Abstract
The atrioventricular node (AVN) has mystified generations of investigators over the last century and continues today to be at the epicenter of debates amongst anatomists, experimentalists, and electrophysiologists. Over the years, discrepancies have remained in regards to correlating components of AVN structure to function, as evidenced by studies from microelectrodes, optical mapping, and the electrophysiology laboratory. Historically, the AVN has been defined by classical histological methods, however with recent advances in molecular biology techniques, a more precise characterization of structure is becoming attainable. Distinct molecular compartments are becoming apparent based on connexin staining and genotyping, providing new insight into previously characterized functional aspects of the AVN and its surrounding structures. Advances in optical mapping have provided a unique opportunity for correlating structure and function - unmasking properties of the native AVN pacemaker and providing further insight into basic mechanisms involved in AV conduction. Additionally, procurement of explanted human hearts have provided a unique opportunity to further characterize the human AVN structurally and functionally with both molecular biology techniques and optical mapping. With the elucidation of basic elements of both structure and function via molecular investigation and optical mapping, new opportunities are becoming apparent in utilizing the unique properties of the AVN for pursuing novel clinical applications relevant to clinical electrophysiology.
The topics explored in this review will encompass the roles of not only the AVN, but also the nodal extensions and surrounding atrial myocardium. Consequently, the term AV junction will be used throughout to describe all tissue components involved in atrial-His conduction.
I. Introduction
The AVN, as initially characterized by Sunao Tawara in 1906, describes a compact spindle-shaped network of cells arranged in a “knoten” (node) connected to the His bundle, both being responsible for the only physiological atrio-ventricular axis of conduction. Tawara’s original monograph, Das Reizleitungssystem des Saugetierherzens (The Stimulus Conducting System of Mammalian Hearts),1 provides an exquisite morphological description of the AV node, His bundle, and surrounding atrial myocardium of nine mammalian species, including humans. Anatomically, the AV node is located within the triangle of Koch,2 a region located at the base of the right atrium defined by the following landmarks: the coronary sinus (CS) ostium, tendon of Todaro (tT), and the septal leaflet of the tricuspid valve (TV). Using the central fibrous body (CFB) as the demarcation between the His bundle and AV node as Tawara suggested, morphologically the AV node can then be further divided into the lower nodal bundle (LNB) and compact node (CN).3 In the LNB, the cells are longer and arranged more parallel to one another, whereas in the CN the cells are small and spindle-shaped with no clear orientation. Extending proximally from the LNB towards the coronary sinus (CS) is the inferior nodal extension, also known as the rightward nodal extension in humans.4 The second nodal extension (or leftward nodal extension), present only in humans, extends from the CN towards the CS, and is usually shorter than the rightward extension.4 While functional and tissue characteristics of the AVJ appear to have similar properties between the rabbit and human, molecular differences do exist in addition to the anatomical ones noted above. From a molecular standpoint, the expression patterns of connexins and likely ion channels are species dependant, with the rabbit expressing both Cx43 and Cx40 while our experience from human AVJ preparations is notable for compartmentalization of Cx43 expression.5 Functionally, several different cell types in the rabbit AVJ have been defined based on action potential morphology from microelectrode recordings including A (atrial) cells, N (nodal) cells, and H (His) cells, as well as intermediate cell types such as AN, NH, and other types.6, 7 To date, no microelectrode recordings of human AVJ preparations have been published.
Clinically, the AVJ plays important roles in coordinating and maintaining appropriate AV conduction, protecting the ventricles from atrial tachyarrhythmias, and functioning as a backup pacemaker in the setting of sinoatrial (SA) node dysfunction. Abnormal impulse propagation in the AVJ can be associated with such arrhythmias as AV nodal reentrant tachycardia (AVNRT) and AV block. In AVNRT, there exists a reentrant circuit contained within the AVJ functionally conducted through the fast and slow pathways,8, 9 first reported by Moe, et al in 1956.10 Initial studies suggested that the reentrant circuit maintaining this arrhythmia was entirely confined to the AV node, however subsequent investigations demonstrated that the fast and slow pathways incorporate atrial tissue, in addition to AV nodal tissue.11 Treatment of AVNRT is commonly achieved through ablation of the slow pathway, which disrupts the aberrant circuit leaving normal nodal conduction to occur through the fast pathway, carrying a less than 1% chance of complete heart block.12, 13
Given the lack of consensus on fundamental issues involving a direct anatomical and functional correlation within the AVJ, a debate has existed involving two different approaches to AVNRT ablation based on utilizing an “anatomical”, or structural, approach versus a “potential”, or functional, approach.14 The “anatomical” approach is based on targeting the anatomical landmarks of the slow pathway, such as the isthmus between the coronary sinus (CS) orifice and the tricuspid valve. The “potential” approach is based on targeting a characteristic low frequency electrical potential signature of the slow pathway from the endocardium, also known as “slow potentials”. A combined approach involving aspects of both methods is increasingly utilized in the electrophysiology laboratory. A truly integrated approach based on a direct correlation between anatomic structure and function, however, is lacking.
As a heterogeneous region with distinct cell types, the AVJ display unique structural and functional properties. The cellular electrophysiology of specific cell types in the region are likely driven by different gene expression programs for connexin and ion channel distribution and are not distinguished by classical histological methods, which do not target these proteins. The important work of McGuire, et al in 1996 was one of the early experimental studies alluding to this. In this study using isolated, blood perfused canine and porcine hearts, microelectrode recordings revealed AV junctional cells located around the tricuspid and mitral annulus that were similar to atrial cells by histology, but resembled nodal cells in terms of cellular electrophysiological properties and displayed the limitations of categorizing cells in within the AVJ via standard histological methods.15 We believe that connexin and ion channel expression are likely to be different in cells in the AVJ that are otherwise indistinguishable from atrial cells from a histological standpoint. Potentially, these new molecular markers can be used to more accurately define structure and correlate function. The challenge remains for anatomists, basic and clinical electrophysiologists to resolve the inherent complexity of structure and function in the AVJ as relating to both normal and abnormal conduction. This challenge can be summed by the following statement from a 1988 review by Meijler and Janse that continues to be applicable today: “we are faced with a paradoxical situation that the most typical nodal action potential has not yet been linked to the most typical nodal cell”.3 Recent advances in imaging modalities and molecular biology techniques, however, are beginning to allow researchers and clinicians alike to unravel the true nature of AV nodal electrophysiology.
II. Structural Overview of the AVJ
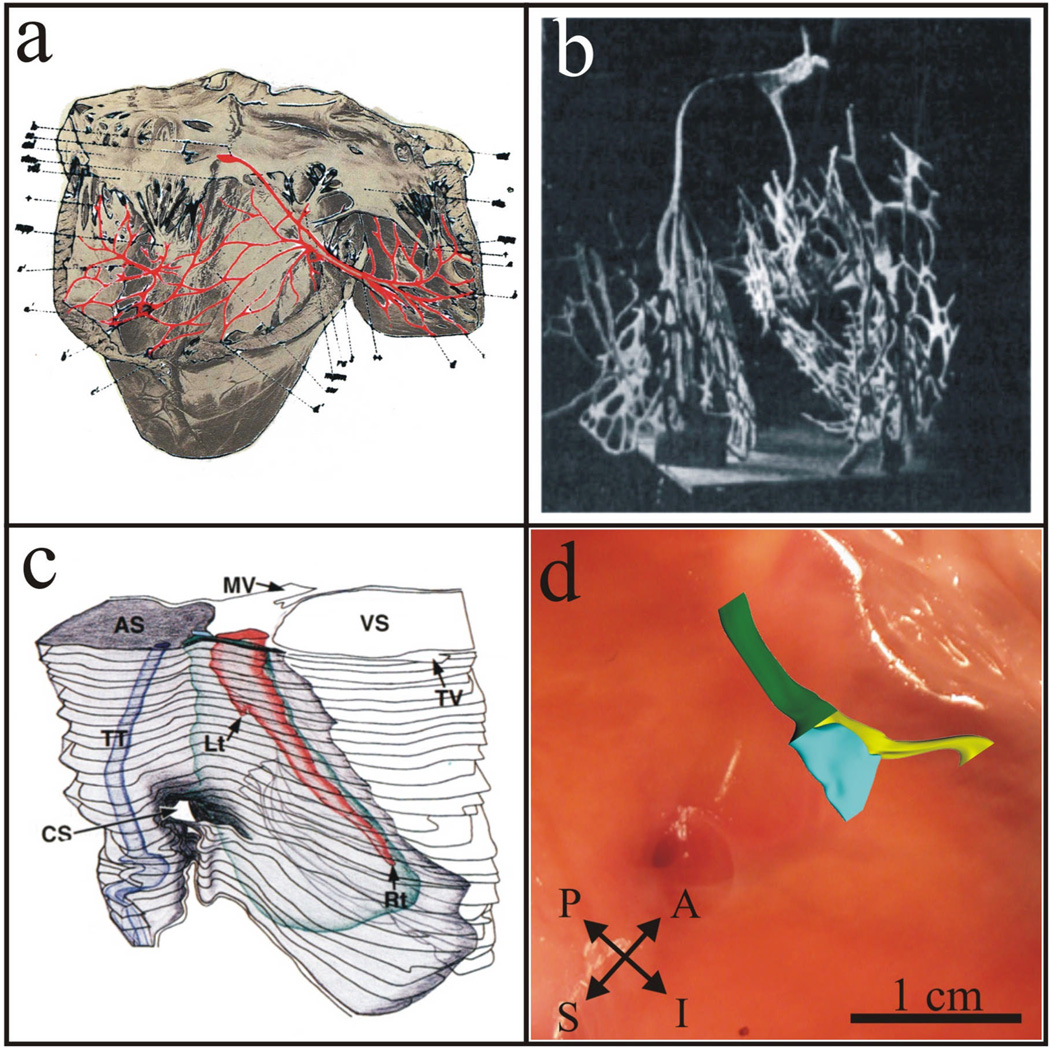
Since the discovery and initial characterization of the AV node by Sunao Tawara in 1906, there has been an evolution in the understanding of the structure of the AVJ and its role in cardiac conduction. Tawara’s discovery of the structure of the AV node, or “knoten”, was garnered from the tedious task of sifting through serial sections, tracing the “knoten” backwards from the His bundle. Figure 1a shows Tawara’s original reconstruction beginning with the AV node at the base of the right atrium and ending with the terminal Purkinje fibers in the right ventricle. In 1909, Lydia DeWitt, a pathologist at the University of Michigan, published a manuscript entitled Observations on the Sino-Ventricular Connecting System of the Mammalian Heart. Using Tawara’s description as her guide, she created an elaborate 3D wax model which was the first, and most complete, reconstruction of the conduction system of several mammalian species at that time (see Figure 1b).16
Figure 1. Evolution of the 3D Structure of the AV Junction.
(a) Sunao Tawara‘s 1906 reconstruction of a bovine conduction system as viewed from the right ventricle.1 (b) Lydia DeWitt’s 1909 3D wax reconstruction of the sino-ventricular system in a calf heart.16 (c) Waki, et al’s 3D anatomical reconstruction of the AV junctional area highlighting the rightward and leftward posterior extensions present in the majority of human hearts.17 (d) Our current understanding of the 3D structure of the AVJ based on expression patterns of Cx43 which delineate two discrete structures.18 Green denotes the His bundle, yellow denotes the LNB and RE (a Cx43-positive region), and blue denotes the CN and LE (a Cx43-negative region).
The identification of nodal extensions and age-related morphological changes brought forth the next stage in elucidating the anatomical structure of the AVJ,4, 17 as shown in Figure 1c. These findings were considered the next major advancement in redefining the human AVJ, leading to a refined understanding of structure and new opportunities in correlating structure and function. The identification of rightward and leftward nodal extensions provided a basis for an anatomical correlate of slow pathway conduction noted in functional studies and refinement in catheter ablation techniques. The description of age-related morphological variability noted in the AVN by Waki, et al17 helped fuel speculations of the possible basis for age-related differences noted in the incidence of AVNRT.
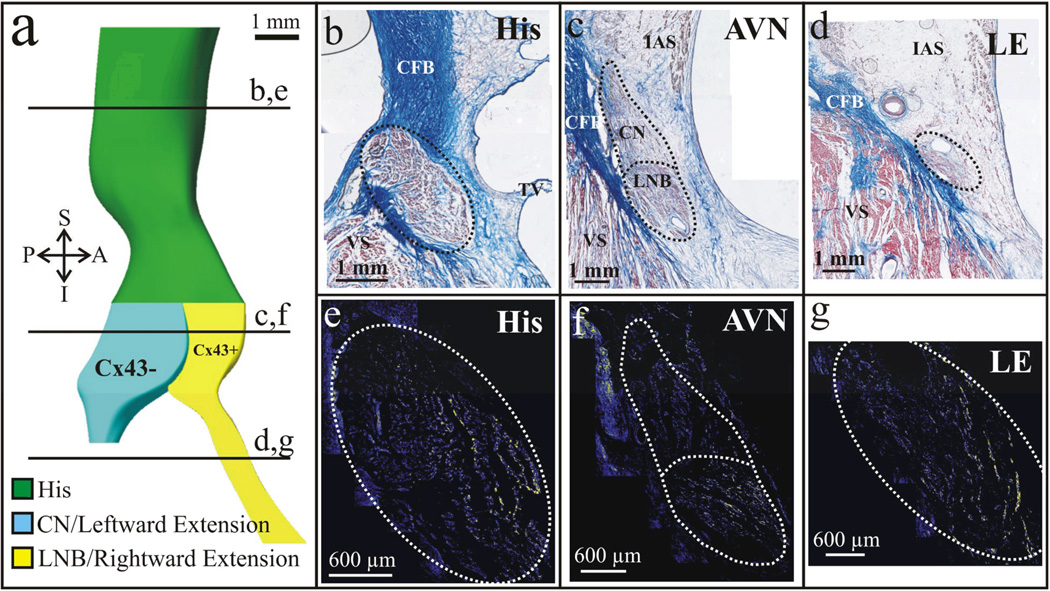
With the advent of new techniques in molecular biology, a more in depth evaluation of the AVJ has been made available, specifically using immunohistochemical methods staining for gap junction proteins involved in cell-to-cell signaling and cardiac conduction. In a recent study, we investigated the expression patterns of connexin 43 (Cx43), a major cardiac gap junction protein, within the AV node and nodal extensions in 4 patients.18 We were able to confirm the presence of rightward and leftward posterior extensions as defined by Inoue-Becker4 in all studied hearts using traditional histological staining methods. In addition, interestingly, we were able to identify two discrete, continuous structures when staining for Cx43: one consisting of the leftward extension and CN (LE/CN) expressing virtually no Cx43, and the other consisting of the rightward extension and LNB (RE/LNB) staining positive for Cx43.18 Using serial histological and immunohistochemical sections we then constructed an updated 3D model of the AVJ reflecting Cx43 expression within the AVJ as noted in Figure 1d & Figure 2a.
Figure 2. Cx43 expression in the Human AVJ.
Histological serial sections revealed the presence of rightward and leftward posterior nodal extensions. In addition, immunofluorescence identified differences in the expression of Cx43 between the two extensions and their corresponding counterparts in the AV node. Immunostaining of the His bundle also identified a gradient of Cx43 expression in line with the differential expressions of the protein in the CN and LNB in this particular preparation. (a) 3D reconstruction of an AVJ from a 58 year old female. (b–d) Histological sections as denoted in (a) by the solid black lines. (e–g) Corresponding immunostained sections for Cx43. Yellow color shows Cx43.
While not extensively evaluated in these particular preparations, there was also evidence of compartmentalization of Cx43 expression extending from the AVJ into the proximal His bundle as visualized in Figure 2b,e. The molecular compartmentalization noted in the AVJ and His bundle, based on Cx43 expression was previously undetectable by traditional histological staining methods and provide insight into the structural organization of the AVJ, His bundle, and remaining components of the cardiac conduction system. This provides us with a picture, although incomplete, of molecular compartmentalization and dissociation as suggested by Cx43 expression in the AVJ involving the LE/CN (Cx43-negative) and RE/LNB (Cx43-positive), as well as evidence suggestive of continuous extension of these compartments based on Cx43 expression patterns into the His bundle. The region, however, is complex from a functional standpoint and is likely more heterogeneous in terms of connexin expression. It is further complicated by the fact that connexins are composed of subunits that can exist in multiple forms (homomeric vs heteromeric) and have multiple interfaces between cells with other forms of connexins’s (homotypic vs heterotypic).19 We have tested for a variety of isoforms of connexin including Cx45, Cx40, and Cx31.9 and unfortunately have failed to obtain a reliable signal with numerous antibodies other than Cx43.
In addition to connexin staining, evaluating different ion channel gene expression patterns will be the next step in using molecular biology techniques to further define the AVJ. We hypothesize that, as noted with Cx43 staining, different gene expression programs will drive protein expression into two distinct molecular compartments, including different isoforms of ion channels (i.e. Cav1.2 vs. Cav1.3), connexins, and receptors possibly providing the molecular basis for longitudinal dissociation and arrhythmia formation within the AVJ. Identification of such distinct molecular markers will further redefine AVJ structure and providing additional insight into function.
III. Functional Overview of the AVJ
Located at the crossroads of the cardiac conduction system, the AVJ functions as an independent pacemaker, a filter for impulses between the atria and ventricles, and a potential arrhythmogenic substrate. With the development experimental techniques initially with microelectrodes and more recently with optical mapping, our understanding of the functional properties characterized by the AVJ have progressed significantly over the last century. Briefly, microelectrodes record intracellular potentials from single cells within larger tissue preparations.7, 20 Optical mapping is based on the use of voltage-dependent fluorescent dye molecules and allows one to record electrical activity in the form of optical action potentials from hundreds to thousands of sites simultaneously within a single preparation.21, 22 The combination of these experimental technologies with traditional techniques, such as electrograms, have subsequently opened the door to increasingly comprehensive functional studies of the heart.
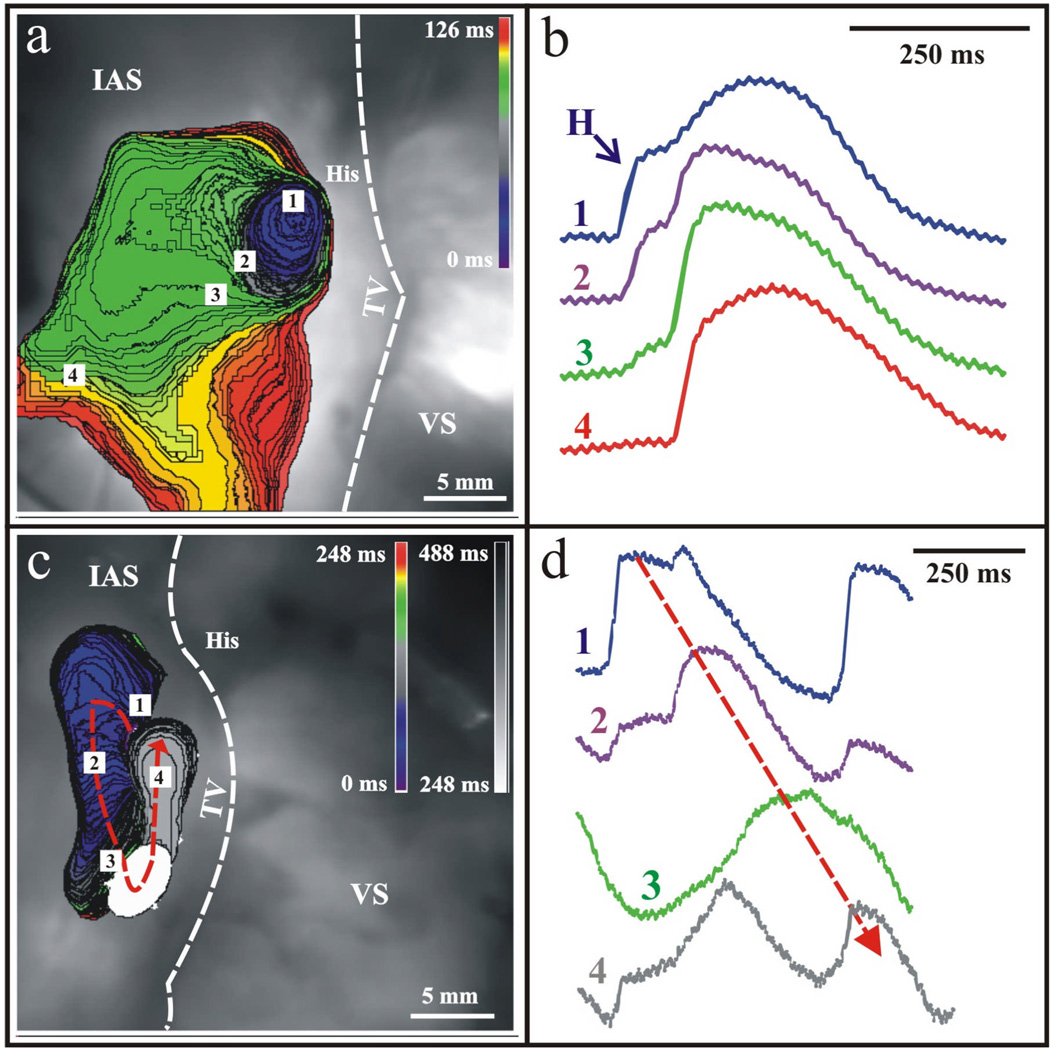
The complex heterogeneity of the region provides the basis for the AVJ to perform its’ unique, independent functions in the heart. From a structural standpoint, as noted previously, a picture is emerging that includes molecular compartmentalization suggested by Cx43 expression that may provide the basis for some of the unique functional properties noted in the AVJ including arrhythmia formation and pacemaker function. Recently, we have investigated the functional bases for junctional rhythm formation in the human heart via optical mapping.23 In earlier studies on the rabbit heart, in the absence of SA nodal impulses, the leading pacemaker was found to have a stable anatomic location, regardless of autonomic modulation, in the inferior nodal extension (INE), which corresponds to the rightward extension (RE) in humans.24 In a separate study, that same leading pacemaker location in the rabbit was found to express high levels of HCN4, the major cardiac pacemaker current responsible for If.5 In contrast, in more recent studies in the human heart, we have found the leading pacemaker to be located in the CN area, as shown in Figure 3a. The structural heterogeneity of the AVJ is further evidenced by the existence of double component optical action potentials as shown in Figure 3b during junctional rhythm. This phenomenon results from the contribution of multiple tissue layers, in this case His bundle activation followed by atrial activation, lying on top of one another in the optical field.25 Additionally, the leading pacemaker location in the human AVJ was found to shift to the RE under autonomic modulation (data not shown).26
Figure 3. Junctional Rhythm and Reentry in the Human AVJ.
Optical mapping with voltage-sensitive dyes revealed the site of origin of the AVJ rhythm to be in the NH region. (a) The leading pacemaker, in this preparation, produced anterograde excitation towards His bundle (not shown) and retrograde excitation towards the compact node. Retrograde excitation spread in the compact AV node and excited the atrium via the fast pathway. (b) Corresponding optical action potentials to the observed junctional rhythm. (c) In another case, retrograde excitation spread over the Cx43-negative leftward nodal extension, turned around and reentered into the Cx43-positive rightward nodal extension. The excitation returned to the site of origin, completing one reentrant circuit. (d) Corresponding optical action potentials confirming the presence of one reentrant circuit as denoted by the dotted arrow.
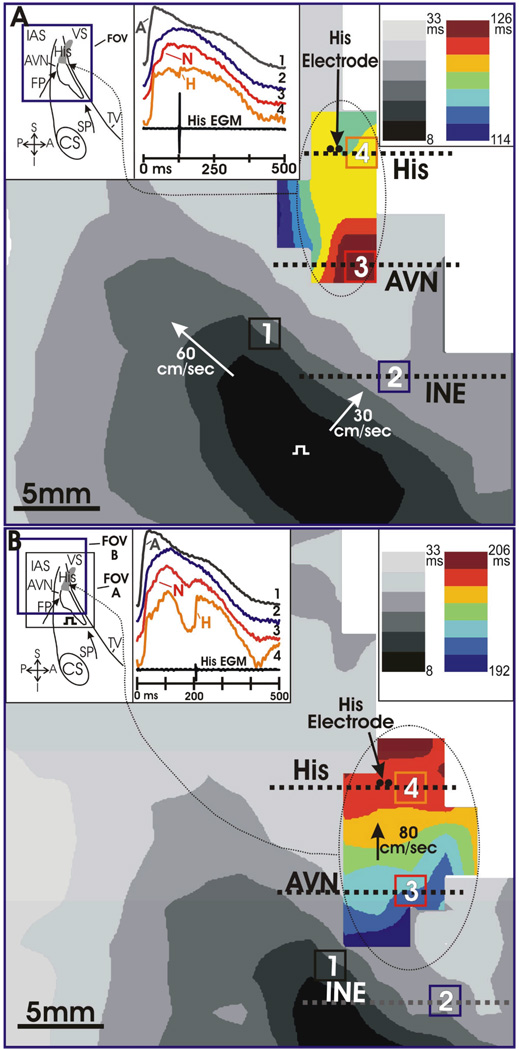
Further evidence of functional longitudinal dissociation is shown in Figure 3c,d and Figure 4. In the example presented in Figure 3c,d, a spontaneous reentrant beat originates in the CN area, similar to where the intrinsic junctional rhythm originates, progresses in a retrograde fashion through the fast pathway compartment, and returns anterogradely through the slow pathway compartment. This is an example of longitudinal dissociation between the fast and slow pathway compartments displaying functional compartmentalization within the AVJ between the Cx43(+) and Cx43(−) regions Recently, we published an example of AV nodal dual pathway characteristics optically mapped for the first time in humans.23 A standard S1–S2 protocol was applied to unmask dual pathway electrophysiology in this preparation. During a routine S1 train at 1000 ms, conduction consistently occurred through the fast pathway compartment, as illustrated in Figure 4a. Then the S1–S2 protocol was decreased incrementally until at an S2 of 600 ms, conduction block occurred in the fast pathway due to its intrinsically long refractory period. In this case, as illustrated in Figure 4b, the impulse was conducted to the His bundle via the slow pathway. It is also important to note the change in amplitude of the His bundle electrogram with a 73% decrease in the size recorded during slow pathway activation as compared to fast pathway activation, as well as change in optical action potential morphology between the two recordings. Interestingly, as seen in the rabbit, this provides evidence of differential His activation and compartmentalization, providing further electrophysiological evidence of functional longitudinal dissociation at the level of the human AVJ and His bundle.27, 28
Figure 4. Functional Longitudinal Dissociation of the AVJ.
Optical action potentials recorded from the AV junction were composed of multiple components. Pacing the atrial myocardium produced a fast wave of excitation that spread across the interatrial septum (IAS) (gray activation maps) that was responsible for the initial upstroke (A) of the OAPs shown. His activation produced an additional hump in OAPs recorded near the His bundle (OAP4), and the colored activation maps were constructed from the His component of these OAPs. The bipolar His electrogram (EGM) indicates His activation at 126 ms in panel A, and at 206 ms in panel B. The bipolar His EGM is 73% smaller in (A) than in (B). The changes in His activation time and His EGM amplitude from panel A to panel B suggest that panel A was fast pathway conduction and panel B was slow pathway conduction. Pacing occurred at time zero at the location marked by a square pulse. Photodiode resolution was 2×2 mm.
IV. Correlating Structure and Function
Dual pathway electrophysiology, one of the hallmarks of the human AVJ, has been widely investigated over the last century. However, unanswered questions still remain in directly correlating anatomical substrates with known functional pathways. Anatomically, there are two pathways consisting of the RE/LNB and the LE/CN that can be identified on a histological and molecular basis as discussed previously and illustrated in Figure 2. Functionally, the AVJ can be described as having two pathways – the slow pathway and the fast pathway, as also discussed previously and shown in Figure 4. It has been shown that the anatomical substrate for the slow pathway involves structures embedded within an isthmus of myocardium located along the tricuspid annulus below the coronary sinus.23, 29, 30 Consequently, evidence exists involving the area of the RE as the anatomical substrate of the slow pathway.8, 31 It is, however, still debated whether slow pathway conduction also includes inferior transitional cells overlaying this nodal extension.11 The fast pathway is less well-defined from an anatomic and structural standpoint. The probable anatomical substrate of this pathway is the transitional cell layers located around the CN at the interface between the CN and transitional cells, which express Cx43 at levels similar to the interatrial septum18 and would functionally support fast conduction in this region.
Despite the need for a structural and functional correlation in the AVJ, the anatomical existence of the substrate for dual pathway electrophysiology is not necessarily indicative of the existence of functional reentry, although required to maintain the reentry circuit. The presumed anatomical bases of the fast and slow pathways are present in the majority of hearts4, 18 despite the relatively low frequency of AVNRT diagnoses. However, 84% of patients undergoing radiofrequency ablation of an accessory pathway with no history of AVNRT, functionally demonstrated the existence of dual pathways.32 Interestingly AVNRT in infants is rare, however, the incidence increases during childhood from 13% in school age children to 50% in older teenagers representing a developmental change that occurs within the AVJ33, 34 likely driven by an increase in size of nodal extension and by an alteration in gene expression of connexins, various ion channel isoforms and receptors responsible for conduction during the ageing process possibly increasing the likelihood of reentry based on gene expression patterns.
It is apparent that both structural and functional compartmentalization exist within the AVJ as revealed by Cx43 expression patterns and functional data obtained from optical mapping and electrogram recordings in the region. From this data, two distinct pathways or “compartments” are becoming apparent within the AVJ, as suggested by Cx43 expression, that appear to be continuous with the His bundle and display distinct conduction properties. While a direct anatomical basis for the functional properties noted in the AVJ remain to be elucidated, we will refer to the region that is Cx43 (−) as the fast pathway compartment and Cx43 (+) region as the slow pathway compartment. These compartments contain the traditional anatomic and functional properties as previously ascribed to the fast and slow pathway but now included is molecular characterization of the region with Cx43 expression providing new “molecular boundaries” within the AVJ. (Figure 5). We do not believe that Cx43 expression is directly responsible for slow pathway conduction, nor the absence of it responsible for fast pathway conduction but rather believe that Cx43 expression provides a starting point for characterizing the region from a molecular standpoint which we are currently pursuing in our laboratory.
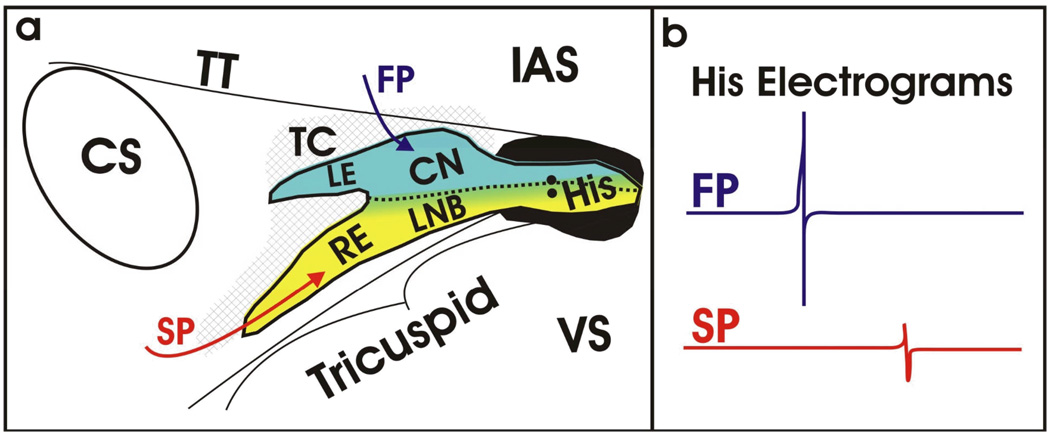
Figure 5. Structural and Functional Understanding of the AVJ.
Based on over a century of research, our current understanding of the AVJ is beginning to blend both function and structure as unified definition as shown in (a). The directional arrows illustrate the functional inputs to the AVJ (fast pathway (FP) in red, slow pathway (SP) in blue) roughly corresponding to the Cx43-positive and –negative regions as defined structurally. (b) Representative His electrograms resulting from activation of the fast and slow pathways, in blue and red, respectively. The significant difference in amplitude results from differential compartments within the AVJ being activated during conduction.
V. Clinical Implications
Although currently the diseased states involving the AVJ are readily treated with either pacemaker placement for conduction disease and ablation for AVNRT, new opportunities for advances in the field of clinical electrophysiology utilizing its unique properties are becoming apparent as the basic elements of this region are slowly being revealed.
An alternative site to RV pacing is desired given the well-established deleterious long term effects noted with RV pacing, including increased incidence of atrial fibrillation, heart failure, hospitalizations, and mortality.35, 36 We have shown previously in rabbit AVJ preparations that AV conduction via slow pathway pacing bypasses the compact node via the LNB and directly activates the His bundle as confirmed by optical mapping and His electrogram recordings.30 Given the distinct excitation pattern for slow pathway activation as compared to fast pathway activation in both rabbit and human preparations and preliminary Cx43 staining suggestive of compartmentalization within the AVJ and His bundle, we propose using the slow pathway compartment as an alternative pacing site that would excite the His bundle independent of traditional fast pathway activation and maintain the much desired synchronized ventricular contraction. Previously direct His bundle pacing has been attempted, however, barriers are present as noted with higher thresholds, lower sensing, and relatively small areas for lead placement which could be attributed to the high fibrous content of the His region.37–39 In contrast, the area of slow pathway conduction or “slow pathway compartment” as suggested by our studies in rabbits and humans, is easily paced and provides an independent pathway for excitation into the His bundle bypassing the compact nodal region. Potentially, this could also be considered an alternative cardiac resynchronization therapy (CRT) by maintaining native His conduction and ventricular synchrony in patients whose conduction disease is proximal to slow pathway compartment insertion into the His bundle. Future clinical electrophysiology studies are warranted in exploring this possible pacing strategy including characterization of appropriate signals, sensitivities, thresholds, optimal sites for lead placement, and lead design.
From animal studies as well as our human preparations, the AVJ pacemaker appears to be predominately located within the region of the slow pathway compartment opening the possibility of genetic and cellular manipulation resulting in modification of existing native AVJ pacemaker function. Potentially, proteins or their encoding genes involved in modulating the pacemaker (HCN4, calcium handling, etc.) and conduction could be delivered within this region altering AVJ pacemaker properties possibly allowing it to drive conduction in the absence of a functioning SA nodal pacemaker.
The role of the autonomic nervous system (ANS) in regulating the AVJ provides another avenue for intervention as it is highly innervated with by both sympathetic and parasympathetic nerves that modulate AVJ function. Recently, Bianchi, et al40 successfully implanted the atrial lead in the postero-septal portion of right atrium (slow pathway compartment) in patients with atrial fibrillation receiving defibrillators. Using high frequency stimulation at this particular pacing site they were able to induce conduction block at the level of the AVJ, providing a method for rate control for rapid ventricular response in atrial fibrillation. Although this specific technique is currently in the proof of principle phase and far from practical clinical use it does reveal feasibility of this site for lead placement and offers promise in terms of lead location and slow pathway pacing.
V. Conclusion
While not yet completely resolved, the discrepancies that exists amongst anatomist, basic and clinical electrophysiologists are slowly dissipating, allowing unique opportunities to once again rethink the nature of the AVJ, as well as re-evaluate its potential role in clinical electrophysiology. Alternative pacing sites within the slow pathway compartment, modulating native AVJ pacemaker function via gene therapy, and manipulation of the ANS within the AVJ are a few of the exciting possibilities that lie ahead with respect to the AVJ. With advances involving microelectrodes, optical mapping, and novel molecular biology techniques we are slowly getting closer to unraveling the secrets of the AVJ.
References
- 1.Tawara S. Das Reizleitungssystem des Saugetierherzens: Eine Anatomisch-Histologische Studie Uber Das Atrioventrikularbundel Und Die Purkinjeschen Faden. Jena: Verlag von Gustav Fischer; 1906. [Google Scholar]
- 2.Koch W. Welche Bedeutung kommt dem Sinusknotten zu? Med Klinik. 1911;7:447–452. [Google Scholar]
- 3.Meijler FL, Janse MJ. Morphology and electrophysiology of the mammalian atrioventricular node. Physiol Rev. 1988;68(2):608–647. doi: 10.1152/physrev.1988.68.2.608. [DOI] [PubMed] [Google Scholar]
- 4.Inoue S, Becker AE. Posterior extensions of the human compact atrioventricular node: a neglected anatomic feature of potential clinical significance. Circulation. 1998;97(2):188–193. doi: 10.1161/01.cir.97.2.188. [DOI] [PubMed] [Google Scholar]
- 5.Dobrzynski H, Nikolski VP, Sambelashvili AT, Greener ID, Yamamoto M, Boyett MR, Efimov IR. Site of origin and molecular substrate of atrioventricular junctional rhythm in the rabbit heart. Circ Res. 2003;93(11):1102–1110. doi: 10.1161/01.RES.0000101913.95604.B9. [DOI] [PubMed] [Google Scholar]
- 6.de Carvalho AP, de Almeida DF. Spread of activity through the atrioventricular node. Circ Res. 1960;8:801–809. doi: 10.1161/01.res.8.4.801. [DOI] [PubMed] [Google Scholar]
- 7.Billette J. Atrioventricular nodal activation during periodic premature stimulation of the atrium. Am J Physiol. 1987;252(1 Pt 2):H163–H177. doi: 10.1152/ajpheart.1987.252.1.H163. [DOI] [PubMed] [Google Scholar]
- 8.Medkour D, Becker AE, Khalife K, Billette J. Anatomic and functional characteristics of a slow posterior AV nodal pathway: role in dual-pathway physiology and reentry. Circulation. 1998;98(2):164–174. doi: 10.1161/01.cir.98.2.164. [DOI] [PubMed] [Google Scholar]
- 9.Hucker WJ, Nikolski VP, Efimov IR. Optical mapping of the atrioventricular junction. J Electrocardiol. 2005;38(4 Suppl):121–125. doi: 10.1016/j.jelectrocard.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Moe GK, Preston JB, Burlington H. Physiologic evidence for a dual A-V transmission system. Circ Res. 1956;4(4):357–375. doi: 10.1161/01.res.4.4.357. [DOI] [PubMed] [Google Scholar]
- 11.McGuire MA, Bourke JP, Robotin MC, Johnson DC, Meldrum-Hanna W, Nunn GR, Uther JB, Ross DL. High resolution mapping of Koch's triangle using sixty electrodes in humans with atrioventricular junctional (AV nodal) reentrant tachycardia. Circulation. 1993;88(5 Pt 1):2315–2328. doi: 10.1161/01.cir.88.5.2315. [DOI] [PubMed] [Google Scholar]
- 12.Jackman WM, Beckman KJ, McClelland JH, Wang X, Friday KJ, Roman CA, Moulton KP, Twidale N, Hazlitt HA, Prior MI, et al. Treatment of supraventricular tachycardia due to atrioventricular nodal reentry, by radiofrequency catheter ablation of slow-pathway conduction. N Engl J Med. 1992;327(5):313–318. doi: 10.1056/NEJM199207303270504. [DOI] [PubMed] [Google Scholar]
- 13.Mitrani RD, Klein LS, Hackett FK, Zipes DP, Miles WM. Radiofrequency ablation for atrioventricular node reentrant tachycardia: comparison between fast (anterior) and slow (posterior) pathway ablation. J Am Coll Cardiol. 1993;21(2):432–441. doi: 10.1016/0735-1097(93)90686-u. [DOI] [PubMed] [Google Scholar]
- 14.Shah D, Haissaguerre M, Gaita F. Slow pathway ablation for atrioventricular nodal reentry. J Cardiovasc Electrophysiol. 2002;13(10):1054–1055. doi: 10.1046/j.1540-8167.2002.01054.x. [DOI] [PubMed] [Google Scholar]
- 15.McGuire MA, de Bakker JM, Vermeulen JT, Moorman AF, Loh P, Thibault B, Vermeulen JL, Becker AE, Janse MJ. Atrioventricular junctional tissue. Discrepancy between histological and electrophysiological characteristics. Circulation. 1996;94(3):571–577. doi: 10.1161/01.cir.94.3.571. [DOI] [PubMed] [Google Scholar]
- 16.DeWitt L. Observations on the Sino-Ventricular Connecting System of the Mammalian Heart. Anat Rec. 1909;III(9):475–497. [Google Scholar]
- 17.Waki K, Kim JS, Becker AE. Morphology of the human atrioventricular node is age dependent: a feature of potential clinical significance. J Cardiovasc Electrophysiol. 2000;11(10):1144–1151. doi: 10.1111/j.1540-8167.2000.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 18.Hucker WJ, McCain ML, Laughner JI, Iaizzo PA, Efimov IR. Connexin 43 expression delineates two discrete pathways in the human atrioventricular junction. Anat Rec (Hoboken) 2008;291(2):204–215. doi: 10.1002/ar.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desplantez T, Halliday D, Dupont E, Weingart R. Cardiac connexins Cx43 and Cx45: formation of diverse gap junction channels with diverse electrical properties. Pflugers Arch. 2004;448(4):363–375. doi: 10.1007/s00424-004-1250-0. [DOI] [PubMed] [Google Scholar]
- 20.Coraboeuf E, Weidmann S. Potential de repos et potentials d'action du muscle cardiaque, mesures a l'aide d'electrodes intracellulaires. C.R. Soc Biol. 1949;143:1329. [Google Scholar]
- 21.Salama G, Morad M. Merocyanine 540 as an optical probe of transmembrane electrical activity in the heart. Science. 1976;191(4226):485–487. doi: 10.1126/science.191.4226.485. [DOI] [PubMed] [Google Scholar]
- 22.Efimov IR, Nikolski VP, Salama G. Optical imaging of the heart. Circ Res. 2004;95(1):21–33. doi: 10.1161/01.RES.0000130529.18016.35. [DOI] [PubMed] [Google Scholar]
- 23.Hucker WJ, Fedorov VV, Foyil KV, Moazami N, Efimov IR. Images in cardiovascular medicine. Optical mapping of the human atrioventricular junction. Circulation. 2008;117(11):1474–1477. doi: 10.1161/CIRCULATIONAHA.107.733147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hucker WJ, Nikolski VP, Efimov IR. Autonomic control and innervation of the atrioventricular junctional pacemaker. Heart Rhythm. 2007;4(10):1326–1335. doi: 10.1016/j.hrthm.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Efimov IR, Mazgalev TN. High-resolution, three-dimensional fluorescent imaging reveals multilayer conduction pattern in the atrioventricular node. Circulation. 1998;98(1):54–57. doi: 10.1161/01.cir.98.1.54. [DOI] [PubMed] [Google Scholar]
- 26.Fedorov VV, Ambrosi CM, Hucker WJ, Glukhov AV, Foyil KV, Wuskell J, Loew LM, Moazami N, Efimov IR. Human AV Junctional Pacemaker Shift Due to Cholinergic and Adrenergic Stimulations: Optical Imaging with a Novel Long Wavelength Voltage-Sensitive Dye; Paper presented at: Circulation; Oct 2008. [Google Scholar]
- 27.Zhang Y, Bharati S, Mowrey KA, Zhuang S, Tchou PJ, Mazgalev TN. His electrogram alternans reveal dual-wavefront inputs into and longitudinal dissociation within the bundle of His. Circulation. 2001;104(7):832–838. doi: 10.1161/hc3301.092804. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Bharati S, Mowrey KA, Mazgalev TN. His electrogram alternans reveal dual atrioventricular nodal pathway conduction during atrial fibrillation: the role of slow-pathway modification. Circulation. 2003;107(7):1059–1065. doi: 10.1161/01.cir.0000051464.52601.f4. [DOI] [PubMed] [Google Scholar]
- 29.Nikolski VP, Jones SA, Lancaster MK, Boyett MR, Efimov IR. Cx43 and dual-pathway electrophysiology of the atrioventricular node and atrioventricular nodal reentry. Circ Res. 2003;92(4):469–475. doi: 10.1161/01.RES.0000059304.97120.2F. [DOI] [PubMed] [Google Scholar]
- 30.Hucker WJ, Sharma V, Nikolski VP, Efimov IR. Atrioventricular conduction with and without AV nodal delay: two pathways to the bundle of His in the rabbit heart. Am J Physiol Heart Circ Physiol. 2007;293(2):H1122–H1130. doi: 10.1152/ajpheart.00115.2007. [DOI] [PubMed] [Google Scholar]
- 31.Inoue S, Becker AE, Riccardi R, Gaita F. Interruption of the inferior extension of the compact atrioventricular node underlies successful radio frequency ablation of atrioventricular nodal reentrant tachycardia. J Interv Card Electrophysiol. 1999;3(3):273–277. doi: 10.1023/a:1009868212415. [DOI] [PubMed] [Google Scholar]
- 32.Lockwood D, Nakagawa H, Jackman WM. Electrophysiologic Characteristics of Atrioventricular Nodal Rentrant Tachycardia: Implications for Reentrant Circuits. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 5th ed. Philadelphia: Saunders Elsevier; 2009. [Google Scholar]
- 33.Ko JK, Deal BJ, Strasburger JF, Benson DW., Jr Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am J Cardiol. 1992;69(12):1028–1032. doi: 10.1016/0002-9149(92)90858-v. [DOI] [PubMed] [Google Scholar]
- 34.Van Hare GF. Developmental aspects of atrioventricular node reentry tachycardia. J Electrocardiol. 2008;41(6):480–482. doi: 10.1016/j.jelectrocard.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, Kutalek SP, Sharma A. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288(24):3115–3123. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 36.Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick D, Sheldon T, Lamas GA. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357(10):1000–1008. doi: 10.1056/NEJMoa071880. [DOI] [PubMed] [Google Scholar]
- 37.Zanon F, Baracca E, Aggio S, Pastore G, Boaretto G, Cardano P, Marotta T, Rigatelli G, Galasso M, Carraro M, Zonzin P. A feasible approach for direct his-bundle pacing using a new steerable catheter to facilitate precise lead placement. J Cardiovasc Electrophysiol. 2006;17(1):29–33. doi: 10.1111/j.1540-8167.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 38.Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation. 2000;101(8):869–877. doi: 10.1161/01.cir.101.8.869. [DOI] [PubMed] [Google Scholar]
- 39.Deshmukh PM, Romanyshyn M. Direct His-bundle pacing: present and future. Pacing Clin Electrophysiol. 2004;27(6 Pt 2):862–870. doi: 10.1111/j.1540-8159.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- 40.Bianchi S, Rossi P, Della Scala A, Kornet L, Pulvirenti R, Monari G, Di Renzi P, Schauerte P, Azzolini P. Atrioventricular (AV) node vagal stimulation by transvenous permanent lead implantation to modulate AV node function: safety and feasibility in humans. Heart Rhythm. 2009;6(9):1282–1286. doi: 10.1016/j.hrthm.2009.05.011. [DOI] [PubMed] [Google Scholar]