Abstract
To enter host cells, vaccinia virus, a prototype poxvirus, can induce transient macropinocytosis followed by endocytic internalization and penetration through the limiting membrane of pinosomes by membrane fusion. Although mature virions (MVs) of the Western reserve (WR) strain do this in HeLa cells by activating transient plasma membrane blebbing, MVs from the International Health Department-J strain were found to induce rapid formation (and lengthening) of filopodia. When the signaling pathways underlying these responses were compared, differences were observed at the level of Rho GTPases. Key to the filopodial formation was the virus-induced activation of Cdc42, and for the blebbing response the activation of Rac1. In addition, unlike WR, International Health Department-J MVs did not rely on genistein-sensitive tyrosine kinase and PI(3)K activities. Only WR MVs had membrane fusion activity at low pH. Inhibitor profiling showed that MVs from both strains entered cells by macropinocytosis and that this was induced by virion-exposed phosphatidylserine. Both MVs relied on the activation of epidermal growth factor receptor, on serine/threonine kinases, protein kinase C, and p21-activated kinase 1. The results showed that different strains of the same virus can elicit dramatically different responses in host cells during entry, and that different macropinocytic mechanisms are possible in the same cell line through subtle differences in the activating ligand.
Keywords: epidermal growth factor receptor, endocytosis, poxvirus, Rho GTPases, virus entry
When the endocytic entry of animal viruses into their host cells was first described, it was assumed that incoming viruses exploit on-going cellular endocytosis processes. More recently, it has become clear that many viruses are not just passive cargo but trigger their own endocytic uptake via cellular signaling pathways (1). Much depends on the nature and physiological state of the host cells and on the interaction of the viruses with their receptors (2, 3).
Vaccinia virus (VACV) belongs to the viruses that actively trigger their endocytic internalization. VACV are large, enveloped, double-stranded DNA viruses belonging to the Poxviridae, and they replicate in the cytoplasm. Two infectious forms of the virus exist: mature virions (MVs), with a single lipid bilayer surrounding a proteinaceous viral core that contains the viral genome, and extracellular virions that are like MVs but surrounded by an additional membrane (4). There are several variants of VACV, including the two strains used in this study, Western reserve (WR) and International Health Department-J (IHD-J).
Entry has mainly been investigated using WR MVs. When bound to the cell surface, the incoming MVs activate a complex signaling pathway involving the small GTPase Rac1, its effector kinase p21-activated kinase 1 (Pak1), and other factors (5). A change in actin dynamics is induced that leads to transient membrane blebbing followed by macropinocytic internalization of virus particles. Because the induction of the signal depends on the presence of exposed phosphatidylserine (PS) in the viral membrane (5–7), it has been concluded that the WR MVs make use of apoptotic mimicry as an entry strategy (5). Penetration of the WR virus core into the cytosol is triggered by low pH in the macropinosome (8).
Previous studies have suggested that entry of IHD-J MVs might be different. Instead of blebs, they seem to induce narrow plasma membrane protrusions (9). The IHD-J virus is also more dependent on glycosaminoglycan binding and does not require acidic vacuolar pH (10). To determine whether the two strains of VACV do, in fact, use different entry strategies, we compared their interaction with HeLa cells and found that the immediate cellular responses to the two strains were quite different. Clearly the same host cells were capable of activating distinct forms of macropinocytosis.
Results
IHD-J MVs Induce Filopodia.
We have previously shown that WR MV entry in HeLa cells is preceded by the activation of a complex signaling pathway that induces major alterations in actin dynamics. This process leads to blebbing of the plasma membrane followed by macropinocytosis that allows internalization of the incoming virus (5).
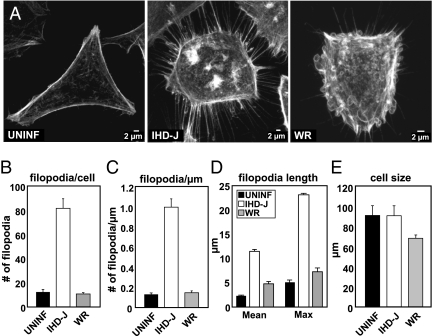
Because it has been reported that instead of blebs, IHD-J MVs induce membrane protrusions (9), we tested the two strains side by side in HeLa cells. A clear difference in host-cell response was detected almost immediately after addition of viruses to cells. Although WR MVs caused the transient formation of numerous actin-containing membrane blebs over the surface of the cells (Fig. 1A, WR), exposure to IHD-J MVs resulted in a dramatic increase in the number and length of filopodia (Fig. 1A, IHD-J). Their number and surface density increased 4- to 5-fold, and the average length 3- to 4-fold compared with those in uninfected or WR MV-treated cells (Fig. 1 B–D). Like the formation of blebs with WR, the extension of the filopodia with IHD-J occurred during the first 30 min of exposure to the virus. Unlike the blebs induced by WR, which were transient, the filopodia remained a permanent feature of the infected cells for many hours (Movie S1, Movie S2, Movie S3, and Fig. S2). The surface area of the cells did not decrease compared with uninfected cells, indicating that the filopodia were not a consequence of cell retraction (Fig. 1E). Thus, the cellular response to the two VACV strains was, indeed, dramatically different.
Fig. 1.
Virus-induced cytoskeletal rearrangement is strain dependent. (A) HeLa cells were left uninfected, or were infected with IHD-J or WR MVs [multiplicity of infection (MOI) = 10]; 30 min after infection, cells were fixed, stained for actin, and imaged. (B–E) Cells treated as in A were subjected to quantitative measurements, including number of filopodia per cell (B), filopodia per micrometer (C), filopodia length (D), and 2D cell size (E). Fifty cells per experiment were analyzed in triplicate and the average displayed with SE.
Role of Rho GTPases.
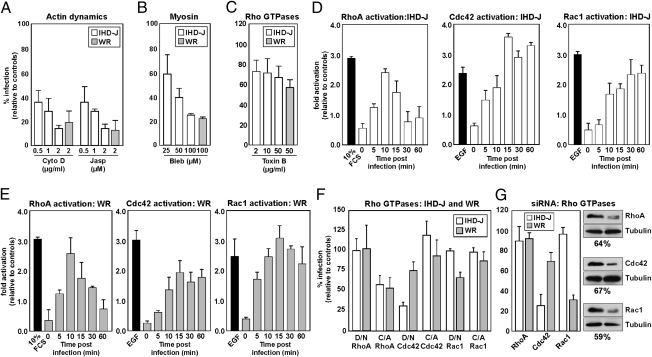
WR MV-induced blebbing, endocytosis, and infection depend on actin, myosin II, and the Rho GTPase Rac1 (5). To test whether this also applied to IHD-J MVs, inhibitors of actin polymerization and depolymerization (cytochalasin D and jasplakinolide) and myosin II (blebbistatin) were first tested. IHD-J and WR MV infection were both efficiently inhibited by these compounds indicating that actin dynamics played a central role in the infection of both viruses (Fig. 2 A and B).
Fig. 2.
Actin dynamics, myosin II, and Cdc42 are required for IHD-J infection. (A–C) Cells were pretreated with various concentrations of cytochalasin D (Cyto D), jasplakinolide (Jasp), blebbistatin A (Bleb), or Toxin B, followed by infection with IHD-J- or WR-EGFP-MVs. Infection was scored by flow cytometry. (D and E) A time course was performed in HeLa cells with IHD-J or WR MVs (MOI = 10). GTPase activities were measured using GTPase-specific G-Lisa assays. (F) HeLa cells were transfected with GFP-tagged WT, D/N, or C/A Rac1, RhoA, or Cdc42. Cells were infected with IHD-J- or WR-mRFP-MVs and analyzed for transfection and infection. (G) HeLa cells were transfected with siRNA targeting RhoA, Cdc42, or Rac1. Cells were infected with IHD-J- or WR-EGFP-MVs and infection quantified at 6 h after infection (Left). The protein knockdown was determined by immunoblot analysis (Right). Tubulin served as a loading control. Experiments were carried out in triplicate and the average displayed with SE.
When all Rho GTPases—including Rho A, Rac1, and Cdc42—that regulate actin dynamics were inhibited using Clostridium difficile toxin B, a decrease of 30 to 40% in infection by IHD-J and WR was observed (Fig. 2C). However, all three GTPases were found to be rapidly and robustly activated when either virus strain was added (Fig. 2 D and E). Activation of RhoA was transient, whereas activation of Rac1 and Cdc42 lasted for more than 60 min with both WR and IHD-J (Fig. 2 D and E). Cdc42 showed the most robust response to IHD-J, with maximal activation reached within 15 min (Fig. 2D); Rac1 was most responsive to WR (Fig. 2E). Thus, it was apparent that by binding to cells both viruses were capable of activating all three GTPases but with some differences.
When the impact of individual Rho GTPases was determined by expressing the constitutive-active (C/A) and dominant-negative (D/N) forms, more distinct differences emerged. MVs of the IHD-J strain were dependent on Cdc42 for infection. The expression of the D/N decreased IHD-J infection by 75%, and the C/A form increased it by 20% (Fig. 2F). Conversely, expression of Rac 1 mutants had no impact on IHD-J infection (Fig. 2F). Both D/N Rac1 and Cdc42 reduced WR infection by 30%; the C/A Rac1 and Cdc42 mutants had little effect (Fig. 2F). Expression of C/A RhoA reduced infection of both viruses by 50%, and the D/N construct had little effect on either virus (Fig. 2F).
To validate the different requirements for the GTPases during infection, we used small interfering RNAs (siRNAs) directed against RhoA, Cdc42, and Rac1. These siRNAs gave 64, 67, and 59% knockdown of these proteins, respectively (Fig. 2G, Right). Knockdown of Cdc42 reduced IHD-J MV infection by 75%, but depletion of Rac1 had no effect (Fig. 2G, Left). For WR MVs, depletion of Rac1 and Cdc42 inhibited infection by 70 and 30%, respectively (Fig. 2G, Left). Knockdown of RhoA had no impact on either IHD-J or WR infection.
These results indicated that Cdc42 was the primary Rho GTPase required for IHD-J MV infection, consistent with its well-documented role in regulating the formation of filopodia (11). We have previously shown that Rac1 is not only required for productive entry of WR MVs, but also for the virus-induced formation of blebs and macropinocytic internalization of virus particles (5). Although Rac1 is the key GTPase promoting infection by WR MVs, the impact of D/N Cdc42 and Cdc42 siRNA suggests that Cdc42 may also be important. That expression of the constitutively active RhoA inhibited infection confirmed previous data on WR virus (12). This inhibitory effect provided a possible explanation for the effect of Toxin B, which only gave partial inhibition of infection; the toxin may have simultaneously increased infection by inactivating RhoA, and decreased it by inhibiting Cdc42 and Rac1.
Epidermal Growth Factor Receptor-TK Activity Is Required for IHD-J and WR MV Entry.
The epidermal growth factor receptor (EGFR) is often associated with activation of Rho family GTPases and actin rearrangement. In addition, its activation is known to trigger macropinocytosis, the endocytic mechanism used by WR MVs for entry into HeLa cells (5, 13).
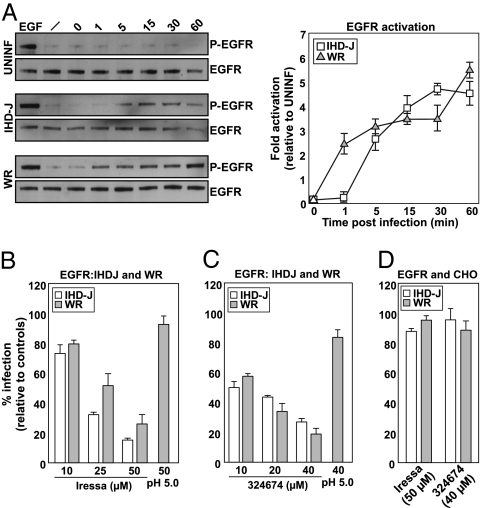
We found that EGFR was rapidly (within 1–5 min) activated by both IHD-J and WR MVs (Fig. 3A) to a 4.5- to 5.5-fold higher level than in uninfected cells, and sustained for at least 60 min. Cycloheximide was used in these experiments to prevent the expression of early viral genes that could influence receptor activity (14).
Fig. 3.
EGFR-TK activity is required for MV infection of HeLa but not CHO cells. (A) Serum-starved HeLa cells were infected with IHD-J or WR MVs (MOI = 10) in the presence of cycloheximide (1 mM). Cells were harvested at the indicated times and lysates analyzed for activated and total EGFR (Left), the fold activation over uninfected cells was determined (Right). (B and C) HeLa cells pretreated with Iressa or 324674 were infected with IHD-J- or WR-EGFP-MVs. Four hours after infection, cells were analyzed by flow cytometry. An acid-mediated bypass (pH 5.0) was performed with WR. (D) CHO cells were pretreated with Iressa or 324674, infected with IHD-J- or WR-EGFP-MVs, and analyzed by flow cytometry. Experiments were carried out in triplicate and the average displayed with SE.
To determine whether EGFR activation was essential for VACV infection in HeLa cells, we tested two inhibitors of this receptor tyrosine kinase, Iressa and 324674 (Calbiochem). Both efficiently inhibited IHD-J and WR MV infection in a dose-dependent fashion (Fig. 3 B and C). To assess whether the inhibitors caused a block in virus entry or later steps in the infection cycle, an acid-mediated bypass experiment was performed using WR MVs (5). Being acid-activated, penetration and infection of WR MVs can be triggered at the plasma membrane, thus allowing the virus to bypass the need for blebbing and endocytosis (5). The experiment showed that brief acidification of cells with bound virus allows infection of cells in the presence of both inhibitors (Fig. 3 B and C, pH 5.0). This result indicated that the EGFR activation is required for VACV entry and not for subsequent cytosolic steps in the replication cycle.
To validate the specificity of the two EGFR inhibitors, we made use of CHO cells. Although these cells lack the EGFR, they support VACV entry, early gene expression, and DNA replication (15). Given that entry must follow a mechanism that does not rely on the EGFR, we reasoned that infection should be insensitive to the two inhibitors if these are specific to the EGFR. CHO cells treated with Iressa or 324674 were, indeed, fully infected by IHD-J and WR MVs (Fig. 3D). The inhibition seen in HeLa cells was therefore likely to be EGFR-specific. We did not pursue the alternative mechanism of entry in CHO cells further, except for observing that the pathway must really be quite different because, unlike HeLa cells, infection of CHO cells was not affected by EIPA, IPA-3, or wortmannin, that inhibit Na+/H+ exchangers, Pak1, and PI(3) kinases, respectively (Fig. S1).
IHD-J MVs Trigger a Signaling Pathway Similar to WR MVs.
Our studies have shown that the signaling cascade that activates blebbing and subsequent macropinocytosis of WR MVs involves serine/threonine-, tyrosine-, and phosphatidylinositide kinases, as well as Na+/H+ exchangers (5). To determine whether these cellular factors were exploited by IHD-J MVs, we compared the effects of a panel of inhibitors on IHD-J and WR MV infection and entry.
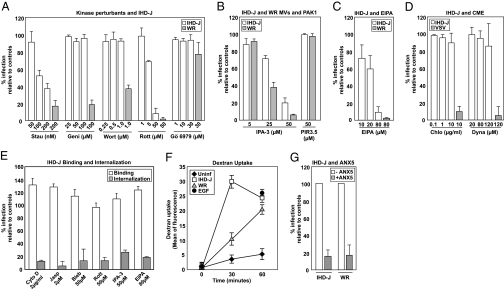
Infection of both strains was inhibited by staurosporine (general kinase inhibitor), rottlerin [inhibitor of protein kinase C (PKC) and other kinases], IPA-3 [PAK1 inhibitor (16)], and EIPA [Na+/H+ exchange, GTPase activation, and macropinocytosis inhibitor (17)] (Fig. 4 A–C). Both strains were also insensitive to Gö6979 (calcium-dependent PKC inhibitor) (Fig. 4A). That infection was insensitive to chlorpromazine (clathrin coat assembly inhibitor), and dynasore [dynamin inhibitor (18)] indicated that internalization did not require clathrin-mediated endocytosis (Fig. 4D). Vesicular stomatitis virus (VSV), which enters via clathrin-coated pits (19), was blocked by both of these inhibitors.
Fig. 4.
IHD-J MVs enter cells by PS-mediated macropinocytosis. (A–C) Cells were pretreated with staurosporine (Stau), genistein (Geni), wortmannin (Wort), rottlerin (Rott), Gö6979, IPA-3 and PIR 3.5, or EIPA and infected with IHD-J- or WR-EGFP-MVs. Four hours after infection, cells were analyzed by flow cytometry. (D) Inhibitor assays were performed as in A. VSV served as a control (22). (E) Binding: IHD-J-EGFP-CORE MVs (MOI = 5) were bound to HeLa cells in the presence of the maximum inhibitor concentrations. Binding was assessed by flow cytometry. Internalization: HeLa cells pretreated with inhibitors were infected with IHD-J-EGFP-CORE MVs. Thirty minutes postinfeciton, external virions were stained with Mab 7D11 and internalized virions quantified. (F) Uptake of 70-kDa dextran was assayed in untreated, IHD-J, or WR MV-infected cells at the indicated times. Cells treated with EGF served as a positive control. (G) Untreated or ANX5-treated IHD-J- or WR-EGPF-MVs were used to infect cells. Four hours after infection, cells were analyzed for infection. Experiments were performed in triplicate and the average displayed with SE.
When the effect of the inhibitors that did block IHD-J infection was analyzed more closely, it was found that none of them blocked IHD-J binding to the cells surface (Fig. 4E, white bars). Rather, a moderate increase in binding was observed. Using an immunofluorescence-based internalization assay, we found that the inhibitors all had a dramatic effect (>75% reduction) on virus internalization (Fig. 4E, gray bars).
Judging by these results, we concluded that the signaling pathways triggered were similar. However, two additional inhibitors showed that they were not identical. Unlike WR MVs, infection by IHD-J MVs was not inhibited by genistein (tyrosine kinase inhibitor), nor by wortmannin [PI(3)-kinase inhibitor] (Fig. 4A). These differences implied that the pathway downstream of the EGFR diverged between the two strains of virus. This finding may explain the differential effects on the Rho GTPases, and hence the different outcome in cellular response.
Macropinocytosis and Apoptotic Mimicry.
The role of the EGFR, the nature of the signaling pathway, the involvement of Pak1 and Na+/H+ exchangers, and the actin-dependence all argued for a macropinocytosis-driven endocytic process for IHD-J. In further support of this, we could show by quantitative analysis of dextran-488 uptake that addition of IHD-J MVs to cells induced a 6-fold elevation in fluid phase internalization within 30 min (Fig. 4F). Like WR MV infection, IHD-J MV infection was inhibited by Annexin 5, a PS-binding protein (Fig. 4G). This finding indicated that IHD-J also has PS exposed in the viral envelope, and that this PS is critical for infection. Taken together, these results made evident that IHD-J entered and infected cells by inducing macropinocytic endocytosis via the exposed PS in the viral envelope.
IHD-J MV Fusion Activity Is Acid-Independent.
It has been reported that the infection by WR MVs is acid-activated, whereas infection by IHD-J MVs is not (10). To confirm and extend this finding, we first tested the ability of IHD-J and WR MVs to mediate the formation of syncytia when exposed to low pH. Formation of syncytia (fusion-from-without) is often used to demonstrate membrane fusion activity of enveloped viruses.
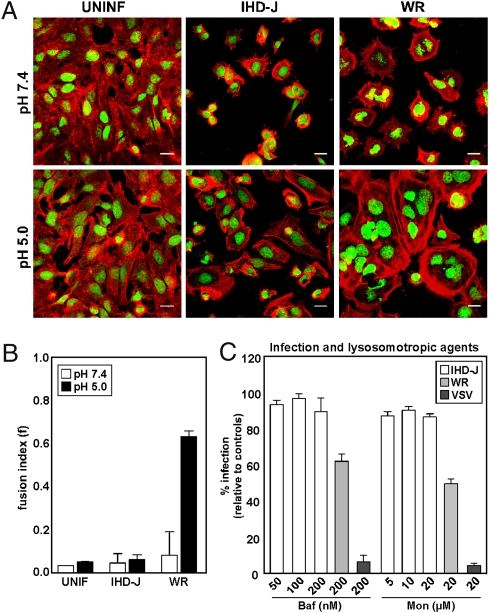
When cell cultures were exposed to IHD-J MVs, no syncytia were formed, irrespective of pH treatment (Fig. 5A, pH 7.4 vs. pH 5.0). In contrast, cells exposed to WR MVs and pH 5.0, showed extensive cell-cell fusion. The extent of syncytia formation in the samples was quantified by determining the fusion index, f (20). The f-values confirmed that contrary to WR MVs, which were highly fusogenic at pH 5.0 (f = 0.66), the IHD-J MVs did not fuse cells at either pH (f = 0.04) (Fig. 5B).
Fig. 5.
IHD-J MV fusion activity is acid-independent. (A) IHD-J- or WR-MVs (MOI = 10) were bound to HeLa cells at 25 °C. Cells were washed and treated with pH 7.4 or pH 5.0 media for 5 min, then incubated for 2 h at 37 °C at neutral pH. Cells were imaged by confocal microscopy for both nuclei and actin. Experiments were performed in triplicate and representative images displayed. (Scale bars, 20 μm). (B) The fusion index (f) for each sample in A was quantified as described in Materials and Methods. (C) HeLa cells were pretreated with various concentrations of Baf or Mon and infected with IHD-J-EGFP-MVs (MOI = 1). Infection was analyzed by flow cytometry. WR MVs and VSV were assayed in parallel. Experiments were performed in triplicate and the average displayed with SE.
To test whether IHD-J MV entry was dependent on endosomal acidification, bafilomycin A1 (Baf), a vacuolar ATPase inhibitor, and monensin A (Mon), a carboxylic ionophore, were used to raise the endosomal pH. IHD-J MVs showed little sensitivity to either inhibitor (Fig. 5C, white bars). With 40 to 50% reduction in infectivity, WR MV entry into HeLa cells proved to be partially sensitive, as previously reported (Fig. 5C, light gray bars) (21). VSV, known to penetrate from early endosomes by acid-activated membrane fusion (19, 22), was inhibited by more than 90% (Fig. 5C, dark gray bars).
We conclude that the membrane fusion activities of IHD-J and WR MVs are different in respect to acid-dependence. Whereas IHD-J can infect cells without acidification, WR MVs possesses a latent acid-activated membrane fusion activity. About half of the infectious particles showed such acid-dependence during HeLa cell infection.
Discussion
The overall effect of WR and IHD-J MVs on HeLa cells was dramatically different. Instead of the transient formation of multiple blebs over the surface of cells seen with WR MVs, IHD-J MVs triggered permanent induction and lengthening of filopodia. At the molecular level, differences were found in the dependence of Rho GTPase, the activation of PI(3)-kinases and genistein-sensitive tyrosine kinases, and the requirement for endosome acidification. That the same HeLa cells could respond to closely related stimuli by activating different macropinocytic programs was evident.
Despite the many differences, it was also apparent that the mechanisms of entry of the two VACV strains had similarities. Both viruses activated the EGFR and triggered a complex signaling cascade that caused a global alteration of actin dynamics. A change in cell behavior was thus induced, including macropinocytic internalization of virus particles. Furthermore, as far as we could determine, both viruses employed an overall entry strategy based on “apoptotic mimicry,” as previously described for the WR MVs (5).
The cellular responses were in each case rapid, with activation of all three Rho GTPases occurring within 5 min. A key trigger was in both cases the exposed PS in the viral envelope, the only lipid in the virus membrane specifically required for infectivity (5–7). Although the cellular PS-sensors involved remain unidentified, PS-receptors have been described mainly in macrophages as components of the machinery needed for endocytosis of apoptotic bodies. Some of these surface proteins bind PS directly, others use bridging molecules to link PS-recognition with receptor activation (23–27). Some contain EGF-like domains that could serve to transactivate the EGFR (24, 27, 28).
Our results clearly defined activation of EGFR as a necessary step in infection of HeLa cells. Blocking this receptor with antibodies and other agents has previously been shown to inhibit VACV infection in L cells (29, 30). However, its role as a VACV receptor has been largely dismissed because the virus infects cells, such as CHO cells, devoid of EGFR (31). Consistent with this, we found that two EGFR inhibitors that blocked infection in HeLa cells had no effect on VACV infection in CHO cells. Because we also failed to observe inhibition by Na+/H+ exchanger- or PAK-1 inhibitors—and in the case of WR MVs, of PI(3)K inhibitors—we concluded that infection of CHO cells does not involve macropinocytosis. Apparently, VACV belongs to a growing group of viruses that seem to make use of different, alternative strategies and pathways to enter cells. Herpes simplex virus is a well-studied example of this; it enters some cell types by endocytosis and others by direct fusion at the plasma membrane (32).
When the signaling pathways downstream of the EGFR were analyzed, it was found that the two strains shared the need for actin, myosin II, Rho GTPases, PAK1, and Na+/H+-exchangers. Although all three of the Rho GTPases were activated within minutes by both viruses, Cdc42 responded most robustly to IHD-J, and Rac1 to WR. Expression of a D/N construct and siRNA silencing showed that Cdc42 was, indeed, critical for infection by IHD-J, consistent with its role as the key regulator in filopodia formation (33). It has been previously reported that Rac1 is required for IHD-J entry (9). Although we confirmed that Rac1 was activated by IHD-J, we saw no effect of D/N and C/A constructs or siRNA silencing on IHD-J infection. In contrast, we have shown previously that Rac1 allows WR MVs to trigger plasma membrane blebbing (5), and have now demonstrated that both Rac1 and Cdc42 contribute to WR MV infection.
Activation of Rho A was transient for both strains. Judging by the effect of a C/A mutant, this activation was actually inhibitory to infection, as previously reported (9). However, under unperturbed conditions, the inhibitory effect was most likely overwhelmed by the activating effects of the other two GTPases. In many systems opposing effects of RhoA, and Rac1 (or Cdc42) have been observed (34, 35).
Several kinase inhibitors blocked entry of both strains, including staurosporin, IPA-3, and rottlerin. However, IHD-J infection differed from WR in being insensitive to moderate concentrations of tyrosine- and PI(3)- kinase inhibitors. Although most forms of macropinocytosis are known to depend on PI(3)-kinases (36), exceptions have been reported (37).
Although differences in the virus particles must be the ultimate explanation for the observed variations in cellular response, we are not aware of any in structure or composition. However, there are functional differences: IHD-J is more dependent on GAGs for infection than WR and less dependent on acidification (10). In respect to acidification, we found that half of the WR MVs were inhibited by agents that elevate vacuolar pH. By assaying for virus-induced cell-cell fusion, we could correlate the acid requirement with viral membrane fusion activity. VACV has a membrane protein complex composed of at least 12 viral proteins involved in viral fusion and its regulation (38, 39). It remains to be determined if variations in these complexes account for the differences seen in IHD-J and WR MV acid dependence and cell-cell fusion activity.
When considering what could cause the differential outcome with the two viruses, the experimental clues we have point toward the differences in GTPase and kinase dependency. On this basis, we speculate that the surface-bound virus particles differ in how they interact with the EGFR. This interaction may lead to differences in receptor clustering and spatial distribution, thereby stimulating the activation of different subsets of kinases. This result, in turn, could cause a downstream divergence in GTPase activation. Other scenarios are also possible, such as a difference in receptors activated in addition to EGFR, and a divergence in the fate of endocytic vacuoles after internalization. Tyrosine- and PI(3)-kinase activities are also required downstream of the GTPases in the signaling pathway triggered by WR VACV MVs, suggesting that they may participate in the regulation of macropinosomes (5).
In summary, we observed that closely related virus particles were capable of eliciting different macropinocytic programs in the same cells. It is known that macropinocytosis can differ between cells (36), but here two distinct responses were observed in the same cells. The plasticity of VACV and its interaction with host cells revealed by our observations demonstrated that the virus is capable of exploiting a repertoire of alternative host-cell responses. This reaction may provide an evolutionary advantage that VACV shares with other viruses known to use multiple entry mechanisms, including herpes viruses and influenza viruses (40).
Materials and Methods
Cells, Viruses, Plasmids, and Reagents.
HeLa and CHO cells were maintained in DMEM (Gibco BRL) supplemented with 10% FCS, glutamax, and nonessential amino acids. Wild-type (IHD-J and WR), XFP-expressing (IHD-J/WR-EGFP/mRFP-MV), and EGFP-tagged A5 (IHD-J/WR-EGFP-CORE) VACV were generated, purified, and titered as previously described (5). Plasmids encoding pEGFP Rac1, RhoA, and Cdc42 variants were obtained from Ian Macara (University of Virginia School of Medicine, Charlottesville, VA). Bafilomycin A1, monensin A, Clostridium difficile toxin B, cytochalasin D, genistein, staurosporine, rottlerin, wortmannin, EIPA, dynasore, chlorpromazine, and cycloheximide were obtained from Sigma-Aldrich, jasplakinolide from Invitrogen, Gö 6979 and 324674 from Calbiochem, Iressa from LC Laboratories, Rhodamine phalloidin from Molecular Probes, and Draq5 from Biostatus Limited. IPA-3 and PIR 3.5 were obtained from Jeffery Peterson (Fox Chase Cancer Center, Philadelphia, PA).
Syncytia Formation.
MVs (MOI = 10) were bound to cells at 25 °C. After washing, media (pH 7.4 or pH 5.0; 20mM Mes) was added for 5 min at 37 °C. Cells were washed and incubated at 37 °C for 2 h. Cells were stained for nuclei and actin. The number of cells and nuclei per image were quantified. A fusion index was calculated using the equation f = [1 − (C/N)], as previously described (20).
GTPase Activation.
MVs were bound to serum-starved cells (MOI = 10) at 25 °C. Cells were washed, shifted to 37 °C, and harvested at the indicated times. G-LISA activation kits (Cytoskeleton, Inc.) were used to measure Cdc42, Rac1, and RhoA activation.
GTPase siRNA.
Cells were transfected with Qiagen control (1027280), RhoA (SI02654211), Cdc42 (SI02757328), or Rac1 (SI02655051) siRNA at a concentration of 20 nM. Forty-eight hours later, cells were infected with EGFP-MVs (MOI = 0.2). Cells were fixed 6 h after infection and analyzed. Antibodies directed against RhoA, Cdc42, and Rac1 (ARH03-A, ACD03-A, ARC03-A; cytoskelton) were used to determine knockdown efficiency.
EGFR Activation.
MVs (MOI = 10) were bound to serum-starved cells at 25 °C. Cells were washed and fed with media containing cycloheximide (1 mM), shifted to 37 °C, and harvested at the indicated times. Cell lysates were analyzed for total (anti-EGFR; Millipore) and phospho-EGFR(Tyr-1173) (clone 9H2; Upstate).
Flow Cytometry.
Inhibitor experiments.
Cells were pretreated with the indicated inhibitors before infection with EGFP-MVs (MOI = 1). Flow cytometry was performed as previously described (5).
D/N or C/A mutants.
Cells were transfected with the indicated plasmids and 18 h later infected with mRFP-MVs (MOI = 1). Cells were analyzed by flow cytometry for infection and transfection.
MV Binding and Internalization.
Binding.
Cells were treated with inhibitors before binding of EGFP-CORE virus (MOI = 5) at 25 °C. Cells were washed and analyzed by flow cytometry.
Internalization.
EGFP-CORE virions (MOI = 40) were bound to cells at 25 °C, washed, and shifted to 37 °C for 30 min. External virions were distinguished using an antibody against L1R (1:10,000; a gift of A. L. Schmaljohn, University of Maryland School of Medicine, Baltimore, MD) followed by Alexa594 secondary antibody. Samples were analyzed using confocal microscopy.
Fluid Phase Uptake.
Cells were bound with EGF (100 ng/ml), IHD-J-, or WR-MVs (MOI = 10), washed, and incubated with 0.5 mg/mL 70kDa FITC-dextran at 37 °C for the indicated times. Surface-bound dextran was removed with (0.1 M sodium acetate, 0.05 M NaCl, pH 5.5), and cells analyzed by flow cytometry.
Supplementary Material
Acknowledgments
A.H. is supported by LipidX, Swiss National Science Foundation, and the European Research Council. J.M. is supported by the European Molecular Biology Organization.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1004618107/-/DCSupplemental.
References
- 1.Marsh M, Helenius A. Virus entry: Open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meier O, Greber UF. Adenovirus endocytosis. J Gene Med. 2004;6(Suppl 1):S152–S163. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- 3.Coyne CB, Shen L, Turner JR, Bergelson JM. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007;2:181–192. doi: 10.1016/j.chom.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss B. In: Fields Virology. Knipe DM, Howley PM, editors. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2905–2946. [Google Scholar]
- 5.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 6.Oie M. Reversible inactivation and reactivation of vaccinia virus by manipulation of viral lipid composition. Virology. 1985;142:299–306. doi: 10.1016/0042-6822(85)90338-1. [DOI] [PubMed] [Google Scholar]
- 7.Laliberte JP, Moss B. Appraising the apoptotic mimicry model and the role of phospholipids for poxvirus entry. Proc Natl Acad Sci USA. 2009;106:17517–17521. doi: 10.1073/pnas.0909376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsley AC, Weisberg AS, Wagenaar TR, Moss B. Vaccinia virus entry into cells via a low-pH-dependent endosomal pathway. J Virol. 2006;80:8899–8908. doi: 10.1128/JVI.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Locker JK, et al. Entry of the two infectious forms of vaccinia virus at the plasma membane is signaling-dependent for the IMV but not the EEV. Mol Biol Cell. 2000;11:2497–2511. doi: 10.1091/mbc.11.7.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bengali Z, Townsley AC, Moss B. Vaccinia virus strain differences in cell attachment and entry. Virology. 2009;389:132–140. doi: 10.1016/j.virol.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladwein M, Rottner K. On the Rho'd: The regulation of membrane protrusions by Rho-GTPases. FEBS Lett. 2008;582:2066–2074. doi: 10.1016/j.febslet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 12.Valderrama F, Cordeiro JV, Schleich S, Frischknecht F, Way M. Vaccinia virus-induced cell motility requires F11L-mediated inhibition of RhoA signaling. Science. 2006;311:377–381. doi: 10.1126/science.1122411. [DOI] [PubMed] [Google Scholar]
- 13.Haigler HT, McKanna JA, Cohen S. Rapid stimulation of pinocytosis in human carcinoma cells A-431 by epidermal growth factor. J Cell Biol. 1979;83:82–90. doi: 10.1083/jcb.83.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzahar E, et al. Pathogenic poxviruses reveal viral strategies to exploit the ErbB signaling network. EMBO J. 1998;17:5948–5963. doi: 10.1093/emboj/17.20.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franke CA, Roseman NA, Hruby DE. Expression and regulation of the vaccinia virus thymidine kinase gene in non-permissive cells. Virus Res. 1985;3:13–17. doi: 10.1016/0168-1702(85)90037-1. [DOI] [PubMed] [Google Scholar]
- 16.Deacon SW, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol. 2008;15:322–331. doi: 10.1016/j.chembiol.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koivusalo M, et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol. 2010;188:547–563. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macia E, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Matlin KS, Reggio H, Helenius A, Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982;156:609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- 20.White J, Matlin K, Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981;89:674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitbeck JC, Foo CH, Ponce de Leon M, Eisenberg RJ, Cohen GH. Vaccinia virus exhibits cell-type-dependent entry characteristics. Virology. 2009;385:383–391. doi: 10.1016/j.virol.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johannsdottir HK, Mancini R, Kartenbeck J, Amato L, Helenius A. Host cell factors and functions involved in vesicular stomatitis virus entry. J Virol. 2009;83:440–453. doi: 10.1128/JVI.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park D, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 24.Park SY, et al. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2008;15:192–201. doi: 10.1038/sj.cdd.4402242. [DOI] [PubMed] [Google Scholar]
- 25.Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 26.Scott RS, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 27.Hanayama R, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 28.Hafizi S, Dahlbäck B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006;273:5231–5244. doi: 10.1111/j.1742-4658.2006.05529.x. [DOI] [PubMed] [Google Scholar]
- 29.Eppstein DA, et al. Epidermal growth factor receptor occupancy inhibits vaccinia virus infection. Nature. 1985;318:663–665. doi: 10.1038/318663a0. [DOI] [PubMed] [Google Scholar]
- 30.Marsh YV, Eppstein DA. Vaccinia virus and the EGF receptor: A portal for infectivity? J Cell Biochem. 1987;34:239–245. doi: 10.1002/jcb.240340403. [DOI] [PubMed] [Google Scholar]
- 31.Hügin AW, Hauser C. The epidermal growth factor receptor is not a receptor for vaccinia virus. J Virol. 1994;68:8409–8412. doi: 10.1128/jvi.68.12.8409-8412.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akhtar J, Shukla D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009;276:7228–7236. doi: 10.1111/j.1742-4658.2009.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakaya M, Tanaka M, Okabe Y, Hanayama R, Nagata S. Opposite effects of rho family GTPases on engulfment of apoptotic cells by macrophages. J Biol Chem. 2006;281:8836–8842. doi: 10.1074/jbc.M510972200. [DOI] [PubMed] [Google Scholar]
- 35.Leeuwen FN, et al. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 37.Jiang J, Kolpak AL, Bao ZZ. Myosin IIB isoform plays an essential role in the formation of two distinct types of macropinosomes. Cell Motil Cytoskeleton. 2009;67:32–42. doi: 10.1002/cm.20419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss B. Poxvirus entry and membrane fusion. Virology. 2006;344:48–54. doi: 10.1016/j.virol.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 39.Turner PC, Moyer RW. An orthopoxvirus serpinlike gene controls the ability of infected cells to fuse. J Virol. 1992;66:2076–2085. doi: 10.1128/jvi.66.4.2076-2085.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 2010 doi: 10.1146/annurev-biochem-060208-104626. 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.