Abstract
Activation of the ErbB family of receptor tyrosine kinases via cognate Epidermal Growth Factor (EGF)-like peptide ligands constitutes a major group of related signaling pathways that control proliferation, survival, angiogenesis and metastasis of breast cancer. In this respect, clinical trials with various ErbB receptor blocking antibodies and specific tyrosine kinase inhibitors have proven to be partially efficacious in the treatment of this heterogeneous disease. Induction of an embryonic program of epithelial-to-mesenchymal transition (EMT) in breast cancer, whereupon epithelial tumor cells convert to a more mesenchymal-like phenotype, facilitates the migration, intravasation, and extravasation of tumor cells during metastasis. Breast cancers which exhibit properties of EMT are highly aggressive and resistant to therapy. Activation of ErbB signaling can regulate EMT-associated invasion and migration in normal and malignant mammary epithelial cells, as well as modulating discrete stages of mammary gland development. The purpose of this review is to summarize current information regarding the role of ErbB signaling in aspects of EMT that influence epithelial cell plasticity during mammary gland development and tumorigenesis. How this information may contribute to the improvement of therapeutic approaches in breast cancer will also be addressed.
Keywords: ErbB, EGF, EMT, Mammary development, Breast cancer
Introduction
The ErbB family of type I receptor tyrosine kinases (RTKs), including Epidermal Growth Factor Receptor (EGFR; ErbB1, HER1), ErbB2 (Neu, HER2), ErbB3 (HER3) and ErbB4 (HER4), are activated by a large family of 14 ligands [1]. ErbB receptors modulate mammary gland development and are often amplified, mutated and/or overexpressed in breast cancer [2, 3]. Since they have important functions in regulating tumor proliferation, survival and metastasis, the ErbB family is a potentially attractive therapeutic target in breast cancer [4]. Co-expression of various ErbB receptor and ligand combinations is frequently associated with a more aggressive phenotype in breast tumors [5]. Increased expression of ErbB receptors, or ligands such as Transforming Growth Factor-α (TGFα), Amphiregulin (AREG), Neuregulin-1 (NRG1), or Cripto-1 (TDGF-1) that are capable of direct or indirect receptor activation, has been associated with an increased incidence of mammary hyperplasia and/or adenocarcinoma development in virgin or multiparous mice [1].
Aggressive breast cancers are generally resistant to current anti-cancer therapies, prone to early recurrence, and metastasize to distant sites, such as brain and lung. These breast cancers likely evolve from less aggressive epithelial-like breast tumors through reactivation of embryonic signaling pathways and programs like epithelial-to-mesenchymal transition (EMT) [6, 7]. EMT arises through a series of epigenetic alterations which converts polarized and adherent epithelial cells to a more motile and invasive mesenchymal-like phenotype. EMT and the converse process of mesenchymal-to-epithelial transition (MET) are fundamental in the development of virtually all organ systems [8, 9]. In cancer, EMT facilitates migration, invasion, and metastasis. Aggressive breast cancers exhibit phenotypic characteristics of EMT. This includes reduced expression of epithelial markers, such as E-cadherin, Occludin, Claudins, Desmoplakin, and epithelial Cytokeratins, and upregulation of mesenchymal markers, such as Vimentin, Smooth Muscle Actin, Fibronectin, and N-cadherin [9]. The downregulation of epithelial markers is a typical consequence of the activity of EMT-associated transcriptional repressors like Snail, Slug, Twist, ZEB1, and/or ZEB2 [9]. Numerous signaling pathways have been described to regulate aspects of EMT in embryonic development and disease, and are discussed elsewhere [8, 9].
Ligand-dependent or independent activation of the ErbB receptors can initiate and sustain various aspects of the EMT pathway in normal and malignant epithelial cells. Importantly, not all contexts of ErbB signaling necessarily promote epithelial cell migration or invasion. This phenomenon appears dependent upon which receptor homodimers or heterodimers are formed, which downstream signaling effectors are activated, and how signaling influences cell–cell and cell–extracellular matrix (ECM) interactions [10]. Additionally, other factors can cooperate with ErbB receptors to influence the invasive and migratory behavior of mammary cells including Transforming Growth Factor-β (TGFβ), Src, and Rho-GTPases [10]. In this review, we discuss the role of ErbB signaling in aspects of EMT that impact epithelial cell plasticity during mammary gland development and breast cancer. Understanding the function of molecular regulators, such as the ErbB pathway, in breast cancer progression is fundamental for the design of more efficient therapeutic strategies.
Role of ErbB Signaling and EMT During Embryogenesis and Prenatal Mammary Gland Development
The processes of EMT and MET are recurring themes during vertebrate embryogenesis [9]. These cellular transitions, as well as signal interactions between adjacent epithelial and mesenchymal tissues, are important in the development of virtually all organ systems. The ErbB signaling pathways participate in diverse developmental events from preimplantation to postnatal development, as indicated by the wide range of developmental defects that are observed in knockout mouse models [11]. Of particular note, ErbB/EGF family members have been implicated in regulating tissue morphogenesis in gastrulation, heart development, and mammary gland morphogenesis [9].
The earliest involvement of the EGF signaling pathway during mammalian development is evident prior to embryo implantation [12]. On the mouse CF-1 genetic background, EGFR is required for maintenance of the inner cell mass, which is a totipotent cell population that will form the epiblast and eventually give rise to all cells that make up the embryo proper. The first recognized EMT in development occurs during gastrulation, when cells of the epithelial epiblast undergo EMT as they migrate through the midline structure called the primitive streak [13]. Migration of these primitive mesenchymal cells (mesendoderm) away from the anterior Node gives rise to the underlying mesoderm and endoderm germ layers. ErbB4 signaling has been shown to regulate convergent extension movements during Xenopus gastrulation [14–16], where mesoderm cells involute and migrate as a contiguous sheet [13]. Direct involvement of an ErbB signaling pathway during mammalian gastrulation has not been described. However, Cripto-1, a member of the Epidermal Growth Factor-Cripto-1/FRL-1/Cryptic (EGF-CFC) subfamily that does not directly bind to any of the four known ErbB receptors but can indirectly activate ErbB4 [17], has an essential role in initiating mesoderm and endoderm formation through Nodal-dependent and independent signaling pathways during mammalian gastrulation [18, 19].
The first organ to develop during vertebrate embryogenesis is the heart. The heart is formed by an overlapping series of cell migration, differentiation, proliferation, EMT and MET. Cardiac progenitor cells are initially specified during the EMT at gastrulation, which then undergo MET and coalesce to form a primitive heart tube that subsequently undergoes looping. Succeeding maturation steps also involve EMT, and include the formation of the trabecular myocardial layer, and the development of the endocardial cushions and valves [20, 21]. Studies across vertebrate species indicate that ErbB signaling is essential for heart morphogenesis [22, 23]. In mice, multiple ErbB receptors and ligands are expressed in the endocardial or myocardial layers of the heart [24–27]. The ErbB2 and ErbB4 receptors, and the ligand NRG1, are required for proper myocardial trabeculation, which is the formation of finger or sheet-like extensions of the myocardium that is critical for the maintenance of blood flow during early cardiogenesis [24, 26, 27], while ErbB2 and NRG1 also participate in cushion formation [28, 29]. The receptors ErbB3 and EGFR, and the Heparin-Binding EGF-like Growth Factor (HB-EGF) ligand, are necessary for cardiac cushion development and valve remodeling that lead to formation of mature cardiac valves [28, 30–32]. The atrioventricular (AV) canal (the region between the atria and ventricles) and the outflow tract cushions are derived from cells of the inner endocardial layer that undergo an EMT into the underlying ECM, called the cardiac jelly. Activation of ErbB2/ErbB3 signaling by NRG1 specifically participates in endocardial cushion EMT via a mechanism involving hyaluronic acid (HA), an important component of the cardiac jelly [28]. Embryos lacking Has2, a major enzyme responsible for HA synthesis, display a complete absence of cardiac jelly and diminished ErbB2/ErbB3 phosphorylation that could be rescued in AV canal explant cultures by addition of HA. Specifically, attenuation of ErbB3 phosphorylation blocked endocardial cushion EMT in wild-type AV canal explants, while NRG1 could rescue endocardial cushion EMT in Has2-/- AV canal explants, indicating a role for ErbB2/ErbB3-NRG1 signaling in modulating cardiac cushion EMT. EGFR and HB-EGF deficient mouse models also display abnormalities in cardiac valve formation [30–32]. HB-EGF does not participate in endocardial cushion EMT however, but rather regulates the proliferation of already transformed mesenchymal cells [32].
ErbB signaling pathways clearly play important roles in heart development and associated EMT, and mutant mouse models for specific ErbB receptors typically exhibit heart and nervous system defects and die prenatally [11]. For this reason, mammary gland development has been a challenge to study in these animals. However, models where the ErbB receptor gene is specifically re-expressed in the heart can rescue embryos from lethality and enable the study of these receptors in mammary gland development.
The mammary gland is a unique organ in that much of its development occurs postnatally Embryonic mammary gland development arrests with only rudimentary glands present [33]. In this regard, development of the mammary gland requires postnatal branching morphogenesis and ductal elongation for complete maturation of the mammary glands which is essential for differentiation and milk protein production during pregnancy and lactation. In mouse, embryonic mammary glands are specified early in development with formation of the milk lines, which are two ridges of stratified epithelium running between the fore- and hind-limb buds on the ventral surface of the embryo. Ectodermal cells along the milk line aggregate at distinct sites and invaginate to form five pairs of mammary buds. Signaling from the epithelial bud induces underlying mesenchymal cells to become dense around the bud, forming the so-called mammary mesenchyme. Epithelial bud cells proliferate, and the bud sprouts through the mesenchyme and branches into the prospective mammary fat pad to form a small ductal tree bearing 10–15 branches [33].
ErbB signaling plays an important role in mediating interactions between the mesenchyme and coalescing mammary bud epithelium as it promotes specification of the mammary bud. In particular, the A/J mouse strain exhibits aberrant positioning and ectopic numbers of mammary buds. This phenotype is produced by mutations in the scaramanga (ska) gene which corresponds to Neuregulin-3 (NRG3), a ligand of the ErbB4 receptor [34]. NRG3 is first expressed in the lateral plate mesoderm underlying the ectoderm where the mammary buds will subsequently develop, immediately prior to the sequential development of each bud. Interestingly, the ErbB4 receptor is also expressed in the lateral plate mesoderm preceding mammary bud formation. In the ska mutant, mammary bud 3 is absent and small ectopic buds often form close to the site of mammary bud 4, indicating a requirement for mesenchymal NRG3 in bud specification in the overlying ectoderm.
It is unlikely that ErbB4 and NRG3 are the only ErbB/EGF family members that participate in embryonic mammary gland formation. A recent study by Wansbury et al describes the expression of all four Neuregulin ligands (NRG1-4) and all ErbB receptors prior to and during formation of the mammary bud in either surface ectoderm or underlying mesoderm or both [35]. Considering that the individual mutant mouse models do not typically exhibit embryonic mammary gland defects, it is likely that some degree of redundancy exists between family members of ligands and receptors, as observed in other aspects of mammary gland development [36].
ErbB Signaling During Postnatal Mammary Gland Development
During the postnatal development of the mouse mammary gland, elements reminiscent of EMT play an important role in regulating cellular migration and the establishment of new tissue. The epithelial cells that make up the developing mammary gland show great plasticity, and are influenced by numerous systemic and local factors including multiple members of the ErbB/EGF family, the ovarian hormones estrogen and progesterone, and other growth factors including FGFs, IGF-2 and Wnt-4 [37]. These factors function through autocrine, paracrine, and juxtacrine mechanisms, with a number of these signals originating from the surrounding mesenchymal tissue including adipocytes as well as neural, lymphoid, and endothelial cells.
Analysis of the temporal expression of EGF family members, such as AREG, TGFα, Betacellulin, HB-EGF, EGF, Epiregulin, and NRG1, indicates all are present during postnatal mammary gland development and have unique expression patterns [38, 39]. EGFR, ErbB2, and later ErbB3, are expressed in the virgin mammary gland [38–40], while all four ErbB receptors are expressed during pregnancy and lactation. Specific mutant mouse models of EGFR, ErbB2, and ErbB4 exhibit defects in aspects of mammary gland morphogenesis, such as ductal elongation and branching, lobuloalveolar development, and milk protein production [40–42].
Penetration of the expanding mammary ducts into the fat pad in the virgin mammary gland, as occurs during allometric outgrowth and side branching, requires ECM degradation and remodeling by ECM-degrading proteases such as Matrix Metalloproteases (MMPs) that are an important component of EMT. Invasion from the terminal end buds (TEBs) as the ducts move into the surrounding stroma requires epithelial cell plasticity and may be due to a process reminiscent of EMT, as EMT-associated transcriptional repressors (Snail and Twist) and MMPs are enriched in TEBs [43]. Stimulation of RTKs such as the ErbB receptors can activate Ras-MAPK signal transduction cascades that upregulate Snail and Slug [44, 45], which repress the transcription of E-cadherin and other cell adhesion molecules [46]. Likewise, numerous MMPs, including MMP2, MMP9 and MT1-MMP, are downstream targets of ErbB signaling pathways [47–49].
AREG is the most abundant EGF ligand during pubertal expansion of the mammary epithelium in the virgin mammary gland [38]. AREG is induced by and required for estrogen-mediated mammary epithelial proliferation, ductal elongation, and TEB formation [50]. In an anchorage-independent culture system, where stem cells survive anoikis and expand to form free-floating mammospheres, AREG mediates the expansion of ductal-limited mammary progenitor cells but not lobule-limited mammary progenitor cells [51]. In this model, AREG signals through EGFR and activates Erk1/2 of the Ras-MAPK pathway to induce mammosphere expansion.
TGFα is expressed throughout mammary development [52]. The highest levels are detected during mammogenesis and involution following pregnancy, which are stages characterized by expansive proliferation and tissue reorganization. TGFα is found in the proliferating cap cells of the TEBs, which are cells required for normal epithelial invasion into the fat pad, and in the surrounding stroma during pubertal growth. TGFα expression is also found during the lobulalveolar stages, although at a reduced level. The epithelial cap cells and myoepithelial cells are descendents of duct-limited mammary progenitor cells. During pregnancy, an increase in TGFα production coincides with extensive mammary epithelial cell proliferation. During subsequent lactation, TGFα levels are 2–3 times higher than levels in mammary glands of virgin mice.
The ligands of the EGF family are proteolytically cleaved from membrane-tethered pro forms. Many EGF family members, such as TGFα, AREG and HB-EGF, are cleaved by TNFα converting enzyme, also known as ADAM17 (A Disintegrin and Metalloproteinase-17) [53–55]. Interestingly, ADAM17 and EGFR knockout mice present similar phenotypes characterized by abnormal pubertal mammary gland development [56, 57]. ADAM17-/- mice still express AREG that normally activates EGFR during mammary gland development, but, in these mice, it is not cleaved from its membrane tethered form and cannot activate EGFR [57]. This suggests that ADAM17 acts upstream of EGFR signaling in mammary gland morphogenesis by regulating ligand activity.
The EGF ligands and the ErbB receptors have multiple and recurring functions throughout mammary development. They are essential for proliferation, invasion, and differentiation of mammary tissue at the major mammary developmental stages. Since these ligands and receptors are involved in proliferation and penetrating growth, it is not surprising that many of these proteins are highly expressed in breast cancer.
ErbB/EGF Signaling and EMT in Breast Cancer
Numerous in vitro and animal models of cellular transformation, tumorigenesis, and metastatic spread have been developed in an attempt to recapitulate and characterize important biologic events which occur during cancer development, to try to identify potential therapeutic targets. A number of biological processes are shared between embryonic development, and the initiation and progression of oncogenesis. One pivotal process that has assumed increased attention is EMT because of its potential to regulate metastasis [58]. The basis for studying EMT in cancer is that the majority of human malignancies are derived from epithelial cells. Most cancer cells, like epithelial cells during embryonic development, undergo physical and biochemical changes that enable them to interact with the surrounding microenvironment, thus facilitating their migration from the site of origin and dissemination to distant tissues and organs [59]. Similar to normal development, epithelial-like cancer cells in the primary tumor can initiate a multi-step process whereby cells downregulate the expression of intracellular adhesion components, such as E-cadherin, Occludin, and Claudins, and upregulate signaling pathways and proteins, such as N-cadherin and Vimentin associated with a more motile, mesenchymal-like phenotype [9]. Such changes lead to alterations in cell polarity and cell–cell adhesion as epithelial cells transition to a mesenchymal-like state.
EMT in cancer cells is often triggered by autocrine and paracrine signals. Numerous growth factors, cytokines and integrin-related interactions can directly or indirectly activate signaling molecules, such as RTKs, Wnt/β-catenin, MAPKs, PI3K/Akt, Ras, ILK, and FAK, capable of inducing EMT [60]. Expression of the ErbB RTK family and their cognate ligands have been detected in diverse human cancers including carcinomas of the lung, colon, ovary, stomach, and breast, and their role during neoplastic transformation and tumor progression has been well documented [2]. Enhanced activity of EGFR and ErbB2 in cancer cells promotes aggressiveness as characterized by an increased rate of recurrence and metastatic spread. Several studies have attempted to associate the expression of individual ErbB receptors with disease progression and have found that the co-expression of different ErbB receptors in breast cancer is more often associated with poor prognosis than the individual expression of specific ErbB receptors and/or EGF-like ligands [2, 5]. For example, in a study group of 242 breast cancer patients, the co-expression of EGFR, ErbB2, and ErbB3 was associated with poor patient survival during a median 15 year follow-up [61]. Given the redundancy of expression of ErbB receptors in cancer, it is clear that the complete picture of ErbB receptor and ligand expression should be taken into consideration for a more accurate assessment of overall signaling activity, when evaluating possible patient therapy options [62].
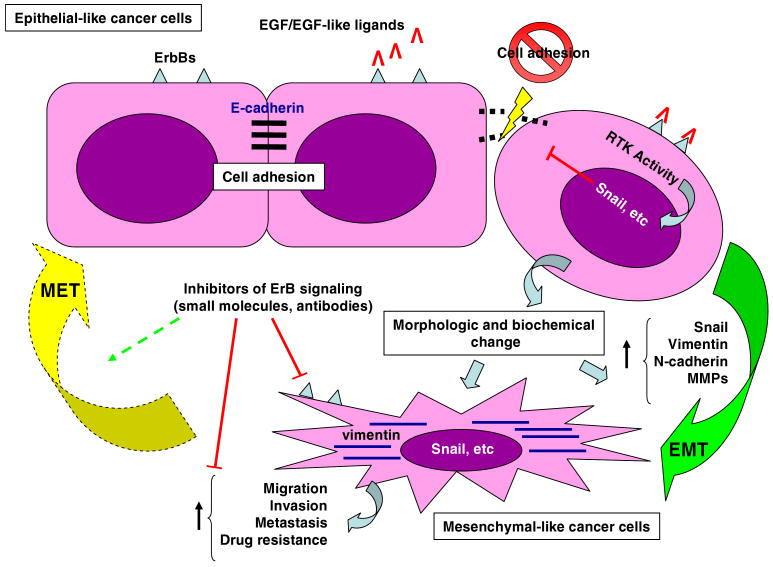
The elevated aggressiveness associated with EGFR activity in cancer cells may be explained in part by the activation of EMT associated events. EGFR activity has been shown to induce tumor cell motility and invasion by regulating the activity of downstream signaling molecules, such as FAK, β-catenin, Ras, Raf, MAPK, and PI3K/Akt [63]. Individual cell migration and invasion is dependent upon the release of cell–cell contacts. The activation of different pathways including ErbB signaling can result in increased activity of transcriptional repressors, such as Snail, ZEB, and Twist, that repress expression of cell adhesion molecules like E-cadherin (as summarized in Fig. 1) [60]. EGFR signaling can induce EMT, invasion, and metastasis in several different types of rodent and human cancer cells, including human breast cancer cells, which can be mediated via STAT3-dependent Twist upregulation [64] or by inducing the expression of Snail and ZEB [65], for example. Importantly, however, the observations that only EGFR/ErbB2 heterodimers, but not homodimers, could induce invasive behavior in mammary epithelial cells [66], and that EGFR over-expression in mice did not induce transformation of the entire mammary epithelium, but caused only focal mammary tumors that were sometimes metastatic [67], together suggest that additional mechanisms likely contribute to ErbB-dependent effects on EMT, cell invasion, and metastasis. Cross-talk with other growth factors such as TGFβ [68, 69], kinases such as Src [70, 71], or cell–matrix signaling molecules such as α6β4 integrin [72, 73], have been described to promote ErbB-dependent effects on mammary tumors.
Figure 1.
Signaling by epithelial growth factor receptors (ErbBs) has been shown to stimulate epithelial to mesenchymal transition (EMT) in epithelial-like cancer cells by activating different intracellular receptor tyrosine kinases (RTK). This often results in increased activity of transcription factors, such as Snail that represses the expression of intracellular adhesion molecules like E-cadherin. As a consequence, these cells become more spindle-shaped, express mesenchyme associated molecules, such as Vimentin, N-cadherin and certain Metalloproteases (MMPs), and assume increased aggressiveness as they become more migratory and invasive. Inhibitors of ErbB signaling have been able to interfere with the EMT process in certain cancer cells. In select cases, reversal of EMT has resulted in increased efficacy of anti-cancer therapy in otherwise EMT-associated drug resistant cancer cells.
Several reports have shown a role for the Cripto-1/Nodal signaling pathway during EMT, which has been implicated in both embryogenesis and cellular transformation [74]. Cripto-1 contains a modified EGF-like motif with 60–70% homology to EGF. Although Cripto-1 is not known to bind directly to any of the known ErbB receptors, it has been shown to indirectly enhance ErbB4 tyrosine kinase activity [17]. An increase in migration and invasion of cervical and breast cancer cells has been associated with Cripto-1 overexpression [75, 76]. Furthermore, studies have described the EMT signature of decreased E-cadherin expression and increased Vimentin and N-cadherin expression in mammary gland hyperplasias and tumors from transgenic mice overexpressing human Cripto-1 [77]. This same study also described biochemical and functional changes associated with EMT in vitro in Cripto-1 overexpressing mouse mammary epithelial cells. The role of Cripto-1 in the induction of EMT in cancer cells may explain, in part, why Cripto-1 expression has been associated with more aggressive behavior in gastric cancer [78, 79], breast cancer [80], ovarian cancer [81], and colon cancer [82]. Considering that Nodal can bind to Cripto-1 during A/P axis orientation and mesoderm formation, it would seem logical that activation of Nodal- and Smad-dependent signaling could also regulate EMT in cancer. In fact, Nodal has been shown to correlate with more advanced stages of melanoma and breast cancer, which may relate to the ability of Nodal to induce migration and invasion in these cells [83, 84]. To further support this, a recent study found that Nodal/Smad2 signaling can positively regulate the expression of Twist, Snail, and Slug during mesoderm formation in Xenopus, and that Twist and Slug in turn can negatively affect expression of the Nodal antagonist, Cerberus [85].
Clearly, EMT represents one mechanism by which activation of ErbB signaling may regulate cancer progression. It is also intuitive that cancer therapy using ErbB-directed small molecule inhibitors and/or blocking antibodies may influence EGF-dependent EMT, and inhibit cancer cell migration and invasion. Unfortunately not all epithelial cancers respond to treatment strategies that block ErbB signaling. Several explanations have been given to account for these low success rates [86]. For instance, acquisition of resistance through mutation of the targeted EGFR has been suggested as a possible explanation in patients that fail to respond to anti-EGFR therapy [87]. Accurate characterization of global ErbB receptor expression in breast cancer and subsequent use of a combination of specific ErbB receptor inhibitors has been shown to increase the anti-cancer effect in select patients [88, 89]. Recently, EMT has been suggested as a key factor in determining the success rates of anticancer therapy. In certain cancers, ErbB receptor inhibitors were more effective in epithelial-like tumor cells than in tumor cells that exhibited a more mesenchymal-like phenotype or that had acquired other EMT characteristics [90–92]. In aggressive inflammatory breast cancer, ErbB receptor inhibition was able to reverse the mesenchymal phenotype of these cancer cells to a less aggressive and potentially more chemotherapy sensitive epithelial phenotype [93].
It is apparent that EMT is no longer a phenomenon limited to explaining the migration of cells to sites of developing tissues and organs during embryogenesis. Clearly, cancer cells that acquire a more mesenchymal phenotype through EMT are more aggressive, are more resistant to conventional chemotherapy and radiotherapy, and are associated with reduced patient survival. Most importantly, future anti-cancer approaches should take into account the status of EMT in individual cancers as reversal of EMT could lead to enhanced sensitivity of cancer cells to specific anti-cancer agents, including ErbB inhibitors. The hope is that such considerations can influence the selection of more efficient treatment options.
Conclusions and Future Perspectives
During embryonic development, one of the key features that results in the exquisite programming of epithelial tissue into a complex organ is EMT. EMT has also provided insight into mechanisms involved in the migration, invasion, and metastatic spread of cancer cells. Indeed, this review has highlighted the importance of the ErbB and EGF families of receptors and ligands in regulating epithelial plasticity and EMT during mammary gland development and breast cancer. Understanding and defining the initial molecular signals leading to the EMT switch in tumor cells would undoubtedly contribute to the earliest possible clinical detection and intervention strategies. Although the use of inhibitors delivered individually to ErbB/EGF targets seems reasonable, limited efficacy suggests that a combinatorial approach would offer improved clinical outcome. Elucidating the steps that initiate the re-emergence of embryonic processes and signaling pathways in cancer, such as those involved in EMT, and understanding the implications of the interactions between transitioning cells and their microenvironment, will ultimately lead to more rational approaches in our arsenal for targeting cancer.
Acknowledgments
Financial Support This work is supported by the U.S. National Institutes of Health (NIH) intramural funding, NIH extramural grants (CA59702 and CA121205) and the Eisenberg Scholar Research Award. We apologize to authors whose work was not mentioned directly.
Abbreviations
- EGF
Epidermal Growth Factor
- EGFR
Epidermal Growth Factor Receptor
- EMT
Epithelial-to-mesenchymal transition
- MET
Mesenchymal-to-epithelial transition
- RTK
Receptor tyrosine kinase
- TGFα
Transforming Growth Factor-α
- TGFβ
Transforming Growth Factor-β
- EGF-CFC
Epidermal Growth Factor-Cripto-1/FRL-1/Cryptic
- AREG
Amphiregulin
- NRG1
Neuregulin-1
- NRG3
Neuregulin-3
- HB-EGF
Heparin-Binding EGF-like Growth Factor
- AV
Atrioventricular
- HA
Hyaluronic acid
- ska
Scaramanga
- ECM
Extracellular matrix
- MMP
Matrix Metalloprotease
- TEB
Terminal end bud
- ADAM17
A Disintegrin and Metalloproteinase-17
Contributor Information
Katharine M. Hardy, Children's Memorial Research Center, Robert H. Lurie, Comprehensive Cancer Center, Northwestern University Feinberg, School of Medicine, 2300 Children's Plaza, Box 222, Chicago, IL 60614, USA
Brian W. Booth, Institute for Biological Interfaces of Engineering, Clemson University, Clemson, SC, USA
Mary J. C. Hendrix, Children's Memorial Research Center, Robert H. Lurie, Comprehensive Cancer Center, Northwestern University Feinberg, School of Medicine, 2300 Children's Plaza, Box 222, Chicago, IL 60614, USA
David S. Salomon, Laboratory of Mammary Gland Biology and Tumorigenesis, Laboratory, National Cancer Institute, Bethesda, MD, USA
Luigi Strizzi, Email: lstrizzi@childrensmemorial.org, Children's Memorial Research Center, Robert H. Lurie, Comprehensive Cancer Center, Northwestern University Feinberg, School of Medicine, 2300 Children's Plaza, Box 222, Chicago, IL 60614, USA.
References
- 1.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, et al. The ErbB receptors and their ligands in cancer: an overview. Curr Drug Targets. 2005;6:243–57. doi: 10.2174/1389450053765879. [DOI] [PubMed] [Google Scholar]
- 3.Troyer KL, Lee DC. Regulation of mouse mammary gland development and tumorigenesis by the ERBB signaling network. J Mammary Gland Biol Neoplasia. 2001;6:7–21. doi: 10.1023/a:1009560330359. [DOI] [PubMed] [Google Scholar]
- 4.Normanno N, Bianco C, De Luca A, Maiello MR, Salomon DS. Target-based agents against ErbB receptors and their ligands: a novel approach to cancer treatment. Endocr Relat Cancer. 2003;10:1–21. doi: 10.1677/erc.0.0100001. [DOI] [PubMed] [Google Scholar]
- 5.McIntyre E, Blackburn E, Brown PJ, Johnson CG, Gullick WJ. The complete family of epidermal growth factor receptors and their ligands are co-ordinately expressed in breast cancer. Breast Cancer Res Treat. 2009 doi: 10.1007s/10549-009-0536-5. [DOI] [PubMed] [Google Scholar]
- 6.Hollier BG, Evans K, Mani SA. The epithelial-to-mesenchymal transition and cancer stem cells: a coalition against cancer therapies. J Mammary Gland Biol Neoplasia. 2009;14:29–43. doi: 10.1007/s10911-009-9110-3. [DOI] [PubMed] [Google Scholar]
- 7.Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11:213. doi: 10.1186/bcr2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–49. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Feigin ME, Muthuswamy SK. ErbB receptors and cell polarity: new pathways and paradigms for understanding cell migration and invasion. Exp Cell Res. 2009;315:707–16. doi: 10.1016/j.yexcr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 11.Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci. 2008;65:1566–84. doi: 10.1007/s00018-008-7440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–4. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 13.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–83. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Nie S, Chang C. Regulation of early Xenopus development by ErbB signaling. Dev Dyn. 2006;235:301–14. doi: 10.1002/dvdy.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nie S, Chang C. Regulation of Xenopus gastrulation by ErbB signaling. Dev Biol. 2007;303:93–107. doi: 10.1016/j.ydbio.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nie S, Chang C. PI3K and Erk MAPK mediate ErbB signaling in Xenopus gastrulation. Mech Dev. 2007;124:657–67. doi: 10.1016/j.mod.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bianco C, Kannan S, De Santis M, Seno M, Tang CK, Martinez-Lacaci I, et al. Cripto-1 indirectly stimulates the tyrosine phosphorylation of erb B-4 through a novel receptor. J Biol Chem. 1999;274:8624–9. doi: 10.1074/jbc.274.13.8624. [DOI] [PubMed] [Google Scholar]
- 18.Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, et al. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–7. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- 19.D'Andrea D, Liguori GL, Le Good JA, Lonardo E, Andersson O, Constam DB, et al. Cripto promotes A-P axis specification independently of its stimulatory effect on Nodal autoinduction. J Cell Biol. 2008;180:597–605. doi: 10.1083/jcb.200709090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner M, Siddiqui MA. Signal transduction in early heart development (II): ventricular chamber specification, trabeculation, and heart valve formation. Exp Biol Med (Maywood) 2007;232:866–80. [PubMed] [Google Scholar]
- 21.Wagner M, Siddiqui MAQ. Signal transduction in early heart development (I): cardiogenic induction and heart tube formation. Exp Biol Med (Maywood) 2007;232:852–65. [PubMed] [Google Scholar]
- 22.Goishi K, Lee P, Davidson AJ, Nishi E, Zon LI, Klagsbrun M. Inhibition of zebrafish epidermal growth factor receptor activity results in cardiovascular defects. Mech Dev. 2003;120:811–22. doi: 10.1016/s0925-4773(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 23.Iwamoto R, Mekada E. ErbB and HB-EGF signaling in heart development and function. Cell Struct Funct. 2006;31:1–14. doi: 10.1247/csf.31.1. [DOI] [PubMed] [Google Scholar]
- 24.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–4. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 25.Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, et al. Notch signaling is essential for ventricular chamber development. Dev Cell. 2007;12:415–29. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–8. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 27.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–90. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 28.Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8:850–5. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- 29.Erickson SL, O'Shea KS, Ghaboosi N, Loverro L, Frantz G, Bauer M, et al. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2- and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 30.Chen B, Bronson RT, Klaman LD, Hampton TG, Wang JF, Green PJ, et al. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat Genet. 2000;24:296–9. doi: 10.1038/73528. [DOI] [PubMed] [Google Scholar]
- 31.Iwamoto R, Yamazaki S, Asakura M, Takashima S, Hasuwa H, Miyado K, et al. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci USA. 2003;100:3221–6. doi: 10.1073/pnas.0537588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. Embo J. 2003;22:2704–16. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watson CJ, Khaled WT. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development. 2008;135:995–1003. doi: 10.1242/dev.005439. [DOI] [PubMed] [Google Scholar]
- 34.Howard B, Panchal H, McCarthy A, Ashworth A. Identification of the scaramanga gene implicates Neuregulin3 in mammary gland specification. Genes Dev. 2005;19:2078–90. doi: 10.1101/gad.338505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wansbury O, Panchal H, James M, Parry S, Ashworth A, Howard B. Dynamic expression of Erbb pathway members during early mammary gland morphogenesis. J Invest Dermatol. 2008;128:1009–21. doi: 10.1038/sj.jid.5701118. [DOI] [PubMed] [Google Scholar]
- 36.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, et al. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–50. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 37.Lanigan F, O'Connor D, Martin F, Gallagher WM. Molecular links between mammary gland development and breast cancer. Cell Mol Life Sci. 2007;64:3159–84. doi: 10.1007/s00018-007-7386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenney NJ, Huang RP, Johnson GR, Wu JX, Okamura D, Matheny W, et al. Detection and location of amphiregulin and Cripto-1 expression in the developing postnatal mouse mammary gland. Mol Reprod Dev. 1995;41:277–86. doi: 10.1002/mrd.1080410302. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder JA, Lee DC. Dynamic expression and activation of ERBB receptors in the developing mouse mammary gland. Cell Growth Differ. 1998;9:451–64. [PubMed] [Google Scholar]
- 40.Sebastian J, Richards RG, Walker MP, Wiesen JF, Werb Z, Derynck R, et al. Activation and function of the epidermal growth factor receptor and erbB-2 during mammary gland morphogenesis. Cell Growth Differ. 1998;9:777–85. [PubMed] [Google Scholar]
- 41.Andrechek ER, White D, Muller WJ. Targeted disruption of ErbB2/Neu in the mammary epithelium results in impaired ductal outgrowth. Oncogene. 2005;24:932–7. doi: 10.1038/sj.onc.1208230. [DOI] [PubMed] [Google Scholar]
- 42.Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci USA. 2003;100:8281–6. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kouros-Mehr H, Werb Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev Dyn. 2006;235:3404–12. doi: 10.1002/dvdy.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze'ev A. Autoregulation of E-cadherin expression by cadherin–cadherin interactions: the roles of beta-catenin signaling, Slug, and MAPK. J Cell Biol. 2003;163:847–57. doi: 10.1083/jcb.200308162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hipp S, Walch A, Schuster T, Losko S, Laux H, Bolton T, et al. Activation of epidermal growth factor receptor results in Snail protein but not mRNA over-expression in endometrial cancer. J Cell Mol Med. 2008;13:3858–67. doi: 10.1111/j.1582-4934.2008.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–61. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 47.Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci. 2002;115:839–48. doi: 10.1242/jcs.115.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kondapaka SB, Fridman R, Reddy KB. Epidermal growth factor and amphiregulin up-regulate matrix metalloproteinase-9 (MMP-9) in human breast cancer cells. Int J Cancer. 1997;70:722–6. doi: 10.1002/(sici)1097-0215(19970317)70:6<722::aid-ijc15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 49.Miettinen PJ, Chin JR, Shum L, Slavkin HC, Shuler CF, Derynck R, et al. Epidermal growth factor receptor function is necessary for normal craniofacial development and palate closure. Nat Genet. 1999;22:69–73. doi: 10.1038/8773. [DOI] [PubMed] [Google Scholar]
- 50.Ciarloni L, Mallepell S, Brisken C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc Natl Acad Sci USA. 2007;104:5455–60. doi: 10.1073/pnas.0611647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Booth BW, Boulanger CA, Anderson LH, Jimenez-Rojo L, Brisken C, Smith GH. Amphiregulin mediates self-renewal in an immortal mammary epithelial cell line with stem cell characteristics. Exp Cell Res. 2010;316:422–32. doi: 10.1016/j.yexcr.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Booth BW, Smith GH. Roles of transforming growth factor-alpha in mammary development and disease. Growth Factors. 2007;25:227–35. doi: 10.1080/08977190701750698. [DOI] [PubMed] [Google Scholar]
- 53.Hinkle CL, Sunnarborg SW, Loiselle D, Parker CE, Stevenson M, Russell WE, et al. Selective roles for tumor necrosis factor alpha-converting enzyme/ADAM17 in the shedding of the epidermal growth factor receptor ligand family: the juxtamembrane stalk determines cleavage efficiency. J Biol Chem. 2004;279:24179–88. doi: 10.1074/jbc.M312141200. [DOI] [PubMed] [Google Scholar]
- 54.Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol. 2004;164:769–79. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sunnarborg SW, Hinkle CL, Stevenson M, Russell WE, Raska CS, Peschon JJ, et al. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J Biol Chem. 2002;277:12838–45. doi: 10.1074/jbc.M112050200. [DOI] [PubMed] [Google Scholar]
- 56.Wiesen JF, Young P, Werb Z, Cunha GR. Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development. 1999;126:335–44. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- 57.Sternlicht MD, Sunnarborg SW, Kouros-Mehr H, Yu Y, Lee DC, Werb Z. Mammary ductal morphogenesis requires paracrine activation of stromal EGFR via ADAM17-dependent shedding of epithelial amphiregulin. Development. 2005;132:3923–33. doi: 10.1242/dev.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Micalizzi DS, Ford HL. Epithelial-mesenchymal transition in development and cancer. Future Oncol. 2009;5:1129–43. doi: 10.2217/fon.09.94. [DOI] [PubMed] [Google Scholar]
- 59.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 60.Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol. 2007;18:1605–19. doi: 10.1093/annonc/mdm070. [DOI] [PubMed] [Google Scholar]
- 61.Wiseman SM, Makretsov N, Nielsen TO, Gilks B, Yorida E, Cheang M, et al. Coexpression of the type 1 growth factor receptor family members HER-1, HER-2, and HER-3 has a synergistic negative prognostic effect on breast carcinoma survival. Cancer. 2005;103:1770–7. doi: 10.1002/cncr.20970. [DOI] [PubMed] [Google Scholar]
- 62.Normanno N, Morabito A, De Luca A, Piccirillo MC, Gallo M, Maiello MR, et al. Target-based therapies in breast cancer: current status and future perspectives. Endocr Relat Cancer. 2009;16:675–702. doi: 10.1677/ERC-08-0208. [DOI] [PubMed] [Google Scholar]
- 63.Barr S, Thomson S, Buck E, Russo S, Petti F, Sujka-Kwok I, et al. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clin Exp Metastasis. 2008;25:685–93. doi: 10.1007/s10585-007-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial–mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–76. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hugo HJ, Wafai R, Blick T, Thompson EW, Newgreen DF. Staurosporine augments EGF-mediated EMT in PMC42-LA cells through actin depolymerisation, focal contact size reduction and Snail1 induction—a model for cross-modulation. BMC Cancer. 2009;9:235. doi: 10.1186/1471-2407-9-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhan L, Xiang B, Muthuswamy SK. Controlled activation of ErbB1/ErbB2 heterodimers promote invasion of three-dimensional organized epithelia in an ErbB1-dependent manner: implications for progression of ErbB2-overexpressing tumors. Cancer Res. 2006;66:5201–8. doi: 10.1158/0008-5472.CAN-05-4081. [DOI] [PubMed] [Google Scholar]
- 67.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–82. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muraoka RS, Koh Y, Roebuck LR, Sanders ME, Brantley-Sieders D, Gorska AE, et al. Increased malignancy of Neu-induced mammary tumors overexpressing active transforming growth factor beta1. Mol Cell Biol. 2003;23:8691–703. doi: 10.1128/MCB.23.23.8691-8703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seton-Rogers SE, Lu Y, Hines LM, Koundinya M, LaBaer J, Muthuswamy SK, et al. Cooperation of the ErbB2 receptor and transforming growth factor beta in induction of migration and invasion in mammary epithelial cells. Proc Natl Acad Sci USA. 2004;101:1257–62. doi: 10.1073/pnas.0308090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muthuswamy SK, Muller WJ. Direct and specific interaction of c-Src with Neu is involved in signaling by the epidermal growth factor receptor. Oncogene. 1995;11:271–9. [PubMed] [Google Scholar]
- 71.Dimri M, Naramura M, Duan L, Chen J, Ortega-Cava C, Chen G, et al. Modeling breast cancer-associated c-Src and EGFR overexpression in human MECs: c-Src and EGFR cooperatively promote aberrant three-dimensional acinar structure and invasive behavior. Cancer Res. 2007;67:4164–72. doi: 10.1158/0008-5472.CAN-06-2580. [DOI] [PubMed] [Google Scholar]
- 72.Falcioni R, Antonini A, Nistico P, Di Stefano S, Crescenzi M, Natali PG, et al. Alpha 6 beta 4 and alpha 6 beta 1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp Cell Res. 1997;236:76–85. doi: 10.1006/excr.1997.3695. [DOI] [PubMed] [Google Scholar]
- 73.Folgiero V, Bachelder RE, Bon G, Sacchi A, Falcioni R, Mercurio AM. The alpha6beta4 integrin can regulate ErbB-3 expression: implications for alpha6beta4 signaling and function. Cancer Res. 2007;67:1645–52. doi: 10.1158/0008-5472.CAN-06-2980. [DOI] [PubMed] [Google Scholar]
- 74.Strizzi L, Bianco C, Normanno N, Salomon D. Cripto-1: a multifunctional modulator during embryogenesis and oncogenesis. Oncogene. 2005;24:5731–41. doi: 10.1038/sj.onc.1208918. [DOI] [PubMed] [Google Scholar]
- 75.Ebert AD, Wechselberger C, Nees M, Clair T, Schaller G, Martinez-Lacaci I, et al. Cripto-1-induced increase in vimentin expression is associated with enhanced migration of human Caski cervical carcinoma cells. Exp Cell Res. 2000;257:223–9. doi: 10.1006/excr.2000.4881. [DOI] [PubMed] [Google Scholar]
- 76.Normanno N, De Luca A, Maiello MR, Bianco C, Mancino M, Strizzi L, et al. CRIPTO-1: a novel target for therapeutic intervention in human carcinoma. Int J Oncol. 2004;25:1013–20. [PubMed] [Google Scholar]
- 77.Strizzi L, Bianco C, Normanno N, Seno M, Wechselberger C, Wallace-Jones B, et al. Epithelial mesenchymal transition is a characteristic of hyperplasias and tumors in mammary gland from MMTV-Cripto-1 transgenic mice. J Cell Physiol. 2004;201:266–76. doi: 10.1002/jcp.20062. [DOI] [PubMed] [Google Scholar]
- 78.Zhong XY, Zhang LH, Jia SQ, Shi T, Niu ZJ, Du H, et al. Positive association of up-regulated Cripto-1 and down-regulated E-cadherin with tumour progression and poor prognosis in gastric cancer. Histopathology. 2008;52:560–8. doi: 10.1111/j.1365-2559.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 79.Zhang JG, Zhao J, Xin Y. Significance and relationship between Cripto-1 and p-STAT3 expression in gastric cancer and precancerous lesions. World J Gastroenterol. 2010;16:571–7. doi: 10.3748/wjg.v16.i5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gong YP, Yarrow PM, Carmalt HL, Kwun SY, Kennedy CW, Lin BPC, et al. Overexpression of Cripto and its prognostic significance in breast cancer: a study with long-term survival. Eur J Surg Oncol. 2007;33:438–43. doi: 10.1016/j.ejso.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 81.D'Antonio A, Losito S, Pignata S, Grassi M, Perrone F, De Luca A, et al. Transforming growth factor alpha, amphiregulin and cripto-1 are frequently expressed in advanced human ovarian carcinomas. Int J Oncol. 2002;21:941–8. [PubMed] [Google Scholar]
- 82.Miyoshi N, Ishii H, Mimori K, Sekimoto M, Doki Y, Mori M. TDGF1 is a novel predictive marker for metachronous metastasis of colorectal cancer. Int J Oncol. 2010;36:563–8. doi: 10.3892/ijo_00000530. [DOI] [PubMed] [Google Scholar]
- 83.Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, et al. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci USA. 2008;105:4329–34. doi: 10.1073/pnas.0800467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–32. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 85.Zhang C, Klymkowsky MW. Unexpected functional redundancy between Twist and Slug (Snail2) and their feedback regulation of NF-kappaB via Nodal and Cerberus. Dev Biol. 2009;331:340–9. doi: 10.1016/j.ydbio.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Normanno N, De Luca A, Maiello MR, Mancino M, D'Antonio A, Macaluso M, et al. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in breast cancer: current status and future development. Front Biosci. 2005;10:2611–7. doi: 10.2741/1725. [DOI] [PubMed] [Google Scholar]
- 87.Baselga J, Albanell J, Ruiz A, Lluch A, Gascon P, Guillem V, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol. 2005;23:5323–33. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 88.Arteaga CL, O'Neill A, Moulder SL, Pins M, Sparano JA, Sledge GW, et al. A phase I–II study of combined blockade of the ErbB receptor network with trastuzumab and gefitinib in patients with HER2 (ErbB2)-overexpressing metastatic breast cancer. Clin Cancer Res. 2008;14:6277–83. doi: 10.1158/1078-0432.CCR-08-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.D'Alessio A, De Luca A, Maiello MR, Lamura L, Rachiglio AM, Napolitano M, et al. Effects of the combined blockade of EGFR and ErbB-2 on signal transduction and regulation of cell cycle regulatory proteins in breast cancer cells. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0649-x. [DOI] [PubMed] [Google Scholar]
- 90.Buck E, Eyzaguirre A, Barr S, Thompson S, Sennello R, Young D, et al. Loss of homotypic cell adhesion by epithelial–mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol Cancer Ther. 2007;6:532–41. doi: 10.1158/1535-7163.MCT-06-0462. [DOI] [PubMed] [Google Scholar]
- 91.Konecny GE, Venkatesan N, Yang G, Dering J, Ginther C, Finn R, et al. Activity of lapatinib a novel HER2 and EGFR dual kinase inhibitor in human endometrial cancer cells. Br J Cancer. 2008;98:1076–84. doi: 10.1038/sj.bjc.6604278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McConkey DJ, Choi W, Marquis L, Martin F, Williams MB, Shah J, et al. Role of epithelial-to-mesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. Cancer Metastasis Rev. 2009;28:335–44. doi: 10.1007/s10555-009-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang D, LaFortune TA, Krishnamurthy S, Esteva FJ, Cristofanilli M, Liu P, et al. Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer. Clin Cancer Res. 2009;15:6639–48. doi: 10.1158/1078-0432.CCR-09-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]