Abstract
Background
The tumor microenvironment is important for progressive and metastatic disease.
Objective
To study the hypothesis that prostate fibroblasts have differential ability to induce castration-resistant prostate cancer (PCa) and metastatic progression and whether this effect might vary depending on the zonal origin of the fibroblast.
Design, setting, and participants
Human prostate fibroblasts from the peripheral (PZ), transition (TZ) and central (CZ) zones of radical prostatectomy specimens (n = 13) were isolated and compared for their ability to promote androgen independence and metastatic progression in androgen-responsive PCa lymph node carcinoma of the prostate (LNCaP) cells in vivo.
Interventions
By coinoculating marginally tumorigenic LNCaP cells with PZ or TZ and by altering host hormonal milieu, a series of tumorigenic and metastatic LNCaP epithelial sublines–P4, P4-2 (derivatives from interaction with PZ), T4, and T4-2 (derivatives from interaction with TZ)–were established and characterized.
Measurements
In vivo and in vitro evaluation of induction of tumor growth and metastatic potential.
Results and limitations
1) LNCaP sublines were permanently altered in their cytogenetic and biologic profiles after cellular interaction with prostate stromal fibroblasts. LNCaP sublines grew faster under anchorage-dependent and -independent conditions, expressed 1–12-fold more prostate-specific antigen in vitro than LNCaP cells, and gained metastatic potential; 2) zonal differences of stromal fibroblasts in their ability to induce the growth and progression of LNCaP tumors as xenografts in mice may exist but need further analysis; 3) PZ-conditioned medium induced more anchorage-independent growth of LNCaP cells in vitro. TZ had a higher growth rate and were more sensitive to dihydrotestosterone.
Conclusions
We demonstrate that prostate fibroblasts have growth inductive potential on PCa cells and affect their subsequent progression to castration resistance and development of a metastatic phenotype.
Keywords: Androgen-independence, Animal model, Bone metastasis, LNCaP, Prostate cancer, Prostate fibroblasts, Stromal-epithelial Interaction, Transition, peripheral and central zones, Tumor microenvironment
1. Introduction
The last decade has brought increased attention to and awareness of prostate cancer (PCa) as a significant public health problem [1]. Development of bone metastases and castration resistance are the hallmarks of advanced PCa. Experimental evidence suggests that reciprocal interactions between PCa epithelial cells and their surrounding microenvironment may be involved in the induction and development of PCa and that modulation of the surrounding stromal compartment might change the biological behaviors of the cancer epithelial cells [2,3].
We have previously demonstrated in a coinoculation model using nontumorigenic PCa lymph node carcinoma of the prostate (LNCaP) and M series osteogenic sarcoma cells [4] that stromal cells can induce tumor growth and confer a survival benefit in the androgen-deprived milieu as well as metastatic potential emphasizing the role of the tumor microenvironment. The LNCaP cell sublines derived from these coinoculation experiments reflect the various steps of PCa progression [5].
Given the pivotal role of stromal cells on PCa progression, we wanted to determine the effect of human prostate-derived fibroblasts on PCa cell biology and behavior. PCa originates most frequently from the peripheral zone (PZ) of the prostate gland, whereas benign prostate hyperplasia (BPH) originates most often from the transition zone (TZ) [6,7]. Therefore we evaluated whether prostate myofibroblasts are able to induce PCa cell growth and whether the zone of origin of the prostate fibroblast is decisive. Furthermore, we wanted to assess whether altering the microenvironment and the hormonal milieu can induce castration resistance and metastatic potential, and to characterize the cytogenetic and biological characteristics of these LNCaP sublines in vivo and in vitro.
2. Materials and methods
2.1. Cell lines
LNCaP (ATCC), C4-2 [4] and NbE rat prostate epithelial cells [8] were used. Cells were grown in T medium (80% Dulbecco’s modified Eagle’s medium (DMEM; Gibco Laboratories, Invitrogen Corp., Carlsbad, CA, USA), 20% F12K (Irvine Scientific, Santa Ana, CA, USA), 3 g/l sodium bicarbonate (NaHCO3), 100 U/l penicillin G, 100 μg/ml streptomycin, 5 μg/ml insulin, 13.6 pg/ml triiodothyronine, 5 μg/ml transferrin, 0.25 μg/ml biotin, 25 μg/ml adenine) with 5% fetal bovine serum (FBS). All cells were free of mycoplasma contamination by reverse transcription-polymerase chain reaction (RT-PCR).
2.2. Isolation of human prostate fibroblasts
Prostate fibroblasts were isolated from radical prostatectomy specimens from defined zonal origin (central zone [CZ], TZ, and PZ) of the prostate glands on the non-tumor-bearing side of the specimen (based on initial biopsy and macroscopic evaluation when clearly delimitable), minced, placed into culture dishes, and cultured in T medium and 10% FBS. Epithelial cells, which adhere more loosely to culture dishes than fibroblasts, were removed by light trypsinization and mechanical irrigation with tissue culture medium. Pure prostate fibroblast cell lines, as judged by morphological and immunohistochemical criteria (vimentin positive but cytokeratin and desmin negative), were obtained after 8–12 rounds of subculturing steps.
2.3. Derivation of LNCaP sublines
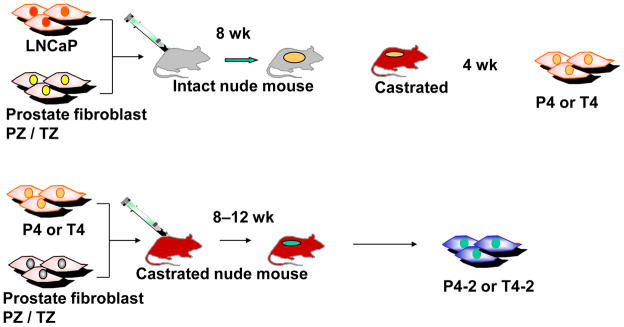
Athymic male nude mice aged 6–8 wk (balb/c; Charles Rivers Laboratories, Wilmington, MA, USA) were used for in vivo tumor growth studies and kept according to approved institutional guidelines. Tumors were induced by subcutaneous coinoculation of 1.0 × 106 LNCaP cells with 1.0 × 106 fibroblasts from TZ, PZ, or CZ. Mice bearing TZ and PZ chimeric tumors were castrated under anesthesia at 8 wk (Fig. 1). Tumors were maintained in castrated hosts for 4 wk. The LNCaP sublines were isolated as described [4]. After five or six passages, pure epithelial derivative cell lines, termed P4 and T4, were established from chimeric tumors as assessed by morphology and positive immunohistochemical staining for androgen receptor, prostate-specific antigen (PSA), and cytokeratins 5 (CK5), 8 (CK8), and 18 (CK18) and negative staining for vimentin.
Fig. 1.
Scheme of the development of the different cell lines.
PZ = peripheral zone; TZ = transition zone.
From the established T4 and P4 LNCaP sublines we derived further the second generation of LNCaP sublines, termed T4-2 and P4-2, from chimeric tumors harvested from castrated mice coinoculated subcutaneously with 1.0 × 106 primary T4 or P4 cells with 1.0 × 106 of their respective TZ or PZ fibroblasts (Fig. 1). Tumors were maintained for 8–12 wk after injection and sublines were derived from tumors as described [4].
2.3. In vivo tumorigenic and metastatic potential of derivative LNCaP sublines
In order to evaluate growth and metastatic potential of derivative LNCaP sublines in vivo, castrated male mice were injected either subcutaneously (4 sites per animal, 4 animals per group) or orthotopically into the dorsal prostate (10 animals per group) with each of the sublines. Orthotopic injections were performed under anesthesia. Animals were followed regularly for weight loss and by x-ray analysis and sacrificed when signs of metastases occurred.
2.4. Intrinsic growth rate of cell lines
In vitro growth of TZ and PZ fibroblasts, as well as LNCaP and its sublines, was determined by crystal violet dye assay [9], and 0.5 × 103 cells were plated and incubated for 9 d. At days 3, 6, and 9, cells were fixed with 1% glutaraldehyde and stained with 0.5% crystal violet. The dye was eluted with 0.5 ml of Sorenson’s solution and 100 μl transferred to a 96-well plate. Absorbance of each well was measured by a Titertek Multiscan TCC/340 (Flow Laboratories, McLean, VA, USA) at 560 nm. Control experiments demonstrated that absorbance reading of eluted dye is directly proportional to the number of live cells per well. Cells were plated in triplicates and experiments performed three times.
2.4. Determination of prostate-specific antigen
Once tumors became measurable, serum PSA values (ng/ml) were determined by microparticle enzyme immunoassay (Abbott IMx clinical analyzer, Abbott Park, IL, USA). PSA expression in cell lines in vitro was measured in the supernatant 3 d after plating of 1 × 104 cells per well in T medium containing 5% FBS. Cells were plated in triplicates and experiments performed twice.
2.5. Histomorphologic and immunohistochemical characterization
Tumor specimens were fixed in 10% neutralized formalin and paraffin embedded. Cells were seeded 5–10 × 103 per well in chambered slides, cultured overnight, and fixed in 10% neutralized formalin. Deparaffinized tissues or fixed cells were subjected to hematoxylin and eosin staining and immunohistochemical analyses. Deparaffinized 4-μm tissue sections or fixed cells were treated with 3% hydrogen peroxide (H2O2); blocked with Super Block (ScyTek Laboratories, Logan, UT, USA); incubated with primary antibody, followed by a reaction with biotinylated goat anti-immunoglobulins (Bio-Genex Laboratories, Jackson, WY, USA); then rinsed and treated with peroxidase-conjugated streptavidin (Bio-Genex). 3,3′-diaminobenzidine and H2O2 were used as substrate. Monoclonal antibodies used were PSA, desmin (Bio-Genex), vimentin, CK18 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and CK5 (Novocastra, Leica Microsystems, Wetzlar, Germany). As negative control, background activity was determined using matching mouse immunoglobulin subtypes or normal rabbit or goat serum.
2.6. Cytogenetic analysis of LNCaP and derivative sublines
Cultures from LNCaP and sublines were exposed to colcemid (0.02 μg/ml) for 40 min at 37°C and the single-cell suspension centrifuged at 1700 rpm for 5 min. The cell pellet was exposed to 0.06 M potassium chloride (KCl) for 15–20 min at 20°C. After centrifugation (1700 rpm for 5 min), cells were fixed in acetic acid:methanol [1:3, volume-to-volume (v/v), 15 min] and then washed three times in the fixative. Conventional air-drying chromosome preparations were made as described [10]. A minimum of five complete karyotypes per cell line were prepared and additional G-banded metaphase spreads were evaluated for the presence or absence of marker chromosomes.
2.7. Growth responsiveness to dihydrotestosterone
Three matched TZ- and PZ-derived fibroblast cell lines were cultured in vitro under serum-free conditions either without (control) or with the addition of 1 nM dihydrotestosterone. Cells were plated in triplicates and experiments performed twice. Growth was measured at day 9 as described [9].
2.8. Conditioned media preparation
Conditioned media (CM) from prostate fibroblast cultures were prepared at 70–80% confluency by downshifting to serum-free T medium. After 48 h, supernatant was removed, lyophilized, and protein concentrations were determined (Bio-Rad Laboratories, Hercules, CA, USA); mean protein concentration was 1.3 mg/ml. Samples were reconstituted in T medium to two times concentration, filtered (0.2 μm), and added to the cell lines at a ratio of 1:1 (v/v) to T medium.
2.9. Clonogenic growth of NbE and, LNCaP cells and LNCaP sublines
Clonogenic growth in vitro reflects tumorigenicity [11]. To determine intrinsic and fibroblast CM-induced clonogenic growth of NbE and of parental and derivative LNCaP cells, 2.5 × 103 cells were trypsinized to single-cell suspensions, resuspended in 0.3% agarose containing T medium and overlaid onto a 12-well plate with 0.6% agarose as the bottom layer. Cells were cultured in T medium alone or with CM from TZ or PZ fibroblasts with media changed twice weekly. Colonies >0.4 mm were scored after 6 wk. Cells were plated in triplicates and experiments performed twice. Data were reported as the average plus or minus standard error of the mean (SEM).
2.10. Statistical analysis
For statistical analysis, Pearson’s chi-square test was used. p < 0.05 was considered statistically significant.
3. Results
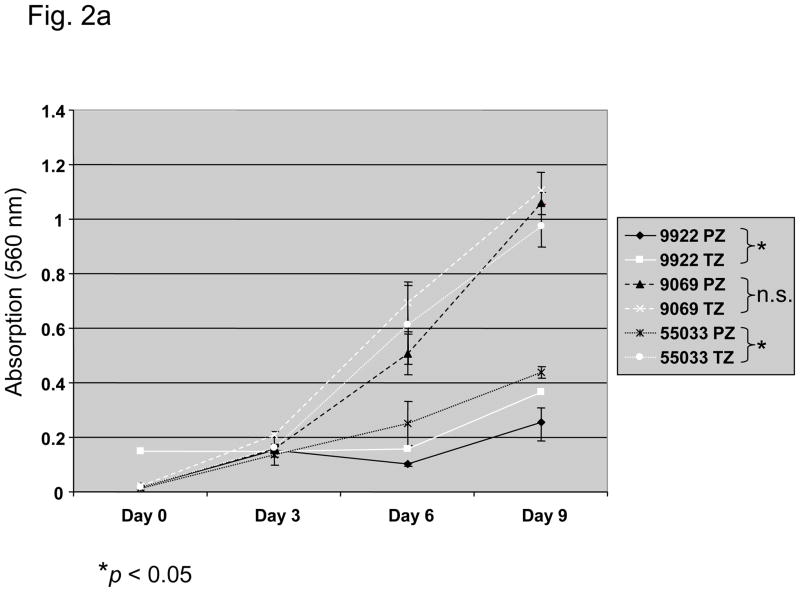
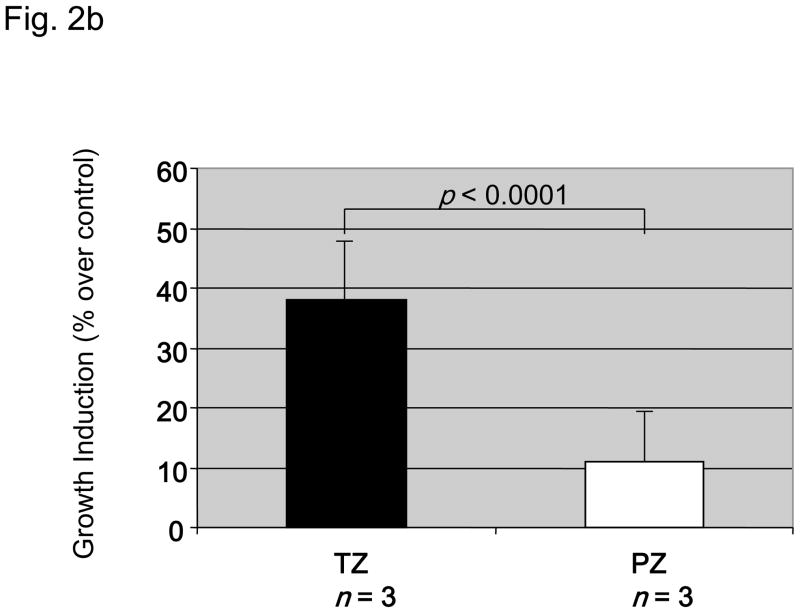
From 17 radical prostatectomy specimens we established one prostate fibroblast cell line derived from the CZ, seven from the TZ, and five from the PZ. Matched cell lines from both TZ and PZ of the same prostate were obtained from three patients. Intrinsic in vitro growth of matched TZ and PZ fibroblasts (TZ1-3 and PZ1-3) is shown in Figure 2a: In two-thirds of paired fibroblast cell lines TZ fibroblasts had a higher intrinsic growth rate than those from the PZ. Mean doubling time of TZ cells was 4 ± 0.87 d as compared to 5.3 ± 0.58 d for PZ cells. Dihydrotestosterone at physiological level exerted a stimulatory effect on the growth of TZ and PZ in vitro with dihydrotestosterone-induced growth responses at day 9 higher in TZ (Fig. 2b). This response was stronger in all TZ of the TZ/PZ pairs tested (data not shown). The level of androgen receptor expression was not assessed in fibroblast cell lines.
Fig. 2.
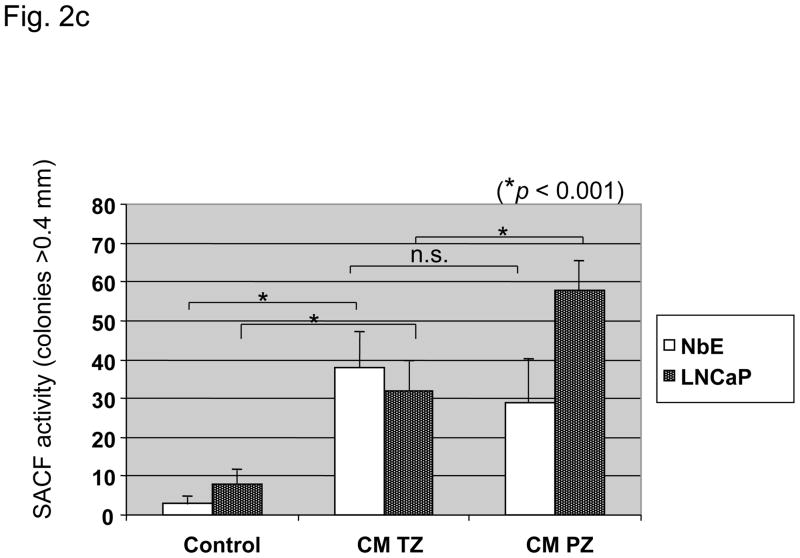
Characteristics of three paired fibroblast cell lines (9922PZ, 9922TZ, 90969PZ, 90969TZ, 55033PZ, 55033TZ) from the transition (TZ) and peripheral zone (PZ) of the prostate of three different patients. (a) Intrinsic in vitro growth curves of paired PZ- and TZ-derived cell lines derived from three patients over 9 d. Culture medium was changed on days 0, 3, and 6. Intrinsic growth of TZ fibroblasts was significantly (p < 0.05) faster than that of PZ fibroblasts in two of three patients, although in 9922 fibroblasts the difference was not large. (b) Dihydrotestosterone-induced growth in three PZ and three TZ fibroblast cell lines. TZ fibroblasts show a significantly (p < 0.0001) stronger growth induction by 1 nM dihydrotestosterone than PZ fibroblasts, expressed as percent over control. (c) In vitro clonogenic assay (soft agar colony formation): Effect of conditioned medium (CM) from three TZ and three PZ fibroblast cell lines on prostatic epithelial (NbE) and lymph node carcinoma of the prostate (LNCaP) cell anchorage-independent growth. PZ CM and TZ CM significantly (p < 0.001) induced anchorage-independent growth of NbE and LNCaP cells. Interestingly, LNCaP cells were significantly (p < 0.001) more sensitive to PZ CM. SACF = soft agarose colony formation.
Fibroblast CM significantly induced anchorage-independent growth of NbE and human LNCaP cells (Fig. 2c). Interestingly, LNCaP cells were induced significantly more by PZ than by TZ CM (p < 0.01), whereas NbE cells responded equally to both.
Coinoculation subcutaneously of 1.0 × 106 LNCaP cells with 1.0 × 106 prostate fibroblasts from CZ, TZ, or PZ resulted in tumor growth of LNCaP cells in 2 of 2 animals injected with CZ fibroblasts, 10 of 12 animals (83%) injected with TZ fibroblasts, and 7 of 11 animals (64%) injected with PZ fibroblasts. Mice injected with either LNCaP or fibroblast cells alone did not form tumors (two animals each).
All human cell lines expressed the androgen receptor as assessed by quantitative RT-PCR (data not shown).
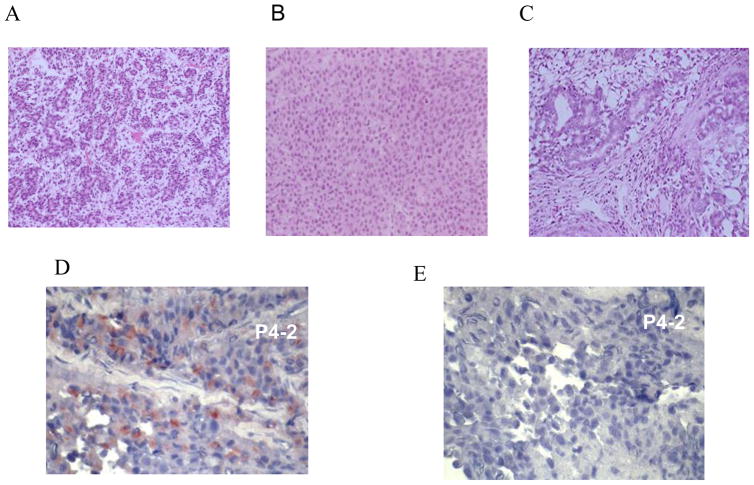
Histomorphologic analysis of these tumors revealed small glandular growth pattern for tumors induced by CZ fibroblasts (Fig. 3a), more solid tumor growth with little stroma for TZ fibroblasts (Fig. 3b), and more diffuse glandular growth with anaplastic features for PZ fibroblasts (Fig. 3c). There were differences between tumors induced by different individual fibroblast cell lines. Immunohistochemical analysis for CK5, CK18, and vimentin indicated that the tumor cells were epithelial (data not shown). Immunohistochemical staining for PSA was positive (Fig. 3d) as compared to control (Fig. 3e). In animals coinoculated with LNCaP and TZ or PZ fibroblasts, 80% expressed PSA in the serum at the time of tumor detection. PSA expression levels in vivo varied. No serial measurements were performed.
Fig. 3.
Representative panel of tumors injected subcutaneously induced by fibroblasts derived from different zones of the prostate. Histomorphologic analysis of tumors induced by human prostate fibroblasts revealed different morphologic patterns with some variation in tumors induced by different fibroblasts: (a) example of a small glandular growth pattern in the tumor induced by central zone (CZ) fibroblasts; (b) a more solid tumor growth with little stroma with transitional zone (TZ) fibroblasts; and (c) diffuse glandular growth with anaplastic features with peripheral zone (PZ) fibroblasts. Immunohistochemical staining for prostate-specific antigen (PSA) in consecutive sections of a P4-2 tumor. (d) Prostate-specific antigen (PSA) antibody (PSA expression red); (e) staining using isotype-specific immunoglobulin as control.
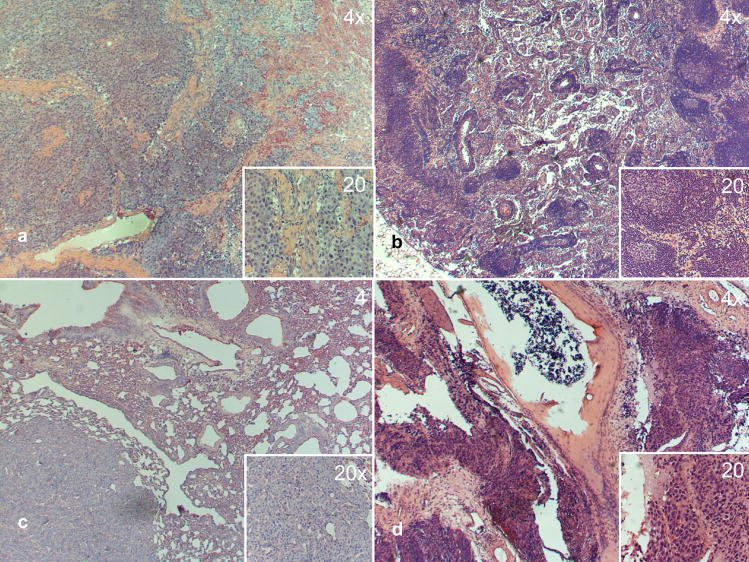
The PCa epithelial cell lines T4 and P4 (Fig. 1) were isolated from LNCaP tumors induced by TZ and PZ fibroblasts from the same patient in the intact host and maintained in the host for further 4 wk after castration. After purification, T4 and P4 cell lines were again coinoculated with the same inductive fibroblast, but this time into a castrated host. T4-2 and P4-2 tumors took 5–12 wk to grow as did control C4-2 tumors. The resulting T4-2 and P4-2 cell lines were able to grow in vivo in the hormone-deprived environment. The tumorigenicity and metastatic potential of the different LNCaP sublines when injected subcutaneously or orthotopically into the castrated host is summarized in Table 1. When injected subcutaneously, P4 and T4-2 had the highest tumorigenicity, whereas T4 alone did not show any tumor growth at all. All derived cell lines were able to grow formed lymph node (Fig. 4b) and bone micrometastases (Fig. 4d) in the castrated host when orthotopically forming tumors were injected in the prostate (Fig. 4a). Primary tumors showed solid invasive growth (Fig. 4a). P4, P4-2, and T4-2 cell lines were able to form lung metastases (Fig. 4c). Liver metastases were found in one mouse injected with P4.
Table 1.
Tumorigenicity and metastatic potential of LNCaP derivative cell lines T4, T4-2, P4, P4-2 injected subcutaneously or orthotopically into castrated hosts
| Tumorigenicity | Metastasis after orthotopic injection | |||||
|---|---|---|---|---|---|---|
| Cell line | Primary | LN | Lung | Liver | Bone | |
| s.c. (%) | ortho. (%) | s.c. (%) | ortho. (%) | |||
| T4* | 0/4 (0) | 10/10 (100) | 10/10 (100) | 0 (0) | 0 (0) | 7/10 (70) |
| T4-2* | 3/4 (75) | 8/9 (89) | 8/9 (89) | 3/9 (33) | 0 (0) | 6/9 (67) |
| P4** | 2/3 (66) | 9/10 (90) | 9/10 (90) | 2/10 (20) | 1/10 (10) | 6/10 (60) |
| P4-2** | 1/4 (25) | 10/10 (100) | 10/10 (100) | 3/10 (30) | 0 (0) | 9/10 (90) |
s.c. = subcutaneously; ortho = orthotopically; LN = lymph node.
T4, T4-2: Cell lines derived from TZ fibroblast induced tumors passaged in vivo once or twice, respectively.
P4, P4-2: Cell lines derived from PZ fibroblast induced tumors passaged in vivo once or twice, respectively.
Fig. 4.
Representative panel of P4, P4-2, T4, T4-2 tumors and metastases (main image, ×4 magnification; insert ×20 magnification): (a) primary tumor P4, solid invasive growth with hypervascularization; (b) lymph node metastasis P4-2, glandular structures in the lymph node tissue; (c) nodular lung metastasis T4-2, solid; (d) bone metastasis P4-2 spinal column with destruction of cortical bone and periosteal growth of tumor cells. Bone metastases were mostly micrometastatic.
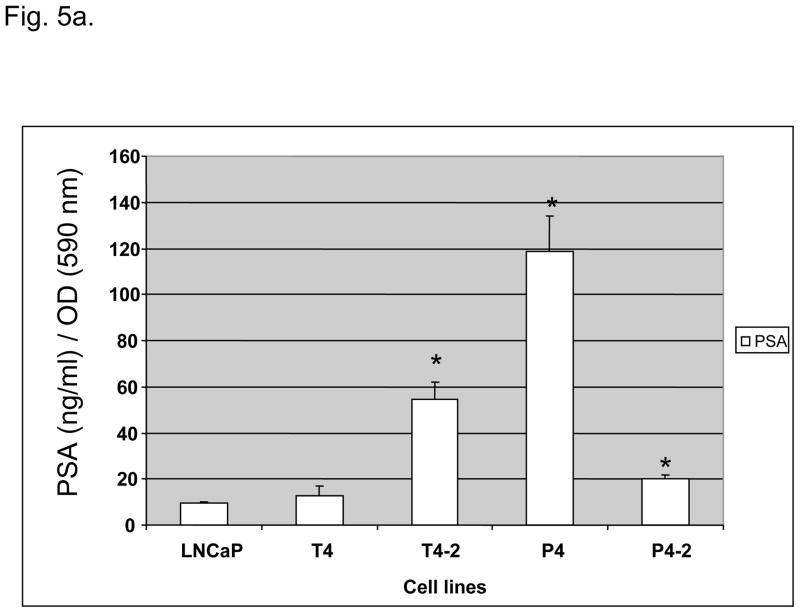
The P4 cell line expressed the highest levels of PSA in vitro, followed by T4-2 and P4-2 (Fig. 5a). In RNA blot analysis all LNCaP sublines expressed messenger RNA for PSA and androgen receptor (data not shown).
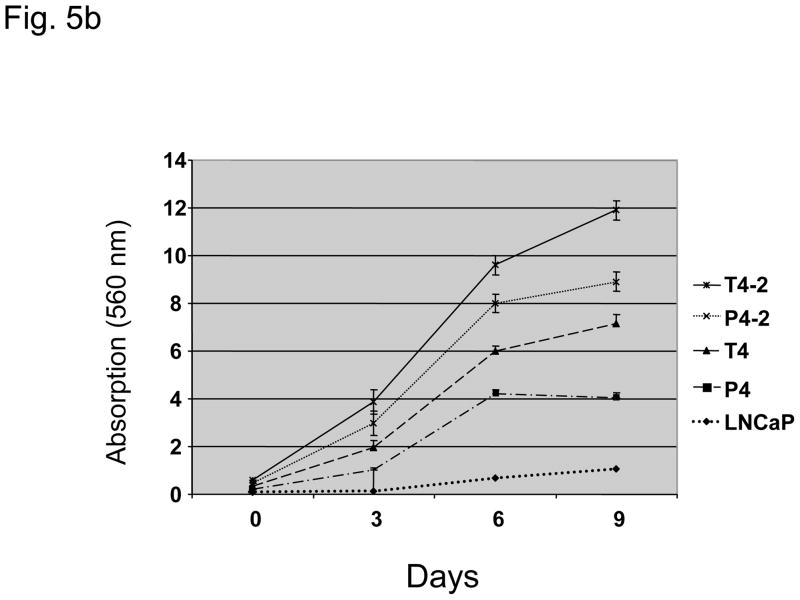
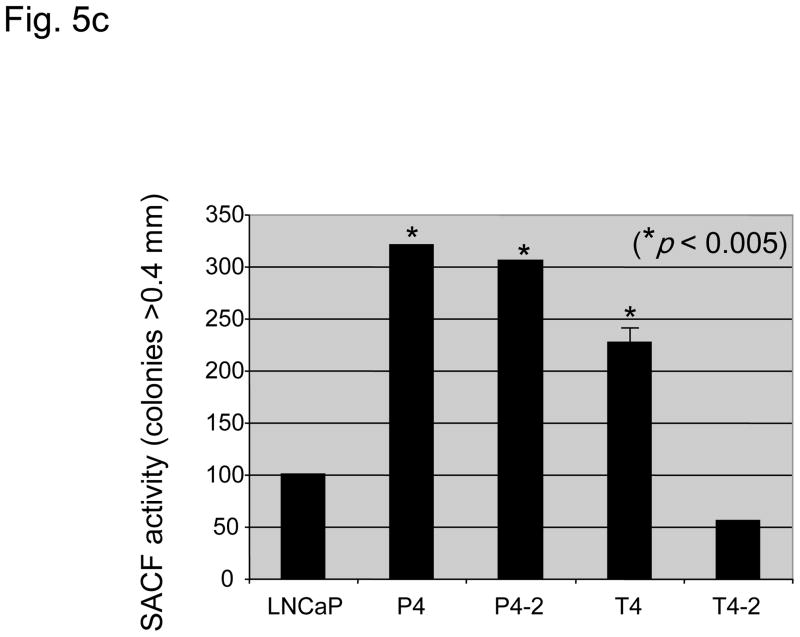
Fig. 5.
(A) Prostate-specific antigen (PSA) levels (nanograms per milliliter) of parental lymph node carcinoma of the prostate (LNCaP) cells and their derivative P and T sublines in relation to cell density (optical density [OD]: 590 nm). *PSA levels significantly increased in T4-2, P4, and P4-2 cells as compared to the parental LNCaP cell line (p < 0.05). (b) Intrinsic in vitro growth of LNCaP sublines as compared to the parental LNCaP cell line. Intrinsic growth rates increased with increased androgen-independence. (c) Clonogenic growth (soft agar colony formation assay) of LNCaP sublines as compared to the parental LNCaP cell line. Cell lines derived from the peripheral zone had a significant stronger anchorage-independent growth. SACF = soft agarose colony formation.
Intrinsic cell growth was highest for T4-2 and P4-2 cell lines (Fig. 5b). Anchorage-independent growth was significantly higher in T4, P4, and P4-2 cells and lower in T4-2 cells as compared to parental LNCaP cells (Fig. 5c).
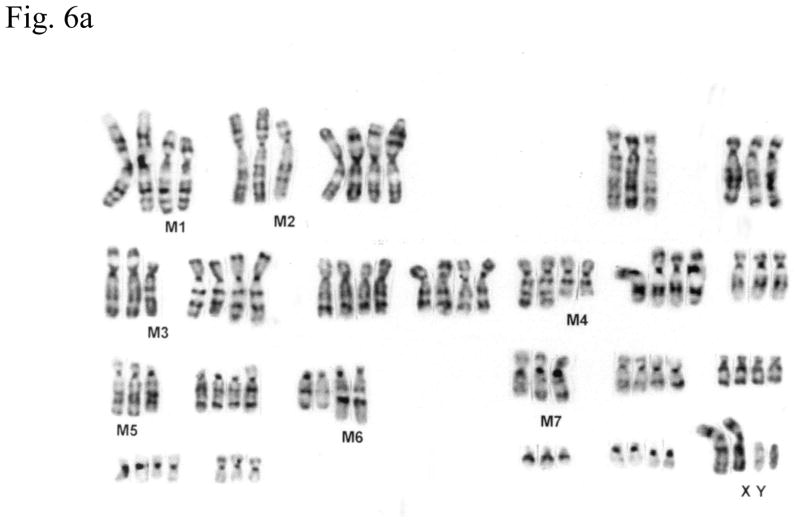
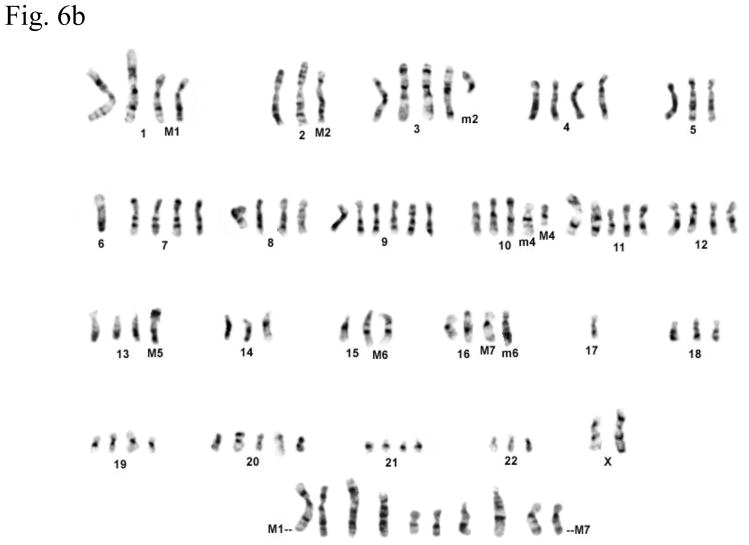
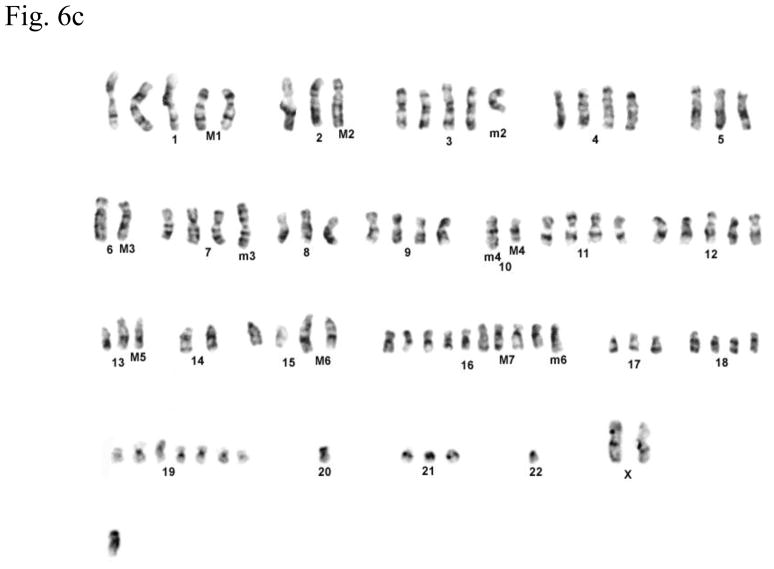
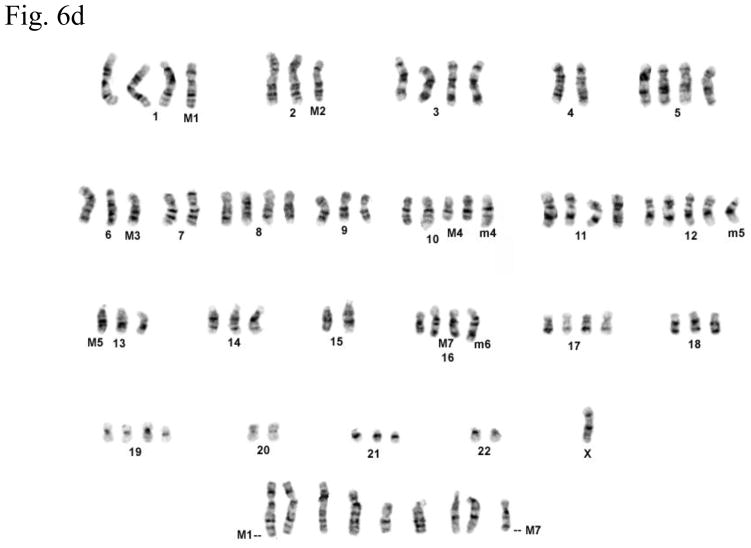
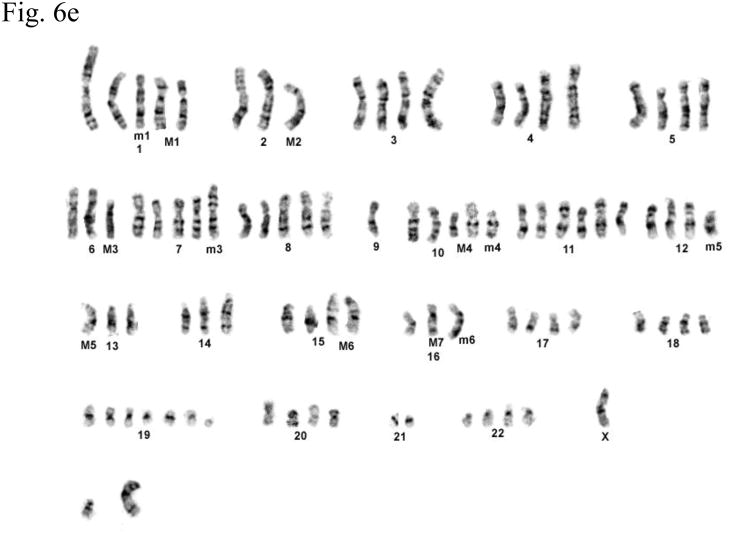
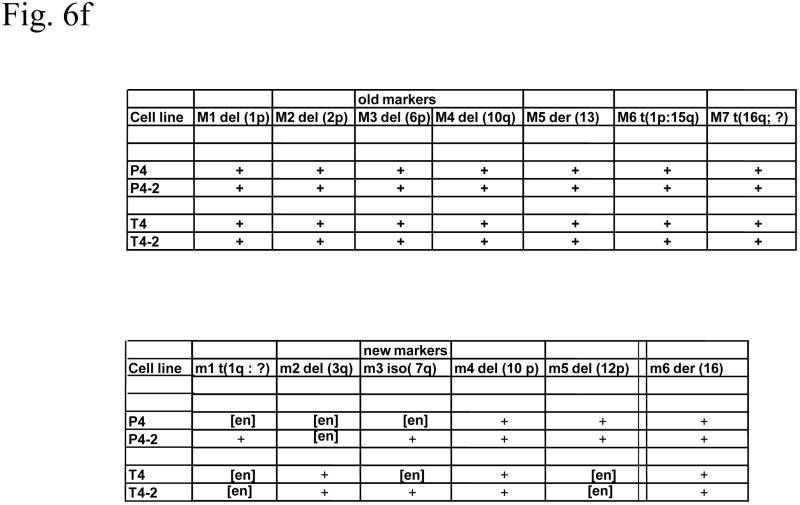
Cytogenetic analysis showed that all four LNCaP sublines are of human origin. The chromosome numbers range between 65 and 90 in all four clones with a modal number of 88 for T4, 87 for T4-2, 83 for P4, and 90 for P4-2 (typical G-banded karyotypes: Fig. 6b–e) as compared to the parental LNCaP cell line (Fig. 6a). Presence of common markers (M1–M7) in all sublines indicated that they were derived from the same parental LNCaP. Of the new markers (m1–m6), only m4 and m6 were shared by all four sublines: m1 was present only in P4-2; m2 was present both in T4 and T4-2; m3, which is an iso7q, was present only in P4-2 and T4-2; m5 was present in both P4 and P4-2. Tentative identification of these marker chromosomes is summarized in Figure 6f.
Fig. 6.
(a) G-banded karyotype of the parental lymph node carcinoma of the prostate (LNCaP) cell line. (b–e) Typical G-banded karyotypes and both types of marker chromosomes (M and m series) from (b) T4; (c) T 4-2; (d) P4; and (e) P4-2 cell lines, respectively. All cell lines are of human origin and have maintained marker chromosomes (M1-7) of the parental LNCaP cell line. Chromosome numbers range from 65 to 90 in all the four clones with a modal number of 88 for T4, 87 for T4-2, 83 for P4, and 90 for P4-2. Among the new markers (m1-6), only m4 and m6 are shared by all four cell lines; m3 is present only in P4-2 and T4-2; m5 is present in both P4 and P4-2 but is absent in T4 and T4-2. (f) Summary of old and new markers in the different cell lines. All marker chromosomes were identified based on their characteristic G-banding patterns and found to be clonal since they were present in more than two metaphases.
4. Discussion
Prostate fibroblast cell lines of defined zonal origin could be cultured from 10 of 17 (60%) radical prostatectomy specimens. Comparison of paired fibroblasts established from TZ and PZ showed a higher intrinsic growth rate and response to dihydrotestosterone for TZ fibroblasts. In contrast, CM from PZ fibroblasts significantly increased anchorage-independent growth of LNCaP cells but not NbE cells when compared to TZ cells, most probably in response to soluble growth factors secreted by PZ cells. In vivo, the PZ-derived LNCaP sublines injected into the dorsal prostate of the mouse appear to be more aggressive in terms of metastatic potential than TZ-derived sublines. It is tempting to put these findings in the clinical context. That PZ fibroblast-induced tumors are more tumorigenic and metastatic is in accordance with the higher prevalence of PCa in the PZ of the prostate gland [12]. The higher intrinsic growth rate and androgen-responsiveness of TZ fibroblasts is in agreement with the development of BPH in the transition zone [13]. Whether these experimental findings in a small sample size indicate true zonal differences in tumor inductive potential remains to be substantiated.
We confirm with prostate-derived fibroblasts the strong inductive potential of the tumor microenvironment as previously shown with bone stromal cells [4,5]. Fibroblasts derived from all zones of the prostate can induce the growth of LNCaP cells, which, by themselves, rarely form tumors. Not only did prostate-derived fibroblasts induce tumor growth in vivo, they also provided a survival advantage to androgen-dependent PCa cells, conferring castration resistance and metastatic potential, emphasizing the role of the tumor microenvironment in cancer growth and progression as suggested by Paget >100 yr ago [14]. Thus, the type of microenvironment (eg, prostate or bone) determines the biologic behaviors of PCa cells and may represent an early metastatic niche [15,16]. This is also shown by the fact that more tumors grew when cells were injected orthotopically than subcutaneously. Based on our data the zonal origin of the inductive fibroblast is of less importance. Zhao et al showed that TZ fibroblasts inhibited the growth of androgen-independent PC-3 cells in vivo [17].
In the LNCaP model of human PCa progression the tumor microenvironment was shown to alter the genotype as observed in clinical human PCa progression [18]. This differs from the observations by Igawa et al [19] and Umekita et al [20], who derived androgen-independent LNCaP subclones by culturing these cells under androgen-deprived conditions alone. The cytogenetic changes reported after long-term culture, however, are not comparable to those after modulation of the stromal microenvironment in mice and do not resemble those of progressive PCa [21]. Given the high level of karyotypic stability reported for LNCaP cells [22], these changes are specific, nonrandom and modulated by the tumor microenvironment in vivo. Together with previously published results where permanent genetic changes were observed in mouse stromal fibroblasts harvested from hosts bearing human PCa xenografts [23], this suggests that tumor-stromal fibroblast interaction is bidirectional and that remarkable plasticity exists in both cancer and stroma cell populations, which are modulated by certain as-yet-undefined mechanisms in vivo, but not in vitro, and which require further analysis.
These genetic and behavioral changes observed in the present study may be due to selective pressure (“adaptive mutations”) and outgrowth of clones able to survive under the new conditions, as has been described for bacteria [24]. There is a growing body of evidence that selected clones that survive under altered environmental conditions or after therapy might have stem cell-like characteristics. The “stem cell model” of metastasis proposes that the primary tumor is composed of a heterogeneous cell population, within which resides a rare population of cancer stem/progenitor-like cells with self-renewal capacity that are responsible for its growth, local invasiveness, and metastatic potential [25]. It is conceivable that normal tissue fibroblasts can provide a protective niche for such cells [26] and maintain a “vicious circle” [27]. The altered stroma in response to cancer epithelium or host inflammatory cells at the site of cancer growth has also been termed “reactive stroma” [28]. The importance of the microenvironmental growth support for the progression of PCa cells makes interference with this growth support an interesting therapeutic strategy. Indeed, suppression of bone turnover by bisphosphonates before colonization of the bone by cancer cells inhibits local growth support and survival of tumor cells [29]. Alternatively, we know that hormonal deprivation increases bone turnover and improves local growth support via soluble factors from bone [30].
The development of castration resistance in T4-2 and P4-2 cells after passaging LNCaP cells harboring a mutation in the androgen receptor in the ligand binding domain in vivo with inductive fibroblasts and altering the endocrine status of the host raises questions about hormonal deprivation therapy for PCa, which has been the standard treatment for >60 yr in patients with advanced disease. Although there is general agreement that it delays disease progression and is effective for palliation of often painful symptoms, controversy exists as to when it should be initiated and whether it substantially prolongs survival [31]. Given the experimental findings discussed above it is conceivable that hormonal therapy may not be beneficial in every clinical case but that treatments targeting the stromal compartment and its response to hormonal treatment may improve long-term survival.
In summary, we demonstrate that prostate fibroblasts have growth inductive potential on PCa cells and affect their subsequent progression to castration resistance and development of a metastatic phenotype. We suggest that this interaction is mainly organ specific but that zonal differences might exist.
Acknowledgments
Funding/Support and role of the sponsor: This study was supported by Swiss National Foundation grants 3200-057118 and 3200-068409 (GNT) and by NIH Grants CA 082739 (HYEZ); CA63341, CA63863, CA 119338, CA 098912, CA 132388, CA 108468, and PC060866 (LWKC).
Radical prostatectomy samples were kindly provided by Dr. L. Pisters, University of Texas, M.D. Anderson Cancer Center, Houston, TX, USA.
Footnotes
Take-home message
The tumor microenvironment is important for disease progression and development of metastatic disease. Prostate fibroblasts can induce prostate cancer cell growth and confer castration resistance and the development of a metastatic phenotype.
Financial disclosures: None.
Author contributions: George N. Thalmann had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thalmann, Sikes, Zhau, Chung.
Acquisition of data: Thalmann, Rhee, Sikes, Pathak, Multani.
Analysis and interpretation of data: Thalmann, Sikes, Pathak, Multani.
Drafting of the manuscript: Thalmann, Zhau.
Critical revision of the manuscript for important intellectual content: Marshall.
Statistical analysis: Thalmann, Sikes.
Obtaining funding: Thalmann, Marshall.
Administrative, technical, or material support: Rhee, Sikes, Multani, Zhau.
Supervision: Marshall, Chung.
Other (specify): None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Murray T, Samuels A, et al. Cancer statistics. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleave ME, Hsieh JT, von Eschenbach AC, Chung LW. Prostate and bone fibroblasts induce human prostate cancer growth in vivo: implications for bidirectional tumor-stromal cell interaction in prostate carcinoma growth and metastasis. J Urol. 1992;147:1151–9. doi: 10.1016/s0022-5347(17)37506-7. [DOI] [PubMed] [Google Scholar]
- 4.Thalmann GN, Anezinis PE, Chang SM, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–81. [PubMed] [Google Scholar]
- 5.Thalmann GN, Sikes RA, Wu TT, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Grignon DJ, Sakr WA. Zonal origin of prostatic adenocarcinoma: are there biologic differences between transition zone and peripheral zone adenocarcinomas of the prostate gland? J Cell Biochem Suppl. 1994;19:267–9. [PubMed] [Google Scholar]
- 7.McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2:35–49. doi: 10.1002/pros.2990020105. [DOI] [PubMed] [Google Scholar]
- 8.Chung LW, Chang SM, Bell C, Zhau HE, Ro JY, von Eschenbach AC. Coinoculation of tumorigenic rat prostate mesenchymal cells with non-tumorigenic epithelial cells results in the development of carcinosarcoma in syngeneic and athymic animals. Int J Cancer. 1989;43:1179–87. doi: 10.1002/ijc.2910430636. [DOI] [PubMed] [Google Scholar]
- 9.Gillies RJ, Didier N, Denton M. Determination of cell number in monolayer cultures. Anal Biochem. 1986;159:103–13. doi: 10.1016/0003-2697(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 10.Pathak S. Chromosome banding techniques. J Reprod Med. 1976;17:25–8. [PubMed] [Google Scholar]
- 11.Passaniti A, Isaacs JT, Haney JA, et al. Stimulation of human prostatic carcinoma tumor growth in athymic nude mice and control of migration in culture by extracellular matrix. Int J Cancer. 1992;51:318. doi: 10.1002/ijc.2910510224. [DOI] [PubMed] [Google Scholar]
- 12.Greene DR, Fitzpatrick JM, Scardino PT. Anatomy of the prostate and distribution of early prostate cancer. Semin Surg Oncol. 1995;11:9–22. doi: 10.1002/ssu.2980110104. [DOI] [PubMed] [Google Scholar]
- 13.Carson C, Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61(suppl 1):2–7. doi: 10.1016/s0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 14.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3. [PubMed] [Google Scholar]
- 15.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66:11089–93. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yilmaz M, Christofori G, Lehembre F. Distinct mechanisms of tumor invasion and metastasis. Trends Mol Med. 2007;13:535–41. doi: 10.1016/j.molmed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Zhao FJ, Han BM, Yu SQ, Xia SJ. Tumor formation of prostate cancer cells influenced by stromal cells from the transitional or peripheral zones of the normal prostate. Asian J Androl. 2009;11:176–82. doi: 10.1038/aja.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyytinen ER, Thalmann GN, Zhau HE, et al. Genetic changes associated with the acquisition of androgen-independent growth, tumorigenicity and metastatic potential in a prostate cancer model. Br J Cancer. 1997;75:190–5. doi: 10.1038/bjc.1997.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Igawa T, Lin FF, Lee MS, Karan D, Batra SK, Lin MF. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate. 2002;50:222–35. doi: 10.1002/pros.10054. [DOI] [PubMed] [Google Scholar]
- 20.Umekita Y, Hiipakka RA, Kokontis JM, Liao S. Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride. Proc Natl Acad Sci USA. 1996;93:11802–7. doi: 10.1073/pnas.93.21.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visakorpi T, Kallioniemi A, Syvanen AC, et al. Genetic changes in primary and recurrent prostate cancer by genomic hybridisation. Cancer Res. 1995;55:342–7. [PubMed] [Google Scholar]
- 22.Van Bokhoven A, Varella-Garcia M, Korch C, et al. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–25. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 23.Pathak S, Nemeth MA, Multani AS, Thalmann GN, von Eschenbach AC, Chung LW. Can cancer cells transform normal host cells into malignant cells? Br J Cancer. 1997;76:1134–8. doi: 10.1038/bjc.1997.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hastings PJ, Bull HJ, Klump JR, Rosenberg SM. Adaptive amplification: an inducible chromosomal instability mechanism. Cell. 2000;103:723–31. doi: 10.1016/s0092-8674(00)00176-8. [DOI] [PubMed] [Google Scholar]
- 25.Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002;3:508–13. doi: 10.1016/s1470-2045(02)00820-3. [DOI] [PubMed] [Google Scholar]
- 26.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 27.Chung LW. Prostate carcinoma bone-stroma interaction and its biologic and therapeutic implications. Cancer. 2003;97(suppl 3):772–8. doi: 10.1002/cncr.11140. [DOI] [PubMed] [Google Scholar]
- 28.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–83. [PubMed] [Google Scholar]
- 29.Van der Pluijm G, Que I, Sijmons B, et al. Interference with the microenvironmental support impairs the de novo formation of bone metastases in vivo. Cancer Res. 2005;65:7682–90. doi: 10.1158/0008-5472.CAN-04-4188. [DOI] [PubMed] [Google Scholar]
- 30.Conde FA, Aronson WJ. Risk factors for male osteoporosis. Urol Oncol. 2003;21:380–3. doi: 10.1016/s1078-1439(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 31.Studer UE, Hauri D, Hanselmann S, et al. Swiss Group for Clinical Cancer Research (SAKK) Bern, Switzerland. Immediate versus deferred hormonal treatment for patients with prostate cancer not suitable for curative local treatment: results of the randomized Swiss trial SAKK 08/88. J Clin Oncol. 2004;22:4109–18. doi: 10.1200/JCO.2004.11.514. [DOI] [PubMed] [Google Scholar]