Abstract
The purpose was to conduct a structured review and meta-analysis to determine the cumulative effect of bilateral arm training on motor capabilities post stroke. Forty-eight stroke studies were selected from three databases with 25 comparisons qualifying for inclusion in our meta-analysis. We identified and coded four types of bilateral arm interventions with 366 stroke patients. A random effects model using the standardized mean difference technique determined a large and significant effect size (0.734; SE = 0.125), high fail-safe N (532), and medium variability in the studies (I2 = 63%). Moderator variable analysis on the type of bilateral training revealed two large and significant effects: (a) BATRAC (0.842; SE = 0.155) and (b) coupled bilateral and EMG-triggered neuromuscular stimulation (1.142; SE = 0.176). These novel findings provide strong evidence supporting bilateral arm training with the caveat that two coupled protocols, rhythmic alternating movements and active stimulation, are most effective.
Keywords: meta-analysis, stroke rehabilitation, bilateral movement training, motor recovery
In a 1977 Psychological Bulletin article, D. J. Glencross advocated that researchers consider integrating central and peripheral processes in the control of skilled movements (Glencross, 1977). Through the years, the central versus peripheral debate subsided; however, integrating input from both sources continues. Moreover, the exact nature of control in skilled movements still drives many research agendas. In fact, many stroke motor recovery interventions integrate input from central and peripheral sources. Re-acquiring upper extremity movements necessary for activities of daily living such as buttoning a shirt or blouse, zipping a jacket, pouring a drink, and buttering bread or toast are essential for making progress toward motor recovery.
To perform any of these four everyday tasks requires coordinating movements on two arms and hands. Thus, a leading question for stroke patients concerns bilateral arm practice: Would bilateral arm training help alleviate some motor dysfunctions and improve motor capabilities? We know that bilateral movements takes advantage of the inherent dependencies between arms; spatial and temporal dependencies (Carson, 2005; Cincotta & Ziemann, 2008; Hallett, 2001a; Hummel, et al., 2005; Lacroix, et al., 2004; Rossini, Calautti, Pauri, & Baron2, 2003). Further, symmetrical bilateral movements are known to activate similar neural distributed networks in both hemispheres. Specific activated areas include the supplementary motor area, sensorimotor cortex, cingulate motor cortex, lateral premotor cortex, superior parietal cortex, and cerebellum (Debaere, Wenderoth, Sunaert, Van Hecke, & Swinnen, 2004; Goldberg, 1985; Jancke, et al., 2000; Nachev, Kennard, & Husain, 2008; Swinnen & Wenderoth, 2004).
In spite of the inherent neural interaction patterns in the two hemispheres when both arms simultaneously move in homologous actions, consistent effective bilateral movement training findings are lacking. A comprehensive review on stroke and bilateral arm training identified contradictory findings (Carson & Swinnen, 2002; Cauraugh & Summers, 2005). Moreover, recent individual stroke rehabilitation and bilateral arm treatment studies found support (Cauraugh, Coombes, Lodha, Naik, & Summers, 2009; Cauraugh, Kim, & Summers, 2008) and failed to find support on the efficacy of bilateral training (Tijs & Matyas, 2006). Further complicating the issue is an initial meta-analysis on stroke rehabilitation and bilateral movements that reported a relatively large effect size (Stewart, Cauraugh, & Summers, 2006). However, perhaps spurious findings were found given the minimal number of studies analyzed (11), and the failure to report (a) a forest plot of the effects, (b) a funnel plot involved in publication bias, or (c) the heterogeneity of individual effect sizes (I2). These conflicting findings warrant a structured review and meta-analysis that includes new statistical techniques to determine the comprehensive effect of motor capabilities as a function of bilateral movement training. Thus, we will attempt to answer an enduring stroke rehabilitation question concerning progress toward recovery: Do bilateral movement training protocols improve motor capabilities in the upper extremities of stroke survivors?
This structured review and meta-analysis focused on studies that investigated contributions of bilateral arm training toward improving upper extremity movements post intervention. Granted, a few studies reported direct comparisons between bilateral and unilateral training, although a majority of the experiments were interested in establishing the efficacy of specific bilateral arm movement protocols versus control groups (i.e., with or without standard care). Thus, our intention was to determine the cumulative effect of bilateral arm movement training regardless of the comparison groups. Even though a considerable amount of evidence comes from unilateral training studies that followed constraint-induced movement therapy (CIMT) guidelines (e.g., EXCITE trial;(Wolf, et al., 2006; Wolf, et al., 2008), we were not concerned with directly comparing forced-use and bilateral arm training.
Methods
Study Selection and Inclusion/Exclusion Criteria
An exhaustive search of the literature was conducted using three databases: (a) ISI web of Knowledge, (b) PubMed Central, and (c) Cochrane Collaboration of systematic reviews. Ten primary key words/phrases guided our search: stroke, bilateral arm training, hemiplegia, hemiparesis, motor recovery/control/function, upper-extremity/limb, neurorehabilitation, bimanual coordination, coupling, and recovery protocols. References from selected studies were carefully inspected to identify studies that were not retrieved in one of our database searches. The systematic searches of the databases were conducted by two authors (NL & SN), and they identified 48 potential research studies (Cauraugh, Coombes, Lodha, Naik, & Summers, 2009; Cauraugh & Kim, 2002; Cauraugh & Kim, 2003a; Cauraugh & Kim, 2003b; Cauraugh & Kim, 2003c; Cauraugh, Kim, & Duley, 2005; Cauraugh, Kim, & Summers, 2008; Chan, Tong, & Chung, 2008; Chang, Tung, Wu, Huang, & Su, 2007; Chang, Tung, Wu, & Su, 2006; Coupar, Van Wijck, Morris, Pollock, & Langhorne, 2007; Cunningham, Stoykov, & Walter, 2002; Desrosiers, Bourbonnais, Corriveau, Gosselin, & Bravo, 2005; Garry, van Steenis, & Summers, 2005; Han, Arbib, & Schweighofer, 2008; Harris-Love, McCombe Waller, & Whitall, 2005; Hesse, Schmidt, & Werner, 2006; Hesse, Schmidt, Werner, & Bardeleben, 2003; Hesse, et al., 2007; Hesse, Schulte-Tigges, Konrad, Bardeleben, & Werner, 2003; Hesse, et al., 2005; Higgins, et al., 2006; Lewis & Byblow, 2004; Lewis & Perreault, 2007; Lin, Chang, Wu, & Chen, 2008; Luft, et al., 2004; Lum, et al., 2006; McCombe Waller, Harris-Love, Liu, & Whitall, 2006; McCombe Waller, Liu, & Whitall, 2008; McCombe Waller & Whitall, 2004; McCombe Waller & Whitall, 2005; McCombe Waller & Whitall, 2008; Morris, et al., 2008; Mudie & Matyas, 1996; Mudie & Matyas, 2000; Mudie & Matyas, 2001; Page, Levine, Teepen, & Hartman, 2008; Platz, Bock, & Prass, 2001; Rice & Newell, 2004; Richards, Senesac, Davis, Woodbury, & Nadeau, 2008; Rose & Winstein, 2005; Stewart, Cauraugh, & Summers, 2006; Stinear, Barber, Coxon, Fleming, & Byblow, 2008; Stinear & Byblow, 2004; Summers, et al., 2007; Tijs & Matyas, 2006; Ustinova, Fung, & Levin, 2006; Whitall, McCombe Waller, Silver, & Macko, 2000).
Keeping in mind that our overall research question concerned post stroke progress toward motor recovery as a function of bilateral arm training, specific inclusion/exclusion criteria for our meta-analysis were observed. In a broad inclusion approach, we cast a wide net across all three stages of stroke recovery for intervention studies that used bilateral arm movements as a training treatment. Typical time frames post stroke define the three stages of recovery: (a) acute, 0 – 1 month post stroke; (b) sub-acute, 1 – 6 months post stroke; and (c) chronic, greater than 6 months post stroke. Twenty-five studies (see Table 1) satisfied inclusion/exclusion criteria as unanimously determined by all authors. Two authors (NL & SN) independently extracted data and coded all studies for meta-analytic techniques. Our coding system recorded: (a) design with groups/subcategories, (b) sample, (c) outcome measures, (d) type of bilateral arm intervention, (e) experimental design, and (f) quality of research. A third author (JC) confirmed the data extraction, and all authors were involved in interpreting the meta-analytic results.
Table 1.
Characteristics of each comparison included in the present meta-analysis (listed alphabetically).
| Study | Total N |
Mean Age: Years |
Mean Time Post Stroke: Months |
Side of Stroke: Hemisphere |
Phase of Stroke |
Pre-treatment Impairment Level |
|---|---|---|---|---|---|---|
| Cauraugh, et al., 2002 | 25 | 63.70 | 39.1 | 13 – Left 12 – Right |
Chronic | Box & Block Test – 18 |
| Cauraugh, et al., 2003 (a) | 26 | 66.40 | 33.6 | 11 – Left 15 – Right |
Chronic | Box & Block Test – 0 s Stimulation – 15.1 5 s Stimulation – 15.1 10 s Stimulation – 22.9 |
| Cauraugh, et al., 2003 (b) | 20 | 63.03 | 33.9 | 11 – Left 9 – Right | Chronic | Upper cutoff limit of 80% motor recovery as assessed by rectified EMG activation patterns |
| Cauraugh, et al., 2005 | 26 | 66.33 | 49.8 | 6 – Left 15 – Right |
21 – Chronic 5 – Healthy Control |
Upper cutoff limit of 80% motor recovery as assessed by rectified EMG activation patterns |
| Cauraugh, et al., 2008 | 16 | 65.82 | 33.7 | 8 – Left 8 – Right |
Chronic | Box & Block Test – 12.5 |
| Cauraugh, et al., 2009 | 30 | 67.23 | 57.8 | 12 – Left 17 – Right |
Chronic | Box & Block Test – Bilateral: Load – 26 Bilateral: No Load – 24.7 Control – 20.0 |
| Chan, et al., 2009 |
20 | 45.50 | 15.1 | 11 – Left 9 – Right |
Chronic | Upper Extremity FMA Score – Treatment Group:18.2 Control Group: 20.0 |
| Chang, et al., 2007 | 20 | 57.10 | 35.4 | 9 – Left 11 – Right |
Chronic | Upper Extremity FMA Score – 32.7 (5 to 55) |
| Desrosiers, et al., 2005 | 41 | 73.25 | 1.1 | 18 – Left 23 – Right |
Sub-acute | Upper Extremity FMA Score – 42.9 |
| Hesse, et al., 2003 | 12 | 63.60 | 9.3 | 7 – Left 5 – Right |
Chronic | Median Modified Ashworth Scale: Wrist – 3 (2-3) & Finger – 3 (3-4) |
| Hesse, et al., 2005 | 44 | 64.70 | 1.4 | 19 – Left 25 – Right |
Sub-acute | Upper Extremity FMA Score – Computerized Arm Training – 7.9 & Electrical Stimulation – 7.3 |
| Higgins, et al., 2006 | 91 | 72.00 | 7.5 | 51 – Left; 39 – Right 1 – Bilateral |
Chronic | Box & Block Test – 26 |
| Lin, et al., 2009 |
60 | 52.14 | 20.6 | 29 – Left 31 – Right |
Chronic | Upper Extremity FMA Score – |
| Distributed Constraint Induced Therapy – 46.05 Bilateral Arm Training – 45.50 Control – 49.75 |
||||||
| Luft, et al., 2004 | 26 | 61.45 | 75.0: M- BATRAC 45.5: M- DMTE |
7 – Left 19 – Right |
Chronic | Upper Extremity FMA Score – BATRAC: 29.6 DMTE: 28.3 |
| Lum, et al., 2006 | 30 | 65.95 | 2.5 | 13 – Left 17 – Right |
Sub-acute | Upper Extremity FMA Score – Robot-Combined: 21.7 Robot-Unilateral: 31.6 Robot-Bilateral: 39.2 Control: 26 |
| Morris, et al., 2008 | 106 | 67.85 | 0.8 | 52 – Left 54 – Right |
Acute | Action Research Arm Test – Bilateral Training: 13.4 Unilateral Training: 18.5 |
| Platz, et al., 2001 | 14 | 55.90 | -- | 7 – Left 7 – Right |
14 – Sub-acute 14 – Healthy Control |
Almost complete or complete recovery from hemiparesis |
| Richards, et al., 2008 | 15 | 64.40 | 65.5 | 8 – Left 6 – Right |
Chronic | Upper Extremity FMA Score – 42.43 (18-64) |
| Stinear, et al, 2004 | 9 | 62.11 | 16.2 | 6 – Left 3 – Right |
3 – Acute 6 – Chronic |
Upper Extremity FMA Score (%) – Acute: 10% - 71% Chronic: 10% - 80% |
| Summers, et al., 2007 | 12 | 61.65 | 61.8 | 3 – Left 8 – Right 1 – Bilateral |
Chronic | Modified Motor Assessment Scale – Unilateral Training: 3.99 Bilateral Training: 3.78 |
| Waller, et al., 2004 |
10 | -- | 30.0 (Median) |
– | Chronic | Upper Extremity FMA Score – 31.25 |
| Waller et al., 2005 |
11 | 58.60 | 78.0 | 11 – Left | Chronic (Left CVA Stroke) |
Upper Extremity FMA Score – 29.5 |
| Waller et al., 2005 |
11 | 64.30 | 98.0 | 11 – Right | Chronic (Right CVA Stroke) |
Upper Extremity FMA Score – 25.0 |
| Waller, et al., 2008 |
18 | 56.01 | 52.5 | 10 – Left 8 – Right |
Chronic | Upper Extremity FMA Score – BATRAC: 35.22 & DMTE: 34.00 |
| Whitall, et al., 2000 | 16 | 63.78 | 78.6 | 7 – Left 7 – Right |
Chronic | Upper Extremity FMA Score – 15.0 |
Twenty-four studies were excluded for one of the following four reasons: (a) case study (Hesse, et al., 2007), (b) review or meta-analysis articles (Coupar, Van Wijck, Morris, Pollock, & Langhorne, 2007; Hesse, Schmidt, & Werner, 2006; Hesse, Schmidt, Werner, & Bardeleben, 2003; McCombe Waller & Whitall, 2008; Stewart, Cauraugh, & Summers, 2006), (c) data extraction problems (Lewis & Byblow, 2004; Mudie & Matyas, 1996; Mudie & Matyas, 2000; Tijs & Matyas, 2006), and (d) missing treatment condition or lack of relevant data (Cauraugh & Kim, 2003c; Chang, Tung, Wu, & Su, 2006; Cunningham, Stoykov, & Walter, 2002; Garry, van Steenis, & Summers, 2005; Han, Arbib, & Schweighofer, 2008; Harris-Love, McCombe Waller, & Whitall, 2005; Lewis & Perreault, 2007; McCombe Waller, Harris-Love, Liu, & Whitall, 2006; Mudie & Matyas, 2001; Page, Levine, Teepen, & Hartman, 2008; Rice & Newell, 2004; Rose & Winstein, 2005; Stinear, Barber, Coxon, Fleming, & Byblow, 2008; Ustinova, Fung, & Levin, 2006).
Further, one study compared treatment and control group effects in two distinct sets of stroke patients: left versus right hemisphere location of the cerebrovascular accident (McCombe Waller & Whitall, 2005). Consequently, we considered each separate comparison as an individual study, and coded the treatment group (bilateral training) and control group (no bilateral training) comparisons in our comprehensive meta-analysis. Thus, our number of coded comparisons equaled 25. Further, control groups (no bilateral arm practice) followed diverse rehabilitation protocols including unilateral training, neuro-developmental therapy, functional movements, dose-matched therapeutic exercises, and placebo electrical stimulation.
Establishing Outcome Measures
Given that a majority of the studies reported more than one motor outcome measure, we extensively discussed establishing continuity across studies by selecting reliable and valid stroke motor outcome data. Consistent with meta-analytic recommendations, we selected only one standard stroke motor outcome measure per study to avoid biasing our findings (Rosenthal, 1995). The standard stroke assessments included: (a) upper extremity section of Fugl-Meyer Assessment, (b) Box and Block manual dexterity test, (c) Modified Motor Assessment Scale, (d) Action Research Arm Test, (e) Modified Ashworth Scale, and (f) Functional Independence Measure. In addition, if data from one of the standard assessment techniques were unavailable, then we coded kinematic and electromyography (EMG) measures such as reaction time, movement time, movement unit, and muscle activation times. Consistent with conventional meta-analytic techniques, analyzing studies with different purposes and treatment goals in synthesizing a set of literature is acceptable under certain conditions (Borenstein, Hedges, Higgins, & Rothstein, 2009; Higgins & Green, 2006). The conditions involve similarities in subjects tested, outcome measures reported, operational definitions, experimental design, and conducting technical tests beyond the overall effect size.
Data Synthesis and Analysis
Rosenthal recommended two essential components for conducting meta-analyses: data synthesis and data analysis (Rosenthal, 1995). Data synthesis involves calculating an individual effect size for each study included in the meta-analysis, and data analysis involves computing an overall effect size of all studies collectively by calculating a standardized mean difference. Standardized mean difference is a robust method of determining effect sizes by computing the difference between means of two groups (e.g., treatment and control) normalized by dividing by a pooled standard deviation. All meta-analysis techniques were conducted using the Comprehensive Meta-Analysis (Biostat. Inc. Version 2) software program.
Heterogeneity Test
An additional meta-analytic technique included computing I2 to determine heterogeneity of the 25 individual effect sizes in our bilateral movement training data. This technique measures heterogeneity as evidenced across the outcome measures of the entire group of studies beyond statistical chance alone. I2 calculates a percentage such that lower values represent smaller variability in outcome measures of selected studies. Representative values define three heterogeneity levels: low = 25%; moderate = 50%; high = 75%.
Publication Bias and Fail-safe N Analysis
Publication bias arises when the probability of publication of a study rises as the effect size of its findings increases. Funnel plots are used to determine the symmetry of publication bias while graphing the effect size of individual studies against the standard error associated with the study. Ideally, plotting the studies shows symmetry across studies of different size and precision with smaller and larger studies scattered uniformly at the base and apex.
Consistent with recommendations to determine stability in the meta-analytic results, we computed the classic fail-safe N analysis (Rosenthal & DiMatteo, 2001). This technique calculates the number of studies with non-significant effects required to nullify the overall effect determined in the current analysis. Larger fail-safe N values increase confidence in the overall effect and validate the stability of the current findings.
Quality Assessment
In the addition to the quantitative analysis of studies, we carried out a quality assessment of the studies. Recommendations on quality assessment include three criteria: (a) treatment randomization, (b) blinding (i.e., treatment group assignment not known by evaluators), and (c) number of drop outs or withdrawals (Higgins & Green, 2006). Table 3 displays the quality assessment values for the studies in our bilateral arm training meta-analysis.
Table 3.
Quality assessments for each comparison included in the meta-analysis.
| Study | Random Assignment |
Single Blind |
Drop Outs |
|---|---|---|---|
| Cauraugh, et al., 2002 | 1 | 1 | 0 |
| Cauraugh, et al., 2003 (a) | 1 | 1 | 0 |
| Cauraugh, et al., 2003 (b) | 1 | 1 | 0 |
| Cauraugh, et al., 2005 | 1 | 1 | 0 |
| Cauraugh, et al., 2008 | 1 | 1 | 0 |
| Cauraugh, et al., 2009 | 1 | 0 | 1 |
| Chan, et al., 2009 | 1 | 0 | 0 |
| Chang, et al., 2007 | 0 | 0 | 0 |
| Desrosiers, et al., 2005 | 1 | 1 | 8 |
| Hesse, et al., 2003 | 0 | 0 | 0 |
| Hesse, et al., 2005 | 1 | 1 | 1 |
| Higgins, et al., 2006 | 1 | 1 | 7 |
| Lin, et al., 2009 | 1 | 1 | 0 |
| Luft, et al., 2004 | 1 | 0 | 5 |
| Lum, et al., 2006 | 1 | 1 | 1 |
| Morris, et al., 2008 | 1 | 1 | 9 |
| Platz, et al., 2001 | 1 | 0 | 0 |
| Richards, et al., 2008 | 0 | 0 | 1 |
| Stinear, et al, 2004 | 0 | 1 | 0 |
| Summers, et al., 2007 | 1 | 0 | 0 |
| Waller, et al., 2004 | 0 | 0 | 1 |
| Waller et al., 2005: Left CVA | 0 | 1 | 0 |
| Waller et al., 2005: Right CVA | 0 | 1 | 0 |
| Waller, et al., 2008 | 1 | 1 | 0 |
| Whitall, et al., 2000 | 0 | 0 | 2 |
Column 2 and 3: 0- False; 1-True
Column 4: number of participants who dropped out or were excluded
Results
Standardized Mean Difference Effect
Twenty-five bilateral arm movement training studies analyzed in a random effects model revealed a large significant cumulative effect = 0.734 (SE = 0.125; p < .0001) with a 95% confidence interval limits of 0.489 to 0.979. Individual weighted effect sizes ranged from –2.148 to 1.851. A negative effect size indicates reduced performance after bilateral training while a positive value is indicative of improvements in motor performance post bilateral intervention. Individual weighted effect sizes are shown in Table 4.
Table 4.
Summary statistics for the 25 comparisons in the meta-analysis.
| Study | Primary Outcome Measure | Participants per Group: Rx / Control |
Standardized Mean Difference |
Confidence Interval (95%) |
|
|---|---|---|---|---|---|
| Cauraugh, et al., 2002 | Box & Block Test (Bilateral + NMS Rx – Control: Unilateral Rx) |
10 / 5 | 1.250 | 0.087 | 2.413 |
| Cauraugh, et al., 2003 (a) | Box & Block Test (Coupled Bilateral + NMS Rx – Control: Bilateral Rx) |
10 / 6 | 1.490 | 0.354 | 2.626 |
| Cauraugh, et al., 2003 (b) | EMG Activation (Bilateral Rx – Unilateral Rx) |
10 / 10 | 1.054 | 0.118 | 1.989 |
| Cauraugh, et al., 2005 | Movement Time (Bilateral Rx – Unilateral Rx) |
11 / 10 | 1.250 | 0.314 | 2.186 |
| Cauraugh, et al., 2008 | Box & Block Test (Pre Rx – Post Rx) |
16 | 1.310 | 0.642 | 1.978 |
| Cauraugh, et al., 2009 | Median Motor Reaction Time (Bilateral No Load Rx – Control: Unilateral Rx) |
10 / 9 | 0.590 | −0.330 | 1.510 |
| Chan, et al., 2009 | Fugl-Meyer Assessment Score (Bilateral + FES Rx – Control: Bilateral Placebo ES) |
10 / 10 | 1.058 | 0.122 | 1.993 |
| Chang, et al., 2007 | Fugl-Meyer Assessment Score (Pre Rx – Post Rx) |
20 | 0.868 | 0.354 | 1.383 |
| Desrosiers, et al., 2005 | Fugl-Meyer Assessment Score (Bilateral Rx – Control: Functional Movements + NDT) |
17 / 16 | −0.316 | −1.003 | 0.371 |
| Hesse, et al., 2003 | Modified Ashworth Scale: Wrist (Pre Rx – Post Rx) |
12 | 0.914 | 0.240 | 1.587 |
| Hesse, et al., 2005 | Fugl-Meyer Assessment Score (Bilateral Rx – ES Control Rx) |
21 / 22 | 0.766 | 0.146 | 1.385 |
| Higgins, et al., 2006 | Box & Block Test (Arm Bilateral Rx – Walking Control) |
47 / 44 | 0.200 | −0.212 | 0.612 |
| Lin, et al., 2009 | Proximal Fugl-Meyer Assessment Score (Bilateral Rx – Control: Functional Movements) |
20 / 20 | 0.885 | 0.236 | 1.535 |
| Luft, et al., 2004 | Fugl-Meyer Assessment Score (BATRAC Rx – DMTE Rx) |
6 / 12 | 1.292 | 0.225 | 2.359 |
| Lum, et al., 2006 | Functional Independence Measure (Robotic Bilateral Rx – Control: NDT) |
5 / 6 | −2.148 | −3.635 | −0.660 |
| Morris, et al., 2008 | Action Research Arm Test (Bilateral Rx – Control: Unilateral Rx) |
51 / 46 | −0.085 | −0.484 | 0.314 |
| Platz, et al., 2001 | Movement Time – Aiming Task (Bilateral Rx – Unilateral Rx) |
7 / 7 | −0.069 | −1.117 | 0.979 |
| Richards, et al., 2008 | Fugl-Meyer Assessment Score (Pre Rx – Post Rx) |
14 | 0.443 | −0.106 | 0.992 |
| Stinear, et al, 2004 | Fugl-Meyer Assessment Score (Pre Rx – Post Rx) |
9 | 0.965 | 0.174 | 1.757 |
| Summers, et al., 2007 | Modified Motor Assessment Scale (Bilateral Rx – Control: Unilateral Rx) |
6 / 6 | 1.851 | 0.498 | 3.203 |
| Waller, et al., 2004 |
Fugl-Meyer Assessment Score (Pre Rx – Post Rx) |
9 | 1.680 | 0.666 | 2.695 |
| Waller et al., 2005: Left CVA |
Fugl-Meyer Assessment Score (Pre Rx - Post Rx) |
11 | 0.645 | −0.004 | 1.295 |
| Waller et al., 2005: Right CVA |
Fugl-Meyer Assessment Score (Pre Rx - Post Rx) |
11 | 0.642 | −0.007 | 1.291 |
| Waller, et al., 2008 |
Paretic Arm Movement Unit (BATRAC Rx – DMTE Rx) |
9 / 9 | 1.377 | 0.349 | 2.404 |
| Whitall, et al., 2000 | Fugl-Meyer Assessment Score (Pre Rx – Post Rx) |
14 | 0.932 | 0.305 | 1.560 |
NMS : Neuromuscular stimulation
EMG: Electromyography
ES: Electrical stimulation
NDT: Neuro-developmental Therapy
BATRAC: Bilateral arm training with rhythmic auditory cueing
DMTE: Dose-matched therapeutic exercises
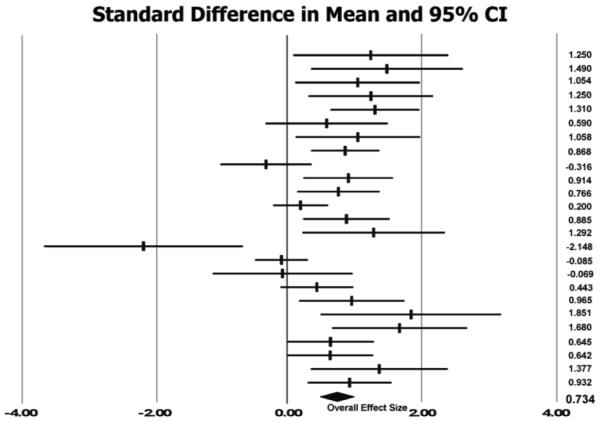
An additional meta-analytic technique is visually displaying the amount of variation between the results of the studies and the cumulative effect size for all studies on a graph. This type of graph is called a forest plot. Figure 1 shows the forest plot of the current analysis. Each horizontal line represents one study with the effect size a tick mark in the center of the line, and the 95% confidence interval at the distal of ends of each line. The numbers in the column on the far right are the individual effect sizes for each of the 25 studies in the meta-analysis. At the bottom of the plot, the black diamond shape represents the cumulative effect sizes and confidence intervals of the analyzed studies. Statistical significance is displayed by examining each line and the overall diamond in relation to the 0.00 vertical line on the x-axis. Any study that does not intercept the vertical line is either a positive or negative effect. The current forest plot is robust with 16 studies demonstrating positive effects, 1 negative effect study, and 8 with no effect. Thus, the effect sizes clearly show that post stroke rehabilitation involving bilateral arm training leads to improved motor capabilities and progress toward recovery.
Figure 1.
Forest plot: random effects model. The column farthest right is the individual effect size for each comparison in alphabetical order (same order as Table 4). Tick marks represent effect sizes, and lengths of the horizontal lines are the 95% confidence intervals. Lines that do not intercept the 0.0 vertical line make a contribution to the cumulative effect. The black diamond at the bottom of the forest plot is the overall effect size.
Heterogeneity Test
Evaluation of the dispersion of effect sizes across the 25 post stroke bilateral arm training comparisons revealed an I2 = 62.81%. This indicated a relatively moderate level of heterogeneity and warranted the application of the random effects model for computing overall effect size in the current meta-analysis. This moderate consistency indicates that the cumulative effect (0.734) is robust across the domain of studies and outcome measures in our meta-analysis (Borenstein, Hedges, Higgins, & Rothstein, 2009).
Publication Bias and Fail-safe N Analysis
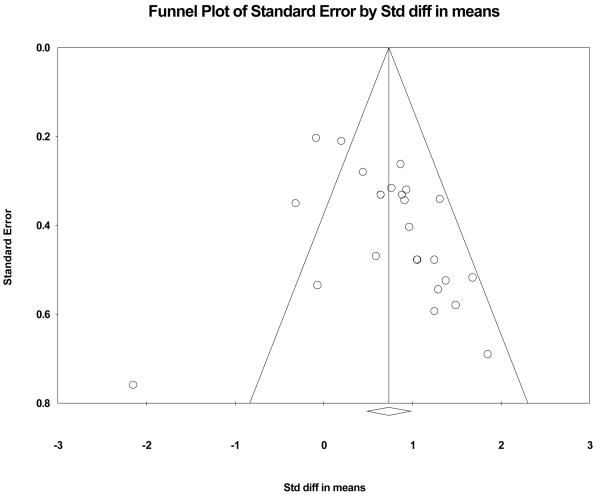
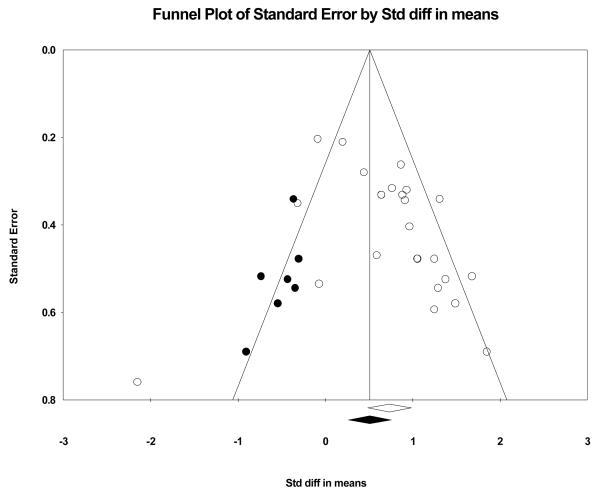
Figure 2 displays the funnel plot examining publication bias by plotting treatment effect size as a function of standard error. Funnel plots evaluating bias in studies are constants in meta-analysis techniques (Higgins & Green, 2006; Borenstein et al., 2009). Each individual study is represented by a circle for the values of an effect size (x-axis) and standard error (y-axis). Because a majority of the circles of the 25 studies are on the right side of the funnel plot (right of the overall effect size vertical line: diamond on x-axis), then a potential bias effect is apparent. The closer to symmetry the circles are in a funnel plot the less concern with publication bias (i.e., only studies that find positive effects are published). The second funnel plot (see Figure 3) balances the studies toward symmetry by adding seven black circles to the left side (negative effects) of the vertical line (cumulative effect) that splits the funnel into two sides. The imputed circles are close approximations to symmetry as quantified by Duval and Tweedie's trim and fill procedure (Borenstein, Hedges, Higgins, & Rothstein, 2009). The black diamond on the x-axis is the recalculated overall effect. Technical experts agree that a perfectly symmetrical funnel plot represents a best estimate of an unbiased overall effect size (Borenstein, Hedges, Higgins, & Rothstein, 2009). Given the characteristics of the two present funnel plots, we are confident in stating that our large cumulative effect (0.734) is an unbiased estimate of stroke motor recovery progress as a function of bilateral arm training.
Figure 2.
Funnel plot evaluating publication bias. Each individual study is represented by a circle: an effect size (x-axis) and standard error (y-axis). The greater the symmetry, the less is the concern with publication bias. Given that a majority of the 25 circles are on the right side of the funnel plot (right of the overall effect size vertical line: diamond on x-axis), then a potential bias effect is apparent.
Figure 3.
Best estimate funnel plot of an unbiased effect (i.e., symmetrical funnel): open circles and diamond represent original 25 comparisons whereas black circles and diamond represent imputed comparisons. This funnel plot balances the studies toward symmetry by adding seven black circles to the left side (negative effects) of the vertical line (cumulative effect).
Rosenthal's classic fail-safe N analysis determined the number of non-significant studies necessary to negate the overall effect. Our analysis found a high fail-safe, N = 532. Thus, our meta-analysis found a robust cumulative effect. Moreover, a large number of null studies (i.e., no significant motor improvements found as a function of bilateral arm training) are required to reduce the effect of bilateral arm training interventions on motor recovery of stroke survivors.
Quality Assessment
As shown in Table 3, the 25 bilateral arm training comparisons displayed a relatively medium quality. Seventeen comparisons reported random assignment to groups whereas only eight failed to describe any randomization procedures. Fifteen studies conducted single blind experiments. Further, the low attrition rate for participants is viewed favorably.
Moderator Variable Analysis
Given that the current meta-analysis findings are based on a broad perspective of bilateral arm training techniques and supplements used during rehabilitation, we conducted a moderator variable analysis. As shown in Table 2, there are four general types of bilateral arm training techniques in the 25 comparisons: (a) pure bilateral – 6, (b) bilateral arm training with rhythmic auditory cueing (BATRAC) – 7, (c) coupled bilateral and EMG-triggered neuromuscular stimulation – 7, and (d) active and/or passive movements, including robotics – 5. These additional analyses elaborate on the contributions of each technique to our cumulative effect. Analyzing the 25 comparisons for a potential moderator variable indicated a significant overall mixed effects model equal to 0.80 (SE = 0.099, p < 0.0001; lower limit = 0.606; upper limit = 0.93). Separately, calculating the contribution of each type of bilateral arm training as moderator in a mixed effects analysis revealed two significant techniques with large effect sizes: (a) BATRAC (0.842, SE = 0.155, p < 0.0001; lower limit = 0.539; upper limit = 1.146) and (b) coupled bilateral and EMG-triggered neuromuscular stimulation (1.142, SE = 0.176, p < 0.0001; lower limit = 0.796; upper limit = 1.488). However, analysis of the active and/or passive movement training studies only indicated a relatively weak trend (0.535, SE = 0.326, p = 0.101; lower limit = −0.104; upper limit = 1.175). Finally, the pure bilateral training studies revealed a small effect (0.268, SE = 0.227) that did not reach significance (p > 0.10; lower limit = −0.178; upper limit = 0.713).
Table 2.
Bilateral arm training characteristics (duration, frequency, and intensity), sessions, total hours, and treatment groups.
| Study [Type of Training] |
Bilateral Arm Training Type, and Duration: One Session |
Treatment Sessions |
Treatment Duration: Total Hours |
Other Groups |
|---|---|---|---|---|
|
Cauraugh, et al., 2002 [Coupled bilateral and EMG-triggered stimulation] |
Bilateral wrist & finger extension with EMG-triggered neuromuscular electrical stimulation: 50 Hz with 200 μs pulse width for 5 s of stimulation to extensor communis digitorum & extensor carpi ulnaris; 1 s ramp up & 1 s ramp down; 16 mA to 29 mA intensity |
30 Trials (30 min)/Session for 3 Sessions/Day for 2 Days/Week for 2 Weeks (12 Sessions) |
6 (360 trials) | Unilateral coupled with EMG-triggered neuromuscular stimulation group & Control with no movement assistance |
|
Cauraugh, et al., 2003 (a) [Coupled bilateral and EMG-triggered stimulation] |
Bilateral wrist & finger extension with EMG-triggered neuromuscular electrical stimulation: 50 Hz with 200 μs pulse width 5 s or 10 s of stimulation to extensor communis digitorum & extensor carpi ulnaris; 1 s ramp up & 1 s ramp down; 17 mA to 28 mA intensity |
30 Trials (30 min)/Session (12 Sessions over 4 Days) |
6 (360 trials) | Bilateral Arm Training with 5 s stimulation group & Control with no movement assistance |
|
Cauraugh, et al., 2003 (b) [Coupled bilateral and EMG-triggered stimulation] |
Bilateral wrist & finger extension with EMG-triggered neuromuscular electrical stimulation: 50 Hz with 200 μs pulse width 5 s of stimulation to extensor communis digitorum & extensor carpi ulnaris; 1 s ramp up & 1 s ramp down; 19 mA to 29 mA intensity; walked around the laboratory after 30 trials |
30 Trials (30 min)/Session (12 Sessions over 4 Days) |
6 (360 trials) | Unilateral coupled with EMG-triggered neuromuscular stimulation group |
|
Cauraugh, et al., 2005 [Coupled bilateral and EMG-triggered stimulation] |
Bilateral wrist & finger extension with EMG-triggered neuromuscular electrical stimulation: 50 Hz with 200 μs pulse width for 5 s of stimulation to extensor communis digitorum & extensor carpi ulnaris; 1 s ramp up & 1 s ramp down; 15 mA to 29 mA intensity |
30 Trials (30 min)/Session (12 Sessions over 4 Days) |
6 (360 trials) | Unilateral coupled with EMG-triggered neuromuscular stimulation group & Healthy Control |
|
Cauraugh, et al., 2008 [Coupled bilateral and EMG-triggered stimulation] |
Bilateral arm training in 2 treatment order: unilateral impaired only & bilateral; wrist/finger extension, elbow extension & shoulder flexion/abduction movements; 5 or 10 s EMG-triggered neuromuscular electrical stimulation & 10 or 25 s rest time between trials; blocked or random practice schedule |
90 min/Session for 4 Days for 5 Protocols |
60 | No other group: pre – post design |
|
Cauraugh, et al., 2009 [Coupled bilateral and EMG-triggered stimulation] |
Bilateral wrist & finger extension with EMG-triggered neuromuscular electrical stimulation: 50 Hz with 200 μs pulse width for 5 s of stimulation to extensor communis digitorum & extensor carpi ulnaris; 1 s ramp up & 1 s ramp down; 15 mA to 29 mA intensity |
30 Trials (30 min)/Session for 3 Sessions/Day for 2 Days/Week for 2 Weeks (12 Sessions) |
6 (360 trials) | Bilateral Arm Training with or without load on unimpaired hand & Control with no movement assistance |
| Chan, et al., 2009 [EMG-triggered stimulation] |
Bilateral arm training with FES to affected hand to facilitate hand opening; 40 Hz & 200 μs pulse width for 8 s (3 s ramp up, 3 s stimulation, 2 s ramp down) |
40 trials (20 min)/Session for 15 Sessions |
5 | Control with no FES assistance |
|
Chang, et al., 2007 [Active and/or Passive] |
Bilateral symmetric arm push and pull movements for 10 s with bilateral force- induced isokinetic arm movement trainer (BFIAMT) at 0.1 Hz cycling frequency; push-pull movement distance ranged from 35 to 45 cm (10 mins conventional rehabilitation + 30 mins BFIAMT) |
40 min /Session, 3 Sessions/Week for 8 Weeks (24 Sessions) |
16 | No other group: pre – post design |
|
Desrosiers, et al., 2005 [Pure] |
Symmetrical bilateral training: symmetrical & asymmetrical bilateral, unilateral for the affected side & unilateral for the less affected side |
45 min/Session for 15 – 20 Sessions for 5 Weeks |
11.25 – 15 | Control Group |
|
Hesse, et al., 2003 [Active and/or Passive] |
Bilateral forearm pronation-supination & wrist flexion-extension movements with 1 degree of freedom arm trainer |
15 min/Day for 3 Weeks (15 Sessions) |
3.75 | No other group: pre – post design |
|
Hesse, et al., 2005 [Active and/or Passive] |
200 cycles of each bilateral forearm pronation-supination & wrist flexion- extension movements with Bi-Manu- Track robotic arm trainer (AT) in 3 modes (passive-passive; active-passive; active-active) |
20 min/Day for 6 Weeks (30 Sessions) |
10 | Electrical Stimulation Control Group |
|
Higgins, et al., 2006 [Pure bilateral] |
Functional bilateral and unilateral tasks: manipulating playing cards, clothes pins, writing exercises |
90 min / Session, 3 Sessions/Week for 6 Weeks |
27 | Control Walking Group |
| Lin, et al., 2009 [Pure bilateral] |
Symmetric & alternating bilateral functional arm movements (lifting 2 cups; picking up 2 pegs; reaching forward or upward to move blocks; grasping & releasing 2 towels; and so on) |
120 min/Day for 3 Weeks |
30 | Distributed Constraint Induced Movement Therapy Group & Control PT Intervention Group |
|
Luft, et al., 2004 [BATRAC] |
Bilateral Arm Training with Rhythmic Auditory Cueing (BATRAC): auditory cues at 0.67 to 0.97 Hz; bilateral push and pull movements in synchrony or alternation (four 5 min movement periods interspersed with 10 min rest) |
60 min/Day, 3 Days/Week for 6 Weeks (18 Sessions) |
18 | Standardized Dose- Matched Therapeutic Exercises (DMTE) |
|
Lum, et al., 2006 [Active and/or Passive] |
Robot-Bilateral Training of 12 targeted reaching activities with Mirror Image Movement Enabler (MIME) |
50 min/Session for 15 sessions over 4 Weeks |
12.5 | Robot-Unilateral Group; Robot-Combined Group; & Control Group |
|
Morris, et al., 2008 [Pure bilateral] |
Bilateral Arm Training: move doweling peg from tabletop to attached to the underside of shelf placed at eye level; move block from table onto a shelf at shoulder level; grasp empty glass, take to the mouth, & return to starting position; point to targets raised 30 cm from the table & positioned at midline, 40 cm to right, & 40 cm to the left of the midline |
20 min/Session, 5 Days/Week for 6 Weeks |
10 | Unilateral Training Control Group |
|
Platz, et al., 2001 [Pure bilateral] |
Bilateral arm training: Fast & accurate aiming movements; fast tapping movements with the index finger; pick up & place small wooden sticks on top of each other |
30 min/Session for 1 Week |
2.5 | Unilateral Training & Healthy Control Group |
|
Richards, et al., 2008 [BATRAC] |
Modified Bilateral Arm Training with Rhythmic Auditory Cueing (modified BATRAC): Bilateral push & pull movements using training device: in- phase and anti-phase movements with metronome cues |
145 min/Session, 4 Sessions/Week for 2 Weeks |
19.33 | No other group: pre – post design |
|
Stinear, et al, 2004 [Active and/or Passive] |
Bilateral rhythmical coordinated wrist flexion-extension where voluntary activation of unaffected hand was used to move the affected hand passively (APBT) |
60 min/Session for 4 Weeks |
20 | No other group: pre – post design |
|
Summers, et al., 2007 [Pure bilateral] |
Bilateral Training: Simultaneous lifting of 2 dowels and placing them on targets located on the shelf. |
50 Trials for 6 Days |
300 Trials | Unilateral Control Group |
| Waller, et al., 2004 [BATRAC] |
Bilateral Arm Training with Rhythmic Auditory Cueing (BATRAC): arms moving together; arms moving alternately at preferred rate (5 min movement periods interspersed with 10 min rest) |
20 min/Session, 3 Sessions/Week for 6 Weeks (18 Sessions) |
6 | No other group: pre – post design |
| Waller et al., 2005 (Left CVA) [BATRAC] |
Bilateral Arm Training with Rhythmic Auditory Cueing (BATRAC): arms moving together; arms moving alternately at preferred rate (5 min movement periods interspersed with 10 min rest) |
20 min/Session, 3 Sessions/Week for 6 Weeks (18 Sessions) |
6 | No other group: pre – post design |
| Waller et al., 2005 (Right CVA) [BATRAC] |
Bilateral Arm Training with Rhythmic Auditory Cueing (BATRAC): arms moving together; arms moving alternately at preferred rate (5 min movement periods interspersed with 10 min rest) |
20 min/Session, 3 Sessions/Week for 6 Weeks (18 Sessions) |
6 | No other group: pre – post design |
| Waller, et al., 2008 [BATRAC] |
Bilateral Arm Training with Rhythmic Auditory Cueing (BATRAC): arms moving together; arms moving alternately at preferred rate (5 min movement periods interspersed with 10 min rest) |
20 min/Session, 3 Sessions/Week for 6 Weeks (18 Sessions) |
6 | Dose-Matched Therapeutic Exercises (DMTE) |
|
Whitall, et al., 2000 [BATRAC] |
Bilateral Arm Training with Rhythmic Auditory Cueing (BATRAC): arms moving together; arms moving alternately at preferred rate (5 min movement periods interspersed with 10 min rest) |
20 min/Session, 3 Sessions/Week for 6 Weeks (18 Sessions) |
6 | No other group: pre – post design |
A second moderator analysis involved stroke patient's impairment levels and functional limitations: What impairment level or functional capability would benefit from bilateral arm training? Across the studies, pre-treatment impairment levels and functional limitations were assessed with a variety of tests: (a) Fugl-Meyer assessment of upper extremity, (b) Box and Block manual dexterity test, (c) Modified Ashworth, and (d) Action Research Arm Test. However, no general consensus was reported in the impairment tests, and the Fugl-Meyer scores ranged from least functional to most functional (5 – 64). Even though we were unable to conduct this planned moderator variable analysis, a partial answer on the type of stroke patient would benefit from bilateral training is that patients who experienced a mild or moderate stroke. General questions on the severity of the stroke patients in the various studies revealed a combination of mild to moderate severity, and severe to marked functional limitations.
Discussion
The current meta-analysis revealed a strong bilateral arm movement training effect and improved motor capabilities post stroke. Indeed, all of the meta-analytic techniques support the conclusion that two specific bilateral arm treatments assist in making progress toward motor recovery. Moreover, a substantial number of training/treatment comparisons (N = 25) as well as participants in the treatment groups (N = 366) contribute to our robust findings. Adhering to strict inclusion/exclusion criteria, coding data rules for common motor outcome measures, and conducting five recommended meta-analytic tests, lead us to the compelling conclusion that the cumulative motor improvement evidence found for the upper extremity is real, rather than spurious.
These findings are important because the cumulative effect of bilateral arm training across the three phases of stroke recovery lends further support to the accumulating evidence that movement-based activities post stroke cause re-learning. Indeed, the current results extend the findings of three distinguished groups of stroke motor recovery researchers lead by Nudo, Cohen, and Hallett who report strong evidence that implicates neural plasticity; multiple improvements in the motor capabilities of the upper extremities after completing movement-based rehabilitation protocols (Chen, Cohen, & Hallett, 2002; Cohen LG & Hallett M, 2003; Hallett, 2001b; Hallett, 2002; Nudo, 1998; Nudo, 1999; Nudo, 2003; Nudo, 2006; Nudo, et al., 2003; Nudo & Milliken, 1996; Nudo, Milliken, Jenkins, & Merzenich, 1996). Neural plasticity refers to permanent changes in synaptic connections or neural ensembles as in establishing a habit state (Cauraugh & Summers, 2005; Cohen LG & Hallett M, 2003; Hallett, 2001b; Hallett, 2002; Hummel & Cohen, 2005; Nudo, 2006). Specifically, the current analysis identified two significant and large effect sizes involved training with BATRAC and coupled bilateral and active stimulation protocols. Movement-based experiences with either the BATRAC or coupled bilateral protocol appear to be essential and sufficient variables for improved motor capabilities.
Moreover, the large significant effects found for BATRAC and coupled bilateral training should be encouraging to therapists and rehabilitation specialists. Additional intervention options may help to significantly decrease the high percentage of stroke patients who continue to display motor dysfunctions one year post. Unfortunately, 60 – 65 % of stroke patients still have motor disabilities 12 months later (Lloyd-Jones, et al., 2008). Incorporating such efficacious bilateral arm movement training protocols into rehabilitation strategies may help to reduce the high percentage of motor disabilities. These two prominent bilateral arm training interventions represent effective strategies in helping stroke patients make progress toward motor recovery, and should be included in a comprehensive program to minimize motor dysfunctions one year post.
The current analysis focused on four specific bilateral arm training protocols, and no direct comparisons with other effective stroke rehabilitation approaches were made. Granted, quantifying motor improvements from CIMT versus bilateral arm practice would be intriguing. However, unilateral movements may generate an interhemispheric inhibition in the ipsilateral hemisphere that prevents mirror movements in the opposite upper limb. In contrast, bilateral movements activate similar neural distributed networks in both hemispheres, allowing mirror movements. Given the importance of mirror movements, future researchers will have to address the two contrasting hemispheric activation patterns (Ramachandran & Altschuler, 2009).
Meta-analytic Techniques
Concerning the meta-analysis technique, one strength is the ease of determining the contribution of potential extreme score effect sizes and calculating a new effect based on the standardized mean difference. Examining the forest plot of effect sizes (Figure 1) reveals one study (Lum, et al., 2006) with a large negative effect (−2.148). No other weighted effect size reached an extreme score area (> 2 SD), thus, we only removed the one study score from our analysis. The overall effect increased to 0.78 when 24 studies were re-analyzed. Consequently, the cumulative bilateral arm training effect is slightly larger without one extreme study with a negative value (or null findings).
Our overall effect is representative of the post stroke bilateral movement training literature. But, excluding 24 studies primarily because of missing treatment conditions (n = 14) and data extraction problems (n = 4) may raise questions about the precision of a meta-analysis. Excluding these studies when some did not support bilateral training would be a concern if the number of comparisons included in our analysis was less than 25. Further, our analysis included 366 treatment participants who experienced one of four bilateral arm practice protocols. Thus, our techniques are precise, robust, and consistent with conventional meta-analyses (Higgins & Green, 2006).
Bilateral Arm Training Conclusions
The moderator variable analysis on the type of bilateral arm training revealed two novel findings. Both the BATRAC and coupled bilateral training and EMG-triggered stimulation techniques produced significant and large effect sizes. These findings are based on seven different studies for each type of protocol. Supplementing bilateral arm movements with either rhythmically paced motion or active stimulation on the impaired arm increased the contribution of these protocols to the mixed design results. Such large and significant contributions to motor recovery were not found with pure bilateral or active and/or passive movement training protocols. Stroke researchers and rehabilitation specialists should be aware of the subtle differences in the four types of bilateral treatments as well as the effectiveness of each treatment. Moreover, likely neurological candidates for these motor improvements are bilateral neural activation patterns and associated mirror neurons (Ramachandran & Altschuler, 2009).
In closing, D. J. Glencross's 32 year old proposition on controlling skilled movements by integrating central and peripheral input still appears relevant (Glencross, 1977). As stroke patients attempt to overcome motor dysfunctions in activities of daily living, practicing bilateral arm training activates both central and peripheral input, and improvements are found.
| Model | Overall Weighted Effect Size |
SE | Confidence Interval (95%) |
Q Statistic |
I2 | Classic Fail-Safe N |
|---|---|---|---|---|---|---|
| Random | 0.734 | 0.125 | 0.489 – 0.979 | 64.53 | 62.81 | 532 |
References
- Borenstein M, Hedges LV, Higgins JPT, Rothstein H. Introduction to meta-analysis. Wiley & Sons; Chichester, West Sussex, United Kingdom: 2009. [Google Scholar]
- Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Research Reviews. 2005;49:641–662. doi: 10.1016/j.brainresrev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Carson RG, Swinnen SP. Coordination and movement pathology: models of structure and function. Acta Psychol (Amst) 2002;110:357–364. doi: 10.1016/s0001-6918(02)00042-2. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Coombes SA, Lodha N, Naik SK, Summers JJ. Upper extremity improvements in chronic stroke: coupled bilateral load training. Restor Neurol Neurosci. 2009;27:17–25. doi: 10.3233/RNN-2009-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauraugh JH, Kim SB. Two coupled motor recovery protocols are better than one: electromyogram-triggered neuromuscular stimulation and bilateral movements. Stroke. 2002;33:1589–1594. doi: 10.1161/01.str.0000016926.77114.a6. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Kim SB. Chronic stroke motor recovery: duration of active neuromuscular stimulation. J Neurol Sci. 2003a;215:13–19. doi: 10.1016/s0022-510x(03)00169-2. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Kim SB. Progress toward motor recovery with active neuromuscular stimulation: muscle activation pattern evidence after a stroke. J Neurol Sci. 2003b;207:25–29. doi: 10.1016/s0022-510x(02)00355-6. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Kim SB. Stroke motor recovery: active neuromuscular stimulation and repetitive practice schedules. J Neurol Neurosurg Psychiatry. 2003c;74:1562–1566. doi: 10.1136/jnnp.74.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauraugh JH, Kim SB, Duley A. Coupled bilateral movements and active neuromuscular stimulation: intralimb transfer evidence during bimanual aiming. Neurosci Lett. 2005;382:39–44. doi: 10.1016/j.neulet.2005.02.060. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Kim SB, Summers JJ. Chronic stroke longitudinal motor improvements: cumulative learning evidence found in the upper extremity. Cerebrovasc Dis. 2008;25:115–121. doi: 10.1159/000112321. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Summers JJ. Neural plasticity and bilateral movements: A rehabilitation approach for chronic stroke. Prog Neurobiol. 2005;75:309–320. doi: 10.1016/j.pneurobio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Chan MK, Tong RK, Chung KY. Bilateral Upper Limb Training With Functional Electric Stimulation in Patients With Chronic Stroke. Neurorehabil Neural Repair. 2008 doi: 10.1177/1545968308326428. [DOI] [PubMed] [Google Scholar]
- Chang JJ, Tung WL, Wu WL, Huang MH, Su FC. Effects of robot-aided bilateral force-induced isokinetic arm training combined with conventional rehabilitation on arm motor function in patients with chronic stroke. Arch Phys Med Rehabil. 2007;88:1332–1338. doi: 10.1016/j.apmr.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Chang JJ, Tung WL, Wu WL, Su FC. Effect of bilateral reaching on affected arm motor control in stroke--with and without loading on unaffected arm. Disabil Rehabil. 2006;28:1507–1516. doi: 10.1080/09638280600646060. [DOI] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Ziemann U. Neurophysiology of unimanual motor control and mirror movements. Clinical Neurophysiology. 2008;119:744–762. doi: 10.1016/j.clinph.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Hallett M. Neural plasticity and recover of function. In: Greenwood RJ, Barnes MP, McMillan TM, Ward CD, editors. Handbook of Neurological Rehabilitation. Psychology Press; Hove, England: 2003. pp. 99–111. [Google Scholar]
- Coupar F, Van Wijck F, Morris J, Pollock A, Langhorne P. Simultaneous bilateral training for improving arm function after stroke (Protocol) Cochrane Database of Systematic Reviews. 2007 doi: 10.1002/14651858.CD006432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Stoykov ME, Walter CB. Bilateral facilitation of motor control in chronic hemiplegia. Acta Psychol (Amst) 2002;110:321–337. doi: 10.1016/s0001-6918(02)00040-9. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage. 2004;21:1416–1427. doi: 10.1016/j.neuroimage.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Desrosiers J, Bourbonnais D, Corriveau H, Gosselin S, Bravo G. Effectiveness of unilateral and symmetrical bilateral task training for arm during the subacute phase after stroke: a randomized controlled trial. Clin Rehabil. 2005;19:581–593. doi: 10.1191/0269215505cr896oa. [DOI] [PubMed] [Google Scholar]
- Garry MI, van Steenis RE, Summers JJ. Interlimb coordination following stroke. Hum Mov Sci. 2005;24:849–864. doi: 10.1016/j.humov.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Glencross DJ. Control of skilled movements. Psychol Bull. 1977;84:14–29. [PubMed] [Google Scholar]
- Goldberg G. Supplementary motor area structure and function: Review and Hypotheses. Behav Brain Sciences. 1985;8:567–616. [Google Scholar]
- Hallett M. Functional reorganization after lesions of the human brain: studies with transcranial magnetic stimulation. Rev Neurol (Paris) 2001a;157:822–826. [PubMed] [Google Scholar]
- Hallett M. Plasticity of the human motor cortex and recovery from stroke. Brain Res Brain Res Rev. 2001b;36:169–174. doi: 10.1016/s0165-0173(01)00092-3. [DOI] [PubMed] [Google Scholar]
- Hallett M. Recent advances in stroke rehabilitation. Neurorehabil Neural Repair. 2002;16:211–217. doi: 10.1177/0888439002016002004. [DOI] [PubMed] [Google Scholar]
- Han CE, Arbib MA, Schweighofer N. Stroke rehabilitation reaches a threshold. PLoS Comput Biol. 2008;4:e1000133. doi: 10.1371/journal.pcbi.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Love ML, McCombe Waller S, Whitall J. Exploiting interlimb coupling to improve paretic arm reaching performance in people with chronic stroke. Arch Phys Med Rehabil. 2005;86:2131–2137. doi: 10.1016/j.apmr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Hesse S, Schmidt H, Werner C. Machines to support motor rehabilitation after stroke: 10 years of experience in Berlin. J Rehabil Res Dev. 2006;43:671–678. doi: 10.1682/jrrd.2005.02.0052. [DOI] [PubMed] [Google Scholar]
- Hesse S, Schmidt H, Werner C, Bardeleben A. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr Opin Neurol. 2003;16:705–710. doi: 10.1097/01.wco.0000102630.16692.38. [DOI] [PubMed] [Google Scholar]
- Hesse S, Schmidt H, Werner C, Rybski C, Puzich U, Bardeleben A. A new mechanical arm trainer to intensify the upper limb rehabilitation of severely affected patients after stroke: design, concept and first case series. Eura Medicophys. 2007;43:463–468. [PubMed] [Google Scholar]
- Hesse S, Schulte-Tigges G, Konrad M, Bardeleben A, Werner C. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects. Arch Phys Med Rehabil. 2003;84:915–920. doi: 10.1016/s0003-9993(02)04954-7. [DOI] [PubMed] [Google Scholar]
- Hesse S, Werner C, Pohl M, Rueckriem S, Mehrholz J, Lingnau ML. Computerized arm training improves the motor control of the severely affected arm after stroke: a single-blinded randomized trial in two centers. Stroke. 2005;36:1960–1966. doi: 10.1161/01.STR.0000177865.37334.ce. [DOI] [PubMed] [Google Scholar]
- Higgins J, Salbach NM, Wood-Dauphinee S, Richards CL, Cote R, Mayo NE. The effect of a task-oriented intervention on arm function in people with stroke: a randomized controlled trial. Clin Rehabil. 2006;20:296–310. doi: 10.1191/0269215505cr943oa. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. The Cochrane Library, Issue 4, 2006. Wiley; Chichester, U.K.: 2006. [Google Scholar]
- Hummel FC, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Drivers of brain plasticity. Curr Opin Neurol. 2005;18:667–674. doi: 10.1097/01.wco.0000189876.37475.42. [DOI] [PubMed] [Google Scholar]
- Jancke L, Peters M, Himmelbach M, Nosselt T, Shah J, Steinmetz H. fMRI study of bimanual coordination. Neuropsychologia. 2000;38:164–174. doi: 10.1016/s0028-3932(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Havton LA, McKay H, Yang H, Brant A, Roberts J, Tuszynski MH. Bilateral corticospinal projections arise from each motor cortex in the macaque monkey: a quantitative study. J Comp Neurol. 2004;473:147–161. doi: 10.1002/cne.20051. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD. Neurophysiological and behavioural adaptations to a bilateral training intervention in individuals following stroke. Clin Rehabil. 2004;18:48–59. doi: 10.1191/0269215504cr701oa. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Perreault EJ. Side of lesion influences bilateral activation in chronic, post-stroke hemiparesis. Clin Neurophysiol. 2007;118:2050–2062. doi: 10.1016/j.clinph.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Lin KC, Chang YF, Wu CY, Chen YA. Effects of Constraint-Induced Therapy Versus Bilateral Arm Training on Motor Performance, Daily Functions, and Quality of Life in Stroke Survivors. Neurorehabil Neural Repair. 2008 doi: 10.1177/1545968308328719. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart Disease and Stroke Statistics--2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, Schulz JB, Goldberg AP, Hanley DF. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. Jama. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Van der Loos M, Shor PC, Majmundar M, Yap R. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: A follow-up study. J Rehabil Res Dev. 2006;43:631–642. doi: 10.1682/jrrd.2005.02.0044. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Harris-Love M, Liu W, Whitall J. Temporal coordination of the arms during bilateral simultaneous and sequential movements in patients with chronic hemiparesis. Exp Brain Res. 2006;168:450–454. doi: 10.1007/s00221-005-0235-3. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Liu W, Whitall J. Temporal and spatial control following bilateral versus unilateral training. Hum Mov Sci. 2008;27:749–758. doi: 10.1016/j.humov.2008.03.006. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Whitall J. Fine Motor Control in Adults With and Without Chronic Hemiparesis: Baseline Comparison to Nondisabled Adults and Effects of Bilateral Arm Training. Arch Phys Med Rehabil. 2004;85 doi: 10.1016/j.apmr.2003.10.020. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Whitall J. Hand dominance and side of stroke affect rehabilitation in chronic stroke. Clin Rehabil. 2005;19:544–551. doi: 10.1191/0269215505cr829oa. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Whitall J. Bilateral arm training: why and who benefits? NeuroRehabilitation. 2008;23:29–41. [PMC free article] [PubMed] [Google Scholar]
- Morris JH, van Wijck F, Joice S, Ogston SA, Cole I, MacWalter RS. A comparison of bilateral and unilateral upper-limb task training in early poststroke rehabilitation: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89:1237–1245. doi: 10.1016/j.apmr.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Mudie MH, Matyas TA. Upper Extremity Retraining Following Stroke: Effects of Bilateral Practice. Neurorehabilitation and Neural Repair. 1996;10:167–184. [Google Scholar]
- Mudie MH, Matyas TA. Can simultaneous bilateral movement involve the undamaged hemisphere in reconstruction of neural networks damaged by stroke? Disabil Rehabil. 2000;22:23–37. doi: 10.1080/096382800297097. [DOI] [PubMed] [Google Scholar]
- Mudie MH, Matyas TA. Responses of the densely hemiplegic upper extremity to bilateral training. Neurorehabil Neural Repair. 2001;15:129–140. doi: 10.1177/154596830101500206. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Role of cortical plasticity in motor recovery after stroke. Neurol Report. 1998;22:61–67. [Google Scholar]
- Nudo RJ. Recovery after damage to motor cortical areas. Curr Opin Neurobiol. 1999;9:740–747. doi: 10.1016/s0959-4388(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. J Rehabil Med. 2003:7–10. doi: 10.1080/16501960310010070. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol. 2006;16:638–644. doi: 10.1016/j.conb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Larson D, Plautz EJ, Friel KM, Barbay S, Frost SB. A squirrel monkey model of poststroke motor recovery. Ilar J. 2003;44:161–174. doi: 10.1093/ilar.44.2.161. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SJ, Levine P, Teepen J, Hartman EC. Resistance-based, reciprocal upper and lower limb locomotor training in chronic stroke: a randomized, controlled crossover study. Clin Rehabil. 2008;22:610–617. doi: 10.1177/0269215508088987. [DOI] [PubMed] [Google Scholar]
- Platz T, Bock S, Prass K. Reduced skilfulness of arm motor behaviour among motor stroke patients with good clinical recovery: does it indicate reduced automaticity? Can it be improved by unilateral or bilateral training? A kinematic motion analysis study. Neuropsychologia. 2001;39:687–698. doi: 10.1016/s0028-3932(01)00005-7. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132:1693–1710. doi: 10.1093/brain/awp135. [DOI] [PubMed] [Google Scholar]
- Rice MS, Newell KM. Upper-extremity interlimb coupling in persons with left hemiplegia due to stroke. Arch Phys Med Rehabil. 2004;85:629–634. doi: 10.1016/j.apmr.2003.08.084. [DOI] [PubMed] [Google Scholar]
- Richards LG, Senesac CR, Davis SB, Woodbury ML, Nadeau SE. Bilateral arm training with rhythmic auditory cueing in chronic stroke: not always efficacious. Neurorehabil Neural Repair. 2008;22:180–184. doi: 10.1177/1545968307305355. [DOI] [PubMed] [Google Scholar]
- Rose DK, Winstein CJ. The co-ordination of bimanual rapid aiming movements following stroke. Clin Rehabil. 2005;19:452–462. doi: 10.1191/0269215505cr806oa. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Writing meta-analytic reviews. Psych Bull. 1995;118:1173–1181. [Google Scholar]
- Rosenthal R, DiMatteo MR. Meta-analysis: recent developments in quantitative methods for literature reviews. Annu Rev Psychol. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Calautti C, Pauri F, Baron J-C. Post-stroke plastic reorganisation in the adult brain. The Lancet Neurology. 2003;2:493–502. doi: 10.1016/s1474-4422(03)00485-x. [DOI] [PubMed] [Google Scholar]
- Stewart KC, Cauraugh JH, Summers JJ. Bilateral movement training and stroke rehabilitation: a systematic review and meta-analysis. J Neurol Sci. 2006;244:89–95. doi: 10.1016/j.jns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Coxon JP, Fleming MK, Byblow WD. Priming the motor system enhances the effects of upper limb therapy in chronic stroke. Brain. 2008;131:1381–1390. doi: 10.1093/brain/awn051. [DOI] [PubMed] [Google Scholar]
- Stinear JW, Byblow WD. Rhythmic bilateral movement training modulates corticomotor excitability and enhances upper limb motricity poststroke: a pilot study. J Clin Neurophysiol. 2004;21:124–131. doi: 10.1097/00004691-200403000-00008. [DOI] [PubMed] [Google Scholar]
- Summers JJ, Kagerer FA, Garry MI, Hiraga CY, Loftus A, Cauraugh JH. Bilateral and unilateral movement training on upper limb function in chronic stroke patients: A TMS study. J Neurol Sci. 2007;252:76–82. doi: 10.1016/j.jns.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Swinnen SP, Wenderoth N. Two hands, one brain: cognitive neuroscience of bimanual skill. Trends Cogn Sci. 2004;8:18–25. doi: 10.1016/j.tics.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Tijs E, Matyas TA. Bilateral training does not facilitate performance of copying tasks in poststroke hemiplegia. Neurorehabil Neural Repair. 2006;20:473–483. doi: 10.1177/1545968306287900. [DOI] [PubMed] [Google Scholar]
- Ustinova KI, Fung J, Levin MF. Disruption of bilateral temporal coordination during arm swinging in patients with hemiparesis. Exp Brain Res. 2006;169:194–207. doi: 10.1007/s00221-005-0136-5. [DOI] [PubMed] [Google Scholar]
- Whitall J, McCombe Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31:2390–2395. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Winstein CJ, Miller JP, Thompson PA, Taub E, Uswatte G, Morris D, Blanton S, Nichols-Larsen D, Clark PC. Retention of upper limb function in stroke survivors who have received constraint-induced movement therapy: the EXCITE randomised trial. Lancet Neurol. 2008;7:33–40. doi: 10.1016/S1474-4422(07)70294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]