Abstract
Purpose
To analyse the combined and updated results from the University of Michigan and University Medical Center Utrecht on normal tissue complication probability (NTCP) of the parotid gland one year after radiotherapy (RT) for head and neck (HN) cancer.
Materials and methods
222 prospectively analyzed patients with various HN malignancies were treated with conventional and intensity-modulated RT. Stimulated individual parotid gland flow rates were measured before RT and one year after RT using Lashley cups at both centers. A flow ratio <25% of pre-treatment was defined as a complication. The data were fitted to the Lyman-Kutcher-Burman (LKB) model.
Results
A total of 384 parotid glands (Michigan: 157; Utrecht: 227 glands) was available for analysis one year after RT. Combined NTCP analysis based on mean dose resulted in TD50 (uniform dose leading to 50% complication probability) of 39.9 Gy and m (steepness of the curve) of 0.40. The resulting NTCP curve had good qualitative agreement with the combined clinical data. Mean doses 25-30 Gy were associated with 17-26% NTCP.
Conclusions
A definite NTCP curve for parotid gland function one year after RT is presented based on mean dose. No threshold dose was observed and TD50 was equal to 40 Gy.
Keywords: Parotid gland function, xerostomia, radiotherapy, NTCP, head-and-neck cancer
Introduction
Radiotherapy (RT) for head and neck (HN) malignancies generally results in a high radiation dose to the major salivary glands. Reduced salivary flow leads to xerostomia and this is a major cause of decreased quality of life in HN cancer survivors (1). The relationship between radiation dose, irradiated volume and the resulting salivary function after RT has been extensively described for the parotid salivary glands.
In these glands, a strong correlation exists between the mean dose to the gland and residual post-RT function (2). The University of Michigan and the University Medical Center Utrecht have published normal tissue complication probability (NTCP) curves that were based on a large cohort of patients (3, 4). Both have used objective parotid salivary flow measurements (using Lashley cups) as a function of the mean dose to study NTCP parameters. However, for parotid gland function one year after RT, different NTCP parameters were obtained. Eisbruch et al. described steep dose-response relationships in a population of 88 HN cancer patients treated with an intensity-modulated radiotherapy (IMRT) technique (3). The TD50 (the dose which results in 50% complication probability for whole parotid gland irradiated uniformly) at one year was determined at 28 Gy. Roesink et al. found no threshold dose in a study of 108 HN patients treated with conventional radiotherapy (CRT) using mostly opposed lateral fields (4). The TD50 at one year in that study was equal to 39 Gy. These differences could have been caused by the use of different RT techniques. Recently, however, it was shown that NTCP parameters for CRT and IMRT one year post-RT are comparable: TD50 being equal to 40 and 38 Gy, respectively (5).
The aim of this study was to analyse the combined, updated results from both institutions in order to arrive at a definitive NTCP curve for parotid gland function one year after RT and to guide clinical decision making.
Patients and methods
Patients and radiotherapy
At the University of Michigan, 92 HN cancer patients treated with primary or postoperative RT between 1994 and 2005 were prospectively evaluated. The parotid gland data (one year post-RT) of the first 54 patients were published previously (3). The remaining 38 patients were also described earlier (6), however not with respect to parotid gland function post-RT. Patients were treated using forward-planned, inverse-planned and beamlet IMRT according to previously detailed methods (3,6,7). The prescribed dose to the gross tumor volume or dissection site was 60-75 Gy in 1.8-2.0 Gy fractions (5 days per week).
In Utrecht, a total of 130 HN cancer patients were prospectively analyzed between 1996 and 2007. These patients’ parotid gland function data (up to one year after RT) were published recently (5). CRT (using opposing lateral beams) was used as well as inverse planned IMRT for the primary or postoperative treatment of various HN tumors. Details on treatment planning and target delineation have been published previously (4,8). The prescribed dose to the gross tumor volume or postoperative tumor bed was 50-70 Gy in 2 Gy fractions using CRT and 69-70 Gy in 2.0-2.3 Gy fractions with IMRT (5 days per week).
For each patient, contrast-enhanced computer-tomography (CT) imaging of the HN region was performed. The left and right parotid glands were delineated on the axial CT slices. Three-dimensional dose distributions in the complete volume of the parotid glands were calculated and converted into dose-volume histograms (DVHs). Each gland was thus analyzed separately. These prospective studies were respectively approved by the Institutional Review Board of the University of Michigan and the Medical Ethical Committee of the University Medical Center Utrecht. Informed consent was obtained from each patient.
Parotid flow measurements
Techniques that were used in Michigan and Utrecht for parotid saliva measurements have been described previously (3,4). The stimulated parotid saliva was collected by Lashley cups after applying citric acid solution (2-5%) on the mobile part of the tongue. Patients were instructed not to eat or drink 60-90 minutes before saliva collection. To avoid the influence of diurnal variation in salivary flow, consecutive measurements were scheduled at the same daytime in each patient. Salivary flow rates were measured before treatment and at one year after RT.
The flow rate at one year was converted into the percentage of the baseline flow rate. A complication was defined for each individual gland as a stimulated parotid flow ratio <25% of the pretreatment flow rate, grade 4 xerostomia according to the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer (RTOG/EORTC) Late Effects Consensus Conference (9).
Normal tissue complication probability model
The parotid flow data were fitted to the NTCP model proposed by Lyman, Kutcher and Burman (LKB model) (10,11). This model is assumed to quantitatively establish the effects of both radiation dose and irradiated volume on the probablility of radiation-induced changes in parotid gland function. Three parameters are present in the sigmoid dose-response relationship described in the LKB model. TD50 is the dose at which a 50% complication probability is seen after uniform parotid gland irradiation and parameter m describes the slope of the NTCP curve. Parameter n accounts for the volume effect of an organ and depends on the tissue organization (10). If n is high (close to or higher than 1) partial sparing of the organ reduces complication probability. This is referred to as a parallel organization of the organ, such as in liver and lungs. The mean dose influences complication probability in this situation. If n approaches zero, the maximum dose influences complication probability. This serial architecture is thought to be present in spinal cord and oesophagus, for example.
NTCP curves that were published previously have used the mean dose (n=1) as descriptive dose parameter (3-5). In the present combined analysis, we also fixed n at 1 and thereby described parotid gland function one year after RT as a function of the mean dose (mean dose model). In addition, we fitted the combined data to the LKB model with n unrestricted in order to investigate if a n value ≠ 1 described the data better (full LKB model).
Statistical analysis
Patient characteristics were analyzed using descriptive statistics (mean, ranges or proportions; where appropriate).
To investigate whether both institutes differed with respect to the NTCP endpoint one year after RT, the relative risk (RR) and accompanying 95% confidence interval (CI) of parotid gland complication probability was calculated for Utrecht versus Michigan. A modified Poisson regression model was used to adjust this relative risk for the mean parotid gland dose (5, 12). The NTCP parameters (TD50, n and m) were determined by a maximum likelihood estimation method described previously (4,13). Before combining the data, separate analyses were performed for the Utrecht and Michigan cohort.
To compare the fits of the mean dose model (n = 1) and the full LKB model (n not restricted) we computed the goodness-of-fit (GOF) using the deviance (Δ). This parameter is defined as minus twice the difference between the log likelihood of the actual fitted model and the log likelihood of the experimental data (14).
All analyses were performed using SPSS version 16 (SPSS Inc., Chicago, IL, USA) except for the modified Poisson regression analysis that was performed using the PROC GENMOD procedure in SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). The two-tailed significance level was set at 0.05. The NTCP-modeling was performed using software developed at the Department of Radiation Oncology at the University Medical Center in Groningen, The Netherlands (13).
Results
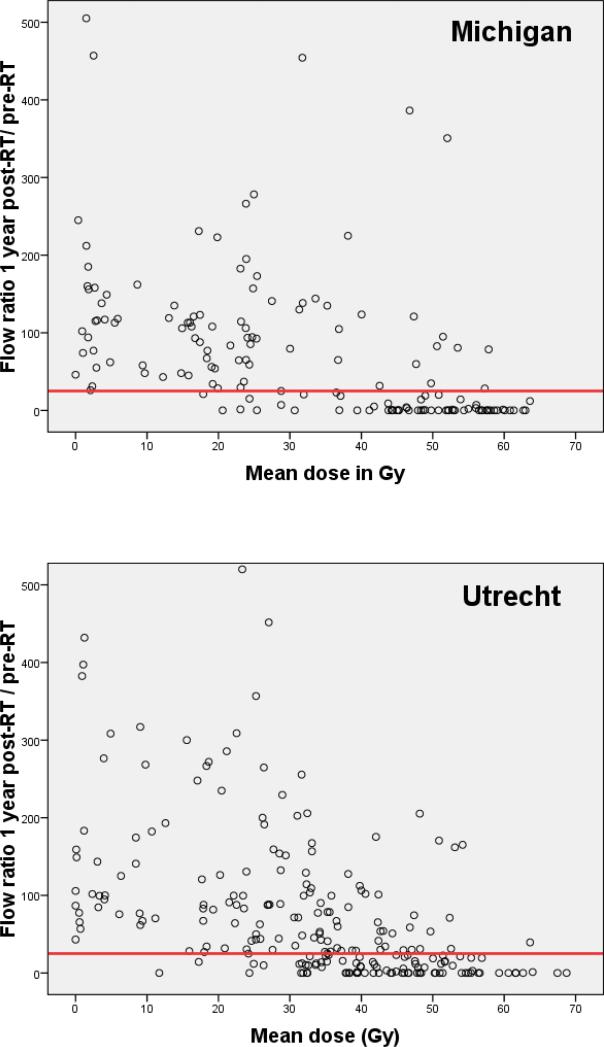
Patient-, tumor- and treatment characteristics of the patients are outlined in Table 1. In total, 384 parotid glands (Michigan: 157; Utrecht: 227) from 222 patients were available for analysis one year after treatment. The patient population in both Michigan and Utrecht included heterogenous tumor sites in order to represent the full range of the mean dose in the parotid gland, which is optimal for NTCP modelling (Figure 1).
Table 1.
Patient and tumor characteristics, n (%).
| Michigan (n = 92) | Utrecht (n = 130) | |
|---|---|---|
| Gender | ||
| Male | 65 (71) | 92 (71) |
| Female | 27 (29) | 38 (29) |
| Age (y) | ||
| Median | 54 | 58 |
| Range | 20-82 | 24-99 |
| Tumor site | ||
| Larynx | 4 (4) | 47 (36) |
| Hypopharynx | 4 (4) | 2 (2) |
| Oropharynx | 53 (58) | 41 (31) |
| Nasopharynx | 4 (4) | 12 (9) |
| Oral cavity | 8 (9) | 11 (8) |
| Nasal cavity | 6 (5) | |
| Salivary glands | 9 (10) | 1 (1) |
| Unknown primary | 4 (4) | 1 (1) |
| Other* | 6 (7) | 9 (7) |
| Stage (AJCC) | ||
| I | 2 (2) | 12 (9) |
| II | 12 (13) | 35 (27) |
| III | 26 (29) | 30 (23) |
| IV | 48 (52) | 36 (28) |
| Recurrent/unknown | 4 (4) | 17 (13) |
| Radiotherapy | ||
| Definitive | 49 (53) | 85 (65) |
| Postoperative | 43 (47) | 45 (35) |
Other: (Michigan) skin 4 patients and maxillary sinuses 2 patients; (Utrecht) Hodgkin/non-Hodgkin lymphoma 4 patients, skin/lip 3 patients, orbita 1 patient, upper trachea 1 patient.
Abbreviation: AJCC = American Joint Committee on Cancer Staging Manual (sixth edition, 2002).
Figure 1.
Parotid flow ratio at one year post-RT as a function of the mean parotid gland dose for Michigan (157 glands) and Utrecht (227 glands). The horizontal line indicates the complication threshold according to RTOG / EORTC grade 4 xerostomia (flow ratio <25% of pre-treatment).
The Michigan and Utrecht cohorts did not differ with respect to the parotid gland complication probability one year after RT, corrected for mean dose (Table 2). Comparable NTCP parameters for both cohorts with overlapping confidence intervals were observed (Table 3). Consequently, the data could be combined to arrive at a single set of NTCP parameters.
Table 2.
Poisson regression analysis for the risk of parotid flow complications one year after RT for Michigan versus Utrecht, corrected for the mean parotid gland dose.
| One year post-RT | Crude RR (95% CI) | Adjusted RR (95% CI)* | p-value† |
|---|---|---|---|
| Michigan vs Utrecht | 1.01 (0.79-1.3) | 0.96 (0.78-1.18) | 0.68 |
| Mean dose (Gy) | 1.05 (1.04-1.06) | <0.0001 |
Adjusted for mean parotid gland dose (in Gy) on the endpoint parotid flow to <25% of pre-treatment.
p-value for the adjusted RR.
Abbreviation: 95% CI = 95% confidence interval; RR = relative risk.
Table 3.
Parameters TD50 and m (95% CI) in terms of mean parotid gland dose (n = 1) for flow data one year post-RT.
| Michigan | Utrecht | |
|---|---|---|
| TD50 (Gy) | 40.5 (36.8-44.1) | 39.7 (37-43.3) |
| m | 0.36 (0.28-0.44) | 0.44 (0.35-0.54) |
Abbreviation: CI = confidence interval; TD50 = the uniform dose to the whole organ resulting in 50% complication probability; m = slope of the NTCP curve.
The combined analysis according to mean dose model (n = 1) resulted in TD50 = 39.9 Gy and m = 0.40. Fitting of the full LKB model (n unrestricted) yielded similar results. Volume dependency parameter n equalled 1.13 in the optimal LKB model fit. This indicates a parallel organization of functional subunits in the parotid gland. There was hardly any difference in goodness-of-fit between both models (expressed as the deviance (Δ), Table 4). At one year post-RT, the mean dose described the probability of flow complications very satisfactory. We chose to describe the NTCP by the mean dose model over the full LKB model because only two parameters had to be fitted (TD50 and m) with comparable goodness-of-fit. Furthermore, it is easy to use in treatment planning.
Table 4.
Combined analysis: parameters TD50, m and n (95% CI) in terms of mean dose (n = 1) and with n unrestricted for flow data one year post-RT. Goodness-of-fit is expressed as the deviance (Δ).
| mean dose (n = 1) | full LKB (n unrestricted) | |
|---|---|---|
| TD50 (Gy) | 39.9 (37.3-42.8) | 39.4 (33.8-41.8) |
| m | 0.40 (0.34-0.51) | 0.42 (0.36-0.58) |
|
n
|
1 |
1.13 (0.75-14.3) |
| Δ | 339.2 | 340.6 |
Abbreviation: CI = confidence interval; TD50 = the uniform dose to the whole organ resulting in 50% complication probability; m = slope of the complication probability curve; n = volume dependency parameter.
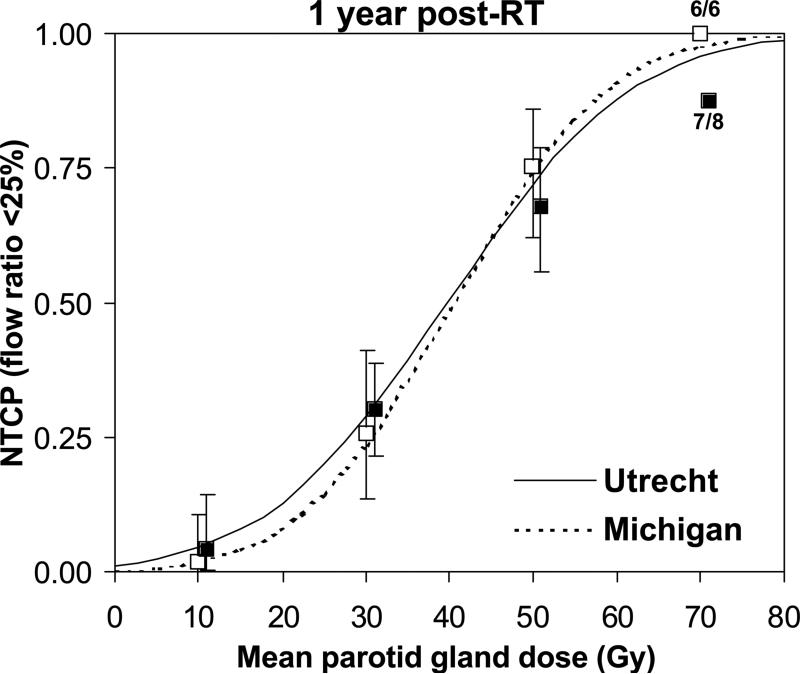
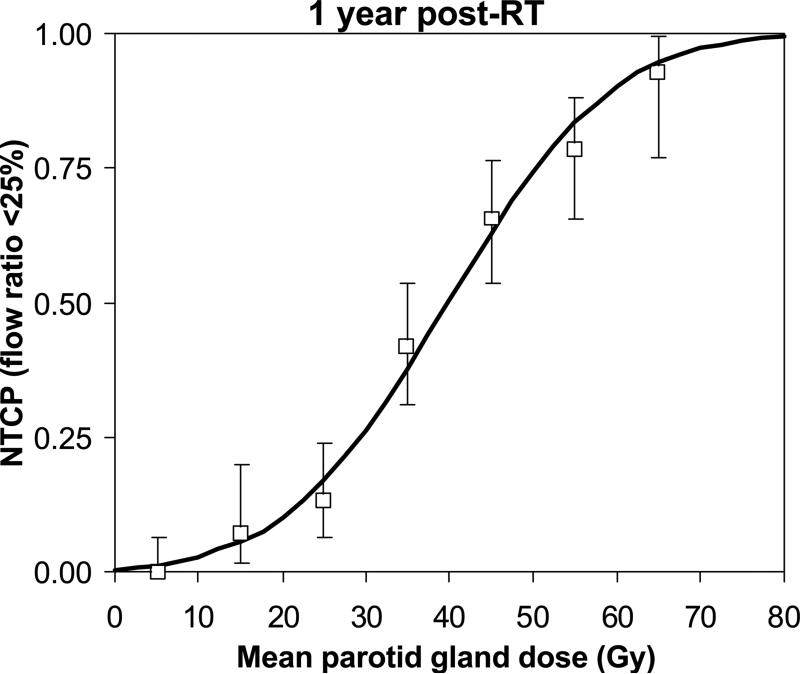
Very similar NTCP curves were observed for the Michigan and Utrecht cohorts separately, confirming the data can be combined (Figure 2). The combined NTCP curve based on mean dose (Figure 3) had good qualitative agreement with the clinical data.
Figure 2.
Normal tissue complication probability (NTCP) curves as a function of the mean parotid gland dose for Michigan (dashed line) and Utrecht (solid line). Clinical NTCP values (using mean dose bins of 20 Gy) are shown forMichigan (open squares) and Utrecht (black squares) including 95% CI.
Figure 3.
Combined Michigan and Utrecht normal tissue complication probability (NTCP) curve as a function of the mean parotid gland dose. Clinical NTCP values (using mean dose bins of 10 Gy) are shown including 95% CI.
Discussion
This study represents the largest series in literature of selective parotid gland function measurements one year after radiotherapy for HN cancer. Based on this analysis, a definite NTCP curve for parotid gland function (one year after RT) is presented for use in clinical practice. No threshold dose was observed. At a mean dose of 39.9 Gy, there is a 50% probability of parotid gland flow reduction to <25% of the pre-radiotherapy outflow. The resulting NTCP parameterization of the combined clinical data is consistent with the presumed parallel organization in the parotid gland. Also, it shows the strong predictive ability of the mean dose on the probability of parotid flow reduction to <25% at one year.
This report represents an update of the data presented by Eisbruch et al. and by Roesink et al. (3,4). Additional patients were included in salivary gland studies at both departments and more advanced RT techniques such as inverse-planned IMRT were used (5,7). The results therefore represent a heterogeneous HN cancer patient population (n=222) treated with both conventional 3D and IMRT techniques. Differences with previously published results probably stem from inhomogeneity in the underlying data, especially in the critical dose range (30-40 Gy). The initial Michigan dataset (3) had little data (n=3) in the 30-40 Gy mean dose range at one year (near the TD50). This probably influenced the Lyman model fit and the steep shape of the resulting NTCP curve. The current updated Michigan dataset (Figure 1) however, contains more data in the critical mean dose range and the individual NTCP curves from both institutions are very similar (Figure 2). Mean parotid gland doses of 25-30 Gy now correspond to 17-26% complication probability one year post-RT. Taken all together, a large cohort of patients and measurements are required to reliably describe the dose-response curve in the parotid gland and in any other organ, for that matter (17). Combining multi-institutional experience is one way to achieve this.
There have been several publications on dose-response modelling in the parotid gland using whole salivary flow and salivary gland scintigraphy in stead of selective parotid flow measurements (18-21). These studies have often used different endpoints which makes it difficult to compare them. Besides the fact that most data originates from relatively small patient cohorts, there are some drawbacks to the techniques mentioned. With whole mouth saliva, individual parotid flow cannot be measured and uncertainty is introduced as the contributions from the submandibular and minor salivary glands are ignored. Scintigraphy is a good indicator of parotid gland function and can detect the gland's ability to collect and excrete saliva to small amounts. It is expensive, however, more invasive and requires hospital equipment. Moreover, in a comparison to determine the best measure for parotid gland function, we found that stimulated flow measurements one year after RT using Lashley cups (complication defined as flow <25% of the pre-RT output) correlated better with mean parotid gland dose than did scintigraphy (22).
In conclusion, when aiming at preservation of parotid gland function after RT for HN tumors, this study shows a gradual increase in NTCP with increasing mean dose. In fact, a treatment planning constraint of 25-30 Gy corresponds to 17-26% complication probability at one year. At 40 Gy mean dose, there is a 50% probability of parotid gland flow reduction to <25% of the pre-RT flow (Figure 3). By combining multi-institutional experience we achieved a large patient cohort which helped to construct a reliable NTCP curve for use in RT practice.
Acknowledgment
The authors would like to thank Carla van Gils, Ph.D. (Julius Center Utrecht) for her contribution on Poisson regression modeling, and Cees Schilstra, Ph.D. (Radiation Oncology, University Medical Center Groningen) for NTCP software development.
Supported by the Dutch Cancer Society (KWF grant UU 2006-3573) and in part by NIH grant CA59827.
This research was presented (oral) at the 50th American Society for Therapeutic Radiology And Oncology (ASTRO) Meeting, Boston, MA, September 21 – 25, 2008; and (oral) at the 27th European Society for Therapeutic Radiology and Oncology (ESTRO) Meeting, Goteborg, Sweden, September 14-18, 2008.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest notification: Any actual or potential conflicts of interests do not exist.
References
- 1.Bjordal K, Kaasa S, Mastekaasa A. Quality of life in patients treated for head and neck cancer: a follow-up study 7 to 11 years after radiotherapy. Int J Radiat Oncol Biol Phys. 1994;28:847–856. doi: 10.1016/0360-3016(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 2.Eisbruch A, Ship JA, Kim HM, et al. Partial irradiation of the parotid gland. Semin Radiat Oncol. 2001;11:234–239. doi: 10.1053/srao.2001.23484. [DOI] [PubMed] [Google Scholar]
- 3.Eisbruch A, Ten Haken RK, Kim HM, et al. Dose, volume and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 4.Roesink JM, Moerland MA, Battermann JJ, et al. Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2001;51:938–946. doi: 10.1016/s0360-3016(01)01717-5. [DOI] [PubMed] [Google Scholar]
- 5.Dijkema T, Terhaard CH, Roesink JM, et al. Large cohort dose-volume response analysis of parotid gland function after radiotherapy: intensity-modulated versus conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1101–1109. doi: 10.1016/j.ijrobp.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 6.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 7.Vineberg KA, Eisbruch A, Coselmon MM, et al. Is uniform target dose possible in IMRT plans in the head and neck? Int J Radiat Oncol Biol Phys. 2002;52:1159–1172. doi: 10.1016/s0360-3016(01)02800-0. [DOI] [PubMed] [Google Scholar]
- 8.Braam PM, Terhaard CH, Roesink JM, et al. Intensity-modulated radiotherapy significantly reduces xerostomia compared with conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:975–980. doi: 10.1016/j.ijrobp.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 9.LENT SOMA Tables Radiother Oncol. 1995;35:17–60. [PubMed] [Google Scholar]
- 10.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res. 1985;8:S13–S19. [PubMed] [Google Scholar]
- 11.Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989;16:1623–1630. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 12.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 13.Schilstra C, Meertens H. Calculation of the uncertainty in complication probability for various dose-response models, applied to the parotid gland. Int J Radiat Oncol Biol Phys. 2001;50:147–158. doi: 10.1016/s0360-3016(00)01553-4. [DOI] [PubMed] [Google Scholar]
- 14.Collet D. Modelling binary data. Chapman & Hall; 1991. [Google Scholar]
- 15.Burlage FR, Pijpe J, Coppes RP, et al. Variability of flow rate when collecting stimulate human parotid saliva. Eur J Oral Sci. 2005;113:386–390. doi: 10.1111/j.1600-0722.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghezzi EM, Lange LA, Ship JA. Determination of variation of stimulated salivary flow rates. J Dent Res. 2000;79:1874–1878. doi: 10.1177/00220345000790111001. [DOI] [PubMed] [Google Scholar]
- 17.Schultheiss TE. The controversies and pitfalls in modeling normal tissue radiation injury/damage. Semin Radiat Oncol. 2001;11:210–214. doi: 10.1053/srao.2001.23479. [DOI] [PubMed] [Google Scholar]
- 18.Chao KS, Deasy JO, Markman, et al. A prospective study of salivary function sparing in patients with head- and- neck cancers receiving intensity-modulated or three-dimentional radiotherapy: initial results. Int J Radiat Oncol Biol Phys. 2001;49:907–916. doi: 10.1016/s0360-3016(00)01441-3. [DOI] [PubMed] [Google Scholar]
- 19.Blanco AI, Chao KS, El Naqa I, et al. Dose-volume modeling of salivary function in patients with head-and-neck cancer receiving radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1055–1069. doi: 10.1016/j.ijrobp.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 20.Münter MW, Karger CP, Hoffner SG, et al. Evaluation of salivary gland function after treatment of head-and-neck tumors with intensity-modulated radiotherapy by quantitative pertechnetate scintigraphy. Int J Radiat Oncol Biol Phys. 2004;58:175–184. doi: 10.1016/s0360-3016(03)01437-8. [DOI] [PubMed] [Google Scholar]
- 21.Tenhunen M, Collan J, Kouri M, et al. Scintigraphy in prediction of the salivary gland function after gland-sparing intensity modulated radiation therapy for head and neck cancer. Radiother Oncol. 2008;87:260–267. doi: 10.1016/j.radonc.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Roesink JM, Schipper M, Busschers W, et al. A comparison of mean parotid gland dose with measures of parotid gland function after radiotherapy for head-and-neck cancer: implications for future trials. Int J Radiat Oncol Biol Phys. 2005;63:1006–1009. doi: 10.1016/j.ijrobp.2005.04.023. [DOI] [PubMed] [Google Scholar]