Abstract
A fetus is inherently antigenic to its mother and yet is not rejected. The T regulatory (Treg) subset of CD4+ T cells can limit immune responses and has been implicated in maternal tolerance of the fetus. Using virgin inbred mice undergoing a first syngenic pregnancy, in which only the male fetuses are antigenic, we demonstrate a maternal splenocyte proliferative response to the CD4+ T cell restricted epitope of the male antigen (H-Y) in proportion to the fetal antigen load. A portion of the maternal immune response to fetal antigens is Treg in nature. The bystander suppressive function of pregnancy-generated Tregs requires the presence of the fetal antigen, demonstrating their inherent antigen specificity. In vivo targeting of diphtheria toxin to kill Tregs leads to a lower fraction of live male offspring and a selective reduction in mass of the surviving males. Thus, Tregs generated in the context of pregnancy function in an antigen-specific manner to limit the maternal immune response to the fetus in a successful pregnancy.
Keywords: denileukin diftitox, Medawar, H-Y
Maternal tolerance of the fetal allograft has been a subject of research since Medawar formalized the apparent paradox of its maintenance (1). It is clear from both animal and human studies that the lack of maternal rejection of the fetus is not due to maternal immunologic ignorance of the fetus (2, 3). There is significant admixing of maternal and fetal cells in the other's compartments (4). Potent mechanisms of immune regulation must be activated during pregnancy to prevent rejection. However, these inhibitory mechanisms must only be directed against antigens of the fetus so that other immune responses can continue to protect the mother.
Mechanisms of peripheral immune tolerance invoke a central role for the regulation of CD4+ T cell activation and function (5). The diverse inhibitory as well as stimulatory roles for subsets of CD4+ T cells (6) implies that fate determination of CD4+ T cells may be an important mechanism limiting the occurrence of autoimmunity in healthy individuals. The robust regulatory capacity of Tregs has led to significant interest in the development and function of these specialized cells. The mechanisms underlying the function of Tregs are of intense interest given their obvious therapeutic potential. Various lines of investigation point toward direct cell contact or soluble mediators (7). Accumulating circumstantial evidence suggests that Tregs can provide an antigen-driven bystander suppression function (8).
Application of the knowledge of Tregs to the maintenance of pregnancy has yielded intriguing observations. Adoptive transfer reconstitution of BALB/c nude mice with lymphocytes depleted of Tregs results in smaller litters from allogenic matings (9). Treg populations peak in peripheral blood lymphocytes during the second trimester of human pregnancies and accumulate in the decidua (10).
To explore the nature of CD4+ T cell responses to fetal antigens, we chose a simple in vivo system: the maternal response of inbred mice during their first pregnancy to a male antigen. Male cells bear a minor transplantation antigen (H-Y), the reaction to which causes female mice to reject tissue grafts from male littermates (11, 12). From transplantation experiments, the CD8 and CD4 T cell epitopes of H-Y have been described (13–15). Details regarding CD4+ responses to H-Y during pregnancy have relied on the artificial system of a reconstituted RAG−/− mouse with T cells carrying a transgenic TCR having specificity for the CD4+ epitope (Dby) (16). We sought to understand the CD4+ T cell reactions in the unperturbed setting.
Results
Previous Pregnancy Imparts CD4+ T Cell Awareness to a Male Antigen.
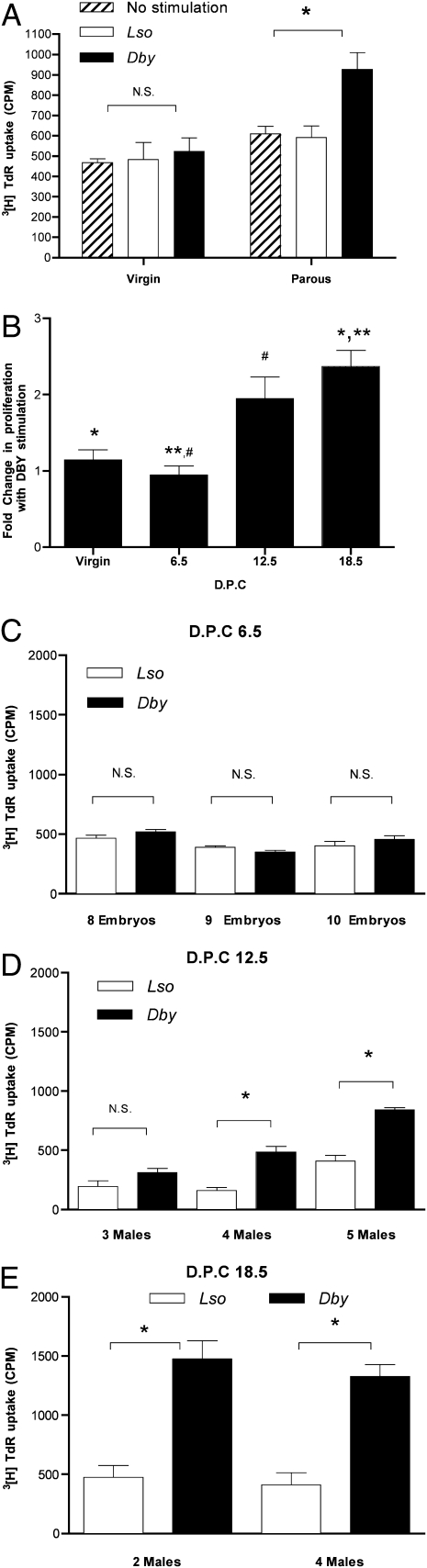
To test the hypothesis that pregnancy generates immunologic awareness of the fetuses, we evaluated inbred female C57BL/6 mice that had previously been pregnant (parous). Using a MHC class II (I-Ab) restricted CD4+ T cell H-Y antigenic peptide epitope (Dby) or a similarly restricted peptide epitope control from listeriolysin (Lso) as a source of stimulation, splenocytes from parous or virgin mice were tested for a proliferative response by 3[H]thymidine uptake (Fig. 1A, Right). The cells from parous mice were found to have a significant increase in proliferation when stimulated with Dby vs. Lso (or no peptide stimulation) reflecting a previous CD4+ T cell encounter with H-Y antigen. As expected, virgin mice do not display such immunologic awareness and do not differ in their (background) proliferative response to Lso as opposed to Dby (Fig. 1A, Left).
Fig. 1.
Pregnancy imparts CD4+ T cell awareness. (A) Splenocytes harvested from virgin (8 weeks old, n = 4) and previously bred (14 weeks old, n = 4) C57BL/6 mice from the same colony: Cellular proliferation assay performed using 1 × 106 cells per mL to the CD4+ T cell peptide epitope of the H-Y antigen presented by I-Ab (10 μM Dby), the I-Ab presented lysteriolysin peptide epitope (10 μM Lso), or no peptide stimulation. Cultures were pulsed with 1 μCi per well 3[H]TdR for the final 18 h of a 72-h culture (cpm ± SD). Statistical difference determined by one-way ANOVA with Bonferroni–Dunn posttest (N.S., nonsignificant; *, P < 0.05). Pregnancy primes maternal CD4+ T cell awareness in proportion to fetal antigenic mass. (B) Splenocytes harvested from virgin 8-week-old C57BL/6 mice (8 weeks, n = 4) and timed first pregnancy of 8-week-old female C57BL/6 × C57BL/6 males (n = 4 each time point) at days post coitus (dpc 6.5, 12.5, 18.5): Cellular proliferation assay performed using 1 × 106 cells per mL to the CD4+ T cell peptide epitope of the H-Y antigen presented by I-Ab (10 μM Dby) or the I-Ab presented lysteriolysin peptide epitope (10 μM Lso). Cultures were pulsed with 1 μCi per well 3[H]TdR for final 18 h of a 72-h culture. Composite fold increase in proliferation with Dby/Lso ± SEM. Statistical difference determined by one-way ANOVA with Bonferroni–Dunn posttest (*, **, #, P < 0.05). (C) Days postcoitus 6.5. Splenocyte proliferative response determined for individual mice ± SD along with fetal gender determination. (D) Days postcoitus 12.5. (E) Days postcoitus 18.5. Statistical difference determined by two-tailed, unpaired Student's t test (N.S., nonsignificant; *, P < 0.05).
We turned to mice in their first pregnancy, examining maternal splenocyte proliferative responses to Dby (vs. Lso) at three gestational time points: days postcoitus (dpc) 6.5, 12.5, and 18.5. Virgin mice did not show a significant proliferative response to Dby compared with Lso (Fig. 1B). At dpc 6.5 in mice with confirmed and enumerated embryos, we did not observe a stimulatory effect of Dby. At dpc 12.5, splenocytes showed a significant response to Dby (compared with Lso) with further increase in net stimulation by dpc 18.5 (Fig. 1B).
Pregnancy Primes Maternal CD4+ T Cell Awareness in Proportion to Fetal Antigenic Mass.
Different mothers carry different numbers of male mice, giving the possibility of seeing a natural dose response. Early in gestation (dpc 6.5), when no detectable maternal CD4+ T cell proliferation in response to Dby was evident (Fig. 1C), we were unable to identify the fetal gender by PCR using either SRY or H-Y amplification, but we believe that this is a result of insufficient antigen because it is statistically unlikely that all of the concepti were female. By dpc 12.5, a detectable splenocyte proliferative response was found to Dby in some pregnancies. At this gestational age, having more male fetuses increased the probability of seeing a significant response to Dby as compared with Lso. A mouse with three male fetuses displayed no significant proliferative response to Dby (Fig. 1D; albeit showing a trend to higher response). A mouse with four male fetuses had a statistically significant response to Dby, and a mouse with five male fetuses had a yet higher response, demonstrating a fetal antigenic dose relationship to the maternal CD4+ T cell response at this gestational age. As the fetal mass increased with advancing gestation, the magnitude of the maternal CD4+ T cell sensitivity to Dby stimulation increased so that two male fetuses were as efficient as four for eliciting a maternal CD4+ T cell proliferative response to Dby (Fig. 1E), although at an earlier gestation, three males were insufficient.
Pregnancy Primes a T Regulatory Response to Male Fetal Antigen.
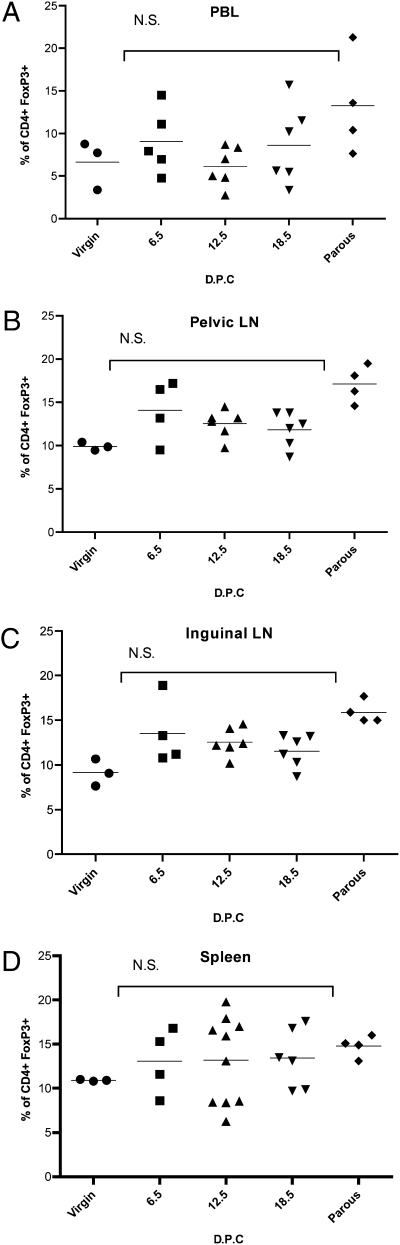
Tregs selectively express the transcription factor FoxP3 (17). To evaluate the role of Tregs induced during pregnancy, we examined changes in the proportion of FoxP3-expressing maternal CD4+ cells in various lymphoid compartments of virgin, first pregnancy, and parous C57BL/6 mice. Cells from various lymphoid organs were evaluated by FACS for the proportion that was CD4+FoxP3+ (Treg). There was a non-statistically significant trend toward increasing Treg proportions with respect to advancing gestational age as compared with virgin mice (Fig. 2). This trend persisted in parous mice. There does not appear to be a significant regional accumulation of Tregs in a specific lymphoid compartment in these syngenic mating, but in more disparate crosses, an increase in the proportion of Tregs has been reported (9).
Fig. 2.
Regional distribution of T regulatory cells in relation to first syngenic mating. Single-cell suspensions from indicated lymphoid organs prepared from virgin (n = 3) or timed first pregnancies from C57BL/6 syngenic mating (dpc 6.5, n = 4; dpc 12.5, n = 6; dpc 18.5, n = 6), or retired from syngenic breeding (n = 4). FACS performed with gating on CD4+ cells to determine the fraction of FoxP3+ cells. Statistical differences determined by one-way ANOVA with Bonferroni–Dunn posttest (N.S., nonsignificant).
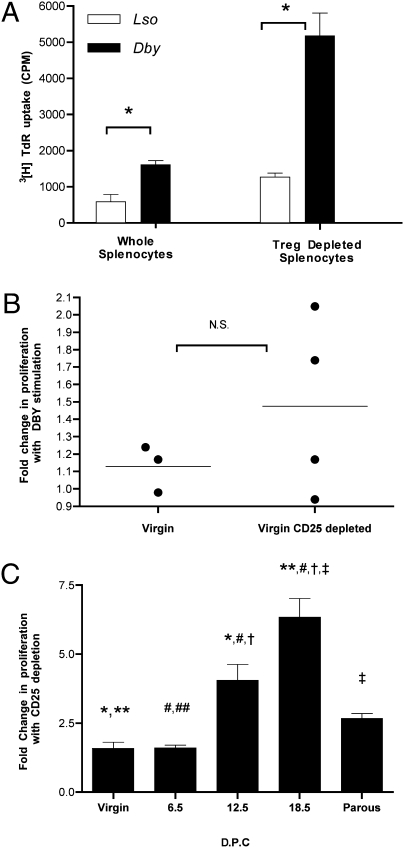
To probe the functional nature of Tregs in pregnant mice, we examined the consequences of depletion of Tregs on the proliferative response of maternal lymphocytes to H-Y antigen. To remove Tregs, we depleted cells bearing CD25 on the cell surface, which should greatly reduce Tregs (18). Day 18.5 whole splenocytes from a C57BL/6 mouse in her first pregnancy with six male fetuses displayed a proliferative response similar to that seen previously for other dpc 18.5 mice (Fig. 1E). After depletion of CD25+ cells, the proliferative response to Dby increased 3-fold (Fig. 3A). In contrast, depletion of CD25+ cells from splenocytes from virgin mice that do not proliferate to Dby (Figs. 1 A and B, and Fig. 3B) did not similarly unmask a proliferative response. With increasing fetal antigenic mass, there is an increased proliferative response to specific fetal antigen (Fig. 1B). Similarly, there is an increasing effect of CD25+ depletion on the augmentation of the proliferative response to Dby in proportion to the fetal antigenic burden (Fig. 3C). In addition, there appears to be a residual augmentation of splenocyte proliferative response to Dby in parous mice with CD25+ cell depletion (Fig. 3C). This last finding suggests that pregnancy develops a lasting functional Treg population in response to the presence of male fetuses. To be sure that we had removed Tregs, we directly characterized the effect of CD25+ depletion on the Treg (CD4+FoxP3+) population. Depletion of CD25+ cells decreased the CD4+FoxP3+ population to a similar extent (80–90%) in all samples tested (Fig. S1).
Fig. 3.
Pregnancy results in a functional T regulatory response in proportion to the fetal mass. (A) Splenocytes harvested from a timed first pregnancy of a 6-week-old C57BL/6 × C57BL/6 male at dpc = 18.5 (six male fetuses). Splenocyte proliferative response determined for individual mice ± SD. (B) Splenocytes from virgin 6-week-old C57BL/6 (no prior plugs). Treg depletion by magnetic bead separation of CD25+ cells. Cellular proliferation assay was performed using 1 × 106 cells per mL to the CD4+ T cell peptide epitope of the H-Y antigen presented by I-Ab (10 μM Dby) or the I-Ab presented lysteriolysin peptide epitope (10 μM Lso). Cultures were pulsed with 1μCi per well 3[H]TdR for the final 18 h of a 72-h culture (cpm ± SD). Statistical difference determined by two-tailed unpaired Student's t test (N.S., nonsignificant; *, P < 0.05). (C) Splenocytes from virgin, timed first pregnancy of C57BL/6 × C57BL/6 (8 weeks old, n = 4 each time point) dpc 6.5, 12.5, and 18.5, or 14-week-old retired breeder (parous). Depletion of CD25+ cells (Treg) by magnetic bead. Composite fold increase in proliferation with Dby (CD25+ depleted)/Dby (whole splenocytes) ± SEM. Statistical difference determined by one-way ANOVA with Bonferroni–Dunn posttest (*, **, #, †, ‡, P < 0.05).
Pregnancy-Induced Treg Suppressive Function Is Antigen Specific.
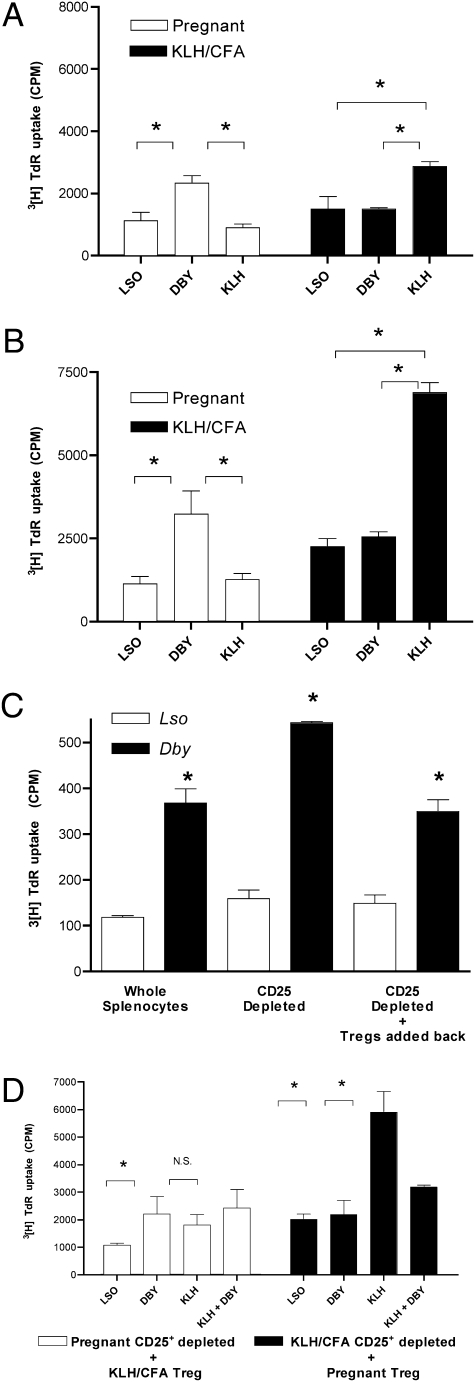
We sought to determine whether Tregs generated during pregnancy were capable of bystander suppression, as has been previously reported in other nonpregnancy systems (7), and whether bystander suppression is an antigen-specific phenomenon. To examine this question, we evaluated the ability of pregnancy-induced Tregs to suppress an in vitro lymphocyte response to a model antigen (KLH) raised in a separate mouse. Splenocytes were isolated from a four-male pregnant mouse on dpc 18.5 and from a virgin female mouse immunized with KLH/CFA 7 days previously. Splenocytes from the pregnant mouse showed a proliferative response when stimulated with Dby vs. Lso. Additionally, these splenocyte cultures from the pregnant mouse did not show a proliferative response to KLH above basal proliferation (Fig. 4A). Conversely, the virgin mouse immunized with KLH/CFA did not show a proliferative response to Dby above the basal level, but did have a significant response to KLH. When the splenocytes from the pregnant mouse were depleted of CD25+ cells (Tregs), the proliferative response to Dby was augmented whereas the responses to Lso (basal proliferation) or KLH were unaffected (Fig. 4B). Similarly, CD25+ cell depletion from splenocyte cultures from KLH/CFA-immunized mice generated a significant increase in proliferation to KLH when compared with nondepleted splenocyte cultures, yet the proliferative responses to Lso or Dby in these virgin mouse cells remained unaffected (Fig. 4B). Of note, we deliberately did not provoke a maximal response to KLH to keep the proliferation in line with the subtle nature of the antipregnancy response.
Fig. 4.
Pregnancy-induced T regulatory cells suppressive function is antigen-specific. (A) Splenocytes harvested from a timed first pregnancy of 6-week-old C57BL/6 × C57BL/6 male at dpc 18.5 (four male fetuses) as well as virgin 6-week-old C57BL/6 female immunized with KLH/CFA 7 days prior. Cellular proliferation assay was performed using 1 × 106 cells per mL to the CD4+ T cell peptide epitope of the H-Y antigen presented by I-Ab (10 μM Dby), the I-Ab presented lysteriolysin peptide epitope (10 μM Lso) or KLH (1 μg/mL). Cultures were pulsed with 1 μCi per well 3[H]TdR for the final 18 h of a 72-h culture. (B) CD25+ reduced splenocytes. (C) Splenocytes harvested from a timed first pregnancy of 6 weeks – old C57BL/6 x C57BL/6 male at dpc 15.5 (three male fetuses). CD25+ cell reduced. CD25+ cells (Treg) added back to CD25+-depleted splenocytes in a ratio of 4:1. (D) CD25+-reduced splenocytes from either the dpc 18.5 mouse (□) or the KLH/CFA-immunized mouse (■) above cocultured with the CD25+ (Treg enriched population) isolated by magnetic bead positive selection from the KLH/CFA-immunized mouse (□) or the pregnant mouse (■) at a ratio of 4:1. Stimuli as indicated (Lso 10 μM, Dby 10 μM, KLH 1 μg/mL). Statistical difference determined by one-way ANOVA with Bonferroni–Dunn posttest (N.S., nonsignificant; *, P < 0.05).
When the CD25+ cells (Tregs) were added back to previously CD25+ depleted splenocytes from the pregnant mouse, the resulting proliferation matched the unperturbed splenocyte culture (Fig. 4C). When the CD25+ cells (Tregs) from the pregnant mouse were cocultured with CD25+-depleted splenocytes from the KLH/CFA-immunized mouse (ratio of Treg/CD25+ depleted splenocytes = 1:4) the culture showed an unchanged proliferative response to KLH despite the physical presence of additional Tregs (Fig. 4D). When Dby and KLH were added to the mixed CD25+ cell–depleted splenocytes from KLH/CFA-immunized mice and CD25+ cells from the pregnant mouse, the proliferative response to KLH was efficiently suppressed (Fig. 4D). Thus, the suppressive effects of the CD25+ cells (Treg) from the pregnant mouse were only manifest in the presence of Dby, reflecting an antigen requirement for activation of the bystander suppressive effect. In contrast, CD25+ cells isolated from KLH immunized mice and cocultured with CD25+-depleted splenocyte cultures from pregnant mice did not show a corresponding ability to perform bystander suppression when activated with KLH and Dby (Fig. 4D). In both cases, a similar absolute number of CD4+FoxP3+ cells were present in the cultures [9,970 pregnancy-derived Tregs vs. 12,800 KLH/CFA-derived Tregs (Fig. S2)]. We did find that limited fetal antigen exposure (one to two male fetuses on dpc 12.5) generated no detectable bystander suppressive effect (Fig. S3 A and B, respectively).
Depletion of Tregs in Vivo During Pregnancy Leads to Rejection of Male Fetuses.
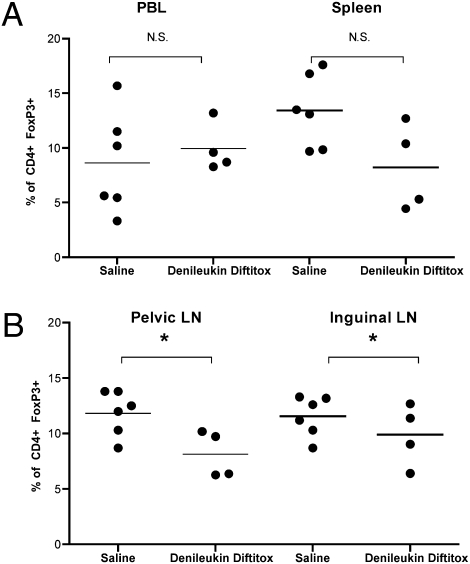
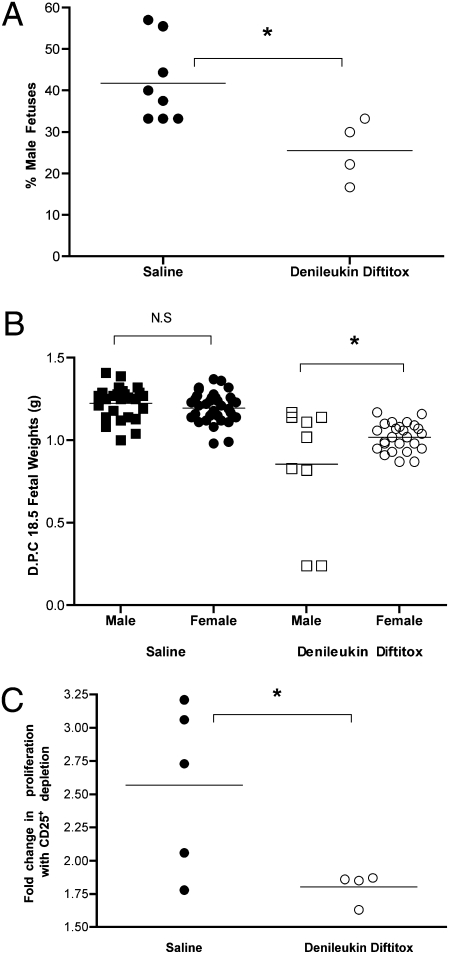
CD25 is the IL-2 receptor protein and denileukin diftitox is a recombinant protein consisting of IL-2 linked to diphtheria toxin (19). This FDA-approved drug (for recurrent cutaneous T cell lymphoma) has been shown in mice to partially deplete (by up to 50%) Tregs in vivo over the course of 3 days (19). We treated C57BL/6 mice in their first pregnancy (syngenic) with three equal doses of the drug on dpc 6.5, 12.5, and 15.5. Evaluation of the Treg populations on dpc 18.5 in various lymphoid organs found that only in the pelvic/periaortic lymph nodes was there a significant decrease in Tregs (Fig. 5). This finding is consistent with the known recovery of Treg populations over the course of 3 days posttreatment (19). However, the pelvic nodes may be particularly susceptible to Treg depletion because these lymph nodes are the draining sites for the peritoneum and, as such, may have had the highest local dose of denileukin diftitox. Despite relatively unchanged Treg populations on dpc 18.5, we did observe adverse pregnancy outcomes in the treated mice vs. saline-treated controls. First, there is a striking reduction in the proportion of males among litters from mice treated with denileukin diftitox (25.6 ± 3.8%) vs. saline-treated controls (41.8 ± 3.4%) (Fig. 6A). Second, the weight of the male fetuses (0.86 ± 0.12 g) was reduced compared with the females (1.02 ± 0.02 g) from mice treated with denileukin diftitox. This observation was dominated by two particularly small males, but many of the male fetuses weighed as much as females. In saline-treated control mice, the weights were equivalent between the male (1.22 ± 0.02 g) and female (1.20 ± 0.01 g) fetuses (Fig. 6B). Our in vitro evaluation of splenocyte response to Dby stimuli compared with Lso from denileukin diftitox–treated mice found that, as would be expected, further depletion of CD25+ cells from splenocyte cultures did not augment the proliferative response to Dby to nearly the same extent as in saline-treated control mice (Fig. 6C). This last finding might indicate that although the population of Tregs overall is unperturbed, the H-Y–specific Tregs were selectively diminished in vivo.
Fig. 5.
In vivo effect of denileukin diftitox on Treg populations in regional lymphoid organs. Shown is FACS analysis of indicated single-cell suspensions immediately ex vivo stained for CD4 and FoxP3 from timed first pregnancy of C57BL/6 × C57BL/6 at dpc 18.5. Saline, n = 6; denileukin diftitox, n = 4. Statistical difference was determined by two-tailed, unpaired Student's t test (N.S., nonsignificant; *, P < 0.05).
Fig. 6.
In vivo reduction in Tregs results in poor pregnancy outcome specific to fetal antigens. (A) Percentage male fetuses determined for each litter of C57BL/6 × C57BL/6 by PCR from Saline-treated (●) (n = 8) or denileukin diftitox–treated (○) mice (n = 4). (B) On dpc 18.5, fetal weights determined for fetuses from the above mating. (C) Splenocytes harvested from the saline-treated (●) (n = 5) or denileukin diftitox–treated (○) (n = 4) mice, and cellular proliferation assay performed using 1 × 106 cells per mL to the CD4+ T cell peptide epitope of the H-Y antigen presented by I-Ab (10 μM Dby) or the I-Ab presented lysteriolysin peptide epitope (10 μM Lso). Cultures were pulsed with 1 μCi per well 3[H]TdR for the final 18 h of a 72-h culture. Depletion of CD25+ cells (Treg) by magnetic bead. Composite fold increase in proliferation with Dby (CD25+ depleted)/Dby (whole splenocytes). Statistical difference was determined by two-tailed, unpaired Student's t test (N.S., nonsignificant; *, P < 0.05).
A pregnant outbred mouse faces an immunologic challenge of dramatic proportions in the foreign nature of the many fetal antigens. By using inbred mice, we have chosen to focus on a single antigenic difference: the maternal response to male fetuses during first pregnancy as evidenced by a recall response to male antigen in cells from parous mice but not those from virgin mice. It is important to note that in our system, we deliberately used virgin (no prior vaginal plug) mice because in some systems mucosal exposure to male antigens is sufficient to prime an immune response (20).
Discussion
As the fetal mass increases with increasing gestational age and number of male concepti, the maternal CD4+ T cell response increases. This maternal CD4+ T cell response to H-Y involves at least two cell types, active helper T cells and Tregs. Removing the Tregs reveals the magnitude of the active T cell response, showing that both forms of response are occurring. The suppressive ability of the Tregs isolated was in proportion to antigenic stimulus (i.e., the mass of fetal antigen). Pregnancy apparently leads to a maternal immune response to the fetus strongly suppressed by the Treg component. This is at least one reason why mothers do not reject their babies as evidenced by compromising the Treg response with denileukin diftitox, which generated selective rejection of male fetuses. It would appear that exposing mice over much of their pregnancy to an environment lacking sufficient Tregs leads to embryo rejection.
To understand the role of antigen in suppression, we examined the bystander effect of antigen-driven Tregs. For the cells developed during pregnancy, antigen was required for them to suppress a KLH response; the simple presence of unstimulated Tregs had no effect. Bystander suppression was not reciprocal: KLH-specific Tregs suppressed a KLH response but even with added antigen did not display bystander activity against the H-Y response. The difference in bystander suppressive capability might relate to the proportion of the Tregs that are specific to the antigen rather than the absolute number of CD4+FoxP3+ cells. Conditions that favor the development of Tregs (pregnancy) likely increase a functional subset of CD4+CD25+FoxP3+ cells without affecting the overall population of Tregs. Immunization with CFA is inherently inflammatory and generates an immune response that may be dominated by effector CD4+ cells, especially at the early time we studied.
The antigen-dependent bystander suppression by pregnancy-induced Tregs is evident in this system, but the requirements of Treg-induced suppression as seen in other systems is controversial (7, 8). Some investigations have found such a requirement where as others have not, but all of these studies have used artificial systems like TCR transgenic mice (7, 21–24). Pregnancy, though a complicated stimulus, produces Tregs as part of a natural response. In all of these cases, the study of Treg functionality has been limited by the inability to deliberately induce an antigen-specific Treg population. We found that as an intrinsic property of pregnancy, a population of Tregs develops suppressive function in proportion to the fetal antigens with which the mother must cope. Furthermore, because the fetal antigen in our simple system is well defined (H-Y), we have been able to demonstrate that the Tregs gain suppressive reactivity to this antigen and are capable of bystander suppression. Pregnancy provides a unique situation in which a population of Tregs can be naturally expanded and their physiologic function explored.
Our findings here provide a framework to investigate two fundamental issues in immune tolerance. First, the highly specific and important role that Tregs play in the success of a pregnancy can be explored to examine such questions as what characteristics of pregnancy lead to a Treg response and the conditions that lead to the breakdown of tolerance in pregnancy-specific diseases. Second, the use of pregnancy to generate a population of Tregs with a defined biological role may facilitate our understanding of this important T cell subset.
Materials and Methods
Mice.
C57BL/6 mice were obtained from an existing breeding colony at Caltech. The colony is housed in a barrier facility used for transgenic mice generation. The mice were subjected to timed matings between previously successful breeder males aged 8–12 weeks and virgin (no prior plugs) females aged 5–6 weeks. The day of vaginal plug observation was denoted as dpc 0.5. Mice were housed and handled in accordance with National Institutes of Health guidelines under Institutional Animal Care and Use Committee–approved protocols.
Reagents.
Keyhole limpet hemocyanin (KLH) was purchased from Calbiochem. Freund's adjuvant and Mycobacterium tuberculosis were obtained from Difco. Complete Freund's adjuvant (CFA) was prepared as 4 mg/mL M. tuberculosis in a 1:1 (vol/vol) emulsion of Freund's adjuvant and PBS. CD4+ T cell I-Ab restricted epitopes for H-Y (Dby608–622) NAGFNSNRANSSRSS (15) and irrelevant I-Ab restricted peptide epitope Listeriolysin O (Lso190–201) were prepared by custom solid-phase synthesis (New England Peptide).
Proliferation Assays.
Spleens were harvested and prepared into a single-cell suspension with a metal screen (300 μm). RBCs were lysed by a room temperature incubation in a hypotonic solution [0.83% NH4Cl, 0.02 M Tris (pH 7.6)]. Cells were plated in round-bottom, 96-well tissue culture plates at 1 × 106 cells per mL in RPMI medium 1640 (GIBCO) supplemented with 10% (vol/vol) heat-inactivated, 5.5 × 10−5 M 2-ME, 100 units/mL penicillin, 100 units/mL streptomycin, and 2.5 mM L-glutamine. Cells were cultured at 37 °C with 5% CO2 in a humidified incubator. Cells assayed for proliferation were pulsed with 1 μCi per well 3[H]thymidine for the final 18 h.
Lymphoid Organ Preparation.
Whole blood was collected by cardiac puncture. Lymphocytes were isolated using a Ficoll gradient. Inguinal and pelvic/periaortic lymph nodes were collected and single-cell suspensions prepared by passing the tissue through a 70-μm polypropylene mesh cell strainer (BD) in PBS on ice.
Flow Cytometry and Cell Separation.
Fluorescently labeled monoclonal antibodies to CD4 and FoxP3 were obtained from BioLegend. Staining was done using the Foxp3 staining buffer set according to the manufacturer's instructions (eBioscience). CD25 depletion was accomplished by magnetically labeled antibody mediated columns according to the manufacturer's instructions (Miltenyi Biotec). Analysis was performed on a BD FACSCalibur with postacquisition analysis performed with FlowJo (Tree Star).
Fetal Gender Identification.
Fetal tissue was placed in 75 μL of base solution [0.025 M NaOH, 0.2 mM EDTA (pH 12)] and incubated at 95 °C for 30 min. To this was added 75 μL of neutralization solution [0.04 M Tris·HCl (pH 5.0)]. The supernatant was used as DNA template in a PCR (Platinum Amplitaq; Invitrogen) with primers for murine SRY, 5′-TTGTCTAGAGAGCATGGAGGGCCATGTCAA-3′ and 3′-AGCCTCCCGATTTCACAGTGTCTCCTCACC-5′. In the same reaction were included control primers for Fabpi, 5′-CCTCCGGAGAGCAGCGATTAAAAGTGT-3′ and 3′-TAGAGCTTTGCCACATCACAGGTCATTCA-5′. The amplicon of SRY is 268 bp and of Fabpi is 455 bp.
In Vivo Treg Depletion.
Mice were treated with denileukin diftitox (Eisai) 100 μg/200 μL PBS or PBS alone i.p. on dpc 6.5, 12.5, and 15.5.
Statistical Analysis.
Differences were statistically analyzed using unpaired Student's t test or analysis of variance (ANOVA) with Bonferroni/Dunn posttest. Analysis was accomplished with PRISM (GraphPad).
Supplementary Material
Acknowledgments
We thank L. Sandoval of the Caltech Office of Laboratory Animal Resources for expert assistance with the timed matings. This work was supported in part by a research grant from the Skirball Foundation (to D.B.). D.A.K. is supported by the National Institutes of Health—Building Interdisciplinary Research Careers in Women's Health (BIRCWH) center at the University of California, Los Angeles (K12 HD001400).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003909107/-/DCSupplemental.
References
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Trowsdale J, Betz AG. Mother's little helpers: Mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 3.Mold JE, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barinaga M. Cells exchanged during pregnancy live on. Science. 2002;296:2169–2172. doi: 10.1126/science.296.5576.2169. [DOI] [PubMed] [Google Scholar]
- 5.Niederkorn JY. See no evil, hear no evil, do no evil: The lessons of immune privilege. Nat Immunol. 2006;7:354–359. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 6.Reiner SL. Development in motion: Helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70:326–336. doi: 10.1111/j.1365-3083.2009.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–271. doi: 10.1038/ni1037. [DOI] [PubMed] [Google Scholar]
- 10.Tilburgs T, et al. Evidence for a selective migration of fetus-specific CD4+CD25bright regulatory T cells from the peripheral blood to the decidua in human pregnancy. J Immunol. 2008;180:5737–5745. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- 11.Eichwald EJ, Silmser CR, Weissman I. Sex-linked rejection of normal and neoplastic tissue. I. Distribution and specificity. J Natl Cancer Inst. 1958;20:563–575. [PubMed] [Google Scholar]
- 12.Weissman IL, Jerabek L, Greenspan S. Tolerance and the H-Y antigen: Requirement for male T cells, but not B cells, to induce tolerance in neonatal female mice. Transplantation. 1984;37:3–6. [PubMed] [Google Scholar]
- 13.Greenfield A, et al. An H-YDb epitope is encoded by a novel mouse Y chromosome gene. Nat Genet. 1996;14:474–478. doi: 10.1038/ng1296-474. [DOI] [PubMed] [Google Scholar]
- 14.Markiewicz MA, et al. Long-term T cell memory requires the surface expression of self-peptide/major histocompatibility complex molecules. Proc Natl Acad Sci USA. 1998;95:3065–3070. doi: 10.1073/pnas.95.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott D, et al. Dendritic cells permit identification of genes encoding MHC class II-restricted epitopes of transplantation antigens. Immunity. 2000;12:711–720. doi: 10.1016/s1074-7613(00)80221-6. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Demir Y, Valujskikh A, Heeger PS. The male minor transplantation anti-gen preferentially activates recipient CD4+ T cells through the indirect presentation pathway in vivo. J Immunol. 2003;171:6510–6518. doi: 10.4049/jimmunol.171.12.6510. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 19.Litzinger MT, et al. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110:3192–3201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock RJ, Faruki S. Assessment of immune responses to H-Y antigen in naturally inseminated and sperm-injected mice using cell-mediated cytotoxicity assays. J Reprod Immunol. 1986;9:187–194. doi: 10.1016/0165-0378(86)90012-4. [DOI] [PubMed] [Google Scholar]
- 21.Szymczak-Workman AL, Workman CJ, Vignali DA. Cutting edge: Regulatory T cells do not require stimulation through their TCR to suppress. J Immunol. 2009;182:5188–5192. doi: 10.4049/jimmunol.0803123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: Induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 23.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur J Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 24.Tonkin DR, He J, Barbour G, Haskins K. Regulatory T cells prevent transfer of type 1 diabetes in NOD mice only when their antigen is present in vivo. J Immunol. 2008;181:4516–4522. doi: 10.4049/jimmunol.181.7.4516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.