Abstract
Objective
We have previously shown that TNFR2 protein is highly upregulated in vascular endothelium in response to ischemia, and a global deletion of TNFR2 in mice blunts ischemia-induced arteriogenesis and angiogenesis. However, the role of endothelial TNFR2 is not defined. In this study, we used endothelial cell (EC)-specific transgenesis of TNFR2 (TNFR2-TG) in mice to determine the in vivo function of TNFR2 in arteriogenesis and angiogenesis.
Methods and Results
In a femoral artery ligation model, TNFR2-TG mice had enhanced limb perfusion recovery and ischemic reserve capacity. TNFR2-TG mice also exhibited significantly enhanced arteriogenesis in the upper limb, while capillary formation and maturation in the lower limb was associated with reduction in cellular apoptosis, and increased proliferation. Consistently, ischemia-induced TNFR2-dependent Bmx-VEGFR2 proangiogenic signaling was augmented in TNFR2-TG mice. To further determine if EC-expressed TNFR2 is sufficient to mediate ischemia-induced angiogenesis, we crossed TNFR2-TG with TNFR2 deficient mice to generate TNFR2-KO/TG mice (KO/TG), which only vascular EC express TNFR2. The EC-expressed TNFR2 partially rescued the defects of TNFR2-KO in ischemia-induced angiogenic signaling and flow recovery.
Conclusion
These in vivo data support a critical role for endothelial TNFR2 in ischemia-mediated adaptive angiogenesis. Therefore, specific expression and activation of TNFR2 in EC may provide a novel strategy for the treatment of vascular diseases such as coronary artery disease and peripheral arterial disease.
Keywords: TNFR2, Arteriogenesis, Angiogenesis, Ischemia, VEGFR2, Bmx
INTRODUCTION
Peripheral arterial disease is a common disease found in 10%–25% of patients over the age of 55 years, and its occurrence and severity are strongly correlated with other cardiovascular risk factors that lead to coronary artery disease and stroke, such as hyperlipidemia, smoking, and endothelial dysfunction 1–3. An animal model has been created by ischemic injury of mouse hind limb, and studies using this mouse model reveal that a reduction in gastrocnemius blood pressure ratios and blood flow correlate with a marked decrease in the number of capillaries surrounding each muscle fiber (capillary/fiber ratio), indicative of the inability to mount an adaptive angiogenic response 4, 5. The availability of genetically deficient or transgenic mice permits the testing of genes required for postnatal hind limb angiogenesis and remodeling. Thus, the ischemia hind limb model is a very useful tool for studying the function of genes critical for inflammatory arteriogenesis and angiogenesis 4, 5. Using this model, we have recently demonstrated that several signaling molecules (TNFR1, TNFR2 and AIP1) involved in TNF-TNF receptor pathways are also involved in ischemia-mediated adaptive angiogenesis 6–8.
The prototype inflammatory cytokine, tumor necrosis factor-α (TNF), primarily utilizes TNFR1 to mediate inflammatory and pathological responses while the role of TNFR2 signaling is not well understood. TNFR1 is ubiquitously expressed while TNFR2 expression is tightly regulated and found predominantly on EC, cardiac myocytes and hematopoietic cells 9, 10. Similar to TNFR1, TNFR2 contains an extracellular domain with characteristic cysteine-rich motifs. However, TNFR2 has a unique cytoplasmic domain with different primary amino acid sequences compared to TNFR1. It is believed that the two receptors initiate distinct signal transduction pathways by interacting with different signaling proteins 9, 10. While TNFR1-mediated signaling has been extensively studied, much less is known about TNFR2 signaling 10. We have identified Bmx (bone marrow tyrosine kinase in chromosome X) (also named Etk-endothelial/epithelial tyrosine kinase) as a TNFR2-responsive tyrosine kinase in EC 11. Furthermore, we have shown that TNFR2-Bmx could associate with and transactivate VEGFR2, a potent angiogenic receptor tyrosine kinase in ECs. This association was critical for EC proliferation, migration and tube formation 12. Consistent with aforementioned in vitro observations, we and others have shown that TNFR2-knockout mice exhibit reduced ischemia-initiated angiogenesis and arteriogenesis compared to wild-type mice in a femoral artery ligation model 7, 13. Similar observations were obtained for Bmx-KO mice 6. Interestingly, TNFR2 protein and its downstream effector, Bmx, are both highly induced in vascular EC but not in other cell types such as myocytes in ischemic tissue 6, 7. These data indicate that vascular TNFR2 may play an important role in ischemia-induced adaptive repair.
However, the role of endothelial TNFR2 in arteriogenesis and angiogenesis has not been directly addressed. To this end, we have created endothelial cell (EC)-specific transgenic mice expressing human TNFR2 (TNFR2-TG). We show that TNFR2-TG mice have enhanced ischemia-mediated arteriogenesis and angiogenesis in a femoral artery ligation model. Moreover, EC-specific expression of TNFR2 in TNFR2 deficient mice is sufficient to augment ischemia-induced Bmx-VEGFR2 signaling and functional flow recovery. Our data support a critical role of endothelial TNFR2 in ischemia-mediated adaptive angiogenesis.
METHODS
The detailed materials and methods are provided in the online supplement. All the methods have been previously published, including generation of the EC-specific transgenic mice 14, 15, mouse hindlimb ischemic model, post-contraction hyperemia 6, 7, histology and immunohistochemistry, gene expression in ischemic muscle and image analysis, immunoblotting for Bmx-VEGFR2 signaling, bone marrow transplant (BMT) 6–8 as well as aortic-ring assay 14.
RESULTS
EC-specific expression of TNFR2
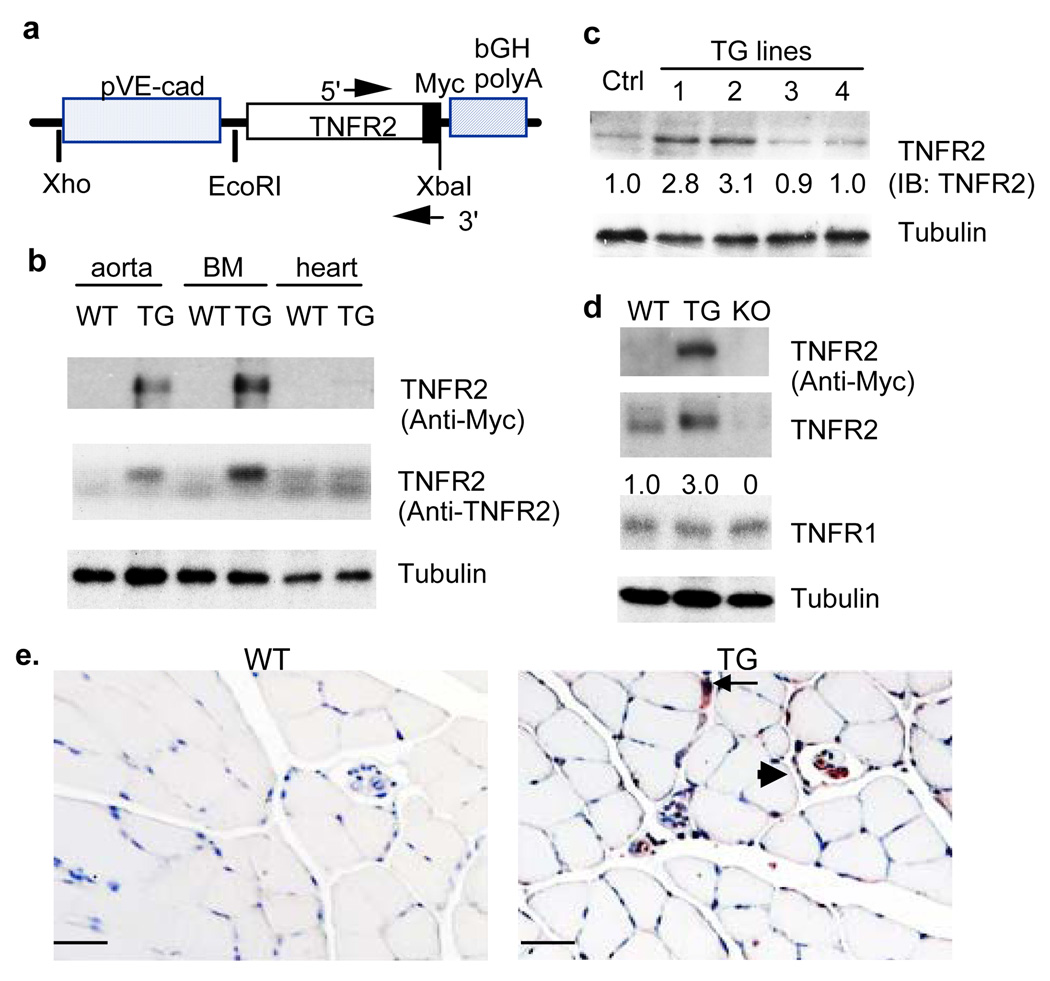
Previously, we have shown that TNFR2 protein is highly upregulated in vascular endothelium in response to ischemia 7. Therefore, we reasoned that a transgene of TNFR2 in EC would increase ischemia-mediated vascular repair. To test this hypothesis, we generated EC-specific transgenic mice by expressing the human TNFR2 with Myc-tag at the C-terminus driven by an EC-specific VE-cadherin promoter 14–16 (TNFR2-TG, Fig.1a). 12 founders were obtained as determined by PCR genotyping (not shown) and Western blot of the tail tissues with anti-TNFR2 antibody (Fig.1b). We chose two lines (#1 and #2 male) to backcross with C57BL/6 mice for more than 6 generations. All experiments were performed with hemizygous TNFR2-TG mice and their non-TG littermates (WT) as controls. The TNFR2 transgene was highly expressed in the aorta and bone marrow as determined by Western blot with anti-Myc and anti-TNFR2 (Fig.1c). Relatively a low level of the TNFR2 transgene was found in the heart despite that cardiac myocyes express a high basal level of endogenous TNFR2 (Fig.1c). This is consistent with the report that the VE-cadherin promoter is less active in adult cardiac vessels compared to developing or newly formed vasculature 17. Total TNFR2 protein (both endogenous and the transgene) in muscle tissue was absent from TNFR2-KO mice, but was 3-fold higher in TNFR2-TG mice than that of WT littermates as determined by Western blotting with anti-TNFR2 antibody (Fig.1d). Furthermore, the TNFR2 transgene was detected in vascular endothelium of both large and small vessels as determined by immunohistochemistry with anti-Myc antibody (Fig.1e). The TNFR2 transgene in EC had no effects on the basal capillary densities in a variety of tissues examined (Supplemental Fig.I for hindlimb and heart). There were no apparent morphological abnormalities in TNFR2-TG mice. Male and female mice appeared equally fertile, and there were no significant differences in growth rate or body weight compared with WT littermates (25.2±4 g for TNFR2-TG vs 24.8±4.0 g for non-transgenic littermates, n=20).
Fig.1. EC-specific expression of TNFR2.
a. Schematic diagram for EC-specific transgenic construct in which expression of the Myc-tagged TNFR2 transgene is driven by a promoter sequence from the VE-cadherin gene (pVE-cad). The bovine growth hormone polyA sequence (bGH polyA) in addition to the 5’ and 3’ primers for genotyping are indicated. b. TNFR2-TG founders. Tails from the TNFR2 transgenic founders were further analyzed for TNFR2 expression by Western blot with anti-Myc (not shown) or anti-TNFR2 antibody. A non-transgenic tail was used as a control. c. Tissue distribution of the TNFR2 transgene. Expression of the TNFR2 protein in different tissues (aorta, bone marrow and heart) was determined by Western blot with anti-Myc or anti-TNFR2. d. Expression of TNFR2 protein in hindlimb muscle. TNFR2 protein in hindlimb was determined by Western blot with anti-Myc or anti-TNFR2 antibody. The relative levels of TNFR2 in TG lines are quantified, with WT as 1.0. TNFR1 was also detected by Western blot with anti-TNFR1. β-tubulin was used as a protein loading control. e. TNFR2 transgene is expressed in the vascular endothelium. The TNFR2 transgene in WT and TNFR2 TG hindlimb sections was detected by immunohistochemistry with anti-Myc antibody. Large vessel and capillary are indicated by arrowhead and arrow, respectively. Scale bar: 100 µM.
TNFR2-TG augments perfusion recovery with enhanced arteriogenesis and angiogenesis in ischemic hindlimbs
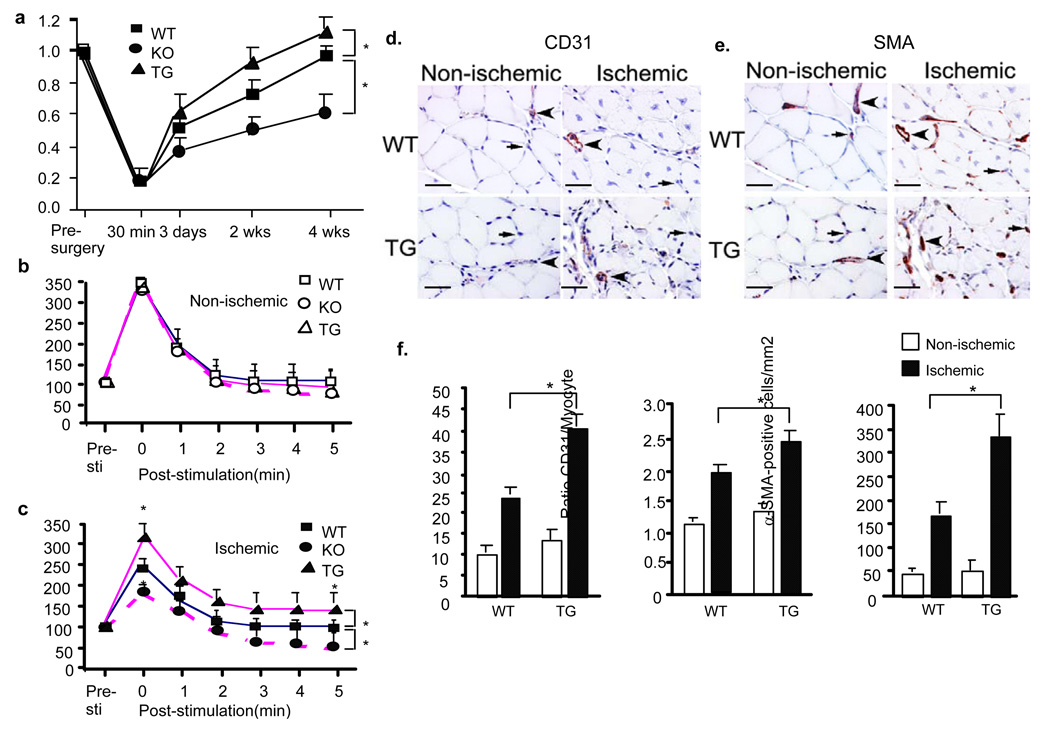
To determine if EC-specific transgene of TNFR2 could augment ischemia-mediated angiogenesis, WT and TNFR2-TG mice were subjected to femoral artery ligation and various analyses at different time points as described previously 6, 7. Blood flow in ischemic and non-ischemic limb perfusion were measured pre-surgery and post-surgery as indicated (30 min, 3 days, 2 weeks and 4 weeks). Before surgery, the ratio of left leg to right leg gastrocnemius blood flow was 1. Post-surgery, blood flow dropped by 80%, and returned to a ratio of 1 over 4 weeks in WT mice. TNFR2-TG mice showed augmented recovery of hindlimb perfusion and flow compared to WT at 2–4 weeks (Fig.2a).
Fig.2. TNFR2-TG augments perfusion recovery with enhanced arteriogenesis and angiogenesis in ischemic hindlimbs.
a. TNFR2-TG mice show augmented recovery of limb perfusion compared to non-transgenic WT mice. Ischemic hindlimb model was performed in WT and TNFR2-TG mice. TNFR2-KO mice were used as controls. Blood flow of ischemic and non-ischemic limb was measured on gastrocnemius muscle at indicated time post-surgery. Ratio of perfusion unit from non-ischemic (left) to ischemic limbs (right) are shown). Data are mean ± SEM, n=10 for each group. *, p<0.05. b–c. TNFR2-TG mice show enhanced postcontraction hyperemia. Adductor muscle groups of mice from pre-surgery (b) and 2 weeks post-surgery (c) were electro-stimulated, and gastrocnemius blood flow was recorded. Both baseline and stimulated lower leg perfusion in legs were measured as an index of the maximal vasodilatory capacity. Data are mean ± SEM, n=5 for each group. *, p<0.05 comparing TNFR2-TG to WT. d–f. 4 weeks after femoral ligation, gastrocnemius muscles were harvested. Capillary density was immunostained with CD31 (an EC marker) and anti-smooth muscle actin (α-SMA). Representative images of CD31(d) and SMA(e) staining are shown. Arterioles and capillaries are indicated by arrowheads and by arrows, respectively. Scale bar: 100 µM. f. Quantification of capillaries (number/mm2 muscle area), ratios of CD31/myocyte and SMA-positive staining (number/mm2 muscle area). Data from 3 sections of each mouse muscle tissue are shown in graphics and n=4 for each strain (total 12 sections). *, p<0.05.
To further define the functional defects in mice, we examined skeletal muscle contraction stimulated hyperemia in the gastrocnemius muscle in WT and TNFR2-TG mice. As seen in the representative traces in Fig.2b, electrical stimulation of the adductor muscle of WT and TNFR2-TG mice results in a marked increase in peak blood flow [compare pre-stimulation (Pre-Sti) to the peak response of stimulation (time 0)]. No difference was detected between WT and TNFR2-TG mice prior to ligation. Next, we measured the same physiological response after limb ischemia in WT and TNFR2-TG mice at 2 weeks post-ischemia. Electrical stimulation of the adductor muscle of WT mice 2 weeks surgery showed only 65% of the peak response measured in the gastrocnemius muscle group compared to pre-ischemia. However, TNFR2-TG mice had a nearly complete recovery in peak blood flow (Fig.2b pre-ischemia, Fig.2c post-ischemia, 95±5%). These data show that TNFR2-TG mice have augmented perfusion recovery in ischemic hindlimbs.
As demonstrated previously 6–8, enhanced clinical recovery and limb perfusion could be due to increased arteriogenesis from existing vessels of the upper limb and/or increased neovascularization/vessel maturation in the lower limb. Consistently, ischemia-induced arteriogenesis in TNFR2-TG mice was significantly increased as measured by microfil casting analysis 6–8 (Supplemental Fig.II). Similarly, angiogenesis and vessel maturation in TNFR2-TG mice were increased in gastrocnemius muscles as measured by immunostaining with anti-CD31 and anti-smooth muscle α-actin (SMA). 4 weeks post-ischemia, CD31-positive capillaries surrounding the skeletal muscle fibers in WT mice were significantly increased (Fig.2d with quantification of capillary number/mm2 and ratio of CD31/myocyte in Fig.2f). Consistent with increased functionally perfused vessels in TNFR2-TG mice, ischemic-induced vessel maturation (SMA-positive vessels, arrowheads) was also increased in TNFR2-TG (Fig.2e with quantification of SMA-positive cells in Fig.2f).
Ischemic-induced cellular survival and proliferation as well as TNFR2-Bmx-VEGFR2 angiogenic signaling are enhanced in TNFR2-TG mice
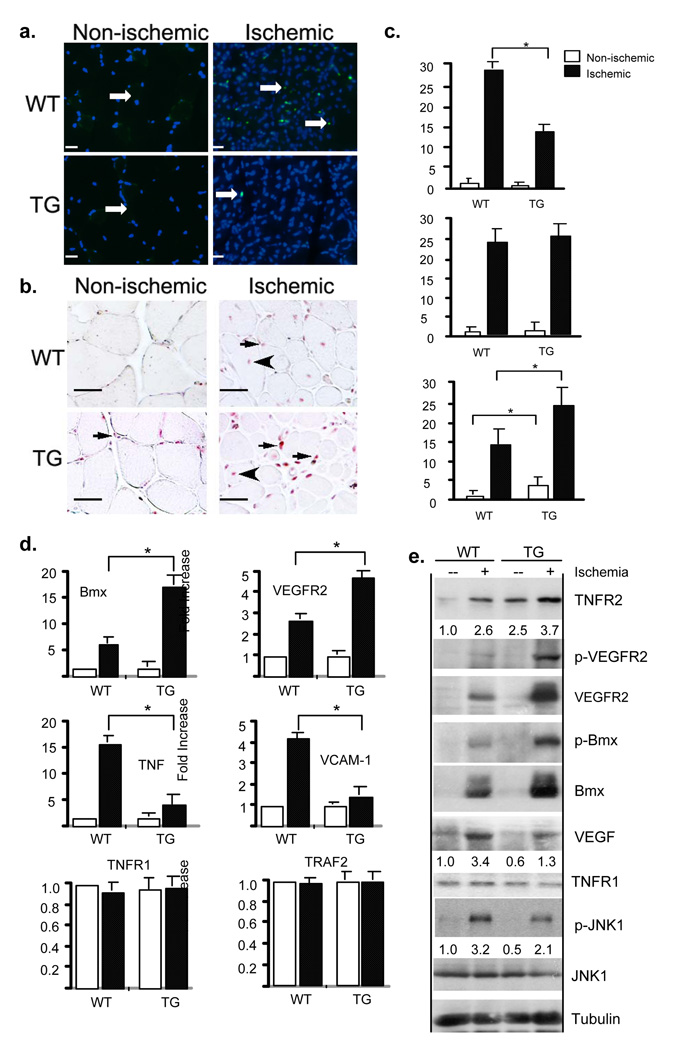
TNFR2 mediates cellular survival and proliferation responses in vitro and in vivo 7, 18. To determine the effects of TNFR2-TG on ischemic-induced cellular proliferative/apoptotic responses, we measured tissue apoptosis by TUNEL assay and cellular proliferation by PCNA staining. Kinetics studies suggested that apoptosis peaked at day 3 post-surgery, and ischemia-induced apoptosis is an early event in the adaptive response. Results showed that ischemia-induced tissue apoptosis was dramatically decreased in TNFR2-TG mice (Fig.3a with quantification in Fig.3c). Cellular proliferation started at day 7 and continued until 4 weeks in the ischemia hindlimb models 7. The TNFR2 transgene increased basal PCNA-positive staining in capillaries (Fig.3b, arrows), consistent with TNFR2-dependent induction of cellular proliferation in EC 7, 18. Both capillaries and myocytes showed PCNA-positive staining upon ischemia (Fig.3b, arrow and arrowhead, respectively), consistent with the increased total numbers of capillaries and muscle fibers at 4 weeks post-ischemia (Fig.2f). Myocytes in ischemic tissue showed central nuclei location (Fig.3b, arrowhead), likely an indicative of regenerative response. No significant differences between WT and TNFR2-TG mice in % of PCNA-positive myocytes were found. However, the number of PCNA-positive EC was significantly increased in TNFR2-TG mice compared to WT mice (Fig.3b with quantification in Fig.3c).
Fig.3. Ischemic-induced cellular survival and proliferation as well as TNFR2-Bmx-VEGFR2 angiogenic signaling are enhanced in TNFR2-TG mice.
a–c. TNFR2 transgene increases ischemia-induced cell survival and proliferation. WT and TNFR2-TG mice were subjected to ischemia ligation, and hindlimb tissues were harvested on day 7. Apoptosis and cellular proliferation in gastrocnemius were determined by TUNEL assay and PCNA staining, respectively. TUNEL-positive nuclei are indicated by white arrows (a). PCNA-positive capillaries and myocytes are indicated by arrows and arrowheads, respectively (b). Total TUNEL-positive cells were counted as number/PF (per field) while % of PCNA-positive EC were counted (c). Data from different mice groups are shown in graphs. n=4 for each strain. *, p<0.05. Scale bar: 100 µM. d–e. TNFR2 transgene increases ischemia-induced Bmx-VEGFR2 angiogenic signaling. WT and TNFR2-TG (n=3) mice were subjected to hindlimb ischemia, and hindlimbs were harvested on day 3 post-surgery as indicated. Gene expression of Bmx, VEGFR2, TNF, VCAM-1, TNFR1 and TRAF2 were determined by qRT-PCR and 18S rRNA was used for normalization. Fold increase (ischemic induction) was calculated by taking non-ischemic WT as 1.0. n=3 for each strain. *, p<0.05. (d). Activation of VEGFR2, Bmx and JNK was determined by Western blot with respective phospho-specific antibodies. Expression of TNFR2, VEGFR2, Bmx and TNFR1 was determined by Western blot with respective antibodies. β-tubulin was used as a control. The relative levels of TNFR2, VEGF and phospho-JNK are quantified, with non-ischemic WT as 1.0 (e). Similar results were obtained from additional two independent mice.
Previously, we have shown that ischemia induces activation of TNFR2-dependent gene expression and signaling complex (TNFR2-Bmx-VEGFR2), which are defective in TNFR2-KO mice 7. To determine if the TNFR2-Bmx-VEGFR2 pathway is upregulated in TNFR2-TG mice, we examined gene expression and activation of TNFR2-dependent signaling molecules. Gene expression of angiogenic factors and their cognate receptors was determined by quantitative RT-PCR. The gene expression in both non-ischemia and ischemic muscles was analyzed, and relative abundance (fold of induction) of each gene is shown by taking non-ischemic WT as 1.0. Ischemia rapidly induces the expression of inflammatory molecules (TNF and VCAM-1) and angiogenic molecules (Ang-2 and VEGF; VEGFR2, TNFR2 and Bmx) on day 3 post-ischemia 6–8. These molecules are known to play important roles in inflammatory angiogenesis 4, 19. Gene expression of VEGFR2 and Bmx was significantly augmented in TNFR2-TG mice (Fig.3d). Interestingly, inflammatory molecules (TNF and VCAM-1) were reduced while TNFR1 and TRAF2 were not significantly altered in TNFR2-TG mice (Fig.3d). Consistently, a reduced infiltration of leukocytes to ischemic hindlimb was observed as determined by immunostaining with the anti-CD45 antibody (Supplemental Fig.III). Activation of TNFR2-dependent signaling pathways was also determined by Western blot with phospho-specific antibodies. Ischemia induced activation of Bmx-VEGFR2 to a greater extent in TNFR2-TG mice compared to WT mice (Fig.3e). The TNFR2-TG mice also showed a greater induction of the total Bmx protein. Notably, phosphorylation of JNK, a TNFR1-dependent apoptotic signaling kinase 11 and the JNK-responsive gene VEGF 20 were slightly decreased in TNFR2-TG mice (Fig.3e).
The TNFR2 transgene in EC is sufficient to mediate ischemia-induced responses
To further determine if the TNFR2 transgene in EC is sufficient to mediate ischemia-induced angiogenesis, we crossed TNFR2-TG with TNFR2 deficient mice to generate TNFR2-KO/TG mice (KO/TG). In these mice, only the TNFR2 transgene is expressed in EC, as determined by genotyping and immunostaining with anti-TNFR2 (Supplemental Fig.IV). Similar to TNFR2-TG, TNFR2-KO/TG had no apparent morphological abnormalities compared to WT and TNFR2-KO mice. Male and female mice appeared equally fertile, and there were no significant differences in growth rate or body weight compared with WT littermates (24.5±3 g for TNFR2-KO vs 24.2±3.0 g for TNFR2-KO/TG, n=15). However, we did observe that the TNFR2 transgene increased basal endothelium-dependent vascular reactivity as determined by aortic ring assay in response to the vasoconstrictor phenylepherine (PE) and vasodilator acetylcholine (Ach) 14. Aortas from TNFR2-KO mice dispayed enhanced constriction in response to PE compared to WT. However, re-expression of TNFR2-TG to KO mice completely restored the normal PE response (Supplemental Fig. Va). To determine if the TNFR2 transgene restores the basal release of eNOS-derived NO, aortic rings from WT, TNFR2-KO and TNFR2-KO/TG mice were preconstricted with a submaximal dose of PE, and the NOS inhibitor L-NAME (100 µM) was added at the peak of the constriction to remove endogenous NO tone. L-NAME caused a further constriction in all vessels, resulting in an increase in isometric tension reflecting the removal of basal NO. The ratio of EC50 by PE in the presence vs absence of L-NAME indicates a basal NO activity 14. The ratio for TNFR2-KO vessels was 2-fold less than WT vessels, suggesting a reduction in a basal NO release. However, the TNFR2 transgene restored the basal NO activity (Fig.Vb). The TNFR2-transgene in TNFR2-KO mice also restored endothelium-dependent vasodilation in response to Ach (Fig.Vc). These effects of TNFR2-transgene on vasomotion occurred selectively in the endothelium because the vasoconstrictive responses to KCl and relaxation in response to the nitric oxide (NO) donor drug SNP were similar to TNFR2-KO (Supplemental Fig.V). The exact mechanism by which TNFR2 regulates NO is unclear. The TNFR2-dependent NO activity and endothelium function could be attributed to the increased VEGFR2 activity in TNFR2-TG mice (see below), an upstream activator of eNOS-NO.
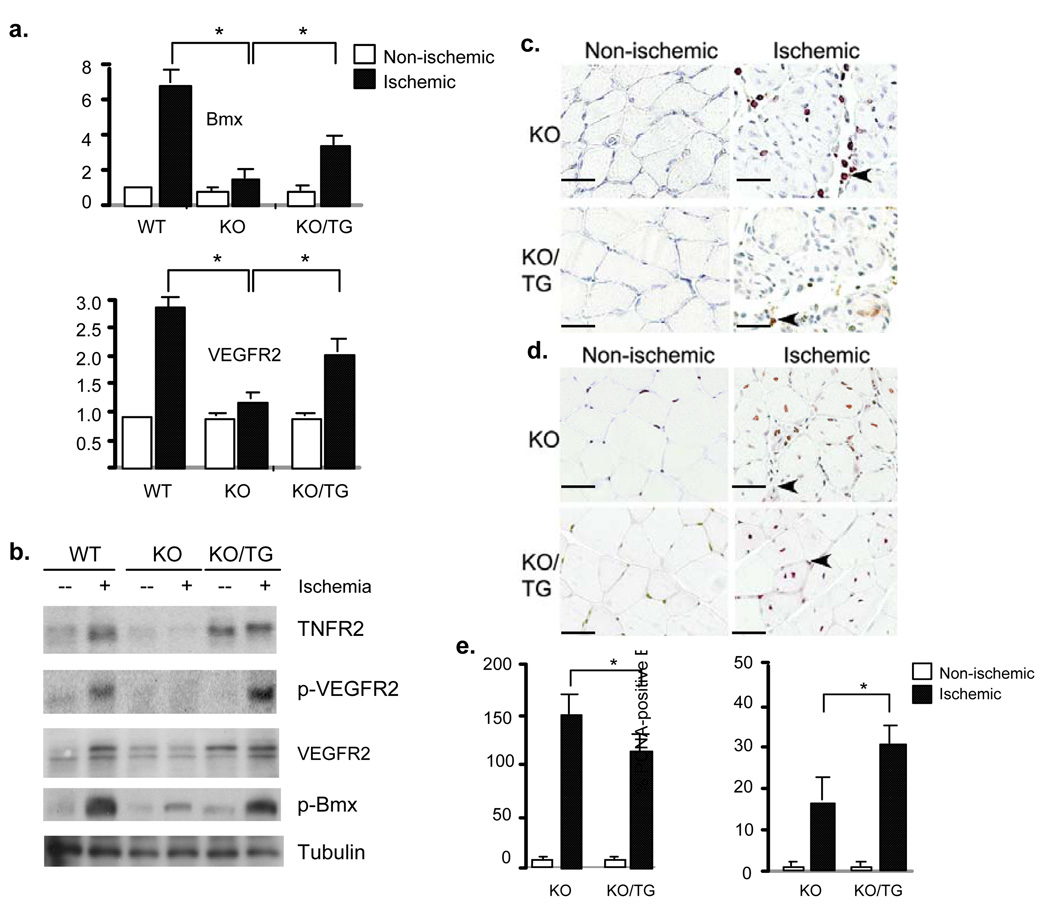
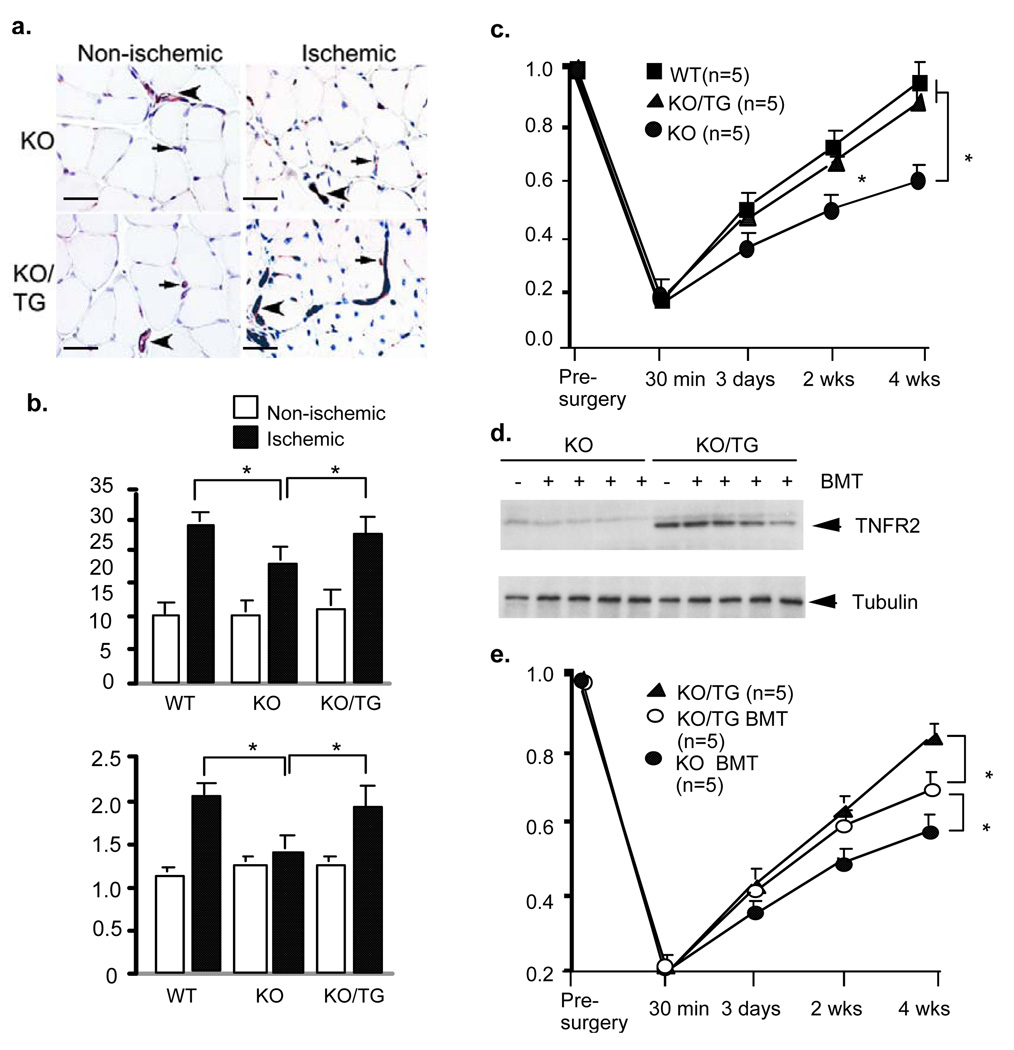
We next examined if the TNFR2 transgene in EC could restore the defects in ischemic responses observed in TNFR2-KO. Consistent with previous findings 7, TNFR2 deletion causes severe impairment in ischemia-induced gene expression of VEGFR2 and Bmx as well as TNFR2-dependent Bmx-VEGFR2 signaling. Indeed, the basal level of TNFR2 in TNFR2-TG driven by VE-cadherin promoter is similar to that induced by ischemia in WT muscle, and the TNFR2 transgene could restore ischemia-induced gene expression of VEGFR2 and Bmx as determined by RT-PCR (Fig.4a) and activation of Bmx-VEGFR2 signaling in hindlimb as determined by Western blot with phospho-specific antibodies (Fig.4b). The increased Bmx-VEGFR2 signaling in TNFR2-KO/TG correlated with reduced infiltration (by CD45 staining) and increased proliferation (by PCNA staining) compared to TNFR2-KO mice (Fig.4c–e).
Fig.4. The TNFR2 transgene in EC is sufficient to mediate ischemia-induced responses.
a–b. Effects of TNFR2 transgene on Bmx-VEGFR2 expression and activation. WT, TNFR2-KO (KO) and TNFR2-KO/TNFR2-TG (KO/TG) mice (n=3 in each group) were subjected to ischemia ligation and hindlimb tissues were harvested on day 3. Relative mRNA levels of Bmx and VEGFR2 are mean ± SEM (fold induction), *, p<0.05 comparing WT and KO/TG to KO mice group (a). Activation of Bmx-VEGFR2 signaling is shown in (b). c–e. On day 7, tissues were harvested, infiltration of immune cells and cellular proliferation in gastrocnemius were determined by immunostaining with anti-CD45 and anti-PCNA, respectively. Infiltrated cells are indicated by arrowheads (c, CD45). PCNA-positive capillaries and myocytes are indicated by arrows and arrowheads, respectively (d). Scale bar: 100 µM. Total CD45-positive cells were counted as number/PF (per field), while % of PCNA-positive cells were counted (e). Data from different mice groups are shown in graphs. n=4 for each strain. *, p<0.05.
The TNFR2 transgene in both intrinsic vasculature and bone marrow contribute to ischemia-induced angiogenesis and flow recovery
Finally, we examined if the TNFR2 transgene in EC could restore angiogenesis and functional flow recovery. WT, TNFR2-KO and KO/TG mice were subjected to femoral artery ligation followed by morphological and functional blood flow analyses as described for WT and TNFR2-TG mice. Results show that EC-expressed TNFR2 rescued the defects in ischemia-induced neovascularization and flow recovery observed in TNFR2-KO (Fig.5a–c). This increased angiogenesis observed in vivo seems to EC autonomous because isolated mouse EC from TNFR2-KO/TG also showed increased migration in response to TNF in vitro compared to TNFR2-KO EC (Supplemental Fig.VI), likely attributed to TNF-induced TNFR2-Bmx-VEGFR2 activation 7, 12. In contrast, TNFR1-dependent JNK signaling was reduced by the TNFR2 transgene as observed in vivo (Supplemental Fig.VI).
Fig.5. The TNFR2 transgenes in both intrinsic vasculature and bone marrow contribute to ischemia-induced angiogenesis and flow recovery.
a–b. Effects of TNFR2 transgene on ischemia-induced angiogenesis and flow recovery. WT, TNFR2-KO (KO) and TNFR2-KO/TNFR2-TG (KO/TG) mice (n=3 in each group) were subjected to ischemia ligation. 4 weeks after femoral ligation, gastrocnemius muscles were harvested. Capillaries were immunostained with CD31 (a). Scale bar: 100 µM. Quantification of capillaries (number/mm2 muscle area) and ratio of CD31/muscle fiber are shown (b). Data from 3 sections of each mouse muscle tissue are shown in graphics and n=4 for each strain (total 12 sections). *, p<0.05. c. Blood flow of ischemic and non-ischemic limb was measured on gastrocnemius muscle at indicated times. Ratio of perfusion unit from non-ischemic (left) to ischemic limbs (right) are shown). N (number) for each strain is shown in parenthesis. Data are mean ± SEM, *, p<0.05. d–e. BMT experiment. TNFR2-KO mice were subjected to lethal irradiation followed by receiving bone marrow cells from KO (KO BMT) or TNFR2-KO/TG mice (KO/TG BMT). Successful BMT was verified by genotyping of TNFR2 transgene six weeks after BMT. The mice were then subjected to femoral artery ligation. 4 weeks post-ischemia, bone marrow was harvested and expression of TNFR2 was determined by Western blot with anti-TNFR2. Bone marrow cells from KO and KO/TG mice without BMT/ligation were used as controls (d). Blood flow of ischemic and non-ischemic limb was measured on gastrocnemius muscle at indicated time post-surgery. Ratio of perfusion unit from non-ischemic (left) to ischemic limbs is shown (e). N (number) for each strain is shown in parenthesis. Data are mean ± SEM, *, p<0.05.
The TNFR2 transgene is expressed at a very high level in bone marrow (see Fig.1). Moreover, we have previously shown that Bmx in bone marrow-derived cells contributes to ischemia-induced arteriogenesis/angiogenesis 6. This led us to determine the role of the TNFR2 transgene in bone morrow in ischemia-induced responses. To this end, we performed bone morrow transplantation (BMT) experiments using TNFR2-KO as recipients. First, TNFR2-KO mice received lethal irradiation followed by transplantation of bone morrow cells from TNFR2-KO or TNFR2-KO/TG to TNFR2-KO mice (KO BMT and KO/TG BMT). 6 weeks after BMT, the mice were then subjected to femoral artery ligation. Success of BMT was verified by genotyping of blood samples with TNFR2-KO or TNFR2-TG-specific primers (not shown), and by Western blot of bone marrow samples with anti-TNFR2 antibody (Fig.5d). KO and KO/TG mice without BMT were used as controls. Both control KO mice and KO BMT mice showed a similar impairment of blood flow recovery, and no significant differences were observed between the two groups. Blood flow recovery was dramatically increased in KO/TG BMT group compared to KO on day 14 and day 28 (Fig.5e). Consistently, KO/TG BMT mice had enhanced ischemia-mediated arteriogenesis and angiogenesis (not shown). However, blood flow recovery on day 28 in KO/TG BMT groups was less compared to control KO/TG (Fig.5e). These data suggest that TNFR2-expressing cells in both intrinsic vasculature and bone marrow contribute to ischemia-mediated vascular repair and functional flow recovery.
DISCUSSION
The rationale to generate TNFR2-TG is that TNFR2 is highly induced in vascular EC in response to ischemia 7. The important finding of this study is that the EC-specific TNFR2 plays a critical role in ischemia-mediated arteriogenesis and angiogenesis. Specifically, EC-specific TNFR2 transgene enhances limb perfusion and ischemic reserve capacity compared to the non-transgenic wild-type mice. Immunohistochemical analyses indicate that TNFR2-TG mice had increased ischemia-initiated EC proliferation, neovascularization and vessel maturation. These functional changes in TNFR2-TG mice correlate with augmented Bmx-VEGFR2 proangiogenic activities, which have been previously shown to be TNFR2-dependent in vascular EC 12. Moreover, the EC-expressed TNFR2 rescues the defects of TNFR2-KO in ischemia-induced angiogenic signaling and flow recovery. Moreover, bone marrow transplant suggests that both TNFR2-expressing cells in intrinsic vasculature and bone marrow contribute to ischemia-mediated angiogenesis and flow recovery. Our data support a critical role of endothelial TNFR2 in ischemia-mediated adaptive angiogenesis.
EC-expressed TNFR2 transgene does not significantly induce inflammation and basal capillary density in a variety of tissues that we examined. There were no apparent morphological abnormalities in TNFR2-TG or TNFR2-KO/TG mice. This is in contrast to TNF-transgene mice 21. Previous data have shown that transgenic mice expressing low levels of soluble TNF cause chronic inflammation and arthritis. This is likely due to systemic activation of TNFR1-dependent signaling 21. EC-specific transgenic mice expressing a membrane-bound form of TNF (mtTNF) also induced constitutive EC activation and inflammatory angiogenesis 22. It is not known whether these responses are TNFR1 and/or TNFR2-dependent despite proposal of mtTNF as a ligand for endogenous TNFR2 in vivo. Interestingly, ischemia-induced inflammatory responses (expression of TNF, TNF-induced JNK MAPK signaling and its responsive genes VCAM-1 and VEGF as well as infiltration of immune cells) are actually reduced in the TNFR2-TG mice. Since TNF-induced inflammatory events are primarily mediated by TNFR1, the current study suggests that TNFR1 signaling in EC may be suppressed by the TNFR2 transgene. The mechanism by which TNFR2 suppresses TNFR1-dependent signaling in vascular EC needs to be further defined. In contrast to the TNFR1-dependent inflammatory signaling, TNFR2-dependent Bmx-VEGFR2 proangiogenic signaling pathways are upregulated. This is consistent with our previous findings that TNFR2 in EC utilizes Bmx to transactivate VEGFR2, a pathway critical for in vitro EC angiogenesis 7. Moreover, we have demonstrated that TNFR2 contains a Bmx-binding domain at the C-terminal 59 aa residues, and a mutant with a deletion of the 59 aa residues (TNFR2–59) failed to activate VEGFR2 and EC angiogenesis, and induces apoptosis in cultured EC 7. Consistently, we failed to obtain an EC-specific transgenic mouse line expressing TNFR2–59. It is conceivable that TNFR2–59 transgene in EC leads embryonic lethality by inducing vascular EC apoptosis.
Our data clearly demonstrate that the TNFR2 transgene in bone marrow-derived cells plays a role in ischemia-induced vascular remodeling. This is supported by the results that bone marrow-derived cells expressing the TNFR2 transgene implanted to TNFR2-KO mice augmented ischemia-induced blood flow recovery compared. However, blood flow recovery on day 28 in KO/TG BMT groups was less compared to control KO/TG. These data suggest that TNFR2-expressing cells in both intrinsic vasculature and bone marrow contribute to ischemia-mediated vascular repair and functional flow recovery. These results are similar to our previous findings that Bmx in bone marrow-derived cells is critical for the early phase of ischemia-induced tissue repair, whereas Bmx in intrinsic vasculature may contribute to the later phase of arteriogenesis/angiogenesis 6. Given that the TNFR2 transgene is expressed in both bone marrow and muscle tissues, it is conceivable that the TNFR2-Bmx-VEGFR2-dependent signaling may enhance EC survival and mobilization in bone marrow while promote EC migration and tube formation in local muscle tissue, contributes to ischemic-induced arteriogenesis and angiogenesis.
ECs are a primary target of TNF and TNF elicits a broad spectrum of biological effects including proliferation, differentiation and apoptosis. It has been shown that anti-TNF therapy targeting both TNFR1 and TNFR2 has been successful in treating inflammatory rheumatoid arthritis but has failed in the treatment of cardiovascular diseases although TNF protein is increased in heart disease 23. Our previous and current studies strongly suggest that TNFR2 signaling plays beneficial roles in the cardiovascular system including ischemia-initiated arteriogenesis (growth of existing collaterals) and angiogenesis (formation of new blood vessels). Therefore, specific expression and activation of TNFR2 in EC may provide a novel strategy for the treatment of vascular diseases such as coronary artery disease and peripheral arterial disease.
Supplementary Material
ACKNOWLEDGEMENTS
a. Source of funding:
This work was supported by grants from NIH grants HL077357, HL-65978, and a National Nature Science Foundation of China (30828032) to W. Min and 30872819 to Y. Luo, a National Cancer Institute (NCI) Ruth Kirschstein Predoctoral Fellowship Award (F31 CA 136316) to D.J.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
b. Disclosure: none.
REFERENCES
- 1.Baumgartner I, Schainfeld R, Graziani L. Management of peripheral vascular disease. Annu Rev Med. 2005;56:249–272. doi: 10.1146/annurev.med.56.082103.104649. [DOI] [PubMed] [Google Scholar]
- 2.Mohler ER., 3rd The effect of risk factor changes on peripheral arterial disease and cardiovascular risk. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:259–263. doi: 10.2174/1568006043335998. [DOI] [PubMed] [Google Scholar]
- 3.Brevetti G, Silvestro A, Di Giacomo S, Bucur R, Di Donato A, Schiano V, Scopacasa F. Endothelial dysfunction in peripheral arterial disease is related to increase in plasma markers of inflammation and severity of peripheral circulatory impairment but not to classic risk factors and atherosclerotic burden. J Vasc Surg. 2003;38:374–379. doi: 10.1016/s0741-5214(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 4.Heil M, Schaper W. Influence of mechanical, cellular, and molecular factors on collateral artery growth (arteriogenesis) Circ Res. 2004;95:449–458. doi: 10.1161/01.RES.0000141145.78900.44. [DOI] [PubMed] [Google Scholar]
- 5.Heil M, Schaper W. Cellular mechanisms of arteriogenesis. Exs. 2005:181–191. doi: 10.1007/3-7643-7311-3_13. [DOI] [PubMed] [Google Scholar]
- 6.He Y, Luo Y, Tang S, Rajantie I, Salven P, Heil M, Zhang R, Luo D, Li X, Chi H, Yu J, Carmeliet P, Schaper W, Sinusas AJ, Sessa WC, Alitalo K, Min W. Critical function of Bmx/Etk in ischemia-mediated arteriogenesis and angiogenesis. J Clin Invest. 2006;116:2344–2355. doi: 10.1172/JCI28123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo D, Luo Y, He Y, Zhang H, Zhang R, Li X, Dobrucki WL, Sinusas AJ, Sessa WC, Min W. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol. 2006;169:1886–1898. doi: 10.2353/ajpath.2006.060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, He Y, Dai S, Xu Z, Luo Y, Wan T, Luo D, Jones D, Tang S, Chen H, Sessa WC, Min W. AIP1 functions as an endogenous inhibitor of VEGFR2-mediated signaling and inflammatory angiogenesis in mice. J Clin Invest. 2008;118:3904–3916. doi: 10.1172/JCI36168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 10.Carpentier I, Coornaert B, Beyaert R. Function and regulation of tumor necrosis factor type 2. Curr Med Chem. 2004;11:2205–2212. doi: 10.2174/0929867043364694. [DOI] [PubMed] [Google Scholar]
- 11.Pan S, An P, Zhang R, He X, Yin G, Min W. Etk/Bmx as a tumor necrosis factor receptor type 2-specific kinase: role in endothelial cell migration and angiogenesis. Mol Cell Biol. 2002;22:7512–7523. doi: 10.1128/MCB.22.21.7512-7523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang R, Xu Y, Ekman N, Wu Z, Wu J, Alitalo K, Min W. Etk/Bmx transactivates vascular endothelial growth factor 2 and recruits phosphatidylinositol 3-kinase to mediate the tumor necrosis factor-induced angiogenic pathway. J Biol Chem. 2003;278:51267–51276. doi: 10.1074/jbc.M310678200. [DOI] [PubMed] [Google Scholar]
- 13.Goukassian DA, Qin G, Dolan C, Murayama T, Silver M, Curry C, Eaton E, Luedemann C, Ma H, Asahara T, Zak V, Mehta S, Burg A, Thorne T, Kishore R, Losordo DW. Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation. 2007;115:752–762. doi: 10.1161/CIRCULATIONAHA.106.647255. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Luo Y, Zhang W, He Y, Dai S, Zhang R, Huang Y, Bernatchez P, Giordano FJ, Shadel G, Sessa WC, Min W. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am J Pathol. 2007;170:1108–1120. doi: 10.2353/ajpath.2007.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai S, He Y, Zhang H, Yu L, Wan T, Xu Z, Jones D, Chen H, Min W. Endothelial-specific expression of mitochondrial thioredoxin promotes ischemia-mediated arteriogenesis and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:495–502. doi: 10.1161/ATVBAHA.108.180349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun JF, Phung T, Shiojima I, Felske T, Upalakalin JN, Feng D, Kornaga T, Dor T, Dvorak AM, Walsh K, Benjamin LE. Microvascular patterning is controlled by fine-tuning the Akt signal. Proc Natl Acad Sci U S A. 2005;102:128–133. doi: 10.1073/pnas.0403198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kogata N, Arai Y, Pearson JT, Hashimoto K, Hidaka K, Koyama T, Somekawa S, Nakaoka Y, Ogawa M, Adams RH, Okada M, Mochizuki N. Cardiac ischemia activates vascular endothelial cadherin promoter in both preexisting vascular cells and bone marrow cells involved in neovascularization. Circ Res. 2006;98:897–904. doi: 10.1161/01.RES.0000218193.51136.ad. [DOI] [PubMed] [Google Scholar]
- 18.Al-Lamki RS, Wang J, Vandenabeele P, Bradley JA, Thiru S, Luo D, Min W, Pober JS, Bradley JR. TNFR1- and TNFR2-mediated signaling pathways in human kidney are cell type-specific and differentially contribute to renal injury. Faseb J. 2005;19:1637–1645. doi: 10.1096/fj.05-3841com. [DOI] [PubMed] [Google Scholar]
- 19.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin G, Liu W, An P, Li P, Ding I, Planelles V, Schwarz EM, Min W. Endostatin gene transfer inhibits joint angiogenesis and pannus formation in inflammatory arthritis. Mol Ther. 2002;5:547–554. doi: 10.1006/mthe.2002.0590. [DOI] [PubMed] [Google Scholar]
- 21.Kollias G. TNF pathophysiology in murine models of chronic inflammation and autoimmunity. Semin Arthritis Rheum. 2005;34:3–6. doi: 10.1016/j.semarthrit.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Rajashekhar G, Willuweit A, Patterson CE, Sun P, Hilbig A, Breier G, Helisch A, Clauss M. Continuous endothelial cell activation increases angiogenesis: evidence for the direct role of endothelium linking angiogenesis and inflammation. J Vasc Res. 2006;43:193–204. doi: 10.1159/000090949. [DOI] [PubMed] [Google Scholar]
- 23.Anker SD, Coats AJ. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int J Cardiol. 2002;86:123–130. doi: 10.1016/s0167-5273(02)00470-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.