Abstract
We applied the Item Specific Deficit Approach (ISDA) to California Verbal Learning Test data obtained from 56 severe, acceleration-deceleration CHI participants and 62 controls. The CHI group demonstrated deficits on all ISDA indices in comparison to controls. Regression analyses indicated that encoding deficits, followed by consolidation deficits, accounted for most of the variance in delayed recall. Additionally, level of acquisition played a partial role in CHI-associated consolidation difficulties. Finally, CHI encoding deficits were largely driven by low semantic clustering during list learning. These results suggest that encoding (primary) and consolidation (secondary) deficits account for CHI-associated verbal memory impairment.
MeSH terms: Traumatic Brain Injury, Memory, Neuropsychology
Episodic verbal memory impairment is among the most profound cognitive deficit produced by closed-head injury (CHI; Mateer, Sohlberg, & Crinean, 1987) and is a robust predictor of unemployment in this population (Brooks, Campsie, Symington, Beattie, & McKinlay, 1987). Studies examining the memory profile of CHI have yielded mixed results, with some suggesting a prominent role for encoding deficits and others suggesting greater consolidation and/or retrieval deficits (Curtiss, Vanderploeg, Spencer, & Salazar, 2001; DeLuca, Schulteis, Madigan, Christodoulou, & Averill, 2000; Vanderploeg, Crowell, & Curtiss, 2001). Although the processes of encoding, consolidation, and retrieval partially overlap, distinctions have been found among them (e.g., Bernard, Desgranges, Platel, Baron, & Eustache, 2001; Greicius et al., 2003; Nyberg et al., 1996; Tulving & Osler 1968; Tulving & Thompson, 1973), with these temporally graded memory processes being partially subserved by separable neuroanatomical units (see Bernard et al., 2001; Greicius et al., 2003; Nielsen-Bohlman & Knight, 1994; Nyberg, Forkstam, Petersson, Cabeza, & Ingvar, 2002; Tulving & Markowitsch, 1998; Nyberg et al., 1996).
Research utilizing traditional memory process indices often contradicts quasi-experimental studies (e.g., Vanderploeg et al., 2001 vs. DeLuca et al., 2000). While quasi-experimental designs may lack the precision to clearly identify specific types of memory deficits, they are more likely to produce more reliable results than studies purely based on traditional memory process indices. Traditional measures of encoding have included learning slope, total learning score, and recall differences between the first and last learning trials. However, these indices are calculated by including performance on learning trial 1. Unfortunately, CHI-related inattention can impair trial 1 scores on list-learning tests (Wiegner & Donders, 1999). Indeed, we found evidence that inattention is associated with total learning in a mixed neurological sample (Wright et al., 2009).
Consolidation deficits are often indexed using forgetting rate. Here, reduced scores on delayed recall compared to the last learning trial is considered to indicate rapid forgetting and, thus, a consolidation deficit. However, this assumes that words remembered at the two comparison points are from the same pool of encoded items. Specifically, different items may be remembered at the last learning trial than at delayed recall, which may be more indicative of a deficit in retrieval than consolidation. Additionally, the assumption that lower scores on delayed recall than during learning reflects poor consolidation, rather than retrieval difficulties, is also problematic. In a recent study, we found that forgetting rate and an index of retrieval ability (recognition discriminability-free recall discrepancy) were moderately correlated in a mixed neurological sample (Wright et al., 2009). Therefore, the forgetting rate index is contaminated by retrieval processes. Another measure that has been used as an indicator of consolidation is proactive interference (PI). PI compares the initial recall of a second list (B) in contrast to the initial recall of a preceding list (A). This measure refers to the normative detriment of new learning following recent learning and is suggested to reflect ongoing consolidation of previously presented information. Thus, lower PI scores are thought to represent disrupted consolidation of the first list. However, PI is likely to be invalid as a measure of consolidation for CHI participants, whose attention difficulties likely impair trial 1 recall of both lists (Wiegner & Donders, 1999).
Recognition discriminability-free recall discrepancies have been used to represent retrieval deficits (Duchnick, Vanderploeg, & Curtiss, 2002; Vanderploeg et al., 2001). However, Wilde, Boake, and Sherer (1995) showed that results using this measure did not correlate with other indicators of retrieval ability (semantically cued recall and recall consistency across learning trials) in CHI participants. In addition, comparing recall to recognition is problematic, as the processes are not equivalent; recall is a product of recollection, whereas recognition performances are comprised of both recollection and familiarity (see Aggleton & Brown, 2006).
Methodological, sampling, and definitional issues in CHI memory research make it difficult to determine a specific memory profile. For example, there is great heterogeneity in the etiology and neuropathological consequences of traumatic brain injury [National Institutes of Health (NIH), 1999; Williamson et al., 1996]. CHI is most often due to acceleration-deceleration events (e.g., motor-vehicle accidents; Esselman & Uomoto, 1995; King, 1997; NIH, 1999; Williamson, Scott, & Adams, 1996), which typically results in diffuse axonal injury (Adams et al., 1989; Adams & Jennett, 2001; Abou-Hamden et al., 1997), although lesions to the ventral frontal and ventral anterior medial temporal areas are fairly common as well (Williamson, Scott, & Adams, 1996; Wilson, Hadlet, Wiedmann, & Teasdale, 1995). Some studies include CHI participants with differing etiologies and injury severities, which likely contributes to the mixed findings in this area of research (see Nakayama, Okumura, Shinoda, Nakashima, & Iwama, 2006; Wallesch et al., 2001). Moreover, differences of opinion with regard to what evidence constitutes a specific memory deficit further complicates matters as it does in other areas in memory research (e.g., experimental amnesia; see Miller & Matzel, 2006; Nader, 2006; Squire, 2006;).
Despite these issues, some consistent themes have emerged. Several studies strongly suggest that verbal memory dysfunction in CHI results from encoding deficits. CHI participants show a decreased learning curve (Blachstein, Vakil, & Hoofien, 1993), reduced semantic clustering (Stallings, Boake, & Sherer, 1995), and lack of spontaneous strategy use during list-learning (Levin & Goldstein, 1986) compared to controls. One of the most compelling pieces of evidence for an encoding deficit was provided by DeLuca et al. (2000). In this study, moderate-to-severe CHI participants and demographically matched controls were administered a modified version of the Selective Reminding Test (SRT; Buschke, 1973), in which all of the items were semantically related. Unrecalled items were presented until all 10 words were recalled on two consecutive trials over a maximum of 15 trials. Seventy percent of the CHI participants (CHI-MET) were able to meet acquisition criteria. The CHI-MET group did not differ in delayed recall or recognition scores from controls but required more learning trials to learn the list of words. Overall, these findings suggest that CHI-associated memory dysfunction results from an encoding deficit.
Other research indicates that CHI participants have a consolidation deficit. Vanderploeg et al. (2001) examined performances of moderate-to-severe CHI participants and healthy acquisition- and demographics- matched control groups on common indices derived from the California Verbal Learning Test (CVLT; Delis, Kramer, Kaplan, & Ober, 1987). They found accelerated forgetting rate for the CHI group as compared to both control groups. In addition, demographically matched controls showed more PI than the CHI group. No differences in encoding (trial 1–5 recall differences; learning slope) or retrieval (i.e., cued-free recall differences; recognition discriminability-free recall discrepancies) were detected. It was suggested that the lower PI and greater forgetting rate exhibited by the CHI participants indicated that their verbal memory impairment is primarily driven by a consolidation deficit.
Other investigators addressed the possibility of retrieval deficits following CHI. This work suggests that a relatively small number of CHI participants suffer from retrieval difficulties (Duchnick, Vanderploeg, & Curtiss, 2002; Curtiss et al., 2001), although these data are largely based on recognition-recall discrepancies. In contrast, using the directed forgetting paradigm (see Basden & Basden 1996; Basden, Basden, & Gargano, 1993), a method for examining inhibitory and retrieval mechanisms (Basden, Basden, & Wright, 2003), we found that severe CHI participants exhibited similar memory retrieval abilities as controls, despite displaying impaired recall performances (Schmitter-Edgecombe, Marks, Wright, &, Ventura, 2004).
While traditional indices reflect aspects of the memory process in CHI participants, they are influenced by inattention, provide inadequate distinctions between estimates of consolidation and retrieval, and result in different conclusions in comparison to quasi-experimental manipulations. We recently developed an item analytic method (the Item Specific Deficit Approach; ISDA; see Wright et al., 2009) that provides separable estimates of encoding, consolidation and retrieval ability within the same list-learning test. Each index is distinguished by specific, independent patterns of item recall across learning and/or delayed recall trials (see below). The ISDA was found to be relatively unaffected by inattention and demonstrated adequate reliability and validity when applied to the CVLT.
The primary goal of the current study was to utilize the ISDA in order to determine the impact of deficits in encoding, consolidation, and/or retrieval in a sample of severe acceleration-deceleration CHI participants. Secondarily, we aimed to determine how reductions in semantic clustering during list acquisition might relate to encoding deficits. Finally, we sought to determine the impact of encoding level on consolidation difficulties.
METHOD
Participants
Data were collected from 63 CHI participants and 63 healthy, age- and education-matched control participants. All participants’ consented to voluntary participation in institutional review board (IRB) approved studies conducted between 1993 and 2004 in Tennessee (Memphis area) or Washington (Spokane/Pullman area). We evaluated symptom validity via two indices derived from the CVLT (recognition discriminability and a discriminant function; see Millis, Putnam, Adams, & Ricker, 1995). Using failure on both indices as an indicator of poor effort, we excluded 7 CHI participants and one control participant from our sample. This resulted in a CHI group comprised of 56 participants and a control group with 62 participants. There were no significant differences between the groups in age, education, estimated IQ, or sex ratio (see Table 1), although the control group contained a larger proportion of minority participants. None of the participants had a significant history of alcohol abuse or illicit substance use. We excluded CHI participants who were younger than 15 years or older than 55 years at the time of injury to control for possible developmental effects. All CHIs resulted from acceleration-deceleration incidents (46 motor vehicle accidents, 8 long falls, 2 pedestrian-automobile collisions). Per medical records (n=54) and/or careful clinical interviewing of participants and a reliable informant (n=56), all CHI participants suffered severe injuries, as indicated by a posttraumatic amnesia >7 days (n=44, M=75.94 days, SD=90.86, range=12.00–547.50 days), and/or a loss of consciousness >36 hrs (n=46, M=817.69hrs, SD=785.49, range=12.00–2,928hrs) (Williamson et al., 1996). All CHI participants were tested at least one year post injury (M=8.20yrs, SD=7.39, range=1–28yrs). Individuals with multiple brain injuries, open head injuries, or other neurological (e.g., stroke, epilepsy, multiple sclerosis) or psychiatric conditions (e.g., depression, psychosis, substance abuse/dependence) discovered during record review or participant and informant interview were excluded.
Table 1.
Demographics and Memory Performances
| Variable | CHI (n=56) | Control (n=62) | Test Statistic | Effect Size |
|---|---|---|---|---|
| Demographics | ||||
| Age (in years) | 34.48 (9.97) | 33.81 (9.98) | t (1, 116) = 0.37 | d = 0.07 |
| Age Range (in years) | 19–54 | 18–57 | - | - |
| Education (in years) | 14.15 (2.32) | 14.53 (1.67) | t (1, 116) = 1.02 | d =0.19 |
| Education Range (in years) | 10–20 | 12–20 | - | - |
| Estimated Premorbid IQa | 106.49 (5.94) | 106.76 (6.17) | t (1, 116) = 0.24 | d =0.04 |
| % Male | 69.64% | 74.19% | χ2 (1, n=118)=0.30 | Øc =0.05 |
| % Caucasian | 96.43% | 69.35% | χ2 (1, n=118)=14.74* | Øc =0.35 |
| CVLT Index | ||||
| Trial 1 Recall | 6.36 (1.93) | 7.87 (2.27) | t (1, 116) = 3.89* | d =0.72 |
| Trials 1–5 Recall | 45.88 (11.46) | 55.50 (10.25) | t (1, 116) = 4.82* | d =0.90 |
| Short-Delay Free Recall | 7.87 (4.04) | 11.47 (2.87) | t (1, 116) = 5.61* | d =1.05 |
| Long-Delay Free Recall | 8.96 (3.69) | 12.09 (2.76) | t (1, 116) = 5.25* | d =0.98 |
| Recognition Discriminability | 87.35 (13.31) | 94.60 (10.44) | t (1, 116) = 4,23* | d =0.62 |
| ISDA Index | ||||
| Encoding Deficit | 6.18 (3.21) | 3.61 (2.43) | t (1, 116) = 4.92* | d =0.92 |
| Consolidation Deficit | 0.23 (0.17) | 0.13 (0.11) | t (1, 116) = 3.95* | d =0.71 |
| Retrieval Deficit | 0.33 (0.21) | 0.21 (0.14) | t (1, 116) = 3.66* | d =0.69 |
Note. Table depicts the group comparisons of demographic and memory variables. Ratio data are displayed as mean values and standard deviations (SD in parentheses) and nominal data are displayed as percentages. Group differences were assessed via t-test or chi-square. A Bonferroni corrected significance value of α < .01 was used for the CVLT and ISDA analyses. Raw values were used for CVLT index comparisons. The ISDA consolidation and retrieval indices were divided by the number of items gained during list-learning to control for acquisition level. CHI=Closed-Head Injury. IQ= Intelligence Quotient. ISDA= Item Specific Deficit Approach.
Premorbid IQ scores were estimated from demographic information (Barona et al., 1984).
p <0.001
Procedure
The CVLT was administered in accordance with standard instructions (Delis et al., 1987). The CVLT is a verbal list-learning test comprised of 16 items that can be grouped into four semantic categories. The list is presented orally to participants over five learning trials. Subsequently, a distractor list is presented and participants are asked to recall the distractor items. Following the distractor trial, participants are administered a short-delayed free recall test, a short-delayed cued recall test, a long-delayed (20-minute) free recall test, a long-delayed cued recall test, and a recognition trial. All CVLT data were coded at the item level.
The ISDA was applied to the CVLT data to derive indices of encoding, consolidation, and retrieval deficits. In a mixed sample of healthy and neurologically compromised participants the ISDA demonstrated several strengths in contrast to traditional memory process indices when applied to CVLT (see Wright et al., 2009). These strengths included an encoding deficit index that was relatively unaffected by attention deficits, separable consolidation and retrieval deficit indices, adequate internal consistency for the characterization of memory impairments, and greater predictive validity in terms of discriminating neurological compromise and impaired performance on the Logical Memory subtest of the WMS-III (Wechsler, 1997). The benefits of the ISDA are largely due to the use of item level patterns of recall. As discussed in greater detail below, item level analyses allow for greater specificity for characterizing participant performances as opposed the total scores used to calculate traditional memory process indices.
The ISDA encoding index reflects low acquisition across learning trials. Here, items recalled less then three times across the five learning trials [the lower half of possible recall outcomes (0, 1, 2) for a given item during list learning] were summed (maximum value = 16). The greater this value, the greater the encoding difficulties are presumed to be. The ISDA encoding deficit index is not particularly reliant on any given learning trial, so it is less likely to be affected by inattention in comparison to more traditional encoding/acquisition indices. Specifically, previous work showed that the ISDA encoding deficit index was not associated with impaired auditory attention (Digit Span), while total learning was related with attention (Wright et al., 2009).
As consolidation deficits are commonly defined by a loss of previously learned material, the ISDA consolidation index is calculated via summing the individual items that were recalled during list learning, but not recalled on any subsequent cued or free recall trial. Therefore, this index is more likely to reflect a loss of encoded information rather than retrieval failures. To control for possible acquisition differences between groups, the consolidation index was divided by the sum of the individual items recalled during list learning. Although retrieval difficulties can theoretically result in a persistent inability to recall a given piece of information, we reasoned that inconsistent recall was probably the most reliable way to assess retrieval deficits.
Within the framework of the ISDA, retrieval deficits are operationally defined as an inconsistent ability to access stored information. That said the ISDA retrieval index was calculated by summing the individual items that were recalled during list learning but inconsistently recalled across delayed recall trials. This value was divided by the sum of the individual items recalled during list learning to control for acquisition level. Since the consistency of individual list items is tracked across delayed recall trials, this index is more likely to reflect retrieval problems with less concern about contamination from consolidation difficulties.
We have previously shown that the ISDA consolidation deficit index is unrelated to the ISDA retrieval deficit index, in contrast to traditional indices of consolidation (forgetting/retention) and retrieval (recognition–recall discrepancies) that were moderately associated with each other (Wright et al., 2009). The ISDA makes use of item level performances across delayed recall trials to provided separable patterns of performance suggestive of consolidation and retrieval difficulties. With regard to the ISDA, each encoded item has three possible delayed recall patterns: 1) the item can be lost at all delayed recall trials (a consolidation problem); 2) variably recalled across delayed recall trials (a retrieval problem); 3) consistently recalled across delayed recall trials (intact recall). The item outcomes for the first two patterns are summed to provide the ISDA consolidation and retrieval deficit indices, respectively. Also, as mentioned above, these values can be divided by the sum of the individual items recalled during list learning to control for differing acquisition levels.
To investigate the role of clustering in possible encoding deficits, semantic and serial clustering scores were calculated for each of the learning trials of the CVLT. Serial clustering is an inferior learning strategy compared to semantic clustering (Delis et al., 1987; Forrester & King, 1971). Semantic and serial clustering indices were derived from the list-based equations presented in Stricker, Brown, Wixted, Baldo, and Delis (2002).
Finally, to investigate the role of the level of encoding in the development of consolidation difficulties, modified ISDA consolidation indices were calculated. Specifically, consolidation deficit indices were calculated for both high acquisition (items recalled 4 or 5 times during learning) and low acquisition (items recalled 1 or 2 times during learning) items.
Data Analysis
A significance level of α<0.05 was adopted, unless otherwise stated. Group differences in demographics and memory performances were evaluated with either t-tests or chi-square analyses. Acquisition corrections to the consolidation and retrieval indices resulted in proportion data. Proportions can artificially reduce variance (see Keppel & Wickens, 2004). For this reason, arcsine-transformed ISDA consolidation and retrieval indices were analyzed. These results did not differ from those obtained with the untransformed values. Therefore, results from untransformed data are presented. Following the determination of verbal memory impairment and specific memory process deficits, hierarchical regression was utilized to determine the impact of memory process deficits on delayed free recall performance of the CHI group. The predictors were entered into the model based on their temporal relationship with each other (i.e., 1. encoding, 2. consolidation, 3. retrieval). Mixed-model ANOVAs were conducted to determine between-group differences in serial and semantic clustering, as well as within-subject differences in clustering over learning trials. Significant within-subjects trial effects were followed up using paired samples t-tests, with Bonferroni corrected p-values. Subsequent to demonstrating declines in semantic clustering in the CHI group, multiple linear regression was used to determine if average semantic clustering and total change in semantic clustering during list learning (the sum trial-to-trial differences in clustering) predicted encoding deficits in the CHI group. Finally, a mixed-model ANOVA was used to determine if the level of item acquisition (high vs. low) affected consolidation difficulties exhibited by the CHI and control participants.
RESULTS
Sample Characteristics
As can be seen in Table 1, the CHI and controls groups were well matched in age, education, estimated premorbid IQ, and sex ratio. The control group contained a larger proportion of minority participants than the CHI group. However, the controls outperformed the CHI group on the CVLT (see below). Hours of posttraumatic amnesia (PTA), years of age, and years post injury were not significantly correlated with CVLT Trials 1–5 Recall (r=−.19, −.21, −.05, respectively; ps> .13), Short-Delay Free Recall (r=−.18, −.12, .08, respectively; ps> .23), nor Long-Delay Free Recall (r=−.23, −.14, .05, respectively; ps> .14) in our CHI group, consistent with the work of Chu et al. (2007) who showed that PTA and age at injury no longer predicted total learning performances on the Rey Auditory Verbal Learning Test beyond one year post injury via mixed-effects modeling in a longitudinal study including a sample of 794 traumatic brain injury survivors.
Memory Performances
The CHI group performed significantly worse than the controls across all of the major indices of the CVLT (see Table 1), with effect sizes ranging from medium to large. Additionally, the CHI group exhibited greater deficits on all of the ISDA indices than the controls, with the group difference on the encoding deficit index representing a large effect and the group differences on the consolidation and retrieval deficit indices indicating medium effects. It should be noted that acquisition-corrected values were used for the consolidation and retrieval deficit indices to control for CHI-related encoding difficulties.
Impact of Memory Process Deficits
Acquisition-corrected values were not used in the regression analyses, since no group comparisons were made. The raw ISDA indices were not strongly associated (rs<.44). The predictors were ordered in the regression by their natural temporal relationship (i.e., 1. encoding, 2. consolidation, 3. retrieval). The encoding index significantly predicted long-delayed free recall (Model 1), FΔ(1, 54)=93.42, accounting for 63% of the variance (see Table 2). The consolidation index added significant predictive power to the model (Model 2), FΔ(1, 53)=32.39, accounting for an additional 14% of the variance. The retrieval index added marginal predictive power to the model (see Model 3), FΔ(1, 52)=5.19, accounting for an additional 2% of the variance. Overall, the ISDA encoding and consolidation indices (Model 2) were the most notable predictors of delayed recall in the CHI group.
Table 2.
Summary of Hierarchical Regression to Determine the Impact of CHI Associated Encoding, Consolidation, and Retrieval Deficits on Long-Delay Free Recall
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Variable | B | SE B | B | SE B | B | SE B |
| Encoding Deficit Index | −.92 | .10 | −.71 | .08 | −.65 | .08 |
| Consolidation Deficit Index | −.73 | .13 | −.79 | .13 | ||
| Retrieval Deficit Index | −.19 | .08 | ||||
| R2 | .63 | .77 | .79 | |||
| F for change in R2 | 93.42** | 32.39** | 5.19* | |||
Note. Raw values were used for regression analysis; the consolidation and retrieval difficulties indices were not corrected for acquisition. CHI=Closed-Head Injury. ISDA=Item Specific Deficit Approach.
p<.05
p<.001
Role of Clustering in Encoding Deficits
To determine any between- or within-group differences in serial clustering over learning trials, a 2 (group) × 5 (learning trial) ANOVA, with repeated measures on the last factor, was conducted. No effect of group, trial, or group × trial interaction was detected, Fs≤ 2.34. These findings indicate that CHI participants (trial 1, M=.33, SD=.77; trial 2, M=.34, SD=.97; trial 3, M=.41, SD=.98; trial 4, M=.48, SD=1.22; trial 5, M=.80, SD=1.40) and controls (trial 1, M=.14, SD=.74; trial 2, M=.26, SD=.96; trial 3, M=.27, SD=1.22; trial 4, M=.31, SD=1.31; trial 5, M=.33, SD=1.35) demonstrated similar patterns of serial clustering over the learning trials.
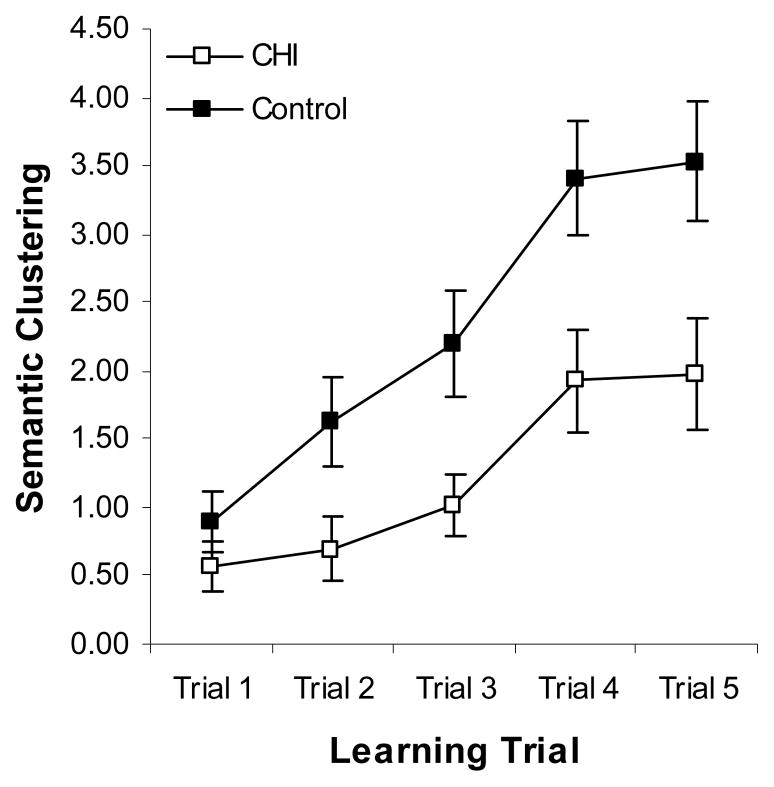
The same analysis was completed for semantic clustering and this revealed a significant effect of group, F(1,116)=7.29, MSe=24.05, ηp2=.06; trial, F(4,116)=39.09, MSe=2.43, ηp2=.25; and a significant group × trial interaction, F(4,116)=2.99, MSe=2.43, ηp2=.03. Follow-up paired samples t-tests (Bonferroni correction: α < .0125) revealed that the controls showed significantly improved semantic clustering between trials 1 and 2, (trial 1, M=.90, SD=1.71; trial 2, M=1.63, SD=2.60), t(1,61)=−3.02, d=.33; trials 2 and 3, (trial 3, M=2.20, SD=2.96), t(1,61)=−2.64, d=.20; and 3 and 4, (trial 4, M=3.41, SD=3.31), t(1,61)=−4.31, d=.39, but their clustering remained stable between trials 4 and 5 (trial 5, M=3.53, SD=3.44), t(1,61)=−0.46, d=.04. Conversely, the CHI group showed a significant improvement between trials 3 and 4, (trial 3, M=1.02, SD=1.70; trial 4, M=1.93, SD=2.85), t(1,55)=−2.98, d=.39, but not between any other contiguous trials, ts≤−1.69, ds≤.18 (trial 1, M=.57, SD=1.31; trial 2, M=.70, SD=1.79; trial 5, M=1.98, SD=3.09). These data indicate that the CHI participants, in comparison to controls, suffered from impairments in the ability to initiate and utilize semantic clustering strategies during list learning (see Figure 1).
Figure 1.
Mean semantic clustering (± SE) by learning trial for the CHI and control groups.
Finally, multiple linear regression was used to determine if 1) average semantic clustering across trials, 2) changes in semantic clustering over trials, or 3) the interaction between the two variables predicted encoding deficits in the CHI group. The model was significant, R2 =.48, F(3,52)=15.73, although only average semantic clustering emerged as a significant predictor, B=−1.19, t=−3.27; change in clustering, B=.02, t=−.09, and average clustering × change in clustering, B=.01, t=−.09, were not significant predictors. It should be noted that average semantic clustering and change in semantic clustering were not intercorrelated (r=0.03). Overall, these results suggest that reduced semantic clustering plays a large role in encoding deficits for verbal material in individuals with severe CHI.
Impact of Encoding on Consolidation
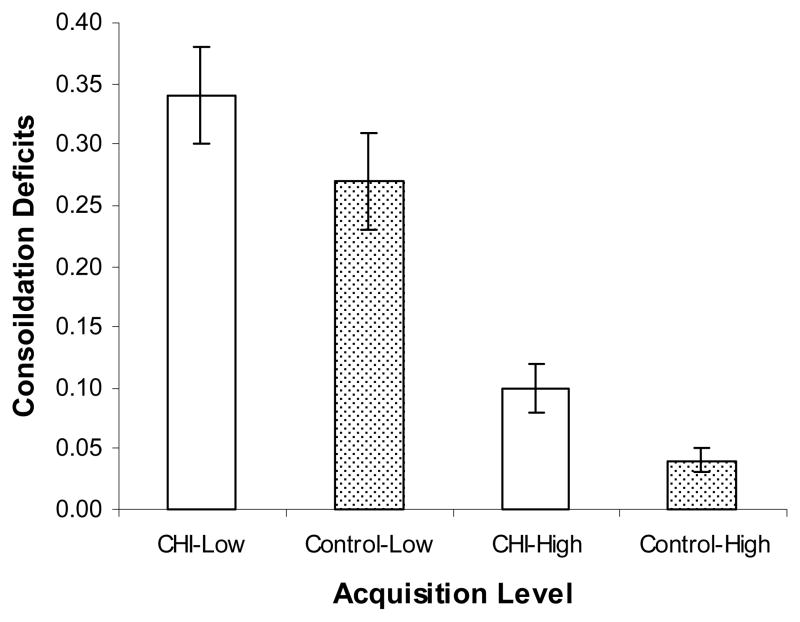
To determine if greater encoding benefited consolidation of list items, ISDA consolidation deficit indices were calculated for both high acquisition (items recalled 4 or 5 times during learning) and low acquisition (items recalled 1 or 2 times during learning); these values reflect the median split of measurable list acquisition, as the maximum recall value for a given item on the CVLT is 5. A 2 (group) × 2 (acquisition level) ANOVA, with repeated measures on the last factor, revealed significant effects of group, F(1,116)=4.43, MSe=0.06, ηp2=.04, and acquisition level, F(1,116)=80.03, MSe=.04, ηp2=.41, but no group × acquisition level interaction, F<1. These results indicated group differences in consolidation across acquisition levels and greater consolidation deficits for items acquired at a low level (CHI: M=0.34, SD=0.30, control: M=0.27, SD=0.29) as opposed to a high level (CHI: M=0.10, SD=0.15, control: M=0.04, SD=0.07) (see Figure 2). Overall, these findings suggest that level of acquisition plays a role in consolidation difficulties, but like the hierarchical regression results presented above, that CHI survivors exhibit consolidation difficulties above and beyond their encoding deficits.
Figure 2.
Mean ISDA consolidation deficit index values (± SE) for high (items recalled 4 or 5 times during learning) and low (items recalled 1 or 2 times during learning) acquisition levels for the CHI and control groups.
DISCUSSION
In the current study, the ISDA was applied to the CVLT to investigate verbal memory dysfunction in a group of severe, acceleration-deceleration CHI participants. The ISDA is an item level technique designed to quantify encoding, consolidation, and retrieval deficits, with demonstrated reliability and validity (Wright et al., 2009). As expected, the CHI participants exhibited gross verbal memory impairments on the CVLT in comparison to controls. On the ISDA indices, the CHI group showed significant deficits in encoding (large effect), consolidation (medium effect), and retrieval (medium effect). Deficits in encoding and, to a lesser extent, consolidation, accounted for the majority of the variance (77%) in CHI participants’ long-delayed free recall; retrieval deficits played a marginal role, accounting for only 2% of the variance. Analysis of item clustering over learning trials indicated that encoding deficits in CHI participants were largely accounted for by reduced initiation and use of semantic clustering. Finally, when accounting for the level of item acquisition, we found that encoding deficits played a partial, but not complete, role in item consolidation. Taken together, these data suggest that encoding deficits, which are driven by reductions in strategic processing, account for the greater part of CHI-related verbal memory impairment, while consolidation deficits play a smaller, but significant role.
In contrast to many studies utilizing traditional memory process indices derived from standard list learning tests (e.g., forgetting rate), our findings are consistent with studies employing quasi-experimental manipulations (DeLuca et al., 2000; Levin & Goldstein, 1986) and list learning characteristics (Blachstein et al., 1993; Stallings et al., 1995) in suggesting that moderate to severe CHI leads to encoding deficits for verbal material. We also found that consolidation deficits played a small, but significant role in CHI-associated memory impairment, unlike DeLuca et al. (2000). Two important factors most likely account for the differences between DeLuca et al.’s findings and our results. First, DeLuca et al. equated CHI and control participants on list acquisition, such that each list item was recalled twice. Second, they employed a modified version of the SRT, in which all of the items were semantically related. Although CHI participants have difficulty spontaneously clustering information, they can benefit from semantic organization inherent in to-be-learned word lists where semantic associates are in contiguous positions (Levin & Goldstein, 1986). Acquisition control, in conjunction with semantic support, likely provided for the reduction of consolidation deficits in DeLuca et al.’s study. Although we retrospectively matched the control and CHI groups on acquisition, we did not provide additional memory support (e.g., category blocking), which likely contributed to the consolidation deficits observed in our CHI sample.
Our sample consisted of severe CHI participants who suffered from acceleration-deceleration injuries. The ventral frontal lobe and ventral anterior medial temporal lobe areas have a higher probability of being damaged following CHI (Adams et al., 1989; Wilson et al., 1995), although lesions to these areas do not uniformly occur. DAI, which occurs in the majority of acceleration-deceleration CHI cases (Adams et al., 1989; Adams & Jennett, 2001; Abou-Hamden et al., 1997; Bigler, 2001; Takaoka et al., 2002; Williamson et al., 1996), can frequently be observed in the corpus callosum, the cerebral lobar white matter, and the white matter of the dorsolateral quadrant of the midbrain (a.k.a., shearing injury triad; Takaoka et al., 2002), and is much more evident in diffusion tensor imaging (Kraus et al., 2007; Wang et al., 2008). Furthermore, the severity of DAI predicts coma duration, arousal level/fatigability, and neurocognitive and functional outcome (Adams et al., 1989; Adams & Jennett, 2001; Auerbach, 1986; Bigler, 2001; Kraus et al., 2007; Wang et al., 2008; Williamson et al., 1996). It has been suggested that DAI is responsible for memory deficits following traumatic brain injury (Levine et al., 2002; Timmerman & Brouwer, 1999). Interestingly, Fork et al. (2005) showed that DAI resulting from CHI, in contrast to other patterns of CHI neuropathology (e.g., focal lesions), was associated with impairments in learning and delayed recall. Convergent evidence suggesting a relationship between diffuse white matter pathology and memory impairment has also been presented. Interestingly, like CHI survivors, individuals with HIV-1 infection and those with multiple sclerosis, two populations suffering from diffuse white matter disruption (Medana & Esiri, 2003), also exhibit deficits in encoding verbal material (Arnett et al., 1997; Gongvatana et al., 2007; Scott et al., 2006; for an excellent review see Rao, 1996). The possibility that diffuse white matter pathology may underlie certain encoding deficits suggests that novel approaches to neurocognitive rehabilitation may hold great promise for CHI survivors, and other neurologic populations, as recent data suggests that axonal regrowth may affect neuropsychological recovery from severe CHI (Voss et al., 2006).
One limitation of this work is that these results may not be generalizable to other types of traumatic brain injury (e.g., focal insult). Additionally, we only evaluated verbal episodic memory, and results may differ for other types of material (e.g., nonverbal memory). Finally, our analysis was limited to data collected in standard fashion via the CVLT. Other modes of item presentation (e.g., category blocking, multimodal input, criterion driven learning trials) may produce varied patterns of recall performances in CHI participants. In summary, our data suggest that verbal memory impairment in severe CHI due to acceleration-deceleration mechanisms primarily results from encoding deficits related to reduced strategic processing, while consolidation deficits play a secondary role. As such, our data are generally consistent with findings from quasi-experimental studies of verbal memory in CHI.
Acknowledgments
Special thanks to Anna Curren for her assistance in data collection and coding. This research was supported in part by a Marchionne Memorial Doctoral Fellowship awarded by Washington State University, an American Psychological Association Dissertation Research Award, a Van Vleet Memorial Doctoral Fellowship, a Vidulich Research Fellowship awarded by The University of Memphis, NICHD grant HD35838 from, NINDS grant NS47690, and NIMH grant MH19535.
References
- Abou-Hamde A, Blumbergs PC, Scott G, Manavis J, Wainwright H, Jones N, et al. Axonal injury in falls. Journal of Neurotrauma. 1997;14:699–713. doi: 10.1089/neu.1997.14.699. [DOI] [PubMed] [Google Scholar]
- Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis, and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Adams JH, Jennett DIG. The structural basis of moderate disability after traumatic brain injury. Journal of Neurology, Neurosurgery & Psychiatry. 2001;71:521–524. doi: 10.1136/jnnp.71.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton AP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends in Cognitive Sciences. 2006;10:455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Arnett PA, Rao SM, Grafman J, Bernardin L, Luchetta T, Binder JR, et al. Executive functions in multiple sclerosis: An analysis of temporal ordering, semantic encoding, and planning abilities. Neuropsychology. 1997;11:535–544. doi: 10.1037//0894-4105.11.4.535. [DOI] [PubMed] [Google Scholar]
- Auerbach SH. Neuroanatomical correlates of attention and memory in traumatic brain injury: an application of neurobehavioral subtypes. The Journal of Head Trauma Rehabilitation. 1986;1:1–12. [Google Scholar]
- Barona A, Reynolds CR, Chastain R. A demographically based index of pre-morbid intelligence for the WAIS-R. Journal of Consulting and Clinical Psychology. 1984;52:885–887. [Google Scholar]
- Basden BH, Basden DR. Directed forgetting: Further comparisons of the item and list methods. Memory. 1996;4:633–653. doi: 10.1080/741941000. [DOI] [PubMed] [Google Scholar]
- Basden BH, Basden DR, Gargano GJ. Directed forgetting in implicit and explicit memory tests: A comparison of methods. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19:603–616. [Google Scholar]
- Basden BH, Basden DR, Wright MJ. Part-list re-exposure and release of retrieval inhibition. Consciousness and Cognition. 2003;12:354–375. doi: 10.1016/s1053-8100(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Bigler ED. Quantitative magnetic resonance imaging in traumatic brain injury. The Journal of Head Trauma Rehabilitation. 2001;16:117–134. doi: 10.1097/00001199-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Bernard F, Desgranges B, Platel H, Baron J, Eustache F. Contributions of frontal and medial temporal regions to verbal episodic memory: A PET study. NeuroReport. 2001;12:1737–1741. doi: 10.1097/00001756-200106130-00044. [DOI] [PubMed] [Google Scholar]
- Blachstein H, Vakil E, Hoofien D. Impaired learning in patients with closed-head injuries: An analysis of components of the acquisition process. Neuropsychology. 1993;7:530–535. [Google Scholar]
- Buschke H. Selective reminding for the analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior. 1973;12:543–550. [Google Scholar]
- Brooks N, Campsie L, Symington C, Beattie A, McKinlay W. The effects of severe head injury on patient and relative within seven years of injury. The Journal of Head Trauma Rehabilitation. 1987;2:1–13. [Google Scholar]
- Chu B, Millis S, Arango-Lasprilla JC, Hanks R, Novack T, Hart T. Measuring recovery in new learning and memory following traumatic brain injury: A mixed-effects modeling approach. Journal of Clinical and Experimental Neuropsychology. 2007;29:617–625. doi: 10.1080/13803390600878893. [DOI] [PubMed] [Google Scholar]
- Curtiss G, Vanderploeg RD, Spencer J, Salazar AM. Patterns of verbal learning and memory in traumatic brain injury. Journal of the International Neuropsychological Society. 2001;7:574–585. doi: 10.1017/s1355617701755051. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober E. California Verbal Learning Test. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- DeLuca J, Schultheis MT, Madigan NK, Christodoulou C, Averill A. Acquisition vs. retrieval deficits in traumatic brain injury: Implications for memory rehabilitation. Archives of Physical Medicine and Rehabilitation. 2000;81:1327–1333. doi: 10.1053/apmr.2000.9390. [DOI] [PubMed] [Google Scholar]
- Duchnick JJ, Vanderploeg RD, Curtiss G. Identifying retrieval problems using the California Verbal Learning Test. Journal of Clinical and Experimental Neuropsychology. 2002;24:840–851. doi: 10.1076/jcen.24.6.840.8405. [DOI] [PubMed] [Google Scholar]
- Ellis HC, Hunt RR. Fundamentals of Human Memory and Cognition. 3. 1983. pp. 84–130. [Google Scholar]
- Esselman PC, Uomoto JM. Classification of the spectrum of mild traumatic brain injury. Brain Injury. 1995;9:417–424. doi: 10.3109/02699059509005782. [DOI] [PubMed] [Google Scholar]
- Fork M, Bartels C, Ebert AD, Grubich C, Synowitz H, Wallesch C. Neuropsychological sequelae of diffuse traumatic brain injury. Brain Injury. 2005;19:101–108. doi: 10.1080/02699050410001726086. [DOI] [PubMed] [Google Scholar]
- Forrester WE, King DJ. Effects of semantic and acoustic relatedness on free recall and clustering. Journal of Experimental Psychology. 1971;88:16–19. [Google Scholar]
- Gongvatana A, Woods SP, Taylor MJ, Vigil O, Grant I the HNRC Group. Semantic clustering inefficiency in HIV-associated dementia. The Journal of Neuropsychiatry and Clinical Neurosciences. 2007;19:36–42. doi: 10.1176/jnp.2007.19.1.36. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Stephan E, Schatzberg AF, Reiss AL, et al. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and Analysis: A Researcher’s Handbook. 4. Upper Saddle River, NJ: Pearson Prentice Hall; 2004. pp. 153–155. [Google Scholar]
- King N. Mild head injury: Neuropathology, sequelae, measurement and recovery. British Journal of Clinical Psychology. 1997;36:161–184. doi: 10.1111/j.2044-8260.1997.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Levin H, Goldstein FC. Organization of verbal memory after severe closed-head injury. Journal of Clinical and Experimental Neuropsychology. 1986;8:643–656. doi: 10.1080/01688638608405185. [DOI] [PubMed] [Google Scholar]
- Levine B, Cabeza R, McIntosh AR, Black SE, Grady CL, Stuss DT. Functional reorganization of memory after traumatic brain injury: A study with H2 15O positron emission topography. Journal of Neurology, Neurosurgery & Psychiatry. 2002;73:173–181. doi: 10.1136/jnnp.73.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateer CA, Sohlberg MM, Crinean J. Focus on clinical research: Perceptions of memory function in individuals with closed-head injury. The Journal of Head Trauma Rehabilitation. 1987;2:74–84. [Google Scholar]
- Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in the human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. Retrieval failure versus memory loss in experimental amnesia: Definitions and processes. Learning & Memory. 2006;13:491–497. doi: 10.1101/lm.241006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis SR, Putnam SH, Adams KM, Ricker JH. The California Verbal Learning Test in the detection of incomplete effort in neuropsychological evaluation. Psychological Assessment. 1995;7:463–471. [Google Scholar]
- Nader K. Unraveling the impenetrable nature of amnesia: A new beginning. Learning & Memory. 2006;13:489–490. [Google Scholar]
- Nakayama N, Okumura A, Shinoda J, Nakashima T, Iwama T. Relationship between regional cerebral metabolism and consciousness disturbance in traumatic diffuse brain injury without large focal lesions: An FDG-PET study with statistical parametric mapping analysis. Journal of Neurology, Neurosurgery & Psychiatry. 2006;77:856–862. doi: 10.1136/jnnp.2005.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT. Electrophysiological dissociation of rapid memory mechanisms in humans. NeuroReport. 1994;5:1517–1521. doi: 10.1097/00001756-199407000-00027. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Cabeza R, Tulving E. PET studies of encoding and retrieval: The HERA model. Psychonomic Bulletin & Review. 1996;3:135–148. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Forkstam C, Petersson KM, Cabeza R, Ingvar M. Brain imaging of human memory systems: Between-systems similarities and within-system differences. Cognitive Brain Research. 2002;13:281–292. doi: 10.1016/s0926-6410(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Rao SM. White matter disease and dementia. Brain & Cognition. 1996;31:250–268. doi: 10.1006/brcg.1996.0044. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Marks W, Wright MJ, Ventura M. Retrieval inhibition in directed forgetting following severe closed-head injury. Neuropsychology. 2004;18:104–114. doi: 10.1037/0894-4105.18.1.104. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Patterson KA, Morgan EE, Heaton RK, Grant I, et al. Recency effects in HIV-associted dementia are characterized by deficient encoding. Neuropsychologia. 2006;44:1336–1343. doi: 10.1016/j.neuropsychologia.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Squire LR. Lost forever or temporarily misplaced? The long debate about the nature of memory impairment. Learning & Memory. 2006;13:522–529. doi: 10.1101/lm.310306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. Journal of Neuroscience. 2002;15:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker JL, Brown GG, Wixted J, Baldo JV, Delis DC. New semantic clustering indices for the California Verbal Learning Test-second edition: Background, rational, and formulae. Journal of the International Neuropsychological Society. 2002;8:425–435. doi: 10.1017/s1355617702813224. [DOI] [PubMed] [Google Scholar]
- Stallings G, Boake C, Sherer M. Comparison of the California Verbal Learning Test and the Rey Auditory Verbal Learning Test in head-injured patients. Journal of Clinical and Experimental Neuropsychology. 1995;17:706–712. doi: 10.1080/01688639508405160. [DOI] [PubMed] [Google Scholar]
- Takaoka M, Tabuse H, Kumura E, Nakajima S, Tsuzuki T, Nakamura K, et al. Semiquantitative analysis of corpus callosum injury using magnetic resonance imaging indicates clinical severity in patients with diffuse axonal injury. Journal of Neurology, Neurosurgery & Psychiatry. 2002;73:289–293. doi: 10.1136/jnnp.73.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman ME, Brouwer WH. Slow information processing after very severe closed head injury: impaired access to declarative knowledge and intact application and acquisition of procedural knowledge. Neuropsychologia. 1999;37:467–478. doi: 10.1016/s0028-3932(98)00085-2. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ. Episodic and declarative memory: Role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Tulving E, Osler S. Effectiveness of retrieval cues in memory for words. Journal of Experimental Psychology. 1968;77:593–601. doi: 10.1037/h0026069. [DOI] [PubMed] [Google Scholar]
- Tulving E, Thompson DM. Encoding specificity and retrieval processes in episodic memory. Psychological Review. 1973;80:352–373. [Google Scholar]
- Vanderploeg RD, Crowell TA, Curtiss G. Verbal learning and memory deficits in traumatic brain injury: Encoding, consolidation, and retrieval. Journal of Clinical and Experimental Neuropsychology. 2001;23:185–195. doi: 10.1076/jcen.23.2.185.1210. [DOI] [PubMed] [Google Scholar]
- Voss HU, Uluç AM, Dyke JP, Watts R, Kobylarz EJ, McCandliss BD, et al. Possible axonal regrowth in late recovery from the minimally conscious state. Journal of Clinical Investigations. 2005;116:2005–2011. doi: 10.1172/JCI27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallesch C, Curio N, Kutz S, Jost S, Bartels C, Synowitz H. Outcome after mild-to-moderate blunt head injury: Effects of focal lesions and diffuse axonal injury. Brain Injury. 2001;15:401–41. doi: 10.1080/02699050010005959. [DOI] [PubMed] [Google Scholar]
- Wang JY, Bakhadirov K, Devous MD, Sr, Abdi H, McColl R, Moore C, et al. Diffusion tensor tractography of traumatic diffuse axonal injury. Archives of Neurology. 2008;65:619–626. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wiegner S, Donders J. Performance on the California Verbal Learning Test after traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 1999;21:159–170. doi: 10.1076/jcen.21.2.159.925. [DOI] [PubMed] [Google Scholar]
- Wilde MC, Boake C, Sherer M. Do recognition-free recall discrepancies detect retrieval deficits in closed-head injury? An exploratory study with the California Verbal Learning Test. Journal of Clinical and Experimental Neuropsychology. 1995;17:849–855. doi: 10.1080/01688639508402434. [DOI] [PubMed] [Google Scholar]
- Williamson DJG, Scott JG, Adams RL. Traumatic brain injury. In: Adams RL, Parsons OA, Culbertson JL, Nixon SJ, editors. Neuropsychology for Clinical Practice: Etiology, Assessment, & Treatment of Common Neurological Disorders. Washington, DC: American Psychological Association; 1996. pp. 9–64. [Google Scholar]
- Wilson JTL, Hadley DM, Wiedmann KD, Teasdale GM. Neuropsychological consequences of two patterns of brain damage shown by MRI in survivors of severe head injury. Journal of Neurology, Neurosurgery & Psychiatry. 1995;59:328–331. doi: 10.1136/jnnp.59.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Woo E, Schmitter-Edgecombe M, Hinkin CH, Miller EN, Gooding AL. The Item-Specific Deficit Approach (ISDA) to evaluating verbal memory dysfunction: Rationale, psychometrics, and application. Journal of Clinical and Experimental Neuropsychology. 2009;31:790–802. doi: 10.1080/13803390802508918. [DOI] [PMC free article] [PubMed] [Google Scholar]