Abstract
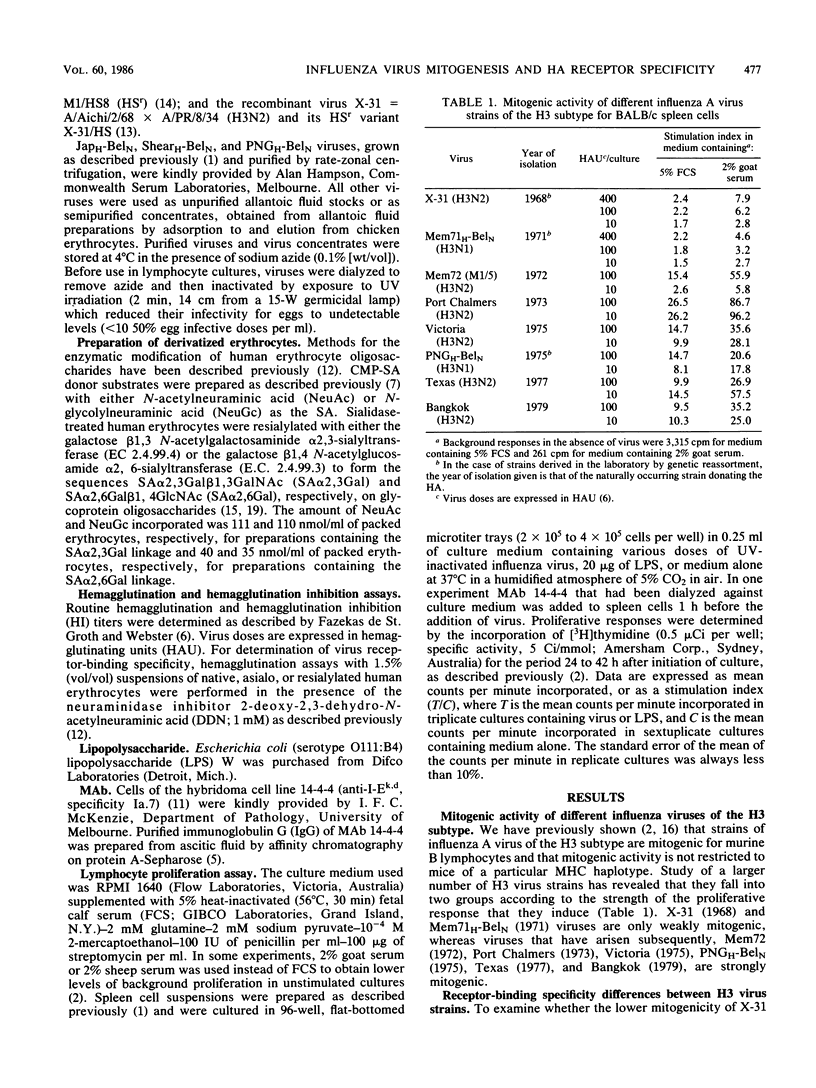
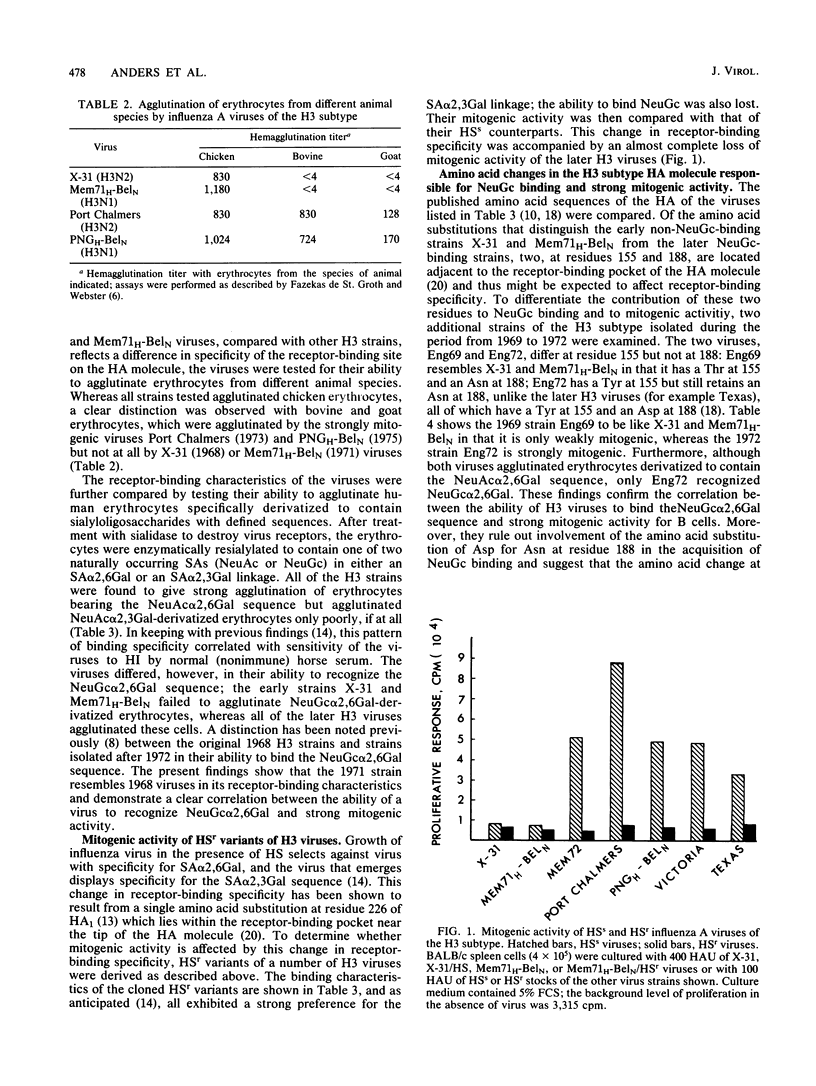
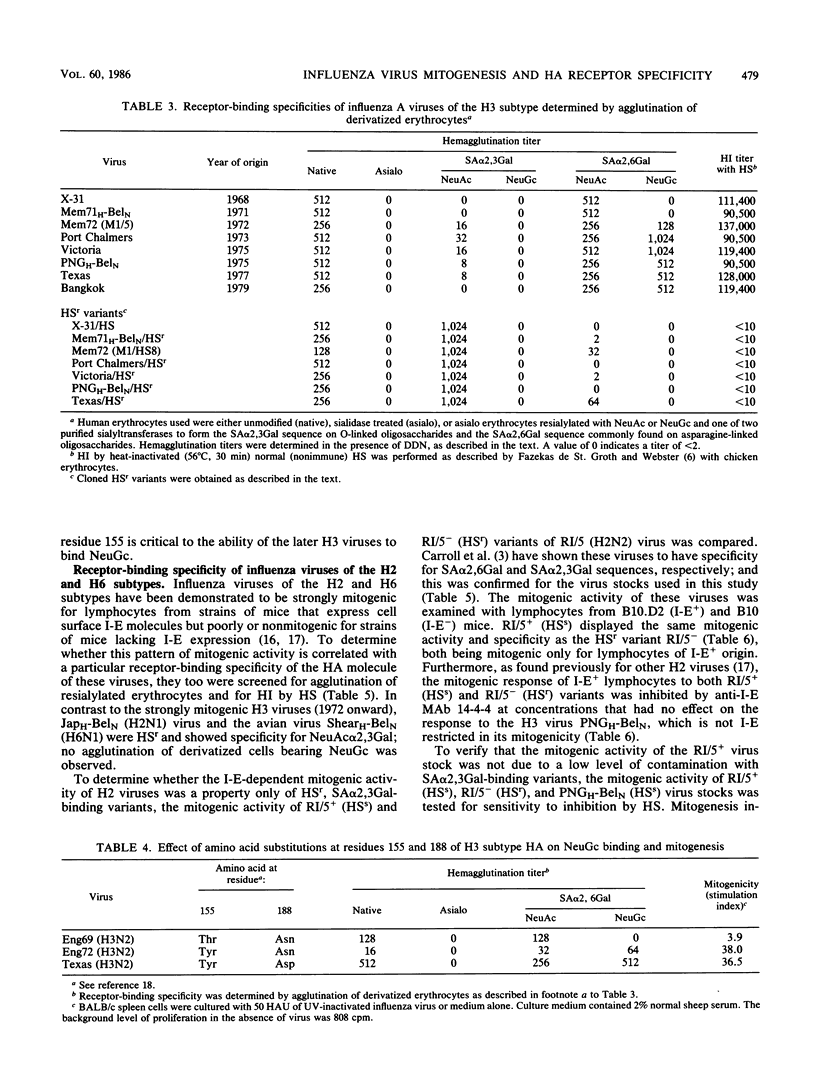
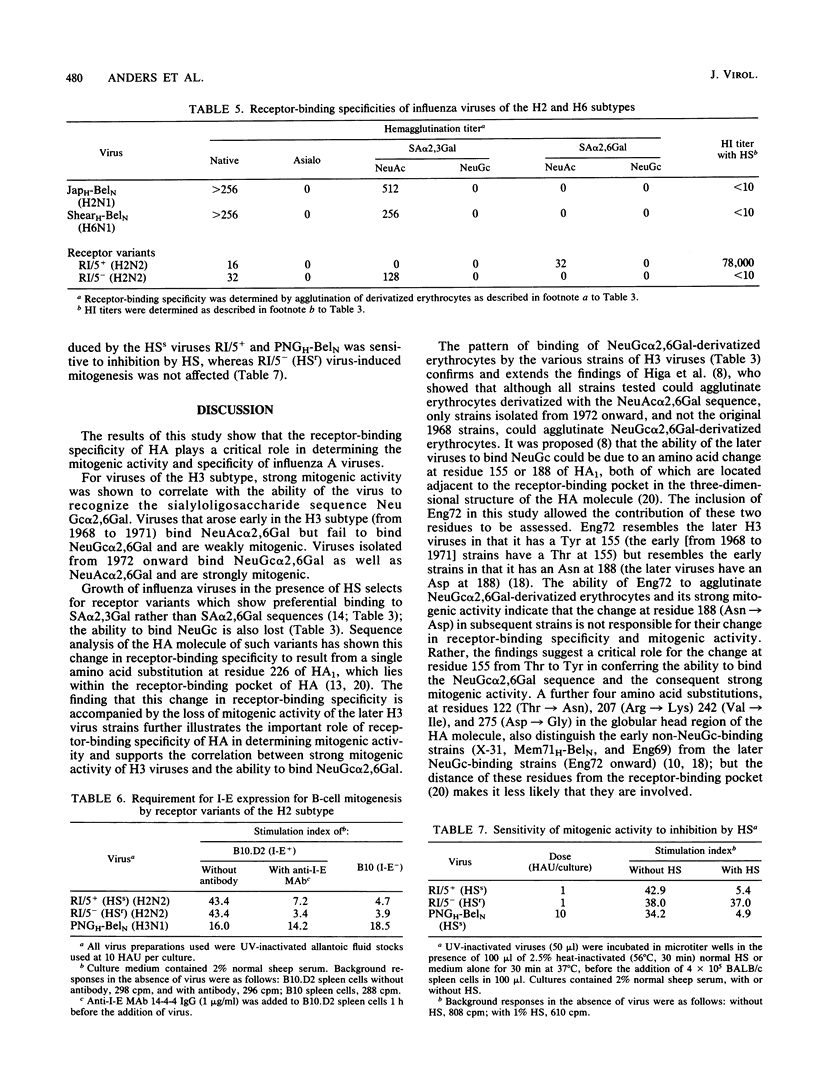
The relationship between the mitogenic activity of influenza type A viruses for murine B lymphocytes and the receptor-binding specificity of their hemagglutinin was examined. Receptor-binding specificity was determined by the ability of the virus to agglutinate erythrocytes that had been sialidase treated and then enzymatically resialylated to contain sialyloligosaccharides with defined sequences. Distinct differences in receptor-binding specificity were observed between strongly and weakly mitogenic viruses of the H3 subtype, with strong mitogenic activity correlating with the ability of the virus to recognize the sequence N-glycolylneuraminic acid alpha 2,6 galactose (NeuGc alpha 2,6Gal). Viruses isolated early in the evolution of the H3 subtype (from 1968 to 1971) are relatively weak mitogens and recognize the sequence N-acetylneuraminic acid alpha 2,6 galactose (NeuAc alpha 2,6Gal) but not NeuGc alpha 2,6Gal. H3 viruses isolated since 1972 are strongly mitogenic, and these viruses recognize both NeuGc alpha 2,6Gal and NeuAc alpha 2,6Gal. The amino acid substitution of Tyr for Thr at residue 155 of HA1 may be critical to this change in receptor-binding specificity and mitogenic activity of the later H3 viruses. Horse serum-resistant variants of H3 viruses, which bind preferentially to the sequence NeuAc alpha 2,3Gal, are poorly mitogenic. Differences were also observed between the receptor-binding specificity of the strongly mitogenic H3 viruses and viruses of the H2 and H6 subtypes, the mitogenic activity of which is limited to strains of mice that express the class II major histocompatibility complex glycoprotein I-E. The results indicate that the receptor-binding specificity of the hemagglutinin plays a critical role in determining the mitogenic activity of influenza viruses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders E. M., Peppard P. M., Burns W. H., White D. O. In vitro antibody response to influenza virus. I. T cell dependence of secondary response to hemagglutinin. J Immunol. 1979 Sep;123(3):1356–1361. [PubMed] [Google Scholar]
- Anders E. M., Scalzo A. A., White D. O. Influenza viruses are T cell-independent B cell mitogens. J Virol. 1984 Jun;50(3):960–963. doi: 10.1128/jvi.50.3.960-963.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. M., Higa H. H., Paulson J. C. Different cell-surface receptor determinants of antigenically similar influenza virus hemagglutinins. J Biol Chem. 1981 Aug 25;256(16):8357–8363. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth, Webster R. G. Disquisitions of Original Antigenic Sin. I. Evidence in man. J Exp Med. 1966 Sep 1;124(3):331–345. doi: 10.1084/jem.124.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa H. H., Paulson J. C. Sialylation of glycoprotein oligosaccharides with N-acetyl-, N-glycolyl-, and N-O-diacetylneuraminic acids. J Biol Chem. 1985 Jul 25;260(15):8838–8849. [PubMed] [Google Scholar]
- Higa H. H., Rogers G. N., Paulson J. C. Influenza virus hemagglutinins differentiate between receptor determinants bearing N-acetyl-, N-glycollyl-, and N,O-diacetylneuraminic acids. Virology. 1985 Jul 15;144(1):279–282. doi: 10.1016/0042-6822(85)90325-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Schauer R., Hahn H. Carbohydrate surface constituents of T cells mediating delayed-type hypersensitivity that control entry into sites of antigen deposition. Immunobiology. 1981;160(2):184–195. doi: 10.1016/S0171-2985(81)80046-0. [DOI] [PubMed] [Google Scholar]
- Newton S. E., Air G. M., Webster R. G., Laver W. G. Sequence of the hemagglutinin gene of influenza virus A/Memphis/1/71 and previously uncharacterized monoclonal antibody-derived variants. Virology. 1983 Jul 30;128(2):495–501. doi: 10.1016/0042-6822(83)90277-5. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983 Jun;127(2):361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Rogers G. N., Pritchett T. J., Lane J. L., Paulson J. C. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology. 1983 Dec;131(2):394–408. doi: 10.1016/0042-6822(83)90507-x. [DOI] [PubMed] [Google Scholar]
- Sadler J. E., Rearick J. I., Paulson J. C., Hill R. L. Purification to homogeneity of a beta-galactoside alpha2 leads to 3 sialyltransferase and partial purification of an alpha-N-acetylgalactosaminide alpha2 leads to 6 sialyltransferase from porcine submaxillary glands. J Biol Chem. 1979 Jun 10;254(11):4434–4442. [PubMed] [Google Scholar]
- Scalzo A. A., Anders E. M. Influenza viruses as lymphocyte mitogens. I. B cell mitogenesis by influenza A viruses of the H2 and H6 subtypes is controlled by the I-E/C subregion of the major histocompatibility complex. J Immunol. 1985 Feb;134(2):757–760. [PubMed] [Google Scholar]
- Scalzo A. A., Anders E. M. Influenza viruses as lymphocyte mitogens. II. Role of I-E molecules in B cell mitogenesis by influenza A viruses of the H2 and H6 subtypes. J Immunol. 1985 Nov;135(5):3524–3529. [PubMed] [Google Scholar]
- Ward C. W. Structure of the influenza virus hemagglutinin. Curr Top Microbiol Immunol. 1981;94-95:1–74. doi: 10.1007/978-3-642-68120-2_1. [DOI] [PubMed] [Google Scholar]
- Weinstein J., de Souza-e-Silva U., Paulson J. C. Sialylation of glycoprotein oligosaccharides N-linked to asparagine. Enzymatic characterization of a Gal beta 1 to 3(4)GlcNAc alpha 2 to 3 sialyltransferase and a Gal beta 1 to 4GlcNAc alpha 2 to 6 sialyltransferase from rat liver. J Biol Chem. 1982 Nov 25;257(22):13845–13853. [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]



