Abstract
Treatment with antiretroviral therapy (ART) has greatly reduced the incidence of dementia. The goal of this longitudinal study was to determine if there are ongoing macrostructural brain changes in human immunodeficiency virus–positive (HIV+) individuals treated with ART. To quantify brain structure, three-dimensional T1-weighted magnetic resonance imaging (MRI) scans were performed at baseline and again after 24 months in 39 HIV+ patients on ART and 30 HIV− controls. Longitudinal changes in brain volume were measured using tissue segmentation within regions of interest and deformation morphometry. Measured by tissue segmentation, HIV+ patients on ART had significantly (all P < .05) greater rates of white matter volume loss than HIV− control individuals. Compared with controls, the subgroup of HIV+ individuals on ART with viral suppression also had significantly greater rates of white matter volume loss. Deformation morphometry confirmed these results with more specific spatial localization. Deformation morphometry also detected greater rates of gray matter and white matter loss in the subgroup of HIV+ individuals with detectable viral loads. These results provide evidence of ongoing brain volume loss in HIV+ individuals on stable ART, possibly suggesting ongoing cerebral injury. The presence of continuing injury raises the possibility that HIV+ individuals—even in the presence of viral suppression in the periphery—are at greater risk for future cognitive impairments and dementia and possibly faster cognitive decline. Therefore, HIV+ individuals on ART should be monitored for cognitive decline, and treatments that reduce ongoing neurological injury should be considered.
Keywords: ART, brain, brain MRI, deformation morphometry, HIV, segmentation
Introduction
The human immunodeficiency virus (HIV) attacks the immune system, resulting in acquired immunodeficiency syndrome (AIDS) associated with high susceptibility to infections, weight loss, and high rates of mortality (Gallo and Montagnier, 2003). Prior to the introduction of effective antiretroviral therapy (ART), severe cognitive impairment, termed HIV-associated dementia (HAD) or AIDS dementia complex (ADC) was commonly observed. Magnetic resonance imaging (MRI) was used to detect HIV-associated changes, worsening with disease severity, in the global brain, basal ganglia, white matter (WM), and gray matter (GM) (Aylward et al, 1993; Di Sclafani et al, 1997; Hestad et al, 1993; Jernigan et al, 1993; Meyerhoff, 2001b,a; 2001b; Osborne, 1994; Pan et al, 1992; Thompson et al, 2005, 2006; Tucker et al, 2004). Progressive cerebral volume loss due to HIV was also observed, as indexed by cerebrospinal fluid (CSF) volume increases and WM volume decreases over time (Stout et al, 1998). Since the introduction of ART, HAD is rarely seen in patients who adhere to prescribed medication schedules. However, there continue to be reports suggesting central nervous system (CNS) involvement in patients treated with ART. These include evidence of neuropsychological impairments (Rothlind et al, 2005; Tozzi et al, 2007), abnormal brain electrical activity related to cognition (Chao et al, 2003), brain changes detected by MRI (Ances et al, 2006) or MR spectroscopy (Chang et al, 2003; Schweinsburg et al, 2005; Roc et al, 2007), alterations in CSF markers of monocyte activation (Neuenburg et al, 2005) and CNS injury, and brain pathology detected at autopsy (Neuenburg et al, 2002). Previous research has not elucidated the extent to which these observations reflect brain alterations due to HIV infection before commencement of ART, or ongoing brain injury due to HIV despite ART. These questions become more pressing as the proportion of HIV+ people living long with ART increases. Even though ART is formidable in keeping the virus in check, its toxicity may burden the immune system and organs already compromised by HIV. Alternatively, because many antiretroviral medications do not cross the blood-brain barrier, ongoing brain injury due to HIV may be present despite virus suppression in the periphery. Thus, brain injury may contribute to the constellation of symptoms now seen in long-term survivors of HIV/AIDS.
Our goal was to determine whether there are ongoing brain volume changes in HIV+ individuals receiving ART, extending previous work (Meyerhoff et al, 2003b). We used two complementary methods to quantify brain volumes: (1) tissue segmentation to measure volumes of GM and WM within regions of interest (ROIs), and (2) deformation morphometry. Deformation morphometry is a technique that is sensitive to detection of disease effects on localized brain anatomy, such as the hippocampus, that might be obscured when looking at measures of tissue volume over large ROIs that include those smaller structures. Deformation morphometry is also useful for examining local tissue volume changes without a priori hypotheses, as it compares tissue volume changes at every location in the image. ROI tissue volumes are more sensitive than deformation morphometry to detecting structural changes that are not spatially consistent. Unlike deformation morphometry, ROI analysis can detect frontal lobe changes even if there are individual variations in location of HIV-related tissue atrophy within the frontal lobe.
Our specific aim was to compare a group of HIV+ patients on ART to a group of HIV− controls to determine the effect of HIV infection, and also to examine whether ongoing brain volume loss was slower in patients who achieved viral suppression compared to patients with detectable viral loads, to examine the presumed protective effects of successful ART. We quantified brain volumes from T1-weighted MRIs and tested the a priori hypothesis of greater rates of GM and WM losses in HIV+ patients compared to HIV− controls. A final aim was to determine whether ongoing tissue loss was related to immune response, by correlating rate of tissue loss with CD4 count or viral load.
Results
Table 1 shows group demographics, including viral loads, CD4 counts, and substance abuse/dependence history. The 39 HIV+ individuals were stable on ART for at least 3 months at both assessments. Of the subjects, 13/39 had detectable viral loads and 21/39 were virally suppressed at both time points. Five individuals did not have viral load testing at one or both time points.
Table 1.
Participant characteristics
| N | Baseline age | Baseline log viral load | Baseline CD4 | Baseline current drinks/month* | Substance abuse or dependence | |
|---|---|---|---|---|---|---|
| HIV− | 30 | 42.3 ± 9.1 | 0 ± 0 | 765 ± 255 | 10 ± 11 | Past marijuana abuse (N = 1) |
| HIV + | 39** | 45.0 ± 6.7 | 2.59 ± 1.37 | 396 ± 205 | 11 ± 17 | Past methamphetamine dependence (N = 3) |
| Current methamphetamine abuse (N = 1) | ||||||
| Past marijuana dependence (N = 2) | ||||||
| Past cocaine dependence (N = 2) | ||||||
| Past poppers dependence (N = 1) | ||||||
| Suppressed | 21 | 44.2 ± 7.6 | 1.70 ± 0.42 | 432 ± 208 | 10 ± 13 | Past methamphetamine dependence (N = 1) |
| Past marijuana dependence (N = 1) | ||||||
| Past cocaine dependence (N = 1) | ||||||
| Past poppers dependence (N = 1) | ||||||
| Viremic | 13 | 46.4 ± 5.7 | 4.10 ± 1.03 | 339 ± 195 | 13 ± 24 | Past methamphetamine dependence (N = 1) |
| Current methamphetamine abuse (N = 1) | ||||||
| Past marijuana dependence (N = 1) | ||||||
| Past cocaine dependence (N = 1) |
Standard drinks/month, standard drink = 13.6 g alcohol.
Five had no viral load measured.
Of the 21 HIV+ participants for whom we have detailed medication history, 7 individuals were on medication regimens that consisted of nucleoside reverse transcriptase inhibitors (NRTIs) and protease inhibitors (PIs) over the entire study period. Five individuals were on NRTI and non-nucleoside reverse transcriptase inhibitor (NNRTI) regimens, and three individuals were on regimens including NRTIs, PIs, and NNRTIs. One individual was taking an NRTI-only regimen. The five remaining individuals had regimens that varied over the study period (all remained on NRTI drugs, but were on and off PIs and NNRTIs). Medication regimens were not compared because of the small numbers of patients in each subgroup.
ROI analyses
Table 2 shows the rates of WM and GM loss, with positive numbers indicating loss and negative numbers gain in cm3/year.
Table 2.
ROI analyses: rates of tissue volume change
| Region | HIV− (N = 26) | HIV+ (N = 28) | Suppressed (N = 14) | Detectable (N = 9) |
|---|---|---|---|---|
| Total white matter | −2.2 ± 2.3 | 5.2 ± 2.2* | 7.6 ± 3.4* | 4.7 ± 3.1 |
| Frontal white matter | −0.3 ± 0.9 | 3.1 ± 0.9* | 3.7 ± 1.4* | 3.2 ± 1.7 |
| Temporal white matter | −0.7 ± 0.6 | 1.0 ± 0.5* | 2.0 ± 0.8* | 0.5 ± 0.6 |
| Parietal white matter | −0.7 ± 0.6 | 1.0 ± 0.5* | 1.6 ± 0.8* | 1.1 ± 0.7 |
| Occipital white matter | −0.6 ± 0.3 | 0.1 ± 0.3 | 0.4 ± 0.4 | −0.1 ± 0.4 |
| Total gray matter | 4.8 ± 2.6 | 2.1 ± 4.0 | −3.7 ± 4.5 | 11.1 ± 9.2 |
| Frontal gray matter | 1.7 ± 1.1 | 1.0 ± 1.7 | −1.5 ± 2.0 | 4.6 ± 3.6 |
| Temporal gray matter | 1.4 ± 0.6 | 0.6 ± 1.0 | −1.2 ± 1.1 | 2.9 ± 2.5 |
| Parietal gray matter | 1.0 ± 0.6 | 0.5 ± 0.9 | −0.7 ± 1.0 | 2.6 ± 2.0 |
| Occipital gray matter | 0.6 ± 0.3 | 0.3 ± 0.4 | −0.3 ± 0.4 | 1.1 ± 0.9 |
Note. Rates are in cc/year. Positive numbers denote rate of tissue loss over time, negative numbers denote rate of tissue gain over time. Significant results are denoted by * (P<.05) and refer to group comparisons with the HIV− control group.
The HIV+ group on ART (N = 28) showed significantly greater loss of WM from the entire brain as well as WM of the frontal, temporal, and parietal lobes, compared to HIV− (N = 26). Compared to the HIV−, the subgroup of virally supresssed HIV+ patients (N = 14) showed significantly higher rates of WM loss in all these ROIs. Because ROI volume estimates were available on only nine patients with detectable viral loads, comparisons with this subgroup are prone to type II error and the reported results considered preliminary. The patients with detectable viral loads (N = 9) did not show significantly greater longitudinal volume loss compared to HIV−. Longitudinal volume loss did not differ significantly between the patients with detectable virus (N = 9) and virally suppressed patients (N = 14). GM volume changes were not significant over time. Although the HIV− group showed a gain in WM volume over time, these gains were not significantly different from zero.
Within all HIV+, lower CD4 counts correlated significantly (all r ≥ .46, all P < .03) with greater rates of volume loss in cerebellum, brainstem, thalamus, and caudate, implying that patients with poorer immune function were losing tissue faster. Because these structures did not differ significantly in group comparisons, this suggests that the variability in immune function within HIV+ obscured accelerated tissue loss within these structures, and that future studies should consider recruiting a population with very low CD4 counts to see if ongoing volume loss occurs in these structures. Within all HIV+, lower CD4 counts also correlated significantly (all r ≤ −.43, all P < .008) with greater rates of CSF gain (implying greater global tissue loss). For correlations with log viral load, only patients with detectable virus were used (as floor levels will skew results): higher log viral loads were correlated with greater volume loss in total GM, and individually within frontal, and parietal GM regions (all r < −.45, all P < .03).
Deformation-based morphometry (DBM) analyses
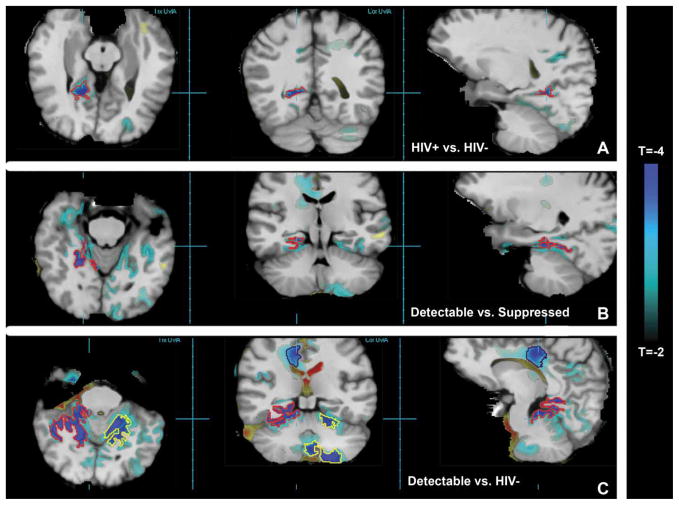
Figure 1A shows T-statistic maps from 28 HIV− versus 38 HIV+, thresholded at |T| ≥ 2. These maps reveal faster rates of tissue volume loss in HIV+ patients over 2 years in parietal, occipital, and cerebellar regions. Cluster analysis revealed a trend in the posterior part of the medial temporal lobe (cluster encompassed by the red contour), with corrected P = .09, where tissue loss was 3.2% faster in HIV+ compared to HIV−.
Figure 1.
The T-statistic map is overlaid on the spatially normalized average MRI. (A) Blue voxels show regions where HIV+ ART patients show greater rate of tissue loss over time compared to HIV− controls, with the red contour delineating a significant cluster (above P = .001) encompassing the posterior temporal region. (B) The red contour delineates a cluster of voxels where HIV+ patients with detectable viral load show greater rate of tissue loss compared to HIV+ suppressed. (C) The red and yellow contours delineate clusters of blue voxels where HIV+ patients with detectable viral load show significantly greater rate of tissue loss compared to HIV−, and the black contour delineates a cluster with a trend to greater rate of tissue loss.
T-statistic maps comparing 28 HIV− versus 21 virally suppressed HIV+ individuals revealed ongoing tissue volume loss in suppressed patients, although no region survived corrections for multiple comparisons. Figure 1B shows accelerated tissue loss in 13 patients with detectable virus compared to virally suppressed individuals. Cluster analyses revealed a significant cluster (red contour) with corrected P < .001, and tissue loss 2.5% faster in detectable versus suppressed. This suggested that individuals with detectable virus were driving the observed accelerated tissue loss in HIV+ versus HIV− participants; therefore, we then compared HIV− to HIV+ patients with detectable virus. Figure 1C shows many regions of accelerated tissue loss in patients with detectable virus, particularly GM deep in the brain that extends into the cerebellum and corpus callosum. Many individual voxels in Figure 1C exceed the permutation testing P = .05 corrected threshold of |T| >5.1, and cluster analysis revealed two significant clusters of voxels showing accelerated tissue loss located deep in the bilateral temporal lobe. Both the red and yellow clusters had corrected P < .001 and tissue loss 4.0% faster in patients with detectable virus versus HIV−. The black cluster in the WM of the corpus callosum had corrected P = .06, and a trend toward 4.1% faster tissue loss.
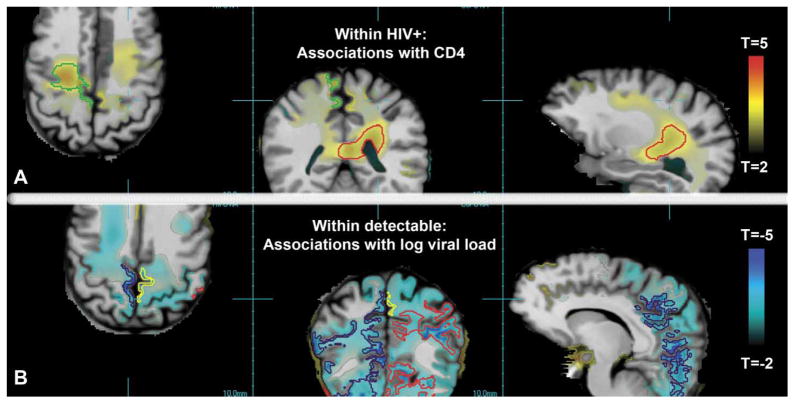
Correlation analysis showed that HIV+ individuals with greater CD4 counts had significantly slower tissue loss, most prominently in the superior frontal lobe and deep WM (Figure 2A). Two significant clusters had corrected P < .001, whereas the cluster outlined in red had tissue loss 1.0% slower and the green cluster 1.3% slower for each 100-count increase in CD4. The relationship between longitudinal tissue loss and log viral load was examined in patients with detectable virus only. Those with greater viral burden had significantly faster tissue loss, most prominently in the GM and WM of the parietal and occipital lobes (Figure 2B). Three clusters had corrected P < .001; the cluster outlined in red showed tissue loss 4.3% faster for each unit increase in log viral load, the black cluster 2.8% faster, and a superior frontal lobe cluster (not shown) 4.3% faster. The yellow cluster had corrected P = .02, indicating tissue loss at 4.5% faster per unit increase in log viral load.
Figure 2.
The T-statistic map is overlaid on the spatially normalized average MRI. (A) Red/yellow voxels show regions where greater rates of tissue loss over time were associated with lower CD4 counts within HIV+ patients, with the green and red contours delineating significant clusters (above P = .001). (B) The red, yellow, and black contours delineate clusters of blue voxels where greater rates of tissue loss over time were associated with higher log viral loads within HIV+ patients with detectable viral load.
Discussion
The major finding is that HIV+ individuals on ART had significantly higher rates of WM atrophy in most brain regions compared to HIV− controls. Virally suppressed patients (by ROI analyses) and patients with detectable peripheral virus (by DBM) also had greater rates of WM atrophy compared to HIV−. DBM also detected greater rates of GM volume loss in HIV+ individuals on ART, in both virally suppressed and those with detectable viral loads. Rates of WM loss in the frontal lobe (by DBM), cerebellum, brainstem, thalamus, and caudate (by ROI) correlated with the CD4 count, so that individuals with low CD4 counts had higher rates of WM loss. Furthermore, higher viral load correlated with higher rates of GM (by ROI and DBM) and WM loss (by DBM). The most likely explanation for these research findings is ongoing progressive brain injury, where the rate of WM volume loss within all HIV+ patients on ART is accelerated compared to normal aging, and the rate of subcortical and cortical GM loss is accelerated in those with the poorest immune status. If this is indeed so, and if accelerated WM and GM volume loss underlie accelerated cognitive impairment, then HIV+ individuals on ART may be at greater risk for future cognitive decline and possibly dementia than normally aging HIV− controls.
Prior to the introduction of ART, accelerated global white matter loss and concomitant CSF volume increase was observed in HIV+ patients longitudinally compared to HIV− controls, with more rapid changes among those with lower CD4 counts (Stout et al, 1998). Accelerated volume loss was not observed in GM regions except for caudate. Our observation of significantly higher rates of WM atrophy in most brain regions in the absence of global GM atrophy, and the association between greater tissue volume loss (or CSF gain) with poor immune status, is consistent with this report. Our results suggest that the introduction of ART has not altered the characteristic progression of cerebral volume loss in HIV as reported in 1998 (i.e., predominantly WM atrophy) (Stout et al, 1998), although ART may have lessened the severity and rapidity of ongoing atrophy compared to pre-ART.
Ongoing brain volume decreases may be indicating ongoing brain injury directly associated with HIV, such as loss or contraction of axons or supporting glial cells. Ongoing brain injury in turn may reflect continued viral infection in the brain, despite effective viral suppression in the periphery. Because of the limited permeability of the blood-brain barrier to many antiretroviral drugs, it has been hypothesized that the brain could serve as a reservoir for HIV (Ellis et al, 2007). Ongoing brain injury might also arise from continued neuronal damage secondary to macrophage activation, in the absence of virus, that might have begun prior to ART (Raposo et al, 2002).
However, it is also possible that progressive reductions of brain volume do not represent ongoing injury directly related to HIV. The continued brain tissue loss observed in this study may be a side effect of ART, either directly through CNS toxicity or indirectly through side effects on other organ systems that impact the brain. In particular, the NNRTI efavirenz is associated with CNS side effects (Fortin and Joly, 2004; Shibuyama et al, 2006), with possible consequent brain tissue loss. PIs are associated with cardiovascular disease (Hsue et al, 2004), which is in turn associated with the presence of lacunar infarcts and abnormal WM in the brain (Chui, 2000). Arguing against this interpretation, we found that higher CD4 counts were associated with less tissue loss over time. Because more effective therapy tends to increase CD4 counts, we infer that ART is associated with less brain tissue loss. Our finding that smaller log viral loads were associated with less tissue loss over time also argues against a damaging effect of ART. In addition, the images of our HIV+ patients did not show evidence of more advanced WM contrast changes or lacunes associated with brain injury due to cardiovascular disease, as compared to the HIV− controls, arguing against PI-associated cardiovascular disease as the mechanism behind accelerated WM loss. According to the neuroradiological MRI reads, only 4/30 HIV− participants and 3/39 HIV+ patients showed abnormal white matter inconsistent with age, and in these 7 participants the amount of abnormal white matter was rated 1 on a 4-point scale (0 = absent, 1 = punctate foci, 2 = early confluence, 3 = large confluent areas). Lacunes were observed in one HIV+ patient and one HIV− participant. A longitudinal comparison between HIV+ patients on ART and HIV+ patients on no ART would determine whether progressive reductions of brain volumes were a side-effect of ART, due to HIV alone, or both. The vast majority of HIV+ patients recruited for this study was on ART, however, so this comparison could not be made.
Taken together, we believe that our findings reflect ongoing brain injury due to HIV infection, despite stable ART. However, another interpretation is that loss of WM volume over time represents a loss of brain edema present at the beginning of the study. We find this explanation less likely, though, because regional WM volumes did not differ significantly between these same HIV+ and HIV− patients at baseline (Meyerhoff et al, 2003a).
Cross-sectional studies have shown structural abnormalities associated with cocaine, marijuana, and methamphetamine use (Berman et al, 2008; Fein et al, 2002; Lim et al, 2008; Matochik et al, 2005; O’Neill et al, 2001). As there was a greater burden of substance abuse and dependence in the HIV+ group (9/39 with current or past abuse or past dependence) compared to the HIV− (1/30), this may also contribute to greater rates of WM volume decrease over time. However, brain tissue volume recovery during abstinence from substances has been reported extensively (Agartz et al, 2003; Cardenas et al, 2007; Pfefferbaum et al, 1995), and the rather long duration since dependence among our participants (range 30 to 336 months) argues that ongoing tissue volume recovery is more likely than ongoing loss due to past substance use. Moreover, a recent cross-sectional study (Tanabe et al, 2009) using voxel-wise analysis reported differences only in orbitofrontal cortex after prolonged abstinence from illicit substances (including cocaine, alcohol, amphetamines, opiates, and marijuana). This suggests that the orbitofrontal cortex may be the only brain region affected by substances in the long term. This region, however, was unaffected in our results, adding further evidence that the greater burden of substance use in the HIV+ group was too little to affect our outcomes. Furthermore, we did not observe baseline volume differences between our groups, suggesting that the level of substance abuse/dependence did not have adverse effects on brain volumes (Meyerhoff et al, 2003a). Only one HIV+ patient was a current substance abuser (methamphetamine), and because methamphetamine abuse has been associated with WM increases (Thompson et al, 2004), our observation of WM decreases over time in the HIV+ group cannot be attributed to that patient. Current substance use was minimal during the course of the study (marijuana use was reported by one HIV− and four HIV+ participants). Because there is not strong evidence for brain structural abnormalities due to marijuana use (Quickfall and Crockford, 2006), the most likely explanation of the ongoing volume change was HIV status, not substance dependence in the distant past.
Several HIV+ participants had obvious ventricular enlargement over time, although we did not observe significant rates of CSF increase in HIV+ patients as a group. Such HIV associated ventricular enlargement may cause compression of deep WM underlying the frontal, parietal, and temporal lobes and no actual cellular loss. Unfortunately, the integrity of WM cannot be assessed using T1-weighted MRI; future studies may be able to address this using diffusion-weighted imaging.
The increased rate of brain tissue loss in HIV+ patients on ART compared to controls was more pronounced in the WM than cortical GM, consistent with HIV initially affecting the subcortex and basal ganglia (Tucker et al, 2004). The ongoing brain loss suggests that the incidence of HAD may increase with the increased life span of these treated HIV+ ART patients. CNS reserves may also decrease over time and increase the risk that these patients will develop Alzheimer’s disease (AD) or vascular dementia, perhaps at an earlier age than their HIV− counterparts.
Except for being HIV+, this was a healthy sample of ART patients, with few cognitive deficits at baseline compared to the HIV− group (Rothlind et al, 2005). Cognitive impairment may eventually emerge, but we lack continued follow-up to detect it. Baseline studies for these patients occurred between January 1999 and February 2002, relatively early in the ART era, and it is possible that newer medication regimens are more effective at preventing CNS injury.
The greatest strength of this work is the longitudinal design. Aside from HIV, many factors influence brain volume (e.g., age, education, prenatal exposure to alcohol, traumatic stress, and more), resulting in highly variable regional brain volumes even among healthy nonimpaired subjects. Because it is impossible to control for everything, the most feasible approach to addressing the problem is to examine the course of HIV longitudinally in a sample matched on as many factors as possible. Here we examined an age- and education-matched sample of men free of chronic substance abuse. In this way, we have fewer confounding factors that might contribute to the observed ongoing injury.
Another strength of this work is the use of two different but complementary methods that both point to ongoing injury due to HIV infection. Although both methods derive tissue volumes from T1-weighted MRIs, the ROI analysis is better suited to detecting changes that occur over a large region, whereas DBM is better suited to detecting spatially localized changes. For example, the ROI analysis revealed ongoing WM but not GM volume loss in the frontal, temporal, and parietal lobes of the brain when comparing HIV+ and HIV−. In contrast, DBM revealed a small region of GM loss but no WM loss in HIV+ versus HIV−. These differing results suggest that HIV-associated WM volume loss is distributed throughout the lobes and does not localize to particular WM tracts, and is thus not detectable using DBM. HIV-associated GM loss appears to be highly localized, and is thus obscured when looking at GM volume at the lobar level in the ROI analysis. Moreover, DBM was more robust to imaging artifact, so more subjects were retained for the DBM analysis. This particularly affected comparisons with the HIV+ ART group with detectable viral loads (N = 13 for DBM, N = 9 for ROI). Small groups may lead to type II errors, where statistical power is not sufficient to detect a true group difference, and this may in part explain why we unexpectedly observed no significant differences between HIV− and patients with detectable viral load using lobar measures of tissue volume, but observed differences using DBM. Using both methods enabled us to maximize the information used in our group comparisons, and reveal both distributed and localized brain volume changes over time due to HIV infection.
In conclusion, these results provide evidence of ongoing tissue loss, primarily in the WM, that may indicate ongoing brain injury in HIV+ patients on ART. This raises the possibility that these patients will be at greater risk for future cognitive impairments and dementia. It may explain the constellation of neurological symptoms seen in HIV+ patients living long on ART. Our findings suggest that HIV+ patients on successful ART should be observed long term, and further development of treatments that reduce the virus within the CNS is needed, including neuroprotective treatments, and formal studies on the relationship between aging, HIV infection, and ART treatment are warranted. We also demonstrated that MRI is sensitive to detection of brain changes over a scan interval of about 2 years, and thus shows promise as a tool for monitoring disease progression longitudinally. As such, quantitative MRI may be important for assessing the brain effects of HIV treatment that includes CNS-penetrating drugs.
Materials and methods
Participants
All participants provided informed consent to a protocol approved by the review boards of both University of California San Francisco (UCSF) and the San Francisco VA Medical Center. Participants were recruited from the community for a study on the effects of HIV infection and chronic alcohol consumption on HIV disease progression. Study procedures included multiple neuropsychological testing and MRI scanning; all tests were performed between January 1999 and May 2004. Participants met the following basic inclusion criteria: 21 to 55 years of age; lifetime consumption of <30 alcoholic drinks/month, with no history of alcohol consumption at >45 drinks/month; HIV+ patients were included if they had been on stable ART for at least 3 months. Exclusion criteria at baseline were delirium, dementia, and amnestic disorders, current substance dependence disorders, schizophrenia or other psychotic disorders, lifetime or current bipolar disorder, current manic, mixed, or hypomanic episodes, current post-traumatic stress disorder (PTSD) or obsessive-compulsive disorder, significant history of head trauma, history of diabetes, stroke, or hypertension, hepatic disease, lack of fluency in English, current opportunistic CNS infection, and recent change in ART (<3 months). Past substance dependence and current or past substance abuse (other than alcohol) were allowed. Patients and controls were studied twice, once after enrollment (baseline) and again after approximately 24 months, during which time the patients were not required to stay on therapy taken at baseline. Plasma viral RNA levels (viral load) were measured at both timepoints using reverse transcriptase–polymerase chain reaction (RT-PCR) (Roche Diagnostics). Ultra-sensitive and standard assays were used, having lower limits of quantitation of 50 and 400 copies/ml, respectively. Patients were classified as virally suppressed (viral loads less than 400 on either assay) or detectable (>400). All male participants from this study who met these criteria and had complete MRI datasets were included in this analysis, yielding 39 HIV+ patients on ART and 30 HIV− controls.
Participants were classified as cognitively impaired if any neuropsychological testing z-score was ≤ −1.5 in two or more separate cognitive “domains.” Domains were motor dexterity and speed, learning/memory, processing speed, working memory/executive function, and motor balance. Using this definition, 8/39 HIV+ patients on ART and 7/30 HIV− controls were classified as cognitively impaired. Because the proportion of participants with cognitive impairment is similar in both groups, we could not identify a subgroup of individuals with HIV-associated neurocognitive impairment at baseline.
MRI acquisition and processing
Coronal T1-weighted images were acquired on a clinical 1.5-tesla MR scanner (Vision; Siemens Medical Systems, Iselin, NJ) using magnetization prepared rapid acquisition gradient echo (TR/TI/TE = 9/300/4 ms, 1 × 1-mm2 in-plane resolution, 1.5-mm slabs); images were acquired orthogonal to the long axis of the hippocampus.
ROI analyses
Three-tissue intensity–based segmentation was applied to images to assign to each voxel a set of probability of WM, GM, or CSF and membership to a ROI, as described in detail in (Cardenas et al, 2005). One or both scans from 15 participants had unacceptable tissue or ROI segmentation and were excluded, leaving 26 HIV− and 28 HIV+ individuals for longitudinal ROI analyses. Total brain ROIs were estimated by summing the individual ROI volumes for each tissue type. This method yielded tissue volumes at each time point, which were used as dependent variables in the statistical analyses, and from which rate of tissue atrophy was estimated. For group comparisons, a range of mixed effects models were fit in R (Pinheiro and Bates, 2000) to the individual ROI volumes from the two time points using maximum-likelihood estimation and compared via likelihood-ratio testing. The final model was optimal for most regions and incorporated age, intracranial volume (ICV), and years from initial scan as covariates. To estimate the effect of HIV, grouping by HIV status (HIV−, HIV+) was incorporated as a fixed effect. In a separate analysis to estimate the protective effect of successful ART, grouping by viral level (suppressed, detectable) was incorporated as a fixed effect. Within group residual variances were homogeneous within groups but heterogeneous across groups. Participant-specific random intercept terms were included and the final model was re-fit using restricted maximum likelihood. Significant results were determined via conditional t tests for the ROI volume by time interaction within the final fitted model. To determine the relationship between rate of brain tissue loss and immune response (CD4 count or viral load), Pearson’s correlation analyses were performed in HIV+ after partialling out age and ICV.
Deformation-based morphometry (DBM) analyses
Robust fluid registration was used to nonlinearly register baseline and 24-month follow-up scans of each participant (Studholme et al, 2006). One or both scans from three individuals were unacceptably noisy and excluded, leaving 38 HIV+ and 28 HIV− individuals for DBM. Maps of longitudinal atrophy in common space were created and filtered as described in (Cardenas et al, 2007). Analysis of covariance (ANCOVA) at each voxel was used to test our first hypothesis that HIV status was associated with greater tissue atrophy rates; the maps of longitudinal change were the dependent variable, group status (i.e., HIV+ or HIV−) was the categorical predictor, and age was a covariate. Subsequent analyses compared HIV− versus viral suppressed, viral suppressed versus detectable, and HIV− versus detectable, in order to determine the effect of successful ART on longitudinal brain loss. Within HIV+, linear regression with baseline CD4 or log viral load was also fit at each voxel, covarying for age, in order to determine the relationship between rate of tissue atrophy and immune response. Permutation testing (Nichols and Holmes, 2002) and nonstationary random field theory (Worsley et al, 2002) were used to correct the statistical maps for multiple comparisons.
Acknowledgments
The authors wish to thank George Fein, the original Principal Investigator of the project, Margaret Chesney for developing assessment tools (quality of life, medication adherence, behavioral), Jennifer Hlavin, Charles McCarthy, Lucy Brysk, and Joselyn Lindgren for recruiting and assessment of the study participants, Evan Williams for MRI acquisition and analysis, and Derek Flenniken for database maintenance. This work was supported in part by NIH grants P01 AA011493, R01 MH65392, and R03 EB 008136 and the Department of Veterans Affairs Research Service.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agartz I, Brag S, Franck J, Hammarberg A, Okugawa G, Svinhufvud K, Bergman H. Mr volumetry during acute alcohol withdrawal and abstinence: a descriptive study. Alcohol Alcohol. 2003;38:71–78. doi: 10.1093/alcalc/agg020. [DOI] [PubMed] [Google Scholar]
- Ances BM, Roc AC, Wang J, Korczykowski M, Okawa J, Stern J, Kim J, Wolf R, Lawler K, Kolson DL, Detre JA. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology. 2006;66:862–866. doi: 10.1212/01.wnl.0000203524.57993.e2. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, Pearlson GD. Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology. 1993:2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Res. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Witt MD, Ames N, Walot I, Jovicich J, DeSilva M, Trivedi N, Speck O, Miller EN. Persistent brain abnormalities in antiretroviral-naive HIV patients 3 months after HAART. Antivir Ther. 2003;8:17–26. [PubMed] [Google Scholar]
- Chao LL, Cardenas VA, Meyerhoff DJ, Rothlind JC, Flenniken DL, Lindgren JA, Weiner MW. Abnormal contingent negative variation in HIV patients receiving antiretroviral therapy. Neuroreport. 2003;14:2111–2115. doi: 10.1097/01.wnr.0000090588.35425.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui HC. Vascular dementia, a new beginning: shifting focus from clinical phenotype to ischemic brain injury. Neurologic clinics of North America. In: DeKosky ST, editor. J Neurol Clin. Vol. 18. Philadelphia: WB Saunders; 2000. pp. 951–978. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Mackay RD, Meyerhoff DJ, Norman D, Weiner MW, Fein G. Brain atrophy in HIV infection is more strongly associated with CDC clinical stage than with cognitive impairment. J Int Neuropsychopharmacol Soc. 1997;3:276–287. [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin C, Joly V. Efavirenz for HIV-1 infection in adults: an overview. Expert Rev Anti Infect Ther. 2004;2:671–84. doi: 10.1586/14789072.2.5.671. [DOI] [PubMed] [Google Scholar]
- Gallo RC, Montagnier L. The discovery of HIV as the cause of AIDS. N Engl J Med. 2003;349:2283–2285. doi: 10.1056/NEJMp038194. [DOI] [PubMed] [Google Scholar]
- Hestad K, McArthur JH, Pan GJD, Selnes OA, Nance-Sproson TE, Aylward E, Mathews VP, McArthur JC. Regional brain atrophy in HIV-1 infection: association with specific neuropsychological test performance. Acta Neurol Scand. 1993;88:112–118. doi: 10.1111/j.1600-0404.1993.tb04201.x. [DOI] [PubMed] [Google Scholar]
- Hsue PY, Giri K, Erickson S, MacGregor JS, Younes N, Shergill A, Waters DD. Clinical features of acute coronary syndromes in patients with human immunodeficiency virus infection. Circulation. 2004;109:316–319. doi: 10.1161/01.CIR.0000114520.38748.AA. [DOI] [PubMed] [Google Scholar]
- Jernigan TJ, Archibald S, hesselink JR, Atkinson JH, et al. Magnetic resonance imaging morphometric analysis of cerebral volume loss in human immunodeficiency virus infection. Arch Neurol. 1993;50:250–255. doi: 10.1001/archneur.1993.00540030016007. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Meyerhoff D. Examining the link between alcohol and HIV/AIDS: studies of CNS effects. Alcohol Res Health. 2001a;25:288–298. [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff D, Cardenas V, Song E, Truran D, Blumenfeld R, Ezekiel F, Studholme C, Rothlind JC, Lindgren JA, Weiner M. Proc Intl Soc Mag Reson Med. Vol. 11. ISMRM; Toronto: 2003a. Brain morphologic changes suggest persistent brain injury in treated HIV-seropositive patients; p. 278. [Google Scholar]
- Meyerhoff DJ. Effects of alcohol and HIV infection on the central nervous system. Alcohol Res Health. 2001b;25:288–298. [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Cardenas V, Studholme C, Blumenfeld R, Truran D, Ezekiel F, Lampiris H, Rothlind J, Lindgren J, Weiner MW. Evidence for brain damage in treated HIV-infected individuals. Neurology. 2003b;60:A186. [Google Scholar]
- Neuenburg JK, Brodt HR, Herndier BG, Bickel M, Bacchetti P, Price RW, Grant RM, Schlote W. HIV-related neuropathology, 1985 to 1999: rising prevalence of HIV encephalopathy in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31:171–177. doi: 10.1097/00126334-200210010-00007. [DOI] [PubMed] [Google Scholar]
- Neuenburg JK, Furlan S, Bacchetti P, Price RW, Grant RM. Enrichment of activated monocytes in cerebrospinal fluid during antiretroviral therapy. AIDS. 2005;19:1351–1359. doi: 10.1097/01.aids.0000181008.39514.ee. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict Biol. 2001;6:347–361. doi: 10.1080/13556210020077073. [DOI] [PubMed] [Google Scholar]
- Osborne A. Diagnostic neuroradiology. St. Louis: Mosby; 1994. [Google Scholar]
- Pan GJD, McArthur JH, Alyward E, Selnes OA, Nance-Sproson TE, Kumar AJ, Mellits ED, McArthur JC. Patterns of cerebral atrophy in HIV-1-infected individuals: results of a quantitative MRI analysis. Neurology. 1992;42:2125–2130. doi: 10.1212/wnl.42.11.2125. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D. Mixed-effects models in S and S-PLUS. New York: Springer-Verlag; 2000. [Google Scholar]
- Quickfall J, Crockford D. Brain neuroimaging in cannabis use: a review. J Neuropsychiatry Clin Neurosci. 2006;18:318–332. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- Raposo G, Moore M, Innes D, Leijendekker R, Leigh-Brown A, Benaroch P, Geuze H. Human macrophages accumulate HIV-1 particles in MHC II compartments. Traffic. 2002;3:718–729. doi: 10.1034/j.1600-0854.2002.31004.x. [DOI] [PubMed] [Google Scholar]
- Roc AC, Ances BM, Chawla S, Korczykowski M, Wolf RL, Kolson DL, Detre JA, Poptani H. Detection of human immunodeficiency virus induced inflammation and oxidative stress in lenticular nuclei with magnetic resonance spectroscopy despite antiretroviral therapy. Arch Neurol. 2007;64:1249–1257. doi: 10.1001/archneur.64.9.noc60125. [DOI] [PubMed] [Google Scholar]
- Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, Weiner MW. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11:70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Gonzalez R, Brown GG, Ellis RJ, Letendre S, Videen JS, McCutchan JA, Patterson TL, Grant I. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J NeuroVirol. 2005;11:356–364. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]
- Shibuyama S, Gevorkyan A, Yoo U, Tim S, Dzhangiryan K, Scott JD. Understanding and avoiding antiretroviral adverse events. Curr Pharm Des. 2006;12:1075–1090. doi: 10.2174/138161206776055796. [DOI] [PubMed] [Google Scholar]
- Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, McCutchan JA, Wallace MR, Atkinson JH, Grant I. Progressive cerebral volume loss in human immunodeficiency virus infection: a longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Arch Neurol. 1998;55:161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- Studholme C, Drapaca C, Iordanova B, Cardenas V. Deformation based mapping of volume change from serial brain MRI in the presence of local tissue contrast change. IEEE Trans Med Imaging. 2006;25:626–639. doi: 10.1109/TMI.2006.872745. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Lu A, Lee SE, Lee JY, Lopez OL, Aizenstein HJ, Toga AW, Becker JT. 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage. 2006;31:12–23. doi: 10.1016/j.neuroimage.2005.11.043. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A. 2005;102:15647–1552. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- Tucker KA, Robertson KR, Lin W, Smith JK, An H, Chen Y, Aylward SR, Hall CD. Neuroimaging in human immunodeficiency virus infection. J Neuroimmunol. 2004;157:153–162. doi: 10.1016/j.jneuroim.2004.08.036. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]