YQKSTEL. (E) Efficiency of MTS labeling was determined by means of accessibility for alkylation by 5-IAF. After MTS labeling, samples were incubated with 5-IAF (250 μM) for 15 min on ice and the relative amounts of 5-IAF-modified TAP were determined by in-gel fluorescence. To determine the maximal labeling capacity by 5-IAF (shown as 100%), TAP was denatured by SDS (2%) for 20 min at room temperature and then labeled with 250 μM of 5-IAF for 3 min before SDS-PAGE (10%) and immunoblotting. Asterisks indicate nonspecific labeling.
We next probed the functional importance of C213 by different thiol-specific reagents. As shown in Fig. 2B, modification of C213 blocks peptide binding to TAP. This effect is most likely caused by steric hindrance, because it is independent of the chemical properties of the reagent. The IC50 of (2sulfonatoethyl)methanethiosulfonate (MTSES) was determined to be 52 ± 7 μM (Fig. 2C), suggesting a quantitative labeling at 10 mM of MTSES. Importantly, the binding activity of TAP could be fully restored by reductive cleavage with β-mercaptoethanol (β-ME) (Fig. 2D). In addition, methanethiosulfonate (MTS) reagents did not affect peptide binding to the CL/CL complex, demonstrating that the effects are specific for C213. Quantitative modification of C213 by MTSES was further demonstrated by subsequent labeling with 5-iodoacetamidofluorescein (5-IAF) and in gel fluorescence detection (Fig. 2E). For reference, 5-IAF did not label the Cys-less TAP complex. These results demonstrate that exclusively C213 is modified and that the microsome preparations are not contaminated by wild-type TAP. In summary, C213 in TAP2 is part of a newly identified binding pocket, in which peptides are fixed in a defined orientation. Thiol-specific modifications prove that this cysteinyl residue is critical for TAP function.
Modulation of the Binding Motif.
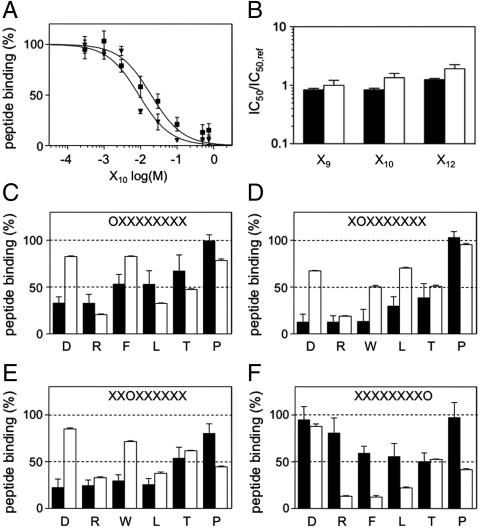
After the important finding that a single residue within TAP2 controls the binding characteristics of the hetrodimeric transporter, we next raised the question whether the C213-modulated epitope selection is based on differences in either the sequence or the length of the peptide. Therefore, we set up a competition assay using the permissive epitope R10T as reporter and peptide libraries of different length as competitors (Fig. 3A). As summarized in Fig. 3B, Cys-less and wild-type TAP have the same affinity toward the 9-, 10-, and 12mer library (IC50 ∼ 10 μM), demonstrating that the epitopes R9L and R10T are not differentiated with respect to their length.
Fig. 3.
C213 in TAP2 controls the substrate specificity. (A) Peptide length specificity of the Cys-less (▾) and wild-type TAP (▪) complex. Competition assays were performed with TAP-containing microsomes (20 μg of total protein), 1 μM of radiolabeled R(125I)YWANATRST (R10T), and increasing concentrations of the peptide library X10. The IC50 of each library was determined by Eq. S2. (B) Peptide length specificity of the Cys-less (black) and wild-type TAP complex (open). The IC50/IC50,ref for competition of radiolabeled R10T was determined as described before. Competition of wild-type TAP by the X9 library served as reference (IC50,ref). (C–F) Cys-less (black) and wild-type TAP (open) display a different binding motif. Competition assays were performed with TAP-containing microsomes (20 μg of total protein) using 1 μM of R(125I)YWANATRST as reporter peptide. The concentration of the sublibraries was set to the IC50 value of the X9 library (10 μM). For comparison, the competition values of human TAP (WT/WT) were taken from Ref. 13. All experiments were performed in triplicates.
It is well established that the three N-terminal and the last C-terminal peptide residues are critical for TAP binding, whereas residues in between do not significantly contribute (12–14). Therefore, we applied a scanning approach of combinatorial peptide libraries focusing on positions 1, 2, 3, and 9. The radiolabeled peptide R10T was used as reporter. The randomized peptide library (X9) served as internal reference, yielding 50% inhibition of peptide binding (IC50) at the given concentration (10 μm). Favored and disfavored sublibraries (i.e., OX8, XOX7, X2OX6, and X8O) are marked by a value below or above 50% binding, respectively. As summarized in Fig. 3 C–F, wild-type and Cys-less TAP have a very different binding motif. Although a negatively charged residue at positions 1, 2, and 3 is highly disfavored for the wild-type TAP, Asp is well accepted by the Cys-less complex. Similarly, bulky hydrophobic residues (Phe, Trp) at positions 1 and 2 are disfavored by wild-type, but preferred by Cys-less TAP. As previously described, epitopes containing proline at position 2 are not recognized by human TAP (12, 13). Surprisingly, Cys-less TAP can accept these peptides. Differences for the C-terminal residue are even more pronounced. Although wild-type TAP has a marked preference for peptides harboring a basic or hydrophobic residue at their C terminus (12, 13, 18), Cys-less TAP is rather promiscuous in this position. Taken together, Cys-less TAP has an altered mode of binding. Compared to wild type, Cys-less TAP is more promiscuous toward the C-terminal residue of the bound peptide and shows a high binding affinity for peptides containing negatively charged residues at the N-terminal positions or aromatic residues at the second position.
Cysteine 213 Promotes a Peptide-Receptive Conformation.
Based on the important finding that the altered binding motif of Cys-less TAP could be allocated to a single residue, we sought to understand the mechanistic details of this effect. Therefore, we compared the binding activities of wild-type, single-Cys, and Cys-less TAP complexes toward the epitope R9L and R10T. In the range of error, the CL/CL(C213) and WT complex displayed similar Kd and Bmax values (Table 1). These results were somewhat expected because the initial transport rates of the CL/CL(C213) complex is comparable to wild-type TAP (see Fig. 1B). By contrast, the Bmax values of the CL/CL, CL/CL(C209), and CL/CL(C197) complex are drastically reduced, whereas the KD values do not differ significantly. Thus, only a limited fraction of the transport complexes is receptive for the epitope R9L. In contrast, the wild-type and Cys-less complexes show very similar Kd and Bmax values for the R10T epitope (Table 1). In conclusion, peptide binding and transport by TAP can be promoted either by the optimal peptide (R10T vs. R9L) or by C213, thus stabilizing a peptide-receptive conformation.
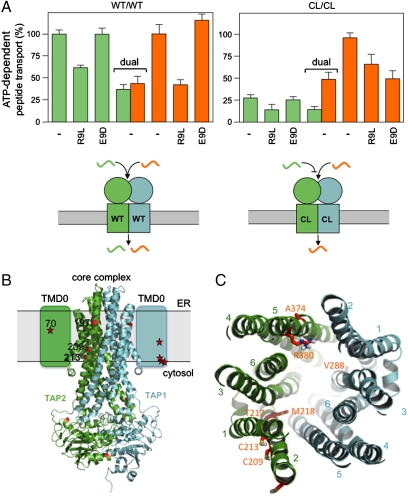
To corroborate this model, we developed a dual-color translocation assay, which allowed us to follow the translocation rate of two epitopes in parallel (Fig. 4A). Peptides R9L-F and R10T-A were labeled with different fluorophores, with no spectral overlap. Notably, the fluorophores attached do not change the peptide-binding affinity to TAP. For R9L-F, the transport activity of Cys-less TAP is drastically reduced in comparison to wild type, whereas R10T-A is translocated equally well by both complexes, confirming our previous results. We next examined the peptide RRYQNSTEL (R9L,) and EPGYTNSTD (E9D), which are high-affinity and nonbinder to wild-type TAP, respectively (13). If R9L is present in equimolar ratio, the transport activity of R9L-F by wild-type and Cys-less TAP is reduced by 50% (black bars). In contrast, the transport activity of R10T-A by wild-type TAP is decreased to 50% as expected, whereas the Cys-less complex has 75% remaining transport activity in the presence of the competitor R9L (open bars). This underlines the existence of R9L-receptive and nonreceptive CL/CL complexes. These findings are finally confirmed by dual-color transport assays. For wild-type TAP, the peptide transport activity is similar (∼50%) for both peptides, whereas for Cys-less TAP, one population transports 50% of R10T, and R9L is transported only by a subpopulation of transporters (∼20%). For wild-type and the R9L-receptive TAP complexes, transport of R9L-F was not affected by the highly disfavored epitope E9D. In contrast, transport of R10T-A by Cys-less TAP was inhibited by E9D, demonstrating that the specificities of the wild-type and Cys-less complexes are drastically different. These results provide further evidence for a receptive and nonreceptive conformation of the Cys-less complex for a given subset of peptides.
Fig. 4.
Dual-color translocation assays reveal restrictions in a shared TAP1/2 interface. (A) ATP-dependent transport of RRYQNSTC(F)L (green bars) and RYWANATRC(A)T (orange bars) by CL/CL and WT/WT were performed as described in Fig. 1A. The competitor peptides RRYKQNSTEL (NST) or EPGYTNSTD (E9D) were applied in equimolar amount. The transport of R9L-F by wild-type TAP was normalized to 100%. A model of the altered binding and translocation modes of wild-type and Cys-less TAP is given below. (B) 3D homology model of the core TAP complex composed of TAP1 (cyan) and TAP2 (green) based on the X-ray structure of Sav1866 (PDB 2HYD) (25, 29). The extra N-terminal domains (TMD0) of each subunit are illustrated schematically. The location of the 10 and 9 cysteines in TAP1 and TAP2, respectively, are indicated in red. (C) Key residues (red) lining the putative substrate-binding pocket and translocation pathway viewed from the ER membrane toward the TMD–NBD interface. The transmembrane helices of core TAP1 and TAP2 are numbered.
Discussion
As the key component of the MHC I peptide-loading complex, TAP translocates a smidgen of the cellular proteome into the ER lumen for the processive assembly of MHC I molecules. Cys-less TAP has been instrumental to determine the membrane topology of the TAP complex by membrane impermeable thiol-specific probes and cysteine scanning approaches (15). Cys-less TAP1 and TAP2 can restore antigen processing and MHC I surface expression in cells lacking either TAP1 or TAP2 (19). However, based on the normalization of the TAP level by immunoblotting and binding assays (Bmax value) using the radiolabeled peptide RR(125I)YWANATRST, the surprising effects on altered epitope selection and transport could not be detected in previous studies. However, (i) by direct comparison of different epitopes, RRYQNSTC(F)L vs. RYWANATRC(A)T, (ii) by generating a systematic set of TAP mutants, (iii) by the use of combinatorial peptide libraries, and (iv) by establishing a dual-transport assay to follow the translocation of different epitopes in parallel, the appealing differences of the wild-type and Cys-less TAP complex became obvious. By comparison of the binding and transport characteristics of wild-type and Cys-less chimera, we could identify Cys 213 in TAP2 as a critical residue modulating the substrate specificity of the TAP complex. This finding is instrumental to our current understanding of solute translocation across cellular membranes. Apart from defects in misfolding and/or trafficking, mutation of a single residue may effect conformational changes as shown for the lactose permease LacY, a member of the major facilitator superfamily. In this case, the mutation C154G results in a conformational arrest of the transporter, which was essential for crystallization and structure determination of this transporter (20, 21).
The functional importance of C213 within TAP2 was investigated by means of thiol-reactive probes. Modifications of C213 by charged or hydrophobic probes resulted in an inactive TAP complex, reflecting the importance of the free thiol side chain of for peptide binding and translocation. Importantly, the inhibition of the TAP complex is reversible as demonstrated by the reductive cleavage of disulfide bonds by hydrophobic reducing reagents (β-ME). More hydrophilic reducing agents, such as DTT, were not able to fully restore TAP activity despite its higher reducing potential, indicating a more hydrophobic environment of this cysteinyl residue. Because modification of C213 results in a reversible switch between an active and inactive translocation complex, we speculated whether this effect is due to a conformational arrest. Reminiscent to these findings, charge-altering mutations at Arg 352 destabilize the open conformation of the cystic fibrosis transmembrane conductance regulator and also alter the ion selectivity filter (22).
Interestingly, the dissociation constant (Kd) of the epitope R9L for Cys-less TAP is in the same range as for the wild-type complex, but the number of binding sites (Bmax value) for CL/CL are drastically reduced. However, for the epitope R10T, Cys-less and wild-type TAP show the same mode of peptide binding and transport. Taken together, these findings exclude a defect in folding and propose a model favoring a peptide-receptive state. This may correspond to an outward-facing or occluded conformation as observed in lactose permease or ABC exporters Sav1866 and MsbA (23–25). Similarly, a mutation in lactose permease (C154G) results in an opening of a hydrophilic pathway to the periplasmic side (20). In case of the yeast multidrug transporter Pdr5, it has been proposed that the kinetics of the translocation cycle of the ABC transporter dictate substrate selection (26). However, in contrast to Pdr5, the ATPase activity of the TAP complex is strictly coupled to substrate binding (27, 28). Here we demonstrate that the initial translocation rate of Cys-less and wild-type TAP do not differ, thus excluding the possibility that kinetic effect of the transport cycle gives rise to this unusual behavior.
Cross-linking studies with the CL/CL(C213) complex revealed that the peptide is oriented in the binding pocket with the C-terminal half pointing toward C213 of TAP2. Remarkably, for the epitope R10T, wild-type and Cys-less TAP display identical binding and transport characteristics. Hence a nonreceptive conformation of CL/CL can be unlocked and fully populated by a subset of epitopes. Competition assays with peptide libraries revealed that wild-type and Cys-less TAP have a distinct but overlapping binding motif. The differences are spotted to the three N-terminal and C-terminal residues of the bound peptide, whereas the peptide length is not critical. In contrast to wild type, the Cys-less complex can accept negatively charged residues at positions 1 and 2 and, in particular, at the C terminus. Mutating cysteine 213 to either alanine or serine may abrogate the electrostatic effect between the thiolate and negatively charged residues of the bound peptide. This may also reflect the local environment, which causes a deprotonation of the sulfhydryl group (pH < pKS). Collectively, these data demonstrate that the cysteinyl residue 213 in TAP2 is crucial for peptide recognition of the antigen translocation machinery. Cys-less and wild-type TAP recognize distinct, but overlapping, sets of peptides.
Based on a homology model derived from the X-ray structure of the ABC exporter Sav1866 (25, 29), C213 is located at the membrane/cytosol interface at the beginning of the cytosolic loop 2 of core TAP2, whereas C197 and C209 are embedded in the ER membrane as part of TM2 (Fig. 4 B and C). Residues 217 (Thr) and 218 (Met) as well as 374 (Ala) and 380 (Arg) of TAP2 have been identified to control the peptide repertoire (30–32). Interestingly, residues 213, 217, 218, and 374 as well as the residue Val 288 in TAP1, recently identified to be involved in substrate sensing and signal transmission (33), form a shared interface for peptide binding.
In summary, C213 of TAP2 is crucial in maintaining a peptide-receptive conformation by providing additional contact sites to orient the peptide in the binding pocket. Although, based on an altered binding motif, a subset of epitopes can compensate the lack of C213, this essential residue stabilizes a peptide-receptive conformation and represents a critical element for epitope selection in the pathway of MHC class I antigen presentation.
Materials and Methods
Details of materials, cloning, expression, membrane preparation, and peptide-binding assays are provided in SI Text.
Single and Dual Peptide Translocation Assays.
TAP-containing membranes were incubated with 1 μM of ATTO565-labeled peptide (RYWANATRC(A)T, R10T-A) and/or Fluorescein-labeled peptide (RRYQNSTC(F)L, R9L-F) in the presence of ATP (3 mM) for 3 min at 32 °C in 50 μL of transport buffer (PBS, 5 mM MgCl2, pH 7.3). The transport reaction was stopped by addition of 500 μL of ice-cold transport buffer supplemented with 10 mM EDTA. After centrifugation, membranes were solubilized with 1 mL of lysis buffer [50 mM Tris·HCl, 150 mM NaCl, 5 mM MgCl2, 2 mM CaCl2, 2 mM MnCl2, 1% octyl phenoxyl(polyethoxyl)ethanol (IGEPAL CA-630); pH 7.3] and incubated for 20 min on ice. Insoluble material was removed by centrifugation. Transported, N-core glycosylated peptides were recovered with 60 μL of concanavalin A-Sepharose (Sigma) by overnight incubation at 4 °C. Following three washing steps with 1 mL of lysis buffer, the peptides were eluted with methyl-α-D-mannopyranoside (200 mM), and quantified with a fluorescence plate reader (λex/em = 485/520 nm or 520/612 nm; Polarstar Galaxy BMG Labtech). Background transport activity was measured in the presence of apyrase (1 U). All experiments were performed in triplicates.
Cysteine Cross-Linking.
TAP-containing membranes (0.5 mg of protein) were incubated with 1.25 μM of radiolabeled single-Cys peptides (C1, C2, C4–C9; cysteine at the indicated position of RRYQKSTEL) and CuPhe (1 mM CuSO4/4 mM 1,10-phenanthroline) in PBS for 5 min at 4 °C. Experiments were performed in the presence or absence of competitor peptide (0.25 mM of RRYQKSTEL). After quenching with 5 mM of NEM, membranes were washed with PBS and collected by centrifugation at 20,000 × g for 8 min at 4 °C. Membranes were solubilized on ice with 42 mM of n-decyl-β-D-maltoside (DM, Glycon) in solubilization buffer (20 mM NaH2PO4, 140 mM NaCl, 15% glycerol; pH 7.4). Insoluble material was removed by centrifugation at 20,000 × g for 30 min at 4 °C. Cross-linked TAP was purified by metal affinity chromatography. In brief, TAP was bound via the His10-tag of TAP1 to Ni-nitrilotriacetate-agarose (Qiagen), washed twice with washing buffer (PBS, 3 mM DM, 15% glycerol; pH 7.0), and eluted with SDS sample buffer. Aliquots were separated by SDS-PAGE (10%) and subjected to autoradiography. TAP expression was determined by immunoblotting.
Supplementary Material
Acknowledgments.
We thank Eckhard Linker and Renate Guntrum for technical assistance and Drs. David Parcej and Andreas Hinz for helpful suggestions on the manuscript. The Deutsche Forschungsgemeinschaft (Research Center SFB 807—Transport and Communication Across Biological Membranes; AB149/1) supported this work.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001308107/-/DCSupplemental.
References
- 1.Lehner PJ, Cresswell P. Recent developments in MHC-class-I-mediated antigen presentation. Curr Opin Immunol. 2004;16:82–89. doi: 10.1016/j.coi.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Rock KL, et al. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 3.Yewdell JW, Haeryfar SM. Understanding presentation of viral antigens to CD8+ T cells in vivo: The key to rational vaccine design. Annu Rev Immunol. 2005;23:651–682. doi: 10.1146/annurev.immunol.23.021704.115702. [DOI] [PubMed] [Google Scholar]
- 4.Wearsch PA, Cresswell P. The quality control of MHC class I peptide loading. Curr Opin Cell Biol. 2008;20:624–631. doi: 10.1016/j.ceb.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abele R, Tampé R. Peptide trafficking and translocation across membranes in cellular signaling and self-defense strategies. Curr Opin Cell Biol. 2009;21:508–515. doi: 10.1016/j.ceb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt L, Tampé R. Structure and mechanism of ABC transporters. Curr Opin Struct Biol. 2002;12:754–760. doi: 10.1016/s0959-440x(02)00399-8. [DOI] [PubMed] [Google Scholar]
- 7.Koch J, Guntrum R, Heintke S, Kyritsis C, Tampé R. Functional dissection of the transmembrane domains of the transporter associated with antigen processing (TAP) J Biol Chem. 2004;279:10142–10147. doi: 10.1074/jbc.M312816200. [DOI] [PubMed] [Google Scholar]
- 8.van Endert PM, et al. A sequential model for peptide binding and transport by the transporters associated with antigen processing. Immunity. 1994;1:491–500. doi: 10.1016/1074-7613(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 9.Meyer TH, van Endert PM, Uebel S, Ehring B, Tampé R. Functional expression and purification of the ABC transporter complex associated with antigen processing (TAP) in insect cells. FEBS Lett. 1994;351:443–447. doi: 10.1016/0014-5793(94)00908-2. [DOI] [PubMed] [Google Scholar]
- 10.Nijenhuis M, Hämmerling GJ. Multiple regions of the transporter associated with antigen processing (TAP) contribute to its peptide binding site. J Immunol. 1996;157:5467–5477. [PubMed] [Google Scholar]
- 11.Nijenhuis M, et al. Identification of a contact region for peptide on the TAP1 chain of the transporter associated with antigen processing. J Immunol. 1996;156:2186–2195. [PubMed] [Google Scholar]
- 12.van Endert PM, et al. The peptide-binding motif for the human transporter associated with antigen processing. J Exp Med. 1995;182:1883–1895. doi: 10.1084/jem.182.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uebel S, et al. Recognition principle of the TAP transporter disclosed by combinatorial peptide libraries. Proc Natl Acad Sci USA. 1997;94:8976–8981. doi: 10.1073/pnas.94.17.8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters B, Bulik S, Tampé R, Van Endert PM, Holzhutter HG. Identifying MHC class I epitopes by predicting the TAP transport efficiency of epitope precursors. J Immunol. 2003;171:1741–1749. doi: 10.4049/jimmunol.171.4.1741. [DOI] [PubMed] [Google Scholar]
- 15.Schrodt S, Koch J, Tampé R. Membrane topology of the transporter associated with antigen processing (TAP1) within an assembled functional peptide-loading complex. J Biol Chem. 2006;281:6455–6462. doi: 10.1074/jbc.M509784200. [DOI] [PubMed] [Google Scholar]
- 16.Abele R, Tampé R. The ABCs of immunology: Structure and function of TAP, the transporter associated with antigen processing. Physiology (Bethesda) 2004;19:216–224. doi: 10.1152/physiol.00002.2004. [DOI] [PubMed] [Google Scholar]
- 17.Procko E, O’Mara ML, Bennett WF, Tieleman DP, Gaudet R. The mechanism of ABC transporters: General lessons from structural and functional studies of an antigenic peptide transporter. FASEB J. 2009;23:1287–1302. doi: 10.1096/fj.08-121855. [DOI] [PubMed] [Google Scholar]
- 18.Heemels MT, Ploegh HL. Substrate specificity of allelic variants of the TAP peptide transporter. Immunity. 1994;1:775–784. doi: 10.1016/s1074-7613(94)80019-7. [DOI] [PubMed] [Google Scholar]
- 19.Heintke S, et al. Functional cysteine-less subunits of the transporter associated with antigen processing (TAP1 and TAP2) by de novo gene assembly. FEBS Lett. 2003;533:42–46. doi: 10.1016/s0014-5793(02)03746-8. [DOI] [PubMed] [Google Scholar]
- 20.Nie Y, Sabetfard FE, Kaback HR. The Cys154-->Gly mutation in LacY causes constitutive opening of the hydrophilic periplasmic pathway. J Mol Biol. 2008;379:695–703. doi: 10.1016/j.jmb.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menick DR, Sarkar HK, Poonian MS, Kaback HR. Cys154 is important for lac permease activity in Escherichia coli. Biochem Biophys Res Commun. 1985;132:162–170. doi: 10.1016/0006-291x(85)91002-2. [DOI] [PubMed] [Google Scholar]
- 22.Cui G, Zhang ZR, O’Brien AR, Song B, McCarty NA. Mutations at arginine 352 alter the pore architecture of CFTR. J Membr Biol. 2008;222:91–106. doi: 10.1007/s00232-008-9105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward A, Reyes CL, Yu J, Roth CB, Chang G. Flexibility in the ABC transporter MsbA: Alternating access with a twist. Proc Natl Acad Sci USA. 2007;104:19005–19010. doi: 10.1073/pnas.0709388104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smirnova I, et al. Sugar binding induces an outward facing conformation of LacY. Proc Natl Acad Sci USA. 2007;104:16504–16509. doi: 10.1073/pnas.0708258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawson RJ, Locher KP. Structure of a bacterial multidrug ABC transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 26.Ernst R, et al. A mutation of the H-loop selectively affects rhodamine transport by the yeast multidrug ABC transporter Pdr5. Proc Natl Acad Sci USA. 2008;105:5069–5074. doi: 10.1073/pnas.0800191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorbulev S, Abele R, Tampé R. Allosteric crosstalk between peptide-binding, transport, and ATP hydrolysis of the ABC transporter TAP. Proc Natl Acad Sci USA. 2001;98:3732–3737. doi: 10.1073/pnas.061467898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herget M, et al. Purification and reconstitution of the antigen transport complex TAP: A prerequisite for determination of peptide stoichiometry and ATP hydrolysis. J Biol Chem. 2009;284:33740–33749. doi: 10.1074/jbc.M109.047779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oancea G, et al. Structural arrangement of the transmission interface in the antigen ABC transport complex TAP. Proc Natl Acad Sci USA. 2009;106:5551–5556. doi: 10.1073/pnas.0811260106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armandola EA, et al. A point mutation in the human transporter associated with antigen processing (TAP2) alters the peptide transport specificity. Eur J Immunol. 1996;26:1748–1755. doi: 10.1002/eji.1830260813. [DOI] [PubMed] [Google Scholar]
- 31.Momburg F, Armandola EA, Post M, Hämmerling GJ. Residues in TAP2 peptide transporters controlling substrate specificity. J Immunol. 1996;156:1756–1763. [PubMed] [Google Scholar]
- 32.Momburg F, et al. Selectivity of MHC-encoded peptide transporters from human, mouse and rat. Nature. 1994;367:648–651. doi: 10.1038/367648a0. [DOI] [PubMed] [Google Scholar]
- 33.Herget M, et al. Mechanism of substrate sensing and signal transmission within an ABC transporter: use of a Trojan horse strategy. J Biol Chem. 2007;282:3871–3880. doi: 10.1074/jbc.M608480200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.