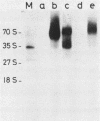
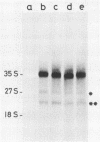
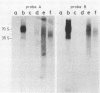
Abstract
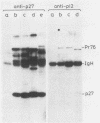
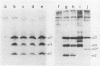
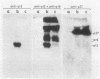
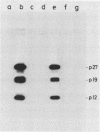
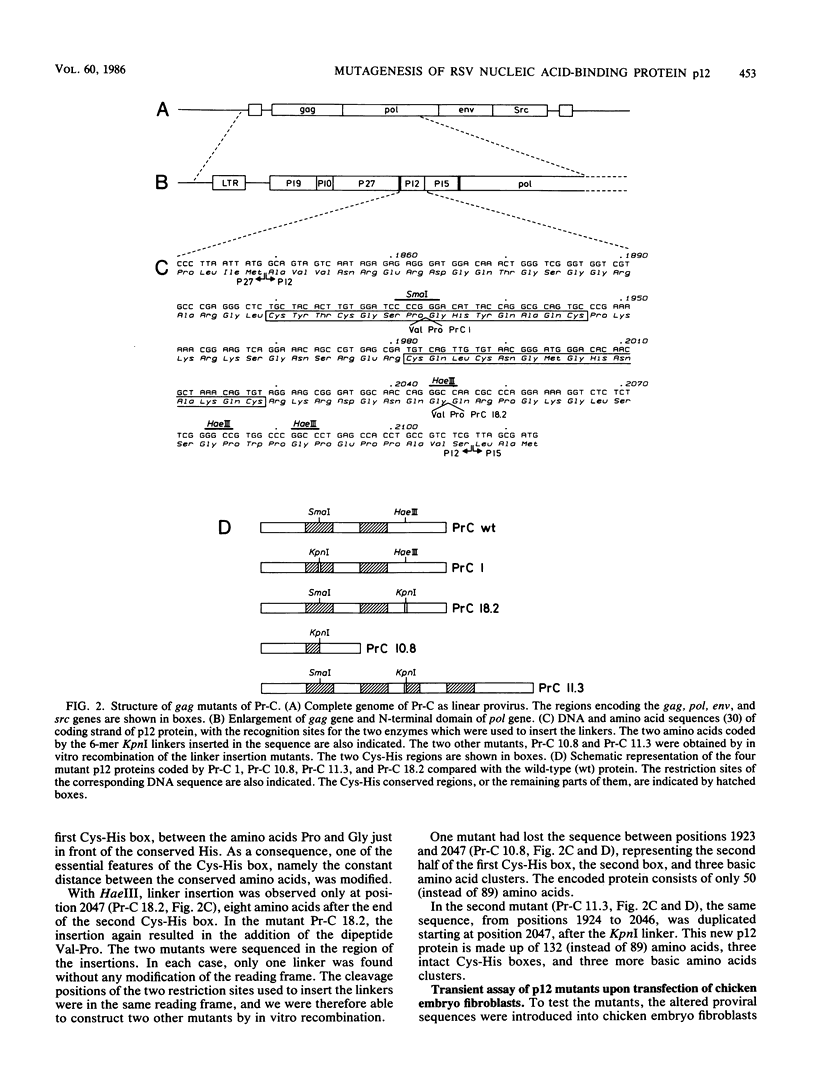
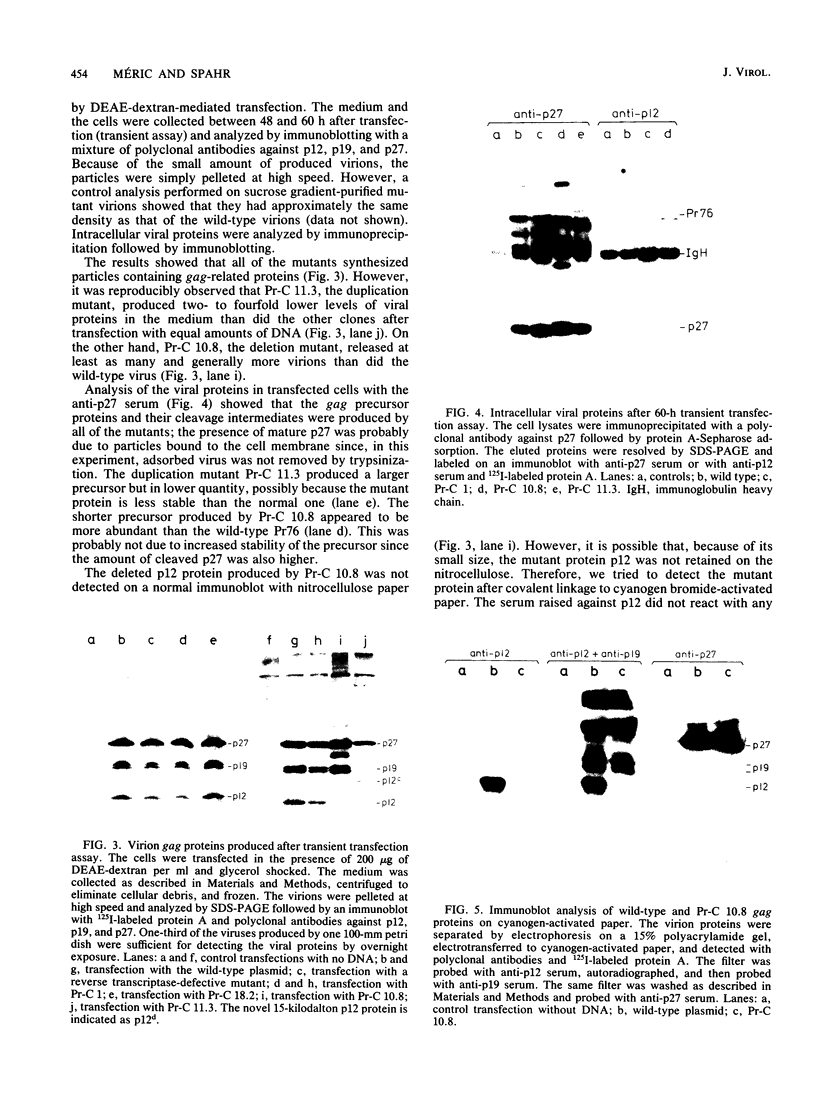
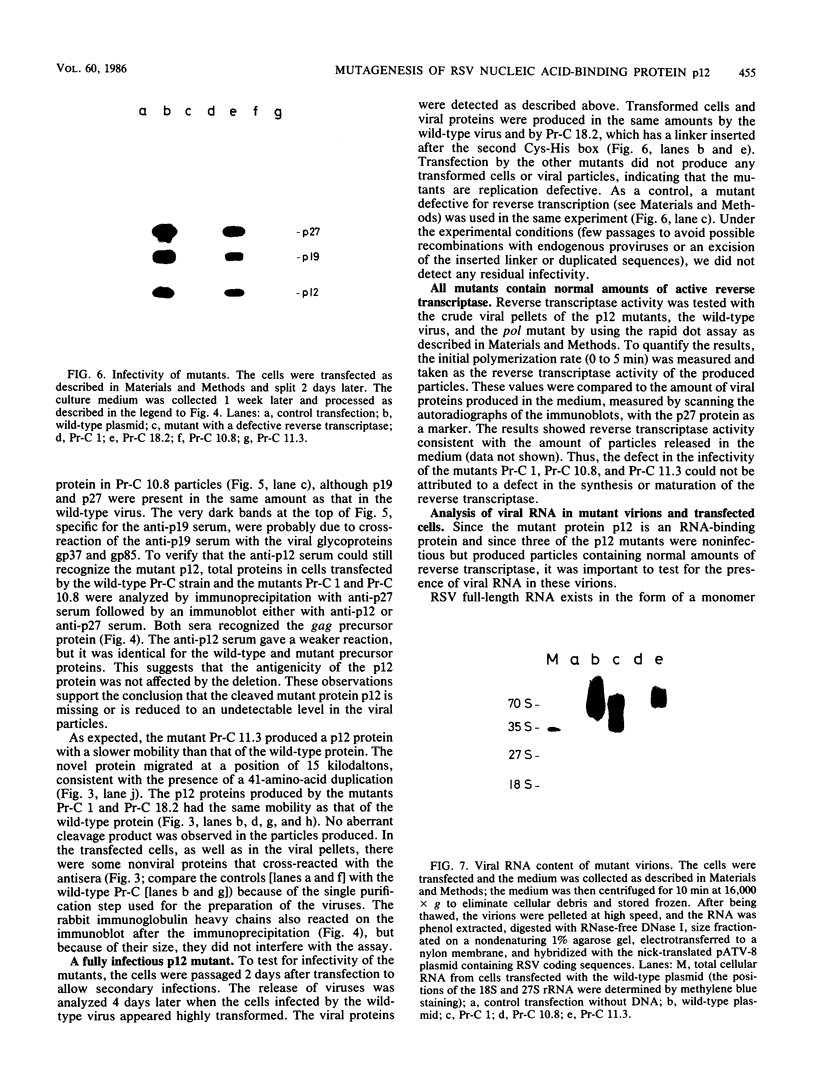
To study the function(s) of the Rous sarcoma virus nucleic acid-binding protein p12, we constructed mutants by using two restriction sites in the p12 proviral coding sequence of the Prague C strain to insert KpnI synthetic linkers. The two restriction sites are in the same reading frame, which allowed us to construct a deletion mutant lacking the two conserved Cys-His regions and a duplication mutant containing three intact Cys-His boxes. These mutant DNAs were transfected into chicken embryo fibroblasts, and the viral particles produced in a transient assay were characterized biochemically and for infectivity. Our results indicate that the Rous sarcoma virus nucleic acid-binding protein p12 is necessary for genomic RNA packaging but not for particle assembly and is implicated in the formation of a stable 70S dimeric RNA. Moreover, the fact that one mutant was apparently able to package normal 70S RNA but was not infectious suggests a role for p12 during the infection process.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Montelaro R. C., Frank H., Schäfer W. Assembly of type C oncornaviruses: a model. Science. 1978 Jan 13;199(4325):183–186. doi: 10.1126/science.202022. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cooper G. M., Okenquist S. Mechanism of transfection of chicken embryo fibroblasts by Rous sarcoma virus DNA. J Virol. 1978 Oct;28(1):45–52. doi: 10.1128/jvi.28.1.45-52.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland T. D., Morgan M. A., Oroszlan S. Complete amino acid sequence of the basic nucleic acid binding protein of feline leukemia virus. Virology. 1984 Feb;133(1):137–145. doi: 10.1016/0042-6822(84)90432-x. [DOI] [PubMed] [Google Scholar]
- Covey S. N. Amino acid sequence homology in gag region of reverse transcribing elements and the coat protein gene of cauliflower mosaic virus. Nucleic Acids Res. 1986 Jan 24;14(2):623–633. doi: 10.1093/nar/14.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. Mutations in gag proteins P12 and P15 of Moloney murine leukemia virus block early stages of infection. J Virol. 1984 Mar;49(3):909–917. doi: 10.1128/jvi.49.3.909-917.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F. Binding sites of viral protein P19 onto Rous sarcoma virus RNA and possible controls of viral functions. J Mol Biol. 1982 Sep 15;160(2):147–161. doi: 10.1016/0022-2836(82)90172-3. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X., Phillips N., Jentoft J., Tuazon P. T., Traugh J. A., Leis J. Site-specific phosphorylation of avian retrovirus nucleocapsid protein pp12 regulates binding to viral RNA. Evidence for different protein conformations. J Biol Chem. 1985 Aug 15;260(17):9941–9947. [PubMed] [Google Scholar]
- Garfinkel D. J., Boeke J. D., Fink G. R. Ty element transposition: reverse transcriptase and virus-like particles. Cell. 1985 Sep;42(2):507–517. doi: 10.1016/0092-8674(85)90108-4. [DOI] [PubMed] [Google Scholar]
- Goff S., Traktman P., Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981 Apr;38(1):239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. W., Schwartzberg P., Goff S. P. Point mutations in the P30 domain of the gag gene of Moloney murine leukemia virus. Virology. 1985 Apr 15;142(1):211–214. doi: 10.1016/0042-6822(85)90435-0. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Omer C. A., Weis J. H., Mitsialis S. A., Faras A. J., Guntaka R. V. Restriction endonuclease and nucleotide sequence analyses of molecularly cloned unintegrated avian tumor virus DNA: structure of large terminal repeats in circle junctions. J Virol. 1982 Apr;42(1):346–351. doi: 10.1128/jvi.42.1.346-351.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Koyama T. Characterization of a Rous sarcoma virus mutant defective in packaging its own genomic RNA: biological properties of mutant TK15 and mutant-induced transformants. J Virol. 1984 Jul;51(1):147–153. doi: 10.1128/jvi.51.1.147-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandjian E. W. UV crosslinking of RNA to nylon membrane enhances hybridization signals. Mol Biol Rep. 1986;11(2):107–115. doi: 10.1007/BF00364822. [DOI] [PubMed] [Google Scholar]
- Leis J., Jentoft J. Characteristics and regulation of interaction of avian retrovirus pp12 protein with viral RNA. J Virol. 1983 Nov;48(2):361–369. doi: 10.1128/jvi.48.2.361-369.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leis J., Johnson S., Collins L. S., Traugh J. A. Effects of phosphorylation of avian retrovirus nucleocapsid protein pp12 on binding of viral RNA. J Biol Chem. 1984 Jun 25;259(12):7726–7732. [PubMed] [Google Scholar]
- Linial M., Medeiros E., Hayward W. S. An avian oncovirus mutant (SE 21Q1b) deficient in genomic RNA: biological and biochemical characterization. Cell. 1978 Dec;15(4):1371–1381. doi: 10.1016/0092-8674(78)90062-4. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Rubin G. M. Complete nucleotide sequence of the Drosophila transposable element copia: homology between copia and retroviral proteins. Mol Cell Biol. 1985 Jul;5(7):1630–1638. doi: 10.1128/mcb.5.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric C., Darlix J. L., Spahr P. F. It is Rous sarcoma virus protein P12 and not P19 that binds tightly to Rous sarcoma virus RNA. J Mol Biol. 1984 Mar 15;173(4):531–538. doi: 10.1016/0022-2836(84)90396-6. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Fine-structure analyses of lipid-protein and protein-protein interactions of gag protein p19 of the avian sarcoma and leukemia viruses by cyanogen bromide mapping. J Virol. 1984 Oct;52(1):145–153. doi: 10.1128/jvi.52.1.145-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugatsch T., Stacey D. W. Identification of a sequence likely to be required for avian retroviral packaging. Virology. 1983 Jul 30;128(2):505–511. doi: 10.1016/0042-6822(83)90279-9. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P., Colicelli J., Gordon M. L., Goff S. P. Mutations in the gag gene of Moloney murine leukemia virus: effects on production of virions and reverse transcriptase. J Virol. 1984 Mar;49(3):918–924. doi: 10.1128/jvi.49.3.918-924.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. The genome-associated, specific RNA binding proteins of avian and mammalian type C viruses. Cell. 1977 Jan;10(1):91–99. doi: 10.1016/0092-8674(77)90143-x. [DOI] [PubMed] [Google Scholar]
- Shiba T., Saigo K. Retrovirus-like particles containing RNA homologous to the transposable element copia in Drosophila melanogaster. Nature. 1983 Mar 10;302(5904):119–124. doi: 10.1038/302119a0. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Bailey J. M. The binding of an avian myeloblastosis virus basic 12,000 dalton protein to nucleic acids. Nucleic Acids Res. 1979 Dec 11;7(7):2055–2072. doi: 10.1093/nar/7.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Snyder P. N. Structure of B77 sarcoma virus RNA: stabilization of RNA after packaging. J Virol. 1975 Nov;16(5):1161–1170. doi: 10.1128/jvi.16.5.1161-1170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykora K. W., Moelling K. Properties of the avian viral protein p12. J Gen Virol. 1981 Aug;55(Pt 2):379–391. doi: 10.1099/0022-1317-55-2-379. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Wight A., Eisenman R. In vitro cleavage of avian retrovirus gag proteins by viral protease p15. Virology. 1979 Oct 15;98(1):154–167. doi: 10.1016/0042-6822(79)90534-8. [DOI] [PubMed] [Google Scholar]
- Voynow S. L., Coffin J. M. Truncated gag-related proteins are produced by large deletion mutants of Rous sarcoma virus and form virus particles. J Virol. 1985 Jul;55(1):79–85. doi: 10.1128/jvi.55.1.79-85.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. R., LoPresti M. B., Setoguchi M., Konigsberg W. H. Amino acid sequence of the T4 DNA helix-destabilizing protein. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4614–4617. doi: 10.1073/pnas.77.8.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]