Abstract
Background and Aims
Homocysteine (Hcy) is a sulfur-containing, non-protein amino acid produced in the metabolic pathway of methionine. Hyperhomocysteinemia is associated with cerebro- and cardiovascular disease in industrialized countries mostly resulting from protein rich diet and sedentary life style. Matrix metalloproteinases are involved in cardiac remodeling, leading to degradation of intercellular junctions, cardiac connexins and basement membranes. The study was designed to investigate the relationship between Hcy, cardiac remodeling, cardiac performance, and rhythm disturbances in an animal model of hyperhomocysteinemia. We tested the hypothesis that induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 leads to connexin 40, connexin 43, connexin 45 expression changes contributing to decreased cardiac performance and disturbed atrioventricular conduction.
Methods and Results
Hcy was added to drinking water of male C57/BL6J mice to achieve moderate Hcy blood levels. ECG was monitored in conscious mice with a telemetric ECG device; echocardiography was used for assessment of left ventricular function. Immunoblotting was used to evaluate matrix metalloproteinase-2, matrix metalloproteinase-9, connexin 40, connexin 43, and connexin 45 expression in cardiac tissue. Animals fed Hcy showed significant prolongation of QRS, QTc, and PR intervals along with reduced left ventricular function. Western blotting showed increased expression of matrix metalloproteinase-2, matrix metalloproteinase-9 and decreased expression of connexin 40, 43, and 45.
Conclusion
Hcy has been identified as a nutritional factor contributing to cardiovascular disease. Cardiac remodelling induced by matrix metalloproteinase-2 and matrix metalloproteinase-9 and decreased expression of connexin 40, 43, and 45 appears to play a role in the pathomechansim of atrioventricular conduction delay and ventricular dilatation in hyperhomocysteinemia.
Keywords: Homocysteine, atrioventricular conduction, long QTc interval, matrix metalloproteinase, cardiac connexin
Introduction
Homocysteine (Hcy) is a sulfur-containing, non-protein amino acid derived from the essential amino acid methionine. Hyperhomocysteinemia has been associated with cerebro- and cardiovascular disease [1, 2]. Physiologic total Hcy (tHcy) blood levels are less than 9 μmol/L, mild to moderate levels range in between 9.1 and 19.9 μmol/L, and levels >20 μmol/L are considered high [2]. Increased Hcy levels are seen in methionine rich diets, e.g., the typical protein rich western diet with a high content of meat, milk, eggs and legumes. Smoking, coffee and alcohol consumption, lack of exercise, advanced age, male gender, and postmenopausal hormone status are factors associated with higher Hcy plasma concentrations [3]. Deficiency in folate, vitamin B12, and vitamin B6 (vitamins catalyzing the reactions in the metabolic pathway of Hcy) in combination or alone are known to lead to hyperhomocysteinemia. Drugs that decrease these vitamins, such as methotrexate and nitrates, are indirectly responsible for high tHcy concentrations [3]. Hcy accumulates with renal impairment as a result of decreased glomerular filtration rate and reduced clearance [4].
Cardiac remodeling is a highly regulated process susceptible to environmental triggers and noxious stimuli [5]. Matrix metalloproteinases reorganize, clean, and clear extracellular matrix [6, 7]. They regulate tissue architecture and activate, deactivate, or modify the activity of signaling molecules, both directly and indirectly. Matrix metalloproteinases are also involved in degradation of intercellular junctions and basement membranes and, therefore, influence cell-to-cell communication [6]. Matrix metalloproteinase-2 and matrix metalloproteinase-9 are cell membrane-linked metalloproteinases involved in vascular remodeling. Their fibronectin domains allow them to connect to cell membranes near cellular junctions. Reorganization around damaged tissue, removal of basement membranes, and disruption of intercellular junctions increase the risk for disturbed electrical conduction in the myocardium [7] and of developing ectopic foci as a result of disruption of cell-to-cell communication and pathologic ion channel function [5]. Several rhythm abnormalities share disorganized electrical conduction because of pathological changes in myocardial structure, extracellular matrix, and conduction system itself, which can ultimately lead to sudden cardiac death [8].
We tested the hypothesis that mild to moderate elevation of Hcy blood levels induce matrix metalloproteinase-2 and matrix metalloproteinase-9 expression, initiate cardiac remodeling, and interfere with connexin 40, 43 and 45 protein expression leading to cardiac rhythm disturbances. To test this hypothesis, we created an animal model of mild to moderate hyperhomocysteinemia, monitored ECGs in unsedated freely moving mice with a telemetric device and used transthoracic two-dimensional (2-D) echocardiography to examine cardiac performance changes. We assessed protein levels of myocardial matrix metalloproteinases (MMP-2 and MMP-9), proteinase involved in cardiac remodeling, and connexins (connexin 40, 43, 45), gap junction proteins involved in cardiac conduction [9].
Methods
Animal model
The University of Louisville’s Institutional Animal Care and Use Committee (IACUC) approved all procedures. Eleven male C57/BL6J mice from Jackson Laboratory (Bar Harbor, ME) 12 weeks old and 25 g in weight were included in this study. Animals were given standard rodent chow and water ad libitum during a 7-day acclimatization period and housed with a 12-hour light/dark cycle at 24°C according to IACUC criteria and guidelines of the University of Louisville. Animals were randomly assigned to the control group (n=5) or the Hcy-enriched diet group (n=6) for 12 weeks. For the 12-week study period, animals in the Hcy-enriched diet group received standard rodent chow and drinking water supplemented with 400 mg DL-Homocystine (Sigma Aldrich, St. Louis, MO) in 1 L water to achieve mild to moderate Hcy blood levels (9.1 to 19.9 μmol/L). Control animals received standard rodent chow and water ad libitum for the 12-week study period.
Homocysteine blood levels
Venous blood samples were taken at the end of the study period after two hours of fasting. Samples were immediately centrifuged at 1000 g for 5 minutes at 4°C to separate plasma from blood cells. Samples were immediately stored at −80°C until analysis. Hcy was measured using a standard protocol by high-performance liquid chromatography (HPLC) with fluorescence detection [10].
Telemetric electrocardiogram (ECG)
A telemetric radio frequency transmitter (DSI product TA10EA-F20, Data Sciences International DSI, St. Paul, MO) with two leads (obtaining lead II reading) was implanted subcutaneously on the animal’s middle back in week 10 of the 12-week study period. Animals were isolated for recovery and observation of vital functions for 120 hours postoperatively. ECG monitoring started 6–7 days postoperatively, provided that surgery-related weight loss was compensated and wound healing was without complications. ECG data were collected and analyzed using data analysis software and hardware for telemetric monitored rodents (Art 3.1, Data Sciences International DSI, St. Paul, MN) Software related set up required defining of time frames with an observation and collection window of 1 second. We defined time periods from 6:00 am until 8:00 am for 5 consecutive days in week 12 of the 12-week study period.
ECG assessment and measurements
Measurements of PQ time, QRS duration, QT time, heart rate corrected QT time as well as R to R intervals were measured in all animals in the observed time period as described in recent publications [11, 12]. QT intervals were corrected for heart rate with Bazett’s [11] formula QTc=QT/√[RR/100].
2-D echocardiography
All animals underwent echocardiographic measurements under sedation (Hewlett Packard Sonos 5500 ultrasound machine, L15/6 Mhz transducer). Tribromoethanol (TBE) 1.25% was injected intraperitoneally, maintaining spontaneous respiration. Assessments started 15 minutes after onset of anesthesia. Animals were placed supine on a heating pad, acoustic gel was applied on the prepared chest. Left ventricular internal dimensions in systole and diastole were assessed in M-mode in left parasternal short and long axis views. Fractional shortening in percent was calculated as [Left ventricular internal dimension in diastole - Left ventricular internal dimension in systole]/Left ventricular internal dimension in diastole × 100. Each animal was assessed upon arrival at 12 weeks of age and at the end of the 12-week study period at 24 weeks of age.
Immunoblotting/Western blot for connexin 40, 43, 45 and matrix metalloproteinase-2 and matrix metalloproteinase-9
Cardiac tissue was snap frozen and stored at −80°C. Samples were lysed in protein extraction buffer (0.1% SDS, 0.5% deoxycholate, 1% Triton-100, 10 mM Tris pH 7.4) with protease inhibitor added (100 nM aprotinin, 1 μM leupeptin and 1 mM PMSF) for Western blot analysis. Bradford protein assay[13] was used to estimate protein amount. Proteins were separated by polyacrylamide gel electrophoresis (SDS-Page) and transferred to polyvinylidene fluoride (PVDF) membrane (BioRad, Hercules, CA). Transfer membranes were blocked and then incubated with primary antibody (anti-connexin 40, 43, and 45 antibodies diluted 1:1,000 [Zymed, Carlsbad, CA] and anti-matrix metalloproteinase-2 and -9 diluted 1:1,000 [Santa Cruz, Biotechnology, Santa Cruz, CA]). Washed membranes were incubated with secondary antibody (goat anti-rabbit IgG HRP diluted 1:3,000, goat anti-mouse IgG HRP diluted 1:3,000 [Santa Cruz Biotechnology, Santa Cruz, CA]). Connexin 40, 43, and 45 as well as matrix metalloproteinase -2 and 9 bands were normalized to β-Actin bands (42 kDa) after detecting with enzymatic chemiluminescence method (Amersham, GE Healthcare, Little Chalfrost, United Kingdom) according to the manufacturer’s instructions. X-ray films with protein bands were scanned and viewed with Un-Scan-It digitizing software Version 5.1 (Silk Scientific, Orem, UT). Arbitrary densitometry units were compared with β-Actin controls.
Statistical analysis
Data means from all animals were compared within the study groups and between the study groups using Student’s t test and for multiple comparisons Student-Newman-Keuls test if indicated. Western blots were repeated four times for each antibody for each sample in the study groups. Results were compared with each other using ANOVA and t-tests, when no homogeneity of variance was obtained by F test. Data are presented as mean ± SE unless otherwise indicated. P values < 0.05 indicate significance.
Results
Homocysteine blood levels
Total Hcy blood levels in serum were measured with HPLC. All animals fasted for two hours prior to sample collection. Control animals had tHcy levels of 5.575 ± 3.216 μmol/L and animals treated with Hcy for 12 weeks had significantly higher tHcy levels of 13.455 ± 2.691 μmol/L (P < 0.05). Fluid intake per animal ranged between 4 and 7 ml per day or an overall average of 2.5 ml per 10 g. Thus, 400 mg Hcy added to 1000 ml led to an average daily Hcy intake of 0.1 mg per g body weight.
2-D echocardiography
Controls showed significantly smaller left ventricular internal dimension in diastole of 2.99 ± 0.1 mm compared with 3.23 ± 0.05 mm in Hcy-treated animals (P <0.05). Controls showed also significantly lower left ventricular internal dimension in systole (1.05 ± 0.04 mm) compared with Hcy-treated animals (1.6 ± 0.05 mm) (P <0.01). These findings resulted in a reduced left ventricular fractional shortening in Hcy-treated animals of 48.11 ± 1.57 % compared with 69.78 ± 1.47 % in Control animals. Control animals showed significantly smaller right ventricular internal dimension in diastole (0.59 ± 0.02 mm) compared with Hcy-treated animals (0.79 ± 0.02 mm) (P<0.05). Table 1 shows the morphometric and echocardiographic measurements.
Table 1.
Morphometric and echocardiographic parameters assessed at the end of 12-week study period in control and Hcy-treated animals
| Parameter | Control (n=5) | Hcy-treated (n=6) | P |
|---|---|---|---|
| Body weight (g) | 27.09 ± 1.85 | 28.91 ± 2.20 | >0.05 |
| Heart weight (g) | 0.154 ± 0.017 | 0.162 ± 0.030 | >0.05 |
| LVIDs (mm) | 1.04 ± 0.04 | 1.60 ± 0.05 | <0.01 |
| LVIDd (mm) | 2.99 ± 0.10 | 3.23 ± 0.05 | <0.05 |
| RVIDd (mm) | 0.59 ± 0.02 | 0.79 ± 0.02 | <0.05 |
| FS (%) | 69.78 ± 1.47 | 48.11 ± 1.57 | <0.01 |
Data presented as mean ± SE. LVIDs, left ventricular inner dimension in systole (mm); LVIDd, left ventricular inner dimension in diastole (mm); RVIDd, right ventricular inner dimension in diastole (mm); FS, fractional shortening (%).
ECG waveforms and heart rate in control animals and Hcy treated animals
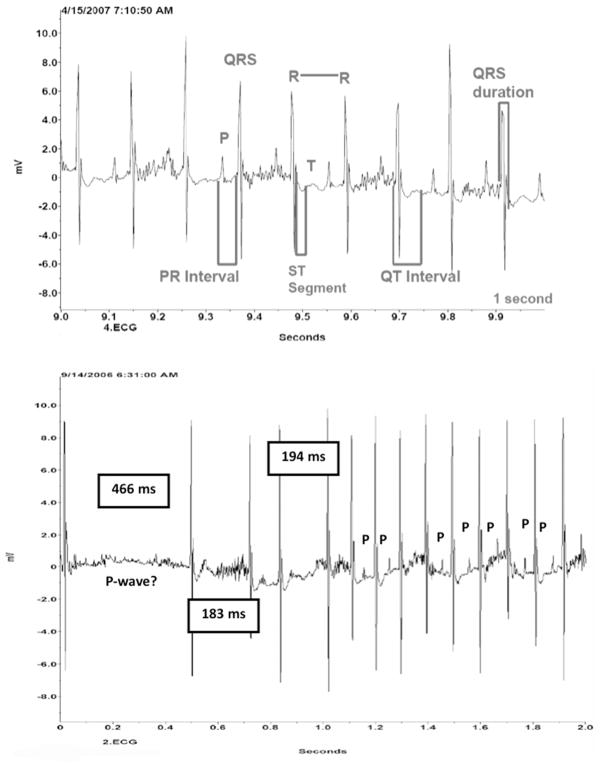
All control animals showed undisturbed atrioventricular conduction with P waves, QRS complex, and T waves in the preselected time frames. Heart rate in control animals was 511 ± 46 beats per minute (bpm) for a 5-day average. The 5-day average heart rate was higher in Hcy-treated animals (548 ± 62 bpm), but the difference was not statistically significant (P=0.105). Both groups showed similar patterns of activity levels or circadian changes in 12-hour day/night cycles over a 5-day average without any statistical differences between the two groups in terms of greater or lesser activity in either night or day cycles. ECG waveforms from a Control animal and an Hcy-treated animal are shown in Figure 1A and 1B. The representative Control animal in Figure 1A shows a regular sinus rhythm with undisturbed atrioventricular conduction. Segments and intervals are illustrated as well. The representative Hcy-treated animal (Figure 1B) shows an atrioventricular conduction delay of 466 ms, which is followed by irregular beats and compensatory rapid beats with an initial loss of P waves. The shorter delay episodes after the initial recorded delay are 183 ms and 194 ms, respectively. P waves appear again after rhythm stabilization. Heart rate in this time frame was reduced to 240 bpm.
Figure 1.
A. Recording of ECG waveform of a representative Control animal showing physiologic segments and intervals. B. Recording of ECG waveform of a representative Hcy-treated animal showing disruption in atrioventricular conduction with loss of P-wave. Length of conduction delay measured in milliseconds in a 2-second time frame.
PR, QRS, QTc and RR measurements in Controls and Hcy-treated animals
PR and QRS interval in Hcy-treated animals was significantly prolonged (P < 0.05) compared with Control animals as displayed in Table 2. QTc in Hcy-treated animals (49.5 ± 1.8 ms) was prolonged more than 10 ms or more than 30% with a statistical significance of P < 0.01 compared with Controls (37.0 ± 0.8 ms). There was no statistically significant difference in R-toR duration between the two study groups in the defined two-hour time frame.
Table 2.
Electrocardiographic data collected in week 12 of the 12-week study period from telemetric monitored control and Hcy-treated animals
| Parameter | Control (n=5) | Hcy-treated (n=6) | P |
|---|---|---|---|
| Heart rate (beats per minute) | 511 ± 46 | 548 ± 62 | >0.05 |
| RR (ms) | 106.5 ± 3.6 | 103.6 ± 3.3 | >0.05 |
| PR (ms) | 33.8 ± 0.7 | 37.6 ± 1.3 | <0.05 |
| QRS (ms) | 17.7 ± 0.8 | 23.9 ± 1.3 | <0.05 |
| QTc (ms) | 37.0 ± 0.8 | 49.5 ± 1.8 | <0.01 |
Heart rate presented in as mean ± SD. RR, R-to-R interval (ms); PR, P-to-R measurements reflecting atrial depolarization (ms); QRS complex, reflecting atrial repolarization and ventricular depolarization (ms); QTc, corrected QT time, reflecting ventricular depolarization and repolarization (RR, PR, QRS and QTc data presented as mean ± SE).
Matrix metalloproteinase-2, matrix metalloproteinase-9, and connexin 40, 43 and 45 protein expression
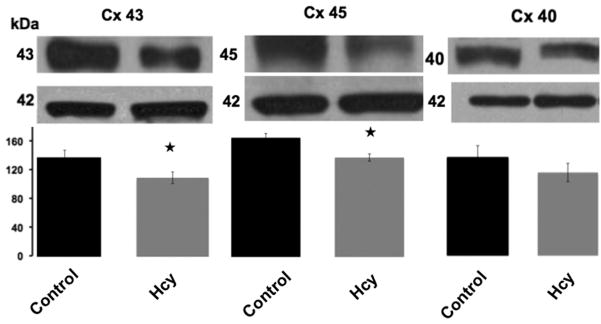
Matrix metalloproteinase-2, matrix metalloproteinase-9, connexin 40, 43 and 45 protein expressions in Controls and Hcy-treated animals were assessed with Western blot. Figure 2 shows increased matrix metalloproteinase-2 (P<0.05) and matrix metalloproteinase-9 (P<0.05) protein expression in animals treated with Hcy compared with Controls. There was a statistically significant decrease in connexin 43 (P< 0.05) and connexin 45 (P< 0.05) expression in Hcy-treated animals compared with Controls and a trend for a decrease in connexin 40 expression (p=0.28, no statistical significance) compared with Controls (Figure 3).
Figure 2.
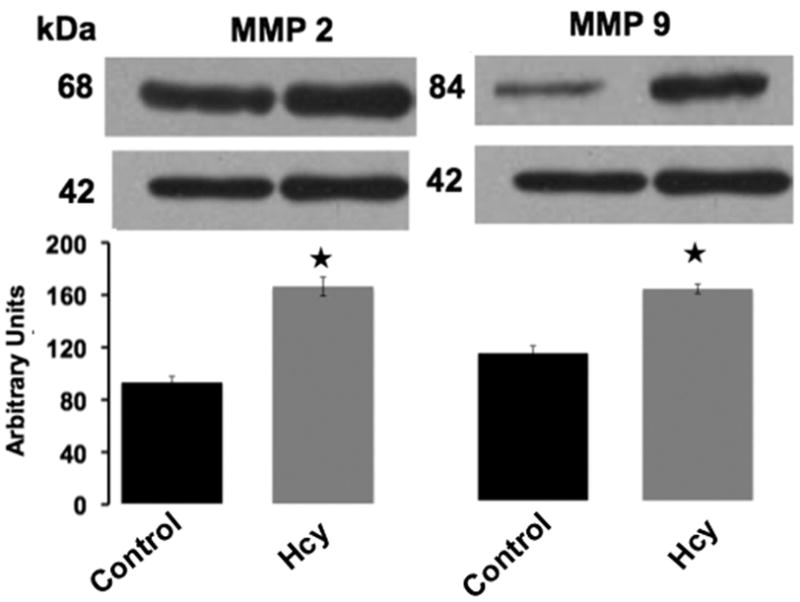
Immunoblotting for matrix metalloproteinase-2 and matrix metalloproteinase-9 expression in myocardial tissue of Control and Hcy-treated animals. Data presented shows statistically increased protein expression in Hcy-treated animals compared with Controls. Data presented as mean ± SE. Stars (★) indicate P < 0.05 compared with Control.
Figure 3.
Immunoblotting for connexin 43, 45 and 40 expression in myocardial tissue of Control and Hcy-treated animals. There was a significant decrease in connexin 43 and connexin 45 expression in Hcy-treated animals. Connexin 40 expression was decreased in Hcy-treated animals; however, it did not reach statistical significance. Data presented as mean ± SE. Stars (★) indicate P < 0.05 compared with Control.
Discussion
This study examined the role of Hcy on cardiac function and atrioventricular conduction in 24-week old male C57/BL6J mice. We hypothesized that mild to moderate elevation of Hcy blood levels induce matrix metalloproteinase-2 and matrix metalloproteinase-9 expression, initiate cardiac remodeling, and interfere with connexin 40, 43 and 45 protein expression leading to cardiac rhythm disturbances.
Total Hcy levels predict cardiac death and death of all causes according to a 10-year follow-up study from Norway [8]. Elevated Hcy levels at hospital admission in patients with acute coronary syndrome strongly predict late cardiac events [14]. Aggressive risk factor modification to prevent cardiac events in patients with renal failure includes lowering of Hcy levels. Nevertheless, patients’ risk for cardiac events is not reduced even with a significant decrease in Hcy levels [4]. This finding suggests that a simple reduction of Hcy blood levels does not address the underlying previous pathological mechanisms that led to Hcy-induced tissue remodeling. The degree of tissue damage over a period of time and the degree of tissue remodeling after Hcy-induced damage remains unpredictable. It is suggested that genetic polymorphisms play a major role in vascular vulnerability in hyperhomocysteinemia and cardiovascular disease [15].
We chose to achieve mild to moderate Hcy blood levels over a 12-week period (Hcy levels of 13.455 ± 2.691 μmol/L) based on results from Nygard’s study in 1997 [2]. Nygard and colleagues [2] found that the adjusted dose-response relation between Hcy and mortality in humans shows a nearly linear correlation from Hcy levels below 5 μmol/L to above 20 μmol/L with a steeper slope above 15 μmol/L. Previous studies show an inverse relationship between Hcy blood levels and left ventricular systolic function in humans[16]. Our findings show significant biventricular cardiac dilatation with Hcy-enriched diet, leading to reduced contractility and fractional shortening after a 12-week period. Telemetric monitoring allowed physiologic monitoring of ECGs in freely moving animals without anesthetic drug interaction on the cardiac conduction system and myocardium. Hcy-treated animals showed loss of QRS complexes and atrioventricular conduction delays followed by compensatory tachycardic episodes. Loss of beats with following compensatory tachycardia in Hcy-treated animals could explain an overall trend to higher heart rate averages (548 ± 62 bpm compared with 511 ± 46 bpm in Control animals). Higher heart rates might also reflect a direct effect of Hcy-induced increase in heart rate as a compensatory mechanism for a decrease in ventricular performance to maintain cardiac output. Hcy-treated animals showed a significant reduction in left ventricular fractional shortening in this study.
PR intervals reflecting atrioventricular conduction were significantly prolonged in animals treated with Hcy compared with Controls. We recorded ECGs in Hcy-treated animals that showed pauses of more than 550 ms with loss of atrioventricular conduction. Pauses of more than 300 ms in Hcy-treated animals decreased the heart rate to less than 240 bpm, which is less than 50% of their average heart rate. Delays of more than 500 ms lower the heart rate to less than 150 bpm minute or 30% of physiologic average. These are severe bradycardic episodes for a mouse. There is potential that Hcy-treated mice experience syncopal events. QRS complex was significantly prolonged in Hcy-treated animals compared with Control. PR prolongation with elevated Hcy blood levels might explain the association of an overall increased risk of atrioventricular rhythm disturbances and atrial fibrillation in patients older than 65 years of age, a group of patients who have also an increased risk for elevated Hcy blood levels [8, 17]. The QT interval was measured and corrected for heart rate (QTc) using Bazett’s equation as previously described [11]. We found highly significant prolongation of QTc interval in Hcy-treated animals compared with Controls. Long QT can lead to torsades de pointes and deteriorate to ventricular fibrillation, which can lead to sudden cardiac death if untreated. The combination of both atrial and ventricular conduction delays increases the risk for fatal arrhythmias.
We suggest that matrix metalloproteinase-induced extracellular matrix remodeling affects expression of connexin 40, 43 and 45, which leads to changes in cardiac gap junction proteins connecting with cardiomyocytes and results in disturbed cell-to-cell communication. Increased cardiac matrix metalloproteinase-2 and matrix metalloproteinase-9 expression coincided with reduced expression of connexin 40, 43 and 45 in this study. Connexin 43 is found in both the atrium and ventricle; whereas, connexin 45 is almost exclusively found in ventricular myocytes. Connexin 43 and connexin 45 were both significantly reduced in our study. Connexin “lateralization,” transmural distribution changes and disorganization of gap junction proteins in presence of cardiac pathologies such as heart failure and dilated atria have been described in recent literature [18, 19]. Connexin 40 expression was reduced, but didn’t reach statistical significance; however, even small reductions of this predominantly atrial gap junction protein involved in atrial impulse propagation in rodents [20] might have led to the significant atrioventricular conduction delays in Hcy-treated animals found in this study. Beauchamp et al. [20] found that deletion of connexin 43 in atrial myocytes led to a concomitant decrease in connexin 40 and decreased propagation velocity in myocytes. These findings support our hypothesis that changes in connexin 40 and reduced connexin 43 influenced atrioventricular conduction. We propose that relocation of connexin 40, 43 and 45 might be a contributing factor to atrioventricular conduction delay and prolonged ventricular depolarization and repolarization in this animal model of hyperhomocysteinemia.
Clinical trials to decrease total Hcy blood levels [21] with dietary supplementation of vitamins B6 and B12 have failed to lower the risk of cardiovascular disease, even though Hcy levels were reduced [22, 23]. The Framingham Study identified Hcy as an independent risk factor for incident stroke in elderly patients [1]. Hcy and its compounds have been identified to play a major role in clot formation, regardless of preexisting cardiac pathologies [14]. Based on these findings, the International Stroke Council guidelines and recommendations for prevention of cerebrovascular events include lowering of Hcy levels along with lowering of triglycerides and cholesterol [24, 25]. Thrombogenic potential [26] and cardiac disease increases the chance for critical cardiac events with possible negative outcome in hyperhomocysteinemia. Elevated Hcy is a marker for negative cardiac outcome and sudden cardiac death in patients with cardiovascular disease [27]. Our findings lead us to the conclusion that slight increases of Hcy blood levels have an impact on the myocardium and cardiac conduction. Activation of matrix metalloproteinases and reduction of cardiac gap junction proteins play a role in the pathomechanism of cardiac remodeling in moderate hyperhomocysteinemia.
Acknowledgments
This work was supported in part by NIH grants HL-71010, HL-74185, HL-88012. The study sponsor was not involved in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. Nancy Alsip, Ph.D., edited this manuscript (Office of Clinical Research Services and Support, University of Louisville).
Abbreviations
- Hcy
Homocysteine
- Met
Methionine
- MMP
matrix metalloproteinases
- Cx
Connexins
- tHcy
total Hcy
Footnotes
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bostom AG, Rosenberg IH, Silbershatz H, Jacques PF, Selhub J, D’Agostino RB, et al. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: the Framingham Study. Ann Intern Med. 1999;131:352–5. doi: 10.7326/0003-4819-131-5-199909070-00006. [DOI] [PubMed] [Google Scholar]
- 2.Nygard O, Nordrehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–6. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 3.Trabetti E. Homocysteine, MTHFR gene polymorphisms, and cardio-cerebrovascular risk. J Appl Genet. 2008;49:267–82. doi: 10.1007/BF03195624. [DOI] [PubMed] [Google Scholar]
- 4.Rakhit DJ, Marwick TH, Armstrong KA, Johnson DW, Leano R, Isbel NM. Effect of aggressive risk factor modification on cardiac events and myocardial ischaemia in patients with chronic kidney disease. Heart. 2006;92:1402–8. doi: 10.1136/hrt.2005.074393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenberger D, Moshal KS, Kartha GK, Tyagi N, Sen U, Lominadze D, et al. Arrhythmia and neuronal/endothelial myocyte uncoupling in hyperhomocysteinemia. Arch Physiol Biochem. 2006;112:219–27. doi: 10.1080/13813450601093443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 8.Retterstol L, Paus B, Bohn M, Bakken A, Erikssen J, Malinow MR, et al. Plasma total homocysteine levels and prognosis in patients with previous premature myocardial infarction: a 10-year follow-up study. J Intern Med. 2003;253:284–92. doi: 10.1046/j.1365-2796.2003.01096.x. [DOI] [PubMed] [Google Scholar]
- 9.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–49. [PubMed] [Google Scholar]
- 10.Malinow MR, Kang SS, Taylor LM, Wong PW, Coull B, Inahara T, et al. Prevalence of hyperhomocyst(e)inemia in patients with peripheral arterial occlusive disease. Circulation. 1989;79:1180–8. doi: 10.1161/01.cir.79.6.1180. [DOI] [PubMed] [Google Scholar]
- 11.Brouillette J, Grandy SA, Jolicoeur P, Fiset C. Cardiac repolarization is prolonged in CD4C/HIV transgenic mice. J Mol Cell Cardiol. 2007;43:159–67. doi: 10.1016/j.yjmcc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Remme CA, Verkerk AO, Nuyens D, van Ginneken AC, van Brunschot S, Belterman CN, et al. Overlap syndrome of cardiac sodium channel disease in mice carrying the equivalent mutation of human SCN5A-1795insD. Circulation. 2006;114:2584–94. doi: 10.1161/CIRCULATIONAHA.106.653949. [DOI] [PubMed] [Google Scholar]
- 13.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 14.Stubbs PJ, Al-Obaidi MK, Conroy RM, Collinson PO, Graham IM, Noble IM. Effect of plasma homocysteine concentration on early and late events in patients with acute coronary syndromes. Circulation. 2000;102:605–10. doi: 10.1161/01.cir.102.6.605. [DOI] [PubMed] [Google Scholar]
- 15.Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288:2023–31. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- 16.Rossi GP, Seccia TM, Pessina AC. Homocysteine, left ventricular dysfunction and coronary artery disease: is there a link? Clin Chem Lab Med. 2007;45:1645–51. doi: 10.1515/CCLM.2007.353. [DOI] [PubMed] [Google Scholar]
- 17.Moshal KS, Singh M, Sen U, Rosenberger DS, Henderson B, Tyagi N, et al. Homocysteine-mediated activation and mitochondrial translocation of calpain regulates MMP-9 in MVEC. Am J Physiol Heart Circ Physiol. 2006;291:H2825–35. doi: 10.1152/ajpheart.00377.2006. [DOI] [PubMed] [Google Scholar]
- 18.Saffitz JE, Laing JG, Yamada KA. Connexin expression and turnover: implications for cardiac excitability. Circ Res. 2000;86:723–8. doi: 10.1161/01.res.86.7.723. [DOI] [PubMed] [Google Scholar]
- 19.Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beauchamp P, Yamada KA, Baertschi AJ, Green K, Kanter EM, Saffitz JE, et al. Relative contributions of connexins 40 and 43 to atrial impulse propagation in synthetic strands of neonatal and fetal murine cardiomyocytes. Circ Res. 2006;99:1216–24. doi: 10.1161/01.RES.0000250607.34498.b4. [DOI] [PubMed] [Google Scholar]
- 21.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 22.Clarke R, Lewington S, Sherliker P, Armitage J. Effects of B-vitamins on plasma homocysteine concentrations and on risk of cardiovascular disease and dementia. Curr Opin Clin Nutr Metab Care. 2007;10:32–9. doi: 10.1097/MCO.0b013e328011aa71. [DOI] [PubMed] [Google Scholar]
- 23.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 24.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–73. [PubMed] [Google Scholar]
- 25.Flaker GC, Belew K, Beckman K, Vidaillet H, Kron J, Safford R, et al. Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149:657–63. doi: 10.1016/j.ahj.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 26.Colucci M, Cattaneo M, Martinelli I, Semeraro F, Binetti BM, Semeraro N. Mild hyperhomocysteinemia is associated with increased TAFI levels and reduced plasma fibrinolytic potential. J Thromb Haemost. 2008;6:1571–7. doi: 10.1111/j.1538-7836.2008.03070.x. [DOI] [PubMed] [Google Scholar]
- 27.Haim M, Tanne D, Goldbourt U, Doolman R, Boyko V, Brunner D, et al. Serum homocysteine and long-term risk of myocardial infarction and sudden death in patients with coronary heart disease. Cardiology. 2007;107:52–6. doi: 10.1159/000093697. [DOI] [PubMed] [Google Scholar]