Abstract
The primary bactericidal domain of CAP37, a cationic antimicrobial protein with potent activity against Gram-negative organisms was previously shown to reside between amino acids 20 through 44 (NQGRHFCGGALIHARFVMTAASCFQ) of the native protein. In this study, we explored the efficacy of four synthetic CAP37 peptide analogs, based on this sequence, against various Candida species including fluconazole-sensitive and -resistant isolates of C. albicans. Three of the peptides demonstrated strong antifungal activity for C. albicans, including fluconazole-resistant isolates of C. albicans and were active against C. guilliermondii, C. tropicalis, C. pseudotropicalis, C. parapsilosis, and C. dubliniensis. The peptides were ineffective against C. glabrata, C. krusei, and Saccharomyces cerevisiae. For C. albicans isolates, the peptides had relatively greater activity against blastoconidia than hyphal forms, although strong antifungal activity was observed with pseudohyphal forms of the various Candida species tested. Kinetic studies demonstrated fungicidal rather than fungistatic activity. These findings indicate that synthetic peptides based on the antimicrobial domain of CAP37 also have activity against eukaryotic organisms suggesting a broader range of activity than originally demonstrated and show for the first time their potent fungicidal activity.
Keywords: CAP37, antibiotic peptides, in vitro fungicidal activity, Candida albicans, innate immunity
INTRODUCTION
CAP37 (cationic antimicrobial protein of Mr 37 kDa), also termed heparin binding protein [1] and azurocidin [2], was originally identified as a component of the oxygen-independent killing mechanism of the human neutrophil (PMN) and demonstrated strong bactericidal activity for Gram-negative organisms, including Salmonella typhimurium, Escherichia coli, and Pseudomonas aeruginosa [3, 4, 5]. Distinct from its effect on bacteria the native CAP37 protein has potent regulatory effects on host cells. It is an effective regulator of cells of the mononuclear phagocytic system such as monocytes [6], microglia [7], and macrophages [8]. It also regulates corneal epithelial [9], endothelial [10, 11], and smooth muscle cell functions [12].
Structure function analysis of CAP37 enabled us to delineate its antibacterial activity to a domain consisting of 25 amino acids corresponding to residues 20 through 44 of the native molecule [13]. A synthetic peptide (CAP3720-44) based on this sequence mimicked the antimicrobial activity of the native molecule [13] and demonstrated an extended range of activity to encompass two Gram-positive organisms, Staphylococcus aureus and Enterococcus faecalis. In vivo experiments demonstrated the efficacy of CAP3720-44 in attenuating the lethal effects of E. coli lipopolysaccharide (LPS) in a rat model of endotoxic shock [14]. The synthetic peptide bound and neutralized the toxic effects of LPS resulting in the attenuation of many of the circulatory and metabolic dysfunctions and lethality associated with sepsis [14]. The combination of sequence, charge, amphipathicity and hydrophobicity appear to be unique to CAP3720-44, since two synthetic peptides, based on cathepsin G and neutrophil elastase, share almost 40% sequence identity in this region and were significantly less effective at killing bacteria and binding LPS [13, 14].
In this study we extended our findings on the antimicrobial repertoire of the synthetic peptide CAP3720-44 to determine whether it possessed antifungal activity as a means to evaluate its effects against eukaryotes. We further explored the importance of the cysteine residues by synthesizing two new analogs of CAP3720-44. One analog had the cysteine at position 26 substituted with a serine residue (CAP3720-44Ser26), and the other analog had the cysteine at position 42 replaced with a serine residue (CAP3720-44Ser42). The activities of these three analogs were compared to a fourth peptide in which both cysteine residues were replaced by serine residues (CAP3720-44ser26/42). The latter peptide had previously been shown to be ineffective against Gram-negative organisms [13]. The various CAP37 peptide analogs were tested for activity against fluconazole-sensitive and –resistant clinical isolates of Candida albicans, a number of other Candida species and two isolates of Saccharomyces cerevisiae to determine its range of activity. Additionally, the efficacy of the peptide on hyphal forms of C. albicans and pseudohyphal forms of C. pseudotropicalis and C. tropicalis was determined along with a series of time-kill studies to establish whether the activity was fungicidal or fungistatic.
MATERIALS AND METHODS
Synthesis of peptides based on CAP37
Peptides were synthesized by tert-butyloxycarbonyl (Boc)/benzyl solid-phase synthesis on an Applied Biosystems peptide synthesizer (model 433A, Foster City, CA) as previously described [6]. PAM-resins were used and activation of Boc-amino acids was accomplished using HOBt/DCC. The side-chain-protected, Boc-L-amino acids included: Boc-Arg(mesitylsulfonyl); Boc-Cys(4-methoxybenzyl) for Cys; Boc-His(dinitrophenyl), Boc-His(benzyloxycarbonyl) or Boc-His(benzoxymethyl) for His; Boc-Ser(benzyl); and Boc-Thr(benzyl). The couplings were performed in either dimethylformamide or N-methylpyrrrolidone/dimethyl sulfoxide as solvents. The peptides were cleaved off the resin and deprotected in liquid HF/anisole/dimethyl sulfide (10:1:0.5) for 1 h at 0°C, precipitated with cold diethyl ether, extracted into 50% aqueous acetic acid, and lyophilized. The crude peptides were dissolved in formic acid/TFA/trifluoroethanol/water (20:1:10:70, v/v) at approximately 15 mg/ml and purified by preparative reversed-phase (RP) HPLC on a Zorbax SB-C18 silica column (22×250 mm, 5 μm particle size, 300 A pore size; Agilent, Palo Alto, CA) equilibrated in 0.1% aqueous TFA and eluted using a linear gradient of acetonitrile in 0.08% aqueous TFA at 25°C. Purity of the peptides was ascertained by analytical RP-HPLC using a C18 silica SUPER-ODS column (2.1×100 mm, 2 μm particle size, 300 A pore size; TosoHaas, Montgomeryville, PA), eluted using a gradient of acetonitrile in 0.1% aqueous TFA and monitored at 214 nm. It confirmed our observations made during preparative RP-HPLC, namely that CAP3720-44 and its derivatives aggregate in highly acidic solutions and do not chromatograph as sharp, distinct peaks on C18 packings; instead they elute as broad peaks in the 40% and 50% acetonitrile concentration range. Counter-ion alternatives to TFA, such as heptafluorobutyric acid or formic acid, and/or alternative elution solvents to acetonitrile, such as 2-propanol or 1-butanol, did not improve the elution profiles of these peptides, and neither did column elution at higher temperatures (up to 65°C). The HPLC fractions were screened by MALDI-TOF mass spectrometry (MS) and those containing the desired product, as determined by its presence as the major base peak in the mass spectra corresponding to the desired product, were pooled. The purified peptides were obtained lyophilized in the form of their TFA salts. The purity and integrity of the peptides was confirmed by MALDI-TOF MS and electrospray ionization MS, and by N-terminal Edman sequencing, using described protocols [15].
Peptides were synthesized predicated on previous findings that a synthetic peptide, CAP3720-44, based on the native amino acid sequence of CAP37 consisting of residues 20 through 44 (NQGRHFCGGALIHARFVMTAASCFQ) had potent bactericidal activity for a number of Gram-negative organisms [13]. CAP3720-44 contains two cysteine residues in positions 26 and 42 which form a disulfide bridge in the native CAP37 molecule [16]. Previously we showed that simultaneous replacement of both cysteines with serine residues (CAP3720-44ser26/42) abrogated antibacterial activity [13]. This peptide is routinely incorporated in our in vitro studies to serve as an inactive or control peptide. The synthesis of CAP3720-44 is technically challenging due to problems related to solubility and aggregation in the purification stage. We thus queried whether a peptide with replacement of a single cysteine would retain its antimicrobial activity yet overcome the purification problems we had encountered. Therefore, in this study, we synthesized two new analogs containing singly substituted cysteine residues. The analog in which the cysteine at position 26 was replaced by a serine is indicated as CAP3720-44ser26 and the analog in which the cysteine at position 42 was replaced by a serine is indicated as CAP3720-44ser42. The replacement of either one or both cysteine residues is considered a very conservative replacement [17]. The peptides used in this study were obtained with cysteine residues in their reduced form. We confirmed, using mass spectrometry that under the conditions used for the assay (pH 5.5, see below) no significant oxidation of the peptides occurred in the stock solutions prior to their use or upon several weeks of storage at −20°C.
Fungal isolates and culture conditions
Isolates used in this study included C. albicans (3153A), three fluconazole-sensitive isolates of C. albicans and three fluconazole-resistant isolates of C. albicans from patients with vulvovaginitis; and a blood isolate of C. albicans obtained from a patient with candidemia. Isolates were stored frozen at −70°C in 10% glycerol and streaked onto Sabouraud Dextrose Agar (SAB, Sigma, St. Louis, MO) plates and maintained on plates at 4°C, with subculturing approximately every 10 days for the duration of the studies. Fluconazole resistance as determined by standard in vitro susceptibility assays was defined as the MIC of >64 μg/ml. Three clinical isolates of C. glabrata; two clinical isolates of C. dubliniensis; single isolates each of C. krusei, C. guilliermondii, C. parapsilosis, C. pseudotropicalis, and C. tropicalis; and two isolates of Saccharomyces cerevisiae were also used in this study and were maintained similarly on SAB plates at 4°C. For use in assays, a single yeast colony was cultured at 33°C in 1% phytone peptone broth (Becton Dickinson, Sparks, MD) with 0.1% D-glucose (Sigma) to stationary phase (~ 18 h or overnight). Blastoconidia were collected and enumerated by trypan blue dye exclusion.
Induction of hyphae
To induce hyphal formation, a single colony of each C. albicans isolate was cultured as above to stationary phase. An aliquot (500 μl) of the stationary phase culture was subcultured in 5 ml of RPMI-1640 (Cellgro Mediatech Inc, Herndon, VA) with 10% fetal calf serum (Invitrogen, Grand Island, NY) for 120 min at 37°C with rotation. Cultures were monitored and assessed for hyphal formation using phase microscopy. The C. albicans isolates under these growth conditions were predominantly mycelium but with some still in transition from germ tube to hyphae [18]. C. pseudotropicalis and C. tropicalis demonstrated pseudohyphal morphology under the growth conditions described. Samples were normalized by optical density reading at 600 nm for addition to the antifungal assays as described below. Enumeration of samples was confirmed by plating100 μl of the culture at the start of the assay (T=0).
Standard antifungal colony forming unit (CFU) assay
A standard CFU assay [2, 4, 13] was performed to assess the antifungal activity of the peptides incorporating the following modifications. Stock solutions (1mg/ml) of all peptides were made in sterile endotoxin-free water for irrigation (Baxter, Deerfield, IL). Subsequent dilutions were all made in tryptone saline pH 5.5 [3]. An aliquot (100 μl) of a stationary phase fungal culture was subcultured further in 5 ml of 1% phytone peptone broth with 0.1% glucose and incubated in a shaking water bath (80 oscillations per min, Precision, Winchester, VA) for 90 min at 33°C to yield a logarithmic culture. Cell cultures under these conditions were found to consist predominantly of blastoconidia as determined by phase contrast microscopy. The optical density was determined and the culture adjusted to 500 blastoconidia/100 μl in tryptone saline pH 5.5 [3]. To 100 μl of the organism suspension in a 96 well sterile polystyrene microtiter plate (Becton Dickinson, Franklin Lakes, NJ) was added 100 μl of the peptide at varying concentrations for an initial dose response and further at final concentrations of 400 μg/ml and 200 μg/ml. In addition, two controls, T=0 and T=240, were set up consisting of 100 μl of organism suspension and 100 μl of tryptone saline. 100 μl from the T=0 control was plated immediately and served to confirm the starting numbers of the organisms used in each assay. The microtiter plate was incubated at 37°C for 4 h and 100 μl of the contents from each well was plated on SAB plates and incubated at 37°C overnight. The CFU were counted and the antifungal activity was expressed as percent inhibition and calculated according to the following equation: [(T240control CFU − test CFU)/T240control CFU] × 100 = % inhibition. T240control CFU was an indication of the number of colonies present following 240 min of incubation in tryptone saline alone in the absence of peptide. Test CFU was determined by counting the number of colonies present after incubation in tryptone saline containing the peptide. Each experimental point was performed in triplicate. Assays were conducted on blastoconidia unless otherwise indicated.
Time-kill assays
Kinetic fungicidal assays were performed using C. albicans (3153A), C. pseudotropicalis, C. glabrata, and S. cerevisiae. CAP3720-44 and control peptide, CAP3720-44ser26/42 at concentrations ranging from 400 to 100 μg/ml were incubated with the four isolates listed above for 2, 5, 15, 30, 60 and 240 min and cell death measured using the standard antifungal CFU assay.
Statistical analysis
Data are presented as mean ± standard error from three or four independent experiments. Statistical analysis of the data was performed employing one-way analysis of variance (ANOVA) followed by Dunnett’s Multiple Comparison Test, Tukey’s Multiple Comparison Test, or Bonferroni Multiple Comparison Test using GraphPad Prism 4.0 (GraphPad Software, Inc., San Diego, CA). P <0.05 was considered significant.
RESULTS
Standardization of in vitro CFU assay
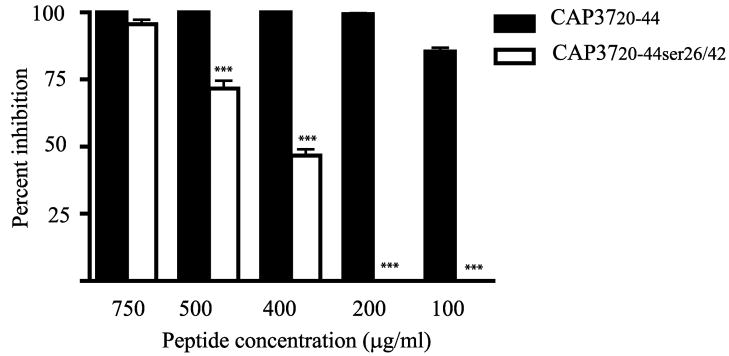
C. albicans (3153A) was used to initiate and standardize the in vitro antifungal assay. We explored a range of peptide concentrations (750, 500, 400, 200 and 100 μg/ml), contact time between peptide and Candida (0.5 1, 2, and 4 h), optimal growth conditions to achieve log phase cultures of Candida (temperature and time, 25°C for 5 h and 33°C for 90 min), and numbers of blastoconidia per well (200, 400, 500, 600, 800, and 1000 CFU) in the standard 4 h assay. Peptides are considered inactive at inhibition of <40%. Results in Figure 1, depict a dose response analysis using 500 CFU C. albicans and a 4 h incubation time with peptide. There was good distinction between the activities of the active (CAP3720-44) and the control peptide (CAP3720-44ser26/42) at 400, 200, and 100 μg/ml. We selected peptide concentrations of 400 (150 μM) and 200 μg/ml (75 μM) for our standard assay. We selected 4 h as the routine time of exposure of Candida to the peptides based on findings that the inactive peptide required 120 to 240 min to reach maximum activity. These data are fully discussed in the detailed time-kill studies described later in the results section and are not duplicated here. Data (not shown) indicated that there was no significant difference between Candida that had been cultured for 5 h at 25°C or for 90 min at 33°C. The 90 minute incubation was more convenient technically and was therefore employed routinely. Analysis of the number of blastoconidia per well indicated that 500 CFU was optimal. The standard assay thus consisted of using 500 CFU per well of Candida that had been grown to log phase at 33°C for 90 minutes and incubated for 4 h at 37°C with peptides at concentrations of 400 and 200 μg/ml. Any variations from these standard conditions are indicated in the relevant figure legends.
FIG 1. Standardization of peptide concentration for antifungal assays.
The antifungal activity of CAP3720-44 and CAP3720-44ser26/42 at concentrations ranging from 750 to 100 μg/ml was evaluated using C. albicans (3153A). Values are expressed as ± standard error of the mean from three independent experiments performed in triplicate. ***P< 0.001 compared to activity of CAP3720-44ser26/42 at the same concentration using ANOVA followed by Bonferroni’s multiple comparison test.
Antifungal activity of CAP37 peptides on various Candida species
Using the standard CFU assay, we analyzed all four peptides for their antifungal activity on a number of different species of Candida and two isolates of S. cerevisiae. Differential activity of the CAP37 peptides was observed depending on the species of Candida tested (Figure 2). CAP3720-44, CAP3720-44ser26, and CAP372044ser42 were significantly more active than CAP3720-44ser26/42 on all isolates of Candida tested (P<0.001). CAP3720-44, CAP3720-44ser26 and CAP372044ser42 were highly effective against C. guilliermondii and C. parapsilosis at concentrations as low as 100 μg/ml (37.5 μM) (Figure 2) compared to control peptide (P<0.001). C. pseudotropicalis, was also extremely sensitive to peptides CAP3720-44, CAP3720-44ser 26, and CAP3720-44ser42 compared to the control peptide (P<0.001). Surprisingly, some activity, although significantly less than with the other three peptides, was also obtained against C. pseudotropicalis with the control peptide CAP3720-44ser26/42, considered as ineffective against Gram-negative organisms. CAP3720-44ser26/42 lacked activity (% inhibition ≤ 40%) against all other Candida species and S. cerevisiae isolates tested. C. tropicalis, C. albicans, and C. dubliniensis were also sensitive to CAP3720-44, CAP3720-44ser26 and CAP3720-44ser42 (P<0.001 compared to CAP3720-44ser26/42). Similar to C. guilliermondii and C. pseudotropicalis, there was no significant difference between the activity of the three active peptides for C. tropicalis and C. albicans. However, CAP3720-44 was significantly less active than CAP3720-44ser26 and CAP3720-44ser42 against C. parapsilosis (P<0.001) and the two isolates of C. dubliniensis (P<0.01 and 0.001). Activity against the three C. glabrata isolates, the single isolate of C. krusei, and the two isolates of S. cerevisiae, was considered minimal to negative when compared to the activity of the peptides against all the other isolates tested.
FIG 2. Activity of CAP37 peptides against various Candida species.
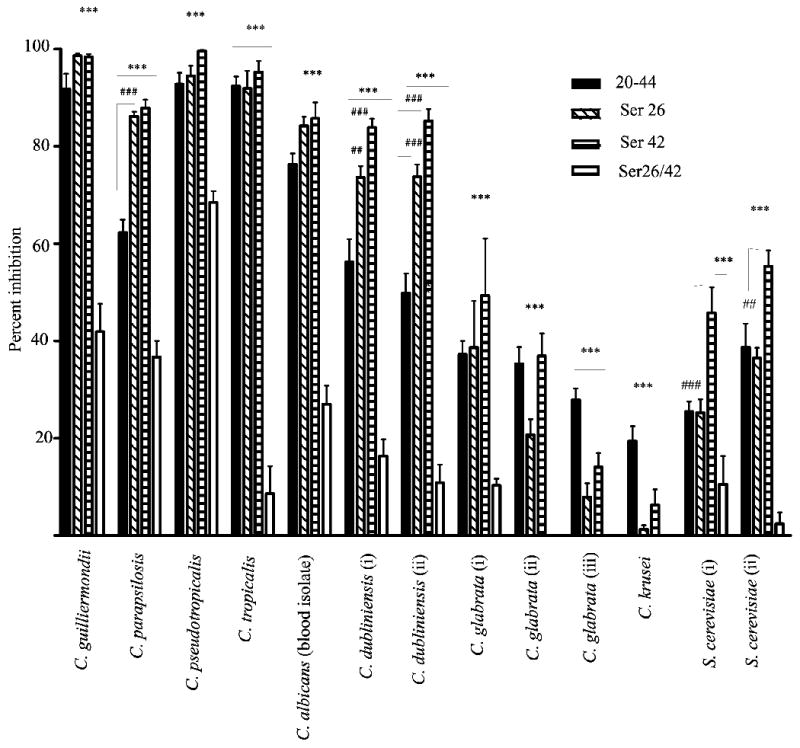
The antifungal activity of CAP37 peptides, CAP3720-44 (20-44), CAP3720-44Ser26 (Ser 26), CAP3720-44Ser42 (Ser 42), and CAP3720-44Ser26/42 (Ser 26/42) was evaluated using a panel of Candida species that included C. guilliermondii, C. parapsilosis, C. pseudotropicalis, C. tropicalis, a blood isolate of C. albicans, two isolates of C. dubliniensis, three isolates of C. glabrata, one isolate of C krusei and two isolates of S. cerevisiae. Data reported here are for a single concentration of the four different peptides. The peptide concentration for C. guilliermondii and C. parapsilosis was 100 μg/ml (37.5 μM) whereas data for all other isolates were obtained using peptide concentrations of 400 μg/ml (150 μM). Values are expressed as ± standard error of the mean from three to four independent experiments performed in triplicate. Statistical analysis of data was performed using ANOVA followed by Tukey’s Multiple Comparison Test., ***P<0.001 represent significance values between control peptide and other peptides for an individual isolate. ##P<0.01 and ###P<0.001 represent significance values between CAP3720-44 and peptides CAP3720-44ser26 and CAP3720-44ser42 for an individual isolate.
Antifungal activity of CAP37 peptides on fluconazole-sensitive and -resistant isolates of C. albicans
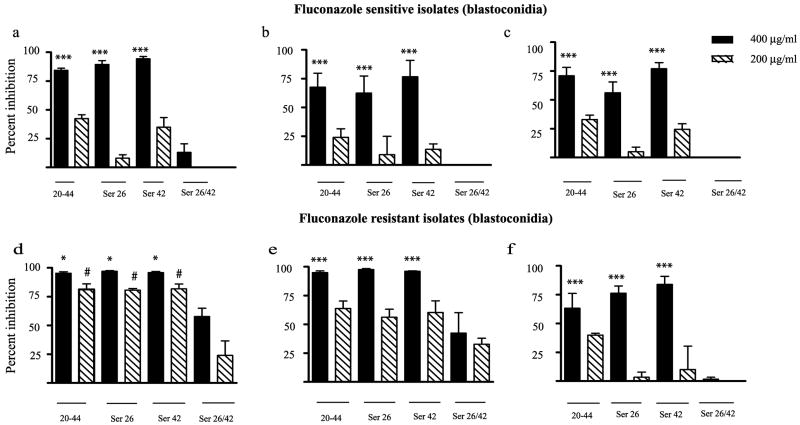
Having established the activity of the CAP37 peptides against the panel of Candida species, we next evaluated their antifungal activity against three fluconazole-sensitive and three fluconazole-resistant clinical mucosal isolates of C. albicans. We found that the peptides CAP3720-44, CAP3720-44ser26 and CAP3720-44ser42 were strongly active against the fluconazole-sensitive isolates (Figure 3a–c) at a concentration of 400 μg/ml (~150 μM) when compared to the activity demonstrated by the control peptide CAP3720-44Ser26/42 (P <0.001). There was no statistical difference (P >0.05), as determined by ANOVA followed by Tukey’s Multiple Comparison Test, between the activities of the three peptides (CAP3720-44, CAP3720-44ser26 and CAP3720-44ser42) at 400 μg/ml for any given fluconazole-sensitive isolate. Furthermore, there was no statistical difference (P >0.05) in the activity of each peptide for the three different fluconazole-sensitive isolates. Activity was negligible with CAP3720-44, CAP3720-44ser26 and CAP3720-44ser42 at 200 μg/ml.
FIG 3. Activity of CAP37 peptides against fluconazole-sensitive and -resistant isolates of C. albicans blastoconidia.
Activity of the four CAP37 peptides, CAP3720-44 (20-44), CAP3720-44Ser26 (Ser 26), CAP3720-44Ser42 (Ser 42), and CAP3720-44Ser26/42 (Ser 26/42) at 400 μg/ml (solid bars) and 200 μg/ml (diagonal striped bars) was evaluated against blastoconidia from three clinical isolates of Candida albicans known to be fluconazole-sensitive (a–c) and three isolates known to be fluconazole-resistant (d–e). The percent inhibition is determined as detailed in the Materials and Methods section. Values are expressed as ± standard error of the mean from three independent experiments performed in triplicate. *P <0.05 and ***P <0.001 compared to activity with control peptide, CAP3720-44ser26/42 at 400 μg/ml and #P<0.05 compared to activity with control peptide at 200 μg/ml using ANOVA followed by Dunnett’s Multiple Comparison Test.
The antifungal activity of peptides CAP3720-44, CAP3720-44Ser26, CAP3720-44Ser42, and CAP3720-44ser26/42 against the three fluconazole-resistant isolates of C. albicans are shown in Figure 3d–f. Peptides CAP3720-44, CAP3720-44Ser26, and CAP3720-44Ser42 were highly active against the three fluconazole-resistant isolates compared with peptide CAP3720-44ser26/42 (P<0.05 with isolate in Figure 3d and P<0.001 with isolates in Figures 3e and 3f). Significant activity (P<0.05) was also obtained with the lower concentration (200 μg/ml or 75 μM) of peptides, CAP3720-44, CAP3720-44Ser26, and CAP3720-44Ser42, with the fluconazole-resistant isolate in Figure 3d. Although, CAP3720-44Ser26/42 peptide at 400 μg/ml was considerably less active than the other peptides with this isolate, it demonstrated moderate activity exhibiting approximately 58% inhibition. There were no significant differences in antifungal activity between each of the three active peptides for any of the fluconazole–resistant isolates.
Antifungal activity of CAP37 peptides on hyphal forms of C. albicans
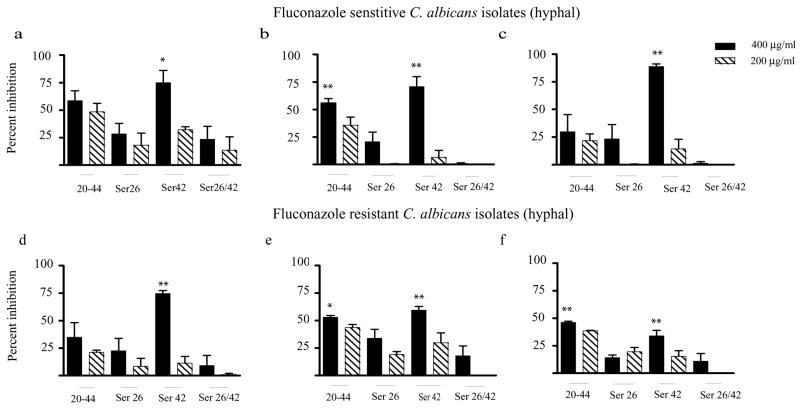
To determine whether CAP37 peptides could inhibit hyphal growth, we evaluated the activity against the same fluconazole-sensitive and -resistant C. albicans isolates following conditions for hyphal development (Figure 4a–f). Of the various CAP37 peptides tested, CAP3720-44ser42 was significantly inhibitory against hyphal forms of all 6 isolates tested, when compared with the control peptide, CAP3720-44ser26/42 (P<0.05 with isolate in Figure 4a, and P<0.01 with all other isolates). CAP3720-44 was significantly inhibitory against the hyphal form of isolates in figures 4b and 4f (P<0.01) and figure 4e (P<0.05). On the other hand, CAP3720-44ser26 was not effective against any of the hyphal forms when compared to activity with the control peptide.
FIG 4. Activity of CAP37 peptides on C. albicans hyphae.
Activity of CAP37 peptides CAP3720-44 (20-44), CAP3720-44Ser26 (Ser26), CAP3720-44Ser42 (Ser42), and CAP3720-44Ser26/42 (Ser26/42) at 400 μg/ml (solid bars) and 200 μg/ml (diagonal striped bars) was evaluated against hyphal forms of the clinical isolates of Candida albicans. The isolates used are identical to those in Figure 3 and were cultured so as to induce hyphal growth. Isolates depicted in Figure 4a–c are fluconazole-sensitive and those in Figure 4d–f are fluconazole-resistant. The percent inhibition was determined as detailed in the Materials and Methods section. Values are expressed as ± standard error of the mean from three independent experiments performed in triplicate. *P<0.05 and **P<0.001 compared to activity with control peptide, CAP3720-44ser26/42 at 400 μg/ml using ANOVA followed by Dunnett’s Multiple Comparison Test.
Antifungal activity of CAP37 peptides on pseudohyphal forms of C. pseudotropicalis and C. tropicalis
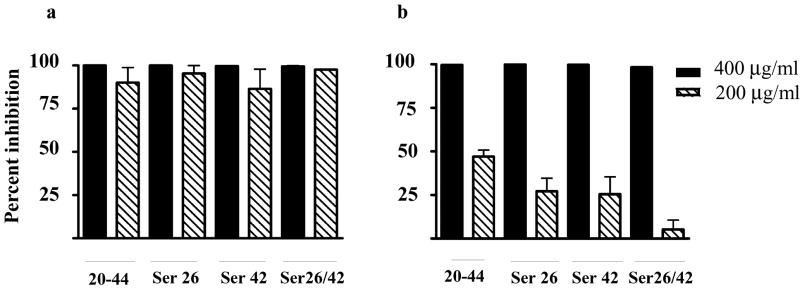
In contrast to the limited activity displayed by the CAP37 peptides against the hyphal forms of C. albicans, we observed potent activity against the pseudohyphal form of C. pseudotropicalis with peptides CAP3720-44, CAP3720-44Ser42, CAP3720-44Ser26, and CAP3720-44ser26/42 at 400 and 200 μg/ml (Figure 5a) and C. tropicalis (Figure 5b) with all four peptides at 400 μg/ml. Limited activity was observed against C. tropicalis using 200 μg/ml (Figure 5b).
FIG 5. Activity of CAP37 peptides on pseudohyphal forms of C. pseudotropicalis and C. tropicalis.
Activity of CAP37 peptides CAP3720-44 (20-44), CAP3720-44ser26 (Ser26), CAP3720-44ser42 (Ser42) and CAP3720-44ser26/42 at 400 (solid bars) and 200 μg/ml (diagonal striped bars) was evaluated using C. pseudotropicalis (a) and C. tropicalis (b) that were induced to form pseudohyphae as indicated in the methods section. The percent inhibition was determined as detailed in the methods section. Values are expressed as ± standard error of the mean from three independent experiments performed in triplicate. P>0.05 compared to activity with control peptide, CAP3720-44ser26/42 at 400 and 200 μg/ml using ANOVA followed by Dunnett’s Multiple Comparison Test.
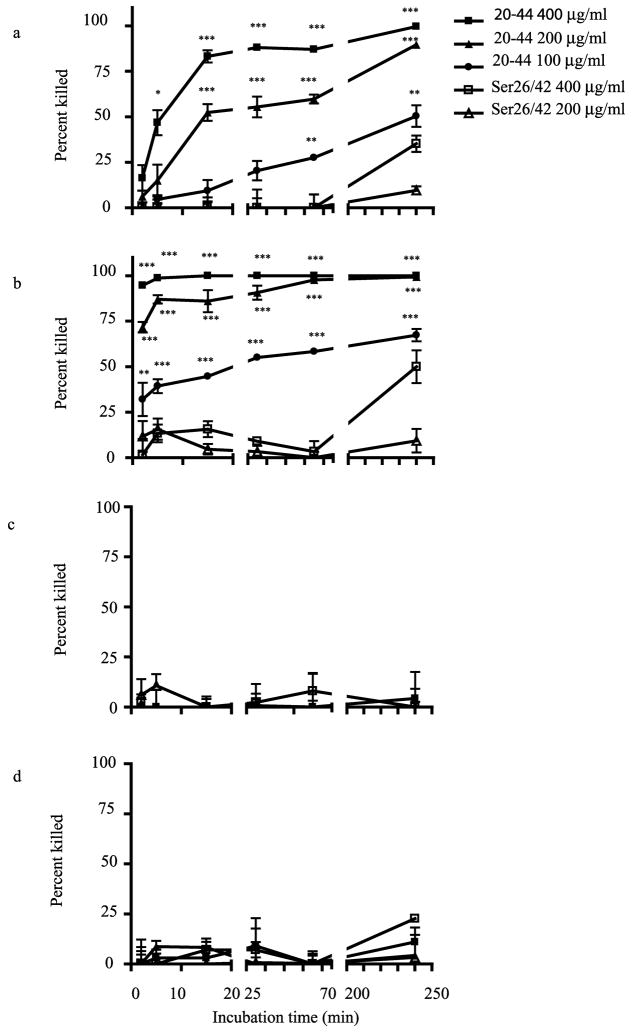
Time-kill studies
Kinetic assays were performed with three different Candida species and one isolate of S. cerevisiae using CAP3720-44 at three concentrations (400, 200 and 100 μg/ml) and CAP3720-44ser26/42 at two concentrations (400 and 200 μg/ml). Antifungal activity of CAP3720-44 at 400 μg/mlfor C. albicans (Figure 6a) showed a dramatic increase between 2 and 15 min and reached a plateau of >85 % between 30 and 60 minutes, reaching maximum antifungal activity thereafter. The lower concentration of CAP3720-44, showed lesser activity at the earlier time points, but displayed significant activity after 240 minutes of incubation. Activity of CAP3720-44 was significantly quicker and greater than CAP3720-44ser26/42 at the comparable concentration. The rapid antifungal activity of CAP3720-44 was more pronounced on C. pseudotropicalis. Significant levels of activity were observed as early as two minutes (Figure 6b). In contrast, and as expected from data depicted in figure 2, the activity of both peptides was negligible against C. glabrata (Figure 6c) and S. cerevisiae (Figure 6d) at all time points tested.
FIG 6. Time kill studies to determine fungicidal activity of CAP37 peptides.
C. albicans (3152A) (a), C. pseudotropicalis (b), C. glabrata (c, isolate iii in Figure 2), and S. cerevisiae (d, isolate ii in Figure 2) were selected for the kinetic studies. The fungal isolates were incubated with active peptide CAP3720-44 (20-44) at concentrations of 400, 200 and 100 μg/ml, and CAP3720-44ser26/42 (Ser26/42) at 400 and 200 μg/ml for 2, 5, 15, 30, 60, and 240 min. CFU were counted and the antifungal activity determined as described in the methods and materials section. Values are expressed as ± standard error of the mean from three or five independent experiments performed in triplicate. *P<0.05, **P<0.01 and ***P<0.001 compared to activity with CAP3720-44ser26/42 using ANOVA followed by Bonferroni’s Multiple Comparison Test.
DISCUSSION
In this study, we demonstrate for the first time that synthetic peptides based on the CAP37 molecule have strong activity against eukaryotic organisms. We had previously shown that the 25 amino acid peptide based on the native sequence had activity against a range of prokaryotic organisms [13]. Here we describe the synthesis of two new CAP37-derived peptide analogs in which the cysteine residues at positions 26 or 42 were replaced by serine residues. Our findings demonstrate significant activity against several Candida species by the peptide based on the native sequence and the two new analogs. The replacement of both cysteines significantly abrogated activity suggesting that a single cysteine residue is needed for the antifungal activity of the peptides. The concentration of CAP37 peptides required to achieve antifungal activity is approximately twice that required for activity against the Gram-negative species S. typhimurium, E. coli, and P. aeruginosa [13], although we used the same number of CFU in both the antifungal and antibacterial assays. This range in activity is not uncommon amongst cationic antimicrobial peptides, which tend to have specificity and greater potency towards certain bacterial species, fungi, parasites and/or viruses. Fungicidal activity on sensitive isolates was relatively rapid requiring between 5 to 240 minutes contact time depending on the organisms. We had previously proposed that the bactericidal mechanism of CAP37 and its antimicrobial peptides on Gram-negative organisms was due to the specific binding to the lipid A component of the endotoxin molecule [13, 14]. The putative target molecule on yeast cells that acts as the receptor for the CAP37 peptides is presently unknown. It is possible that charge, hydrophobicity, and other non-specific factors alone may contribute to this binding. Alternatively, it is possible that there may be a specific binding site on yeast for CAP37. It has recently been demonstrated that salivary histatin-5 kills C. albicans through a process that requires binding to Ssa1/2 proteins on the cell surface [19]. Clearly the structure function analysis of this complex peptide and its mode of action on bacteria and fungi require further exploration.
Our data showed that the peptides exhibited potent activity against C. guilliermondii and C. parapsilosis at lower concentrations (37.5 μM) compared to that for the other species. C. parapsilosis is most often isolated from critically ill patients in intensive care units who typically have indwelling catheters and devices while undergoing treatment [20, 21]. Almost 100% of the starting inocula of C. pseudotropicalis and C. tropicalis were killed with peptide concentrations of 150 μM. C. tropicalis is being increasingly isolated from blood cultures of patients with leukemias, other neoplasias, and those in intensive care units [22]. Of the peptides, CAP3720-44Ser42 and CAP3720-44ser26 were more effective against C. dubliniensis than peptide CAP3720-44 which was based on the native sequence. C. dubliniensis is a recently identified species that is seldom found in healthy persons but tends to be found as the causative agent of oropharyngeal infections mainly in HIV-infected individuals [23]. The peptides were ineffective against C. glabrata, C. krusei and S. cerevisiae. C. glabrata and C. krusei are isolated with increasing frequency from blood stream infections and C. krusei in particular appears to be associated with hematological malignancies [24, 25, 26].
Noteworthy were our findings that CAP3720-44, CAP3720-44Ser26, and CAP3720-44 Ser42 peptides were very effective against the fluconazole resistant mucosal isolates. Overall, fluconazole-resistant and – sensitive species were inhibited nearly equivalent by the peptides. These findings are particularly pertinent to the recent emergence of fluconazole-resistant isolates of C. albicans and other Candida species that will require treatment with new therapeutics with novel or alternative mechanisms of action [23, 27]. In addition to the important finding that CAP37 peptides had activity against fluconazole-resistant mucosal isolates of C. albicans, the peptides showed impressive activity against pseudohyphae. On the other hand, activity of the peptides against the hyphal forms was modest compared to the respective blastoconidia forms. It is unclear whether the differences between pseudohyphae and hyphae are due to the morphological forms or the species. Obviously, further studies will be needed to answer this question. A critical finding was that the activity of the peptides on susceptible isolates was fungicidal rather fungistatic. This was shown both by kinetic studies as well as vital staining (data not shown). This is an important consideration if CAP37 peptides are to be pursued as potential therapeutics, since a number of current antifungal drugs are fungistatic and not fungicidal.
Besides CAP37, several cationic antimicrobial proteins including the defensins, hCAP18/LL37, bactericidal permeability increasing protein (BPI), and lactoferrin [5, 28, 29] are active against C. albicans [30, 31]. Another well known candidacidal molecule is salivary histatin-5 [32, 33] which is active on C. albicans blastospores at a concentration of 15 μM [34]. In addition, synthetic peptides based on naturally occurring proteins such as the first cationic domain of the amino-terminus of human lactoferrin, Lfpep, a lactoferricin H-derived synthetic peptide, and Kaliocin-1 based on residues 153-183 of the human lactoferrin molecule, also show strong fungicidal activity [35, 36] in the range of 50 to 150 μM levels [35]. Synthetic histatin analogs based on the C-terminal fungicidal domain of histatin-5 are effective against C. albicans [37], C. krusei, fluconazole-resistant isolates of C. glabrata and Aspergillus fumigatus [38]. Similar to these antifungal peptides, optimal conditions for in vitro killing by CAP37 peptides required the use of media containing low salt concentrations [36] and pH of 5.5 [39].
The use of natural cationic peptides as novel therapeutics in the treatment of infections is gaining enthusiasm in the scientific and biotechnology communities. The major drawback to conventional antibiotics is the rapidity with which microorganisms can gain multiple resistance patterns. Although the exact mechanism as to how these cationic antimicrobial peptides, including the CAP37 peptides kill microorganisms is not entirely known, it is generally believed that membrane interaction and disruption occurs. Histatin-5 has been shown to cause small membrane defects, whereas LL-37 causes massive disruption of C. albicans cell membranes [40]. Although the mode of action of the CAP37 peptides has yet to be determined it appears to be different from the mechanism of action of azole-based drugs, since it kills fluconazole-resistant and -sensitive isolates equally well.
CAP37 has been traditionally considered a PMN-derived protein since it is constitutively expressed in the granules of these cells. However, more recently we have demonstrated the presence of an inducible form of CAP37 in endothelial cells lining the vasculature, smooth muscle cells, corneal epithelial cells, and squamous epithelial cells of the skin, [9, 10, 12, 41, 42]. Others have also demonstrated a wide repertoire of epithelial antimicrobial peptides in the skin [43, 44, 45, 46), mucosal surfaces lining the gastrointestinal tract [47, 48], oral surfaces [49, 50], respiratory tract [51, 52, 53], and genitourinary tract [48, 54]. These antimicrobial proteins are ideally located to serve as the first line of defense against invading pathogens. To date no cytotoxicity for mammalian cells, by CAP37 peptides, has been demonstrated. Whether expression of CAP37 occurs in mucosal linings of the host in response to Candida infections or whether the induction of CAP37 is compromised in mucosal and epithelial surfaces in patients with recurrent candidiasis, suggesting a physiological role for CAP37 in the protection of the host against fungal infections, is currently unknown. The study reported here describes for the first time that CAP37 peptides and analogs based on the native sequence of CAP37 possess potent fungicidal activity and suggest a more broad-spectrum antimicrobial activity for the molecule than originally proposed. Its precise role in host defense against Candida infections will require investigation in appropriate animal models of Candidiasis.
Acknowledgments
This study was supported in part by Department of Pathology funds to (HAP), and Public Health Service grants AI28018, EY015534 to (HAP), and DE12178 and AI32556 to (PLF) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: None
References
- 1.Flodgaard HE, Ostergaard E, Bayne S, et al. Covalent structure of two novel neutrophile leucocyte-derived proteins of porcine and human origin. Neutrophile elastase homologues with strong monocyte and fibroblast chemotactic activities. Eur J Biochem. 1991;197:535–547. doi: 10.1111/j.1432-1033.1991.tb15942.x. [DOI] [PubMed] [Google Scholar]
- 2.Gabay JE, Scott RW, Campanelli D, et al. Antibiotic proteins of human polymorphonuclear leukocytes. Proc Natl Acad Sci (USA) 1989;86:5610–5614. doi: 10.1073/pnas.86.14.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shafer WM, Martin LE, Spitznagel JK. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984;45:29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shafer WM, Martin LE, Spitznagel JK. Late intraphagosomal hydrogen ion concentration favors the in vitro antimicrobial capacity of a 37-kilodalton cationic granule protein of human neutrophil granulocytes. Infect Immun. 1986;53:651–655. doi: 10.1128/iai.53.3.651-655.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spitznagel JK. Antibiotic proteins of human neutrophils. J Clin Invest. 1990;86:1381–1386. doi: 10.1172/JCI114851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira HA, Shafer WM, Pohl J, Martin LE, Spitznagel JK. CAP37, a human neutrophil-derived chemotactic factor with monocyte specific activity. J Clin Invest. 1990;85:1468–1476. doi: 10.1172/JCI114593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira HA, Ruan X, Kumar P. Activation of microglia: a neuroinflammatory role for CAP37. GLIA. 2003;41:64–72. doi: 10.1002/glia.10167. [DOI] [PubMed] [Google Scholar]
- 8.Morgan JG, Pereira HA, Sukiennicki T, Spitznagel JK, Larrick JW. Human neutrophil granule cationic protein CAP37 is a specific macrophage chemotaxin that shares homology with inflammatory proteinases. Adv Ex Med Biol. 1991;305:89–96. doi: 10.1007/978-1-4684-6009-4_11. [DOI] [PubMed] [Google Scholar]
- 9.Ruan X, Chodosh J, Callegan MC, et al. Corneal expression of the inflammatory mediator CAP37. Invest Ophthalmol Vis Sci. 2002;43:1414–1421. [PubMed] [Google Scholar]
- 10.Lee TD, Gonzalez ML, Kumar P, Chary-Reddy S, Grammas P, Pereira HA. CAP37, a novel inflammatory mediator. Its expression in endothelial cells and localization to atherosclerotic lesions. Am J Pathol. 2002;160:841–848. doi: 10.1016/S0002-9440(10)64907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee TD, Gonzalez ML, Kumar P, Grammas P, Pereira HA. CAP37, a neutrophil-derived inflammatory mediator augments leukocyte adhesion to endothelial monolayers. Microvasc Res. 2003;66:38–48. doi: 10.1016/s0026-2862(03)00010-4. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez ML, Ruan X, Kumar P, Grammas P, Pereira HA. Functional modulation of smooth muscle cells by the inflammatory mediator CAP37. Microvasc Res. 2004;67:168–181. doi: 10.1016/j.mvr.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Pereira HA, Erdem I, Pohl J, Spitznagel JK. Synthetic bactericidal peptide based on CAP37: a 37 kDa human neutrophil granule-associated cationic antimicrobial protein chemotactic for monocytes. Proc Natl Acad Sci (USA) 1993;90:4733–4737. doi: 10.1073/pnas.90.10.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brackett DJ, Lerner MR, Lacquement MA, He R, Pereira HA. A synthetic lipopolysaccharide-binding peptide based on the neutrophil-derived protein CAP37 prevents endotoxin-induced responses in conscious rats. Infect Immun. 1997;65:2803–2811. doi: 10.1128/iai.65.7.2803-2811.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubalek F, Edmondson DE, Pohl J. Synthesis and characterization of a collagen model. dO-phosphohydroxylysine-containing peptide. Anal Biochem. 2002;306:124–134. doi: 10.1006/abio.2002.5693. [DOI] [PubMed] [Google Scholar]
- 16.Pohl J, Pereira HA, Martin NM, Spitznagel JK. Amino acid sequence of CAP37, a human neutrophil granule-derived antibacterial and monocyte-specific chemotactic glycoprotein structurally similar to neutrophil elastase. FEBS Lett. 1990;272:200–204. doi: 10.1016/0014-5793(90)80484-z. [DOI] [PubMed] [Google Scholar]
- 17.Moroder L. Isoteric replacement of sulfur with other chalcogens in peptides and proteins. J Peptide Science. 2005;11:187–214. doi: 10.1002/psc.654. [DOI] [PubMed] [Google Scholar]
- 18.Kadosh D, Johnson AD. Induction of the Candida albicans filamentous growth program by relief of transcriptional repression: A genome-wide analysis. Mol Biol. 2005;16:2903–2912. doi: 10.1091/mbc.E05-01-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vylkova S, Li XS, Berner JC, Edgerton M. Distinct antifungal mechanisms: beta defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob Agents Chemother. 2006;50:324–331. doi: 10.1128/AAC.50.1.324-331.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn DM, Mukherjee PK, Clark TA, et al. Candida parapsilosis. Characterization in an outbreak setting. Emerging Infect Dis. 2004;10:1074–1081. doi: 10.3201/eid1006.030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen MH, Peacock JE, Tanner DC, et al. Therapeutic approaches in patients with candidemia: evaluation in a multicenter prospective observational study. Arch Intern Med. 1995;155:2429–2435. [PubMed] [Google Scholar]
- 22.Warn PA, Sharp A, Morrissey G, Denning DW. In vivo activity of micafungin in a persistently neutropenic murine model of disseminated infection caused by Candida tropicalis. J Antimicrob Agents Chemother. 2002;50:1071–1074. doi: 10.1093/jac/dkf247. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan DJ, Morgan GP, Pinjon E, et al. Comparison of the epidemiology, drug resistance mechanisms, and virulence of Candida dubliniensis and Candida albicans. FEMS Yeast Res. 2004;4:369–376. doi: 10.1016/S1567-1356(03)00240-X. [DOI] [PubMed] [Google Scholar]
- 24.Clark TA, Hajjeh RA. Recent trends in the epidemiology of invasive mycoses. Curr Opin Infect Dis. 2002;15:569–574. doi: 10.1097/00001432-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Hobson RP. The global epidemiology of invasive Candida infections – is the tide turning? J Hosp Infect. 2003;55:159–168. doi: 10.1016/j.jhin.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Rapp RP. Changing strategies for the management of invasive fungal infections. Pharmacotherapy. 2004;24:4S–28S. [PubMed] [Google Scholar]
- 27.Jabra-Rizk MA, Falkler WA, Meiller TF. Fungal biofilms and drug resistance. Emerging Infect Dis. 2004;10:14–19. doi: 10.3201/eid1001.030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelial. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 29.Hancock REW. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 30.Hoover DM, Wu Z, Tucke K, Lu W, Lubkowski J. Antimicrobial characterization of human β-defensin 3 derivatives. Antimicrob Agents Chemother. 2003;47:2804–2809. doi: 10.1128/AAC.47.9.2804-2809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selsted ME, Szklarek D, Ganz T, Lehrer RI. Activity of rabbit leukocyte peptides against Candida albicans. Infect Immun. 1985;49:202–206. doi: 10.1128/iai.49.1.202-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edgerton M, Koshlukova SE, Araujo MWB, Patel RC, Dong J, Bruenn JA. Salivary histatin 5 and human neutrophil defensins 1 kill Candida albicans via shared pathways. Antimicrob Agents Chemother. 2000;44:3310–3316. doi: 10.1128/aac.44.12.3310-3316.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai H, Bobek LA. Studies of the mechanism of human salivary histatin-5 candidacidal activity with histatin-5 variants and azole-sensitive and resistant Candida species. Antimicrob Agents Chemother. 1997;41:2224–2228. doi: 10.1128/aac.41.10.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai H, Raj PA, Bobek LA. Candidacidal activity of recombinant human salivary histatin-5 and variants. Infect Immun. 1996;64:5000–5007. doi: 10.1128/iai.64.12.5000-5007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lupetti A, Paulusma-Annema A, Welling MM, Senesi S, Van Dissel JT, Nibbering PH. Candidacidal activities of human lactoferrin peptides derived from the N terminus. Antimicrob Agents Chemother. 2000;44:3257–3263. doi: 10.1128/aac.44.12.3257-3263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viejo-Diaz M, Andrés MT, Fierro JF. Different anti-Candida activities of two human lactoferrin-derived peptides, Lfpep and Kaliocin-1. Antimicrob Agents Chemother. 2005;49:2583–2588. doi: 10.1128/AAC.49.7.2583-2588.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helmerhorst EJ, Van ‘T Hof W, Veerman ECI, Simoons-Smit I, Nieuw Amerongen AV. Synthetic histatin analogues with broad spectrum antimicrobial activity. Biochem J. 1997;326:39–45. doi: 10.1042/bj3260039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helmerhorst EJ, Reijnders IM, Van ‘T Hof W, Simoons-Smit I, Veerman ECI, Nieuw Amerongen AV. Amphotericin B- and Fluconazole-resistant Candida spp., Aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrob Agents Chemother. 1999;43:702–704. doi: 10.1128/aac.43.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Y-Q, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun. 2002;70:6524–6533. doi: 10.1128/IAI.70.12.6524-6533.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Den Hertog AL, Van Marle J, Van Veen HA, et al. Candidacidal effects of two antimicrobial peptides: histatin 5 causes small membrane defects, but LL-37 causes massive disruption of the cell membrane. Biochem J. 2005;388:689–695. doi: 10.1042/BJ20042099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira HA, Kumar P, Grammas P. Expression of CAP37, a novel inflammatory mediator in Alzheimer’s disease. Neurobiol Aging. 1996;17:753–759. [PubMed] [Google Scholar]
- 42.Pereira HA, Kumar P, Lerner MR, Brackett DJ. Inducible expression of the inflammatory protein CAP37 in the epidermis during wound healing. In: Robinson JW, editor. Focus on Protein Research. Hauppauge, NY: Nova Biomedical Publications; 2004. pp. 127–144. [Google Scholar]
- 43.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 44.Oren A, Ganz T, Liu L, Meerloo T. In human epidermis, beta defensin 2 is packaged in lamellar bodies. Exp Mol Pathol. 2003;74:180–182. doi: 10.1016/s0014-4800(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 45.Shirafuji Y, Oono T, Kanzaki H, Hirakawa S, Arata J. Detection of cryptdin in mouse skin. Clin Diagn Lab Immunol. 1999;6:336–340. doi: 10.1128/cdli.6.3.336-340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sorensen OE, Cowland JB, Theilgaard-Monch K, Liu L, Ganz T, Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J Immunol. 2003;170:5583–5589. doi: 10.4049/jimmunol.170.11.5583. [DOI] [PubMed] [Google Scholar]
- 47.Fellermann K, Stange EF. Defensins – innate immunity at the epithelial frontier. Eur J Gastroenterol Hepatol. 2001;13:771–776. doi: 10.1097/00042737-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Frohm-Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Stahle-Backdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dale BA. Periodontal epithelium: a newly recognized role in health and disease. Periodontal. 2003;30:70–78. doi: 10.1034/j.1600-0757.2002.03007.x. [DOI] [PubMed] [Google Scholar]
- 50.Dale BA, Krisanaprakornkit S. Defensin antimicrobial peptides in the oral cavity. J Oral Pathol Med. 200;30:321–327. doi: 10.1034/j.1600-0714.2001.300601.x. [DOI] [PubMed] [Google Scholar]
- 51.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 52.Hiemstra PS. Epithelial antimicrobial peptides and proteins: their role in host defense and inflammation. Pediatr Respir Rev. 2001;2:306–310. doi: 10.1053/prrv.2001.0165. [DOI] [PubMed] [Google Scholar]
- 53.Huttner KM, Bevins CL. Antimicrobial peptides as mediators of epithelial host defense. Pediatr Res. 1999;45:785–794. doi: 10.1203/00006450-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Malm J, Sorensen O, Persson T, et al. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations and is attached to spermatozoa. Infect Immun. 2000;68:4297–4302. doi: 10.1128/iai.68.7.4297-4302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]